Sustainable Epoxy Composites with UV Resistance Based on New Kraft Lignin Coatings
Abstract
1. Introduction
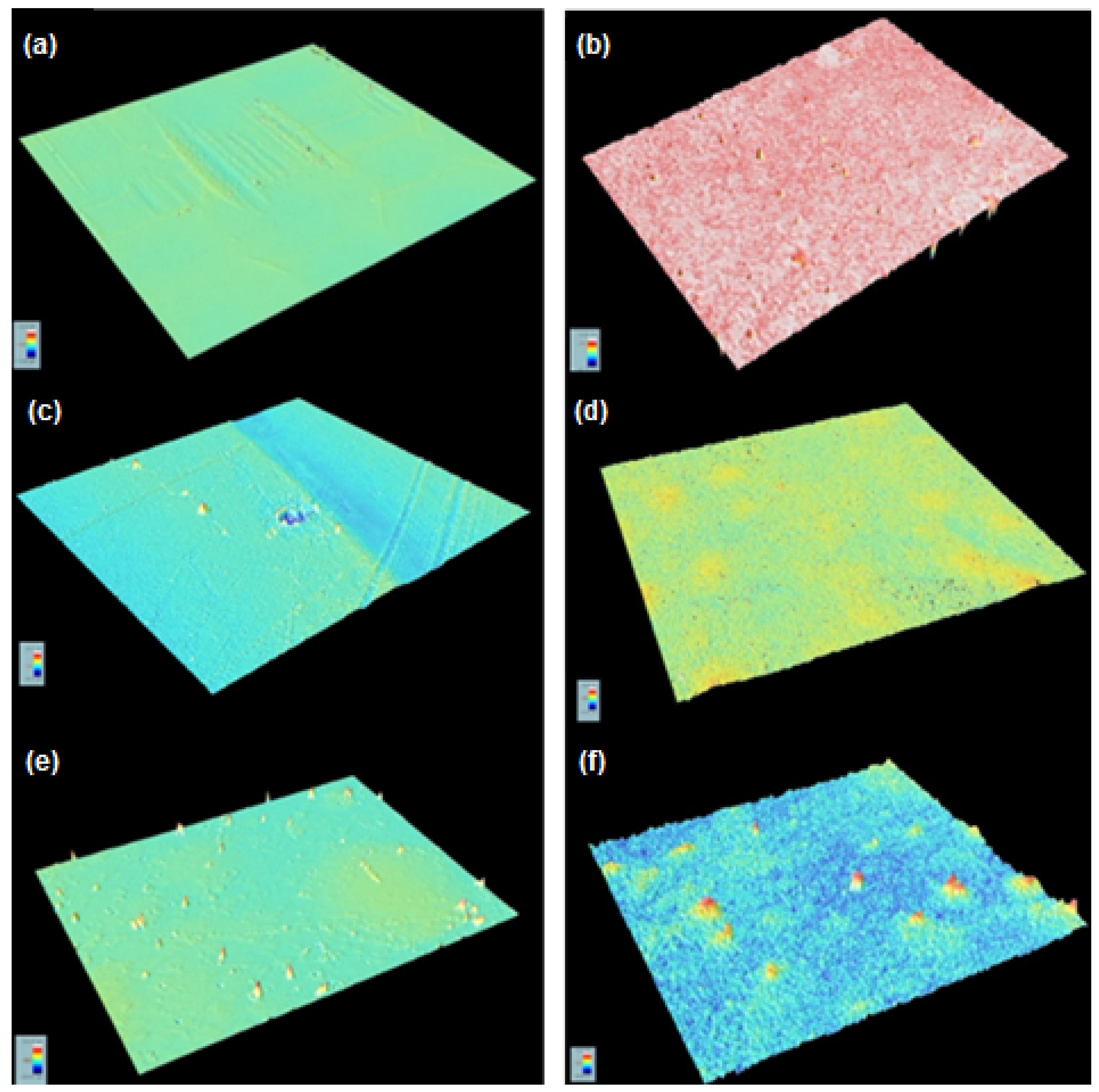
2. Results
3. Materials and Methods
3.1. Epoxy Resin System
3.2. Kraft Lignin
3.3. Kraft Lignin Characterization
3.4. Biobased Resin System and Gelcoat Formulation Characterization
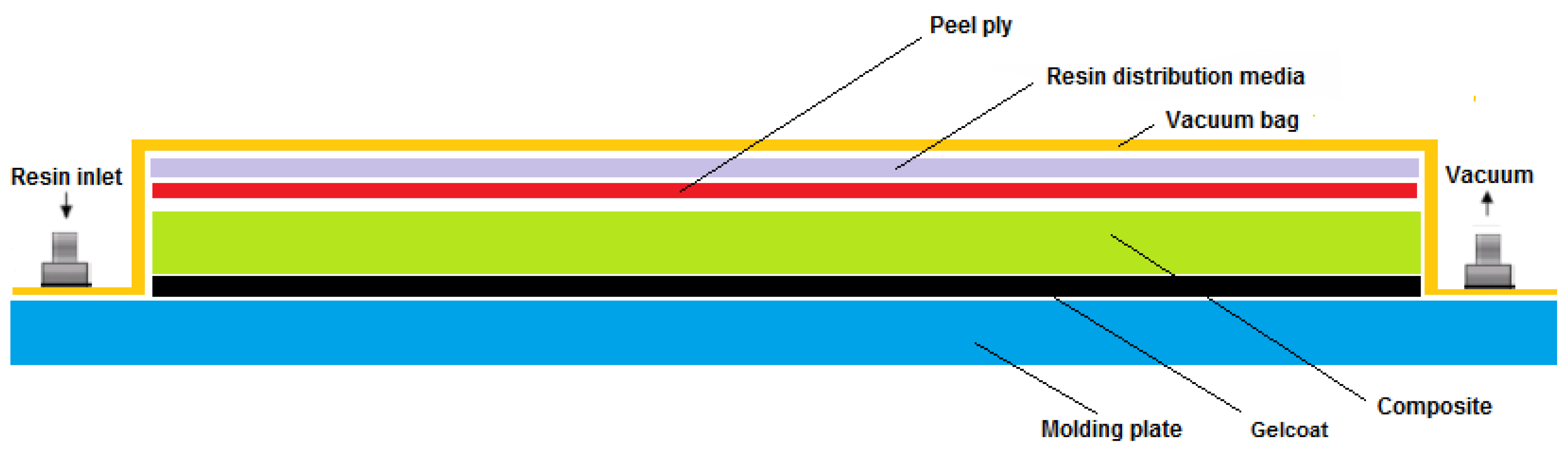
3.5. Composite Development and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matta, A.; Yadavalli, V.R.; Manas, L.; Kadleckova, M.; Pavlinek, V.; Sedlacek, T. Surface Treatments’ Influence on the Interfacial Bonding between Glass Fibre Reinforced Elium® Composite and Polybutylene Terephthalate. Materials 2024, 17, 3276. [Google Scholar] [CrossRef] [PubMed]
- Capretti, M.; Del Bianco, G.; Giammaria, V.; Boria, S. Natural Fibre and Hybrid Composite Thin-Walled Structures for Automotive Crashworthiness: A Review. Materials 2024, 17, 2246. [Google Scholar] [CrossRef] [PubMed]
- Bulińska, S.; Sujak, A.; Pyzalski, M. From Waste to Renewables: Challenges and Opportunities in Recycling Glass Fibre Composite Products from Wind Turbine Blades for Sustainable Cement Production. Sustainability 2024, 16, 5150. [Google Scholar] [CrossRef]
- Broniewicz, M.; Halicka, A.; Buda-Ożóg, L.; Broniewicz, F.; Nykiel, D.; Jabłoński, Ł. The Use of Wind Turbine Blades to Build Road Noise Barriers as an Example of a Circular Economy Model. Materials 2024, 17, 2048. [Google Scholar] [CrossRef] [PubMed]
- Diniță, A.; Ripeanu, R.G.; Ilincă, C.N.; Cursaru, D.; Matei, D.; Naim, R.I.; Tănase, M.; Portoacă, A.I. Advancements in Fiber-Reinforced Polymer Composites: A Comprehensive Analysis. Polymers 2024, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Capretti, M.; Giammaria, V.; Santulli, C.; Boria, S.; Del Bianco, G. Use of Bio-Epoxies and Their Effect on the Performance of Polymer Composites: A Critical Review. Polymers 2023, 15, 4733. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Aguilar, K.; Sánchez-Safont, E.; Rodríguez, A.; Marín, A.; Candal, M.V.; Cabedo, L.; Gamez-Perez, J. Valorization of Agricultural Waste Lignocellulosic Fibers for Poly(3-Hydroxybutyrate-Co-Valerate)-Based Composites in Short Shelf-Life Applications. Polymers 2023, 15, 4507. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, P.; Ranjan, R.; DeSouza, V.; Bhat, R.; Patil, S.; Maddodi, B.; Shivamurthy, B.; Perez, T.C.; Naik, N. Enhanced Wear Resistance in Carbon Nanotube-Filled Bio-Epoxy Composites: A Comprehensive Analysis via Scanning Electron Microscopy and Atomic Force Microscopy. J. Compos. Sci. 2023, 7, 478. [Google Scholar] [CrossRef]
- Atmakuri, A.; Palevicius, A.; Vilkauskas, A.; Janusas, G. Review of hybrid fiber based composites with nano particles—Material properties and applications. Polymers 2020, 12, 2088. [Google Scholar] [CrossRef]
- Oladele, I.O.; Omotosho, T.F.; Adediran, A.A. Polymer-based composites: An indispensable material for present and future applications. Int. J. Polym. Sci. 2020, 1, 8834518. [Google Scholar] [CrossRef]
- Peerzada, M.; Abbasi, S.; Lau, K.T.; Hameed, N. Additive manufacturing of epoxy resins: Materials, methods, and latest trends. Ind. Eng. Chem. Res. 2020, 59, 6375–6390. [Google Scholar] [CrossRef]
- Pappa, C.; Feghali, E.; Vanbroekhoven, K.; Triantafyllidis, K.S. Recent advances in epoxy resins and composites derived from lignin and related bio-oils. Curr. Opin. Green Sustain. Chem. 2022, 38, 100687. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Santos, M.; Cernadas, T.; Ferreira, P.; Alves, P. Advances in the development of biobased epoxy resins: Insight into more sustainable materials and future applications. Int. Mater. Rev. 2022, 67, 119–149. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, A.; Mulla, S.I.; Pant, D.; Sharma, T.; Kumar, A. Lignin as potent industrial biopolymer: An introduction. In Lignin: Biosynthesis and Transformation for Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–15. [Google Scholar]
- Agustiany, E.A.; Rasyidur Ridho, M.; Rahmi DN, M.; Madyaratri, E.W.; Falah, F.; Lubis, M.A.R.; Solihat, N.N.; Syamani, F.A.; Karungamye, P.; Sohail, A.; et al. Recent developments in lignin modification and its application in lignin-based green composites: A review. Polym. Compos. 2022, 43, 4848–4865. [Google Scholar] [CrossRef]
- Li, C.; Wang, B.; Zhou, L.; Hou, X.; Su, S. Effects of lignin-based flame retardants on flame-retardancy and insulation performances of epoxy resin composites. Iran. Polym. J. 2022, 31, 949–962. [Google Scholar] [CrossRef]
- Gouveia, J.R.; Garcia, G.E.; Antonino, L.D.; Tavares, L.B.; Dos Santos, D.J. Epoxidation of kraft lignin as a tool for improving the mechanical properties of epoxy adhesive. Molecules 2020, 25, 2513. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Xiao, L.P.; Li, X.Y.; Xiao, W.Z.; Yang, Y.Q.; Sun, R.C. Renewable and flexible thermosetting epoxies based on functionalized biorefinery lignin fractions. Mater. Today Sustain. 2021, 15, 100083. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Synthesis of renewable thermoset polymers through successive lignin modification using lignin-derived phenols. ACS Sustain. Chem. Eng. 2017, 5, 5059–5066. [Google Scholar] [CrossRef]
- Liu, G.; Jin, C.; Huo, S.; Kong, Z.; Chu, F. Preparation and properties of novel bio-based epoxy resin thermosets from lignin oligomers and cardanol. Int. J. Biol. Macromol. 2021, 193, 1400–1408. [Google Scholar] [CrossRef]
- Nicastro, K.H.; Kloxin, C.J.; Epps, T.H., III. Potential lignin-derived alternatives to bisphenol A in diamine-hardened epoxy resins. ACS Sustain. Chem. Eng. 2018, 6, 14812–14819. [Google Scholar] [CrossRef]
- Zhen, X.; Li, H.; Xu, Z.; Wang, Q.; Zhu, S.; Wang, Z.; Yuan, Z. Facile synthesis of lignin-based epoxy resins with excellent thermal-mechanical performance. Int. J. Biol. Macromol. 2021, 182, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, X.; Chen, P.; Sun, G.; Li, Y.; Han, Y.; Wang, X.; Li, J. High-performance adhesives modified by demethylated lignin for use in extreme environments. New J. Chem. 2023, 47, 6721–6729. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Q.; Lu, M.; Meng, H.; Qu, Z.; Xu, C.A.; Jiao, E. Synthesis of a novel lignin-based epoxy resin curing agent and study of cure kinetics, thermal, and mechanical properties. J. Appl. Polym. Sci. 2021, 138, 50523. [Google Scholar] [CrossRef]
- Over, L.C.; Grau, E.; Grelier, S.; Meier, M.A.; Cramail, H. Synthesis and characterization of epoxy thermosetting polymers from glycidylated organosolv lignin and bisphenol A. Macromol. Chem. Phys. 2017, 218, 1600411. [Google Scholar] [CrossRef]
- Pan, H.; Sun, G.; Zhao, T.; Wang, G. Thermal properties of epoxy resins crosslinked by an aminated lignin. Polym. Eng. Sci. 2015, 55, 924–932. [Google Scholar] [CrossRef]
- Xue, B.; Tang, R.; Xue, D.; Guan, Y.; Sun, Y.; Zhao, W.; Tan, J.; Li, X. Sustainable alternative for bisphenol A epoxy resin high-performance and recyclable lignin-based epoxy vitrimers. Ind. Crops Prod. 2021, 168, 113583. [Google Scholar] [CrossRef]
- Zhen, X.; Li, H.; Xu, Z.; Wang, Q.; Xu, J.; Zhu, S.; Yuan, Z. Demethylation, phenolation, and depolymerization of lignin for the synthesis of lignin-based epoxy resin via a one-pot strategy. Ind. Crops Prod. 2021, 173, 114135. [Google Scholar] [CrossRef]
- Wang, X.; Leng, W.; Nayanathara, R.O.; Caldona, E.B.; Liu, L.; Chen, L.; Zhang, X. Anticorrosive epoxy coatings from direct epoxidation of bioethanol fractionated lignin. Int. J. Biol. Macromol. 2022, 221, 268–277. [Google Scholar] [CrossRef]
- Li, T.; Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuels Bioprod. Biorefining 2018, 12, 756–787. [Google Scholar] [CrossRef]
- Del Río, J.C.; Gutiérrez, A.; Hernando, M.; Landín, P.; Romero, J.; Martínez, Á.T. Determining the influence of eucalypt lignin composition in paper pulp yield using Py-GC/MS. J. Anal. Appl. Pyrolysis 2005, 74, 110–115. [Google Scholar] [CrossRef]
- Anderson, E.M. Differences in S/G ratio in natural poplar variants do not predict catalytic depolymerization monomer yields. Nat. Commun. 2019, 10, 2033. [Google Scholar] [CrossRef] [PubMed]
- Chen, L. Study on pyrolysis behaviors of non-woody lignins with TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2015, 113, 499–507. [Google Scholar] [CrossRef]
- Barzegari, M.R.; Alemdar, A.; Zhang, Y.; Rodrigue, D. Thermal Analysis of Highly Filled Composites of Polystyrene with Lignin. Polym. Polym. Compos. 2013, 21, 357–366. [Google Scholar] [CrossRef]
- Nair, S.S.; Kuo, P.Y.; Chen, H.; Yan, N. Investigating the effect of lignin on the mechanical, thermal, and barrier properties of cellulose nanofibril reinforced epoxy composite. Ind. Crops Prod. 2017, 100, 208–217. [Google Scholar] [CrossRef]
- Zhe, W.; Qingnan, W.; Shuai, Z.; Yang, Z.; Bo, X. Study on Mechanical Properties and Fracture Mechanisms of Lignin Fiber/epoxy Resin Composites. Mater. Plast. 2013, 60, 13–24. [Google Scholar] [CrossRef]
- Wang, H.; Lin, W.; Qiu, X.; Fu, F.; Zhong, R.; Liu, W.; Yang, D. In Situ Synthesis of Flowerlike Lignin/ZnO Composite with Excellent UV-Absorption Properties and Its Application in Polyurethane. ACS Sustain. Chem. Eng. 2018, 6, 3696–3705. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to non-renewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- UNE-EN ISO 14125/AC:2002; Fibre-Reinforced Plastic Composites—Determination of Flexural Properties. (ISO 14125:1998/Cor. 1:2001); UNE: Madrid, Spain, 2002.
- UNE EN ISO 4892-3:2016; Plastics—Methods of Exposure to Laboratory Light Sources—Part 3: Fluorescent UV Lamps. UNE: Madrid, Spain, 2016.
- UNE EN ISO 11664-1:2011; Colorimetry—Part 1: CIE Standard Colorimetric Observers. UNE: Madrid, Spain, 2011.
- UNE ISO 2813:2015; Paints and Varnishes—Determination of Gloss Value at 20º, 60º and 85º (ISO 2813:2014). UNE: Madrid, Spain, 2015.
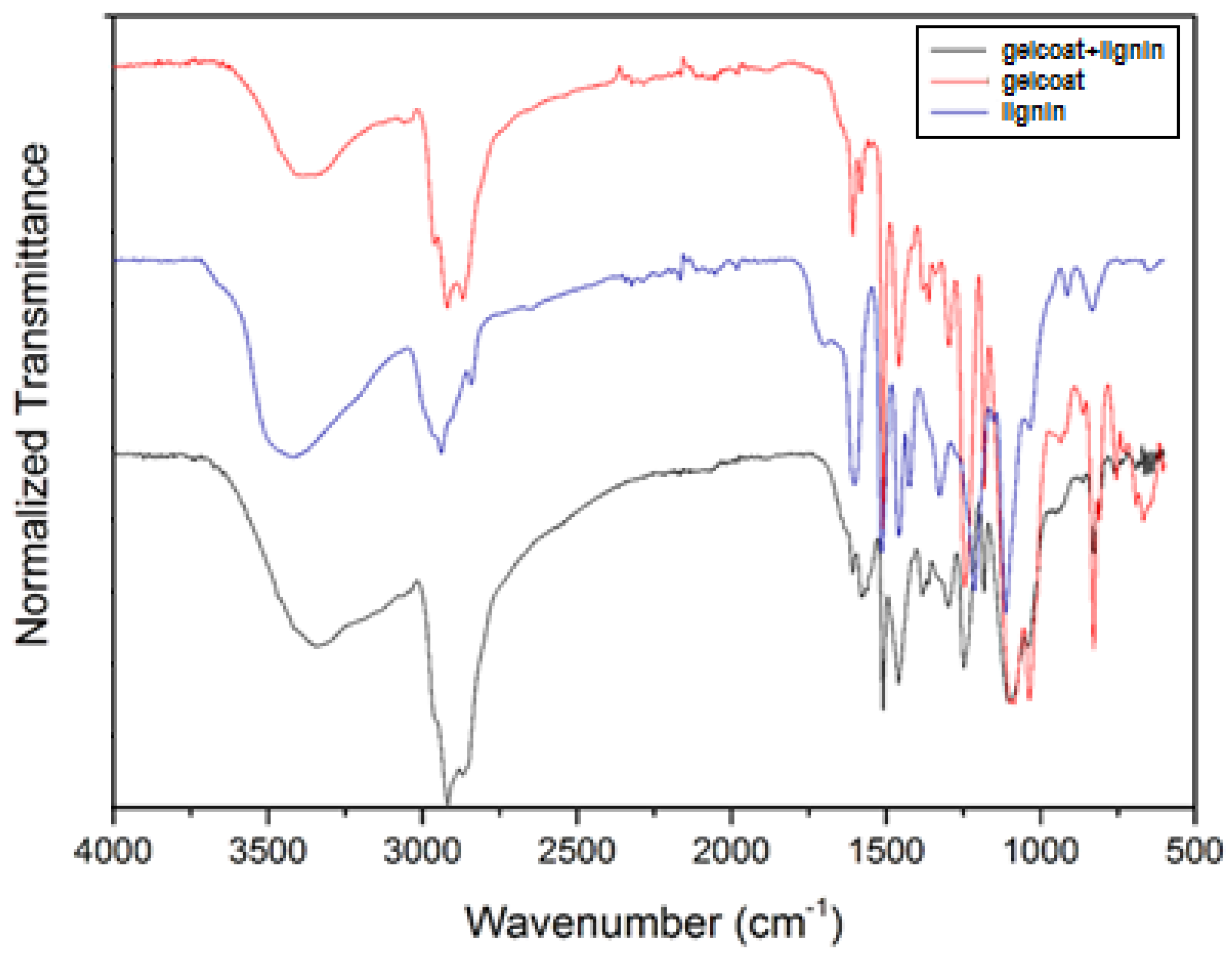
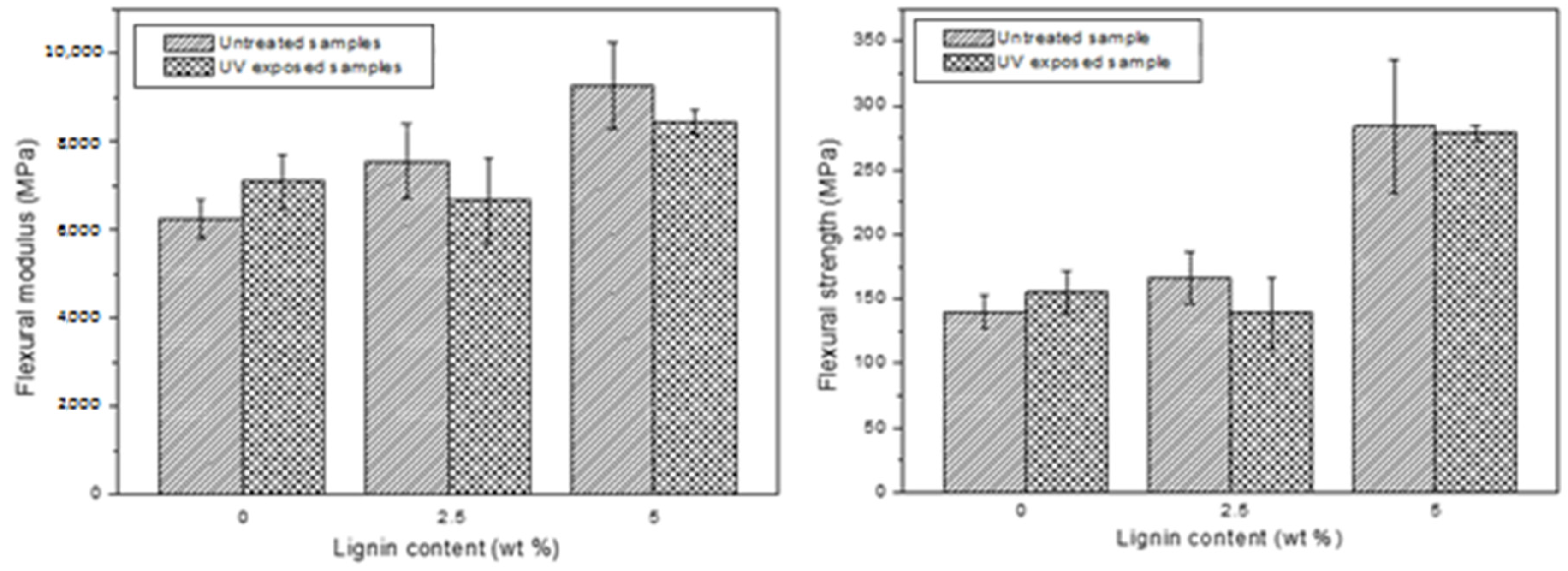
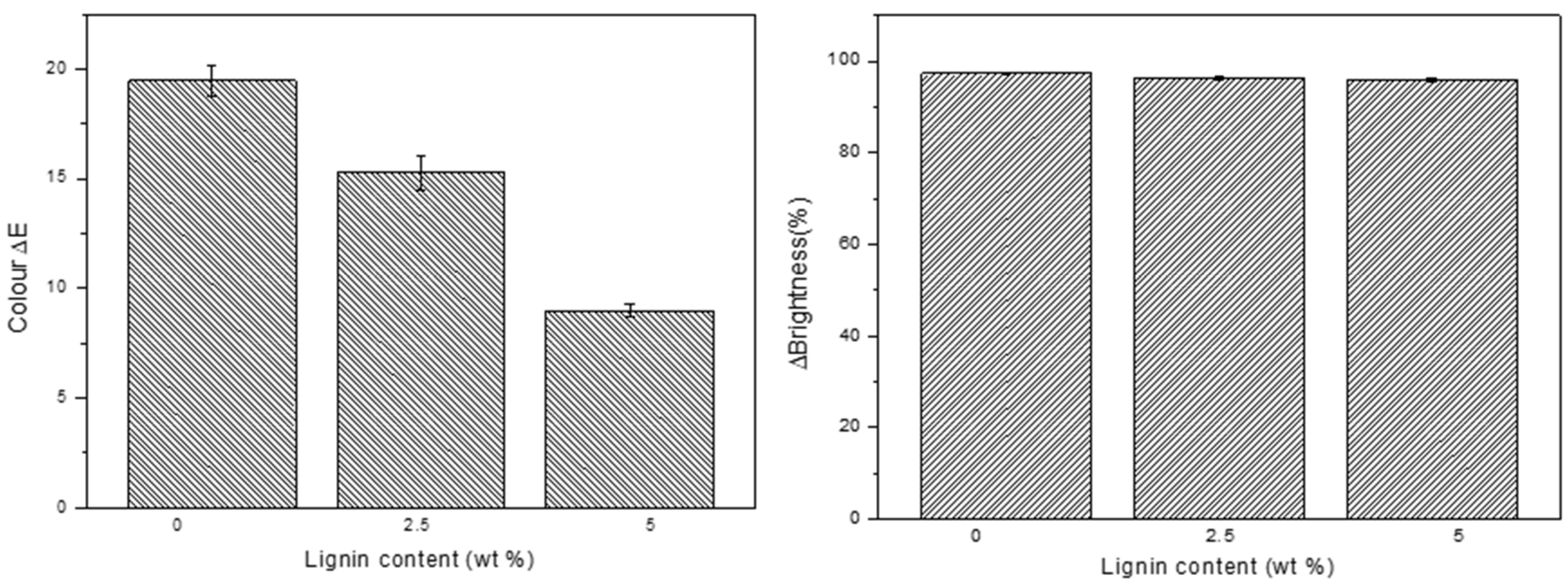

| Aliphatic OH | C5-Substituted OH | Guaiacyl OH | p-Hydroxyphenyl OH | Carboxylic Acid OH | S/G Ratio |
|---|---|---|---|---|---|
| 2.04 | 4.63 | 0.28 | 1.56 | 0.28 | 16.38 |
| Sample | % C | % H | % N | % S | % O (by Difference) |
|---|---|---|---|---|---|
| KL (5% wt.) | 61.63 | 5.63 | 0.13 | 6.60 | 26.01 |
| KL + SGi128 | 41.11 | 6.16 | 14.15 | 2.56 | 36.02 |
| Wavenumber (cm−1) | Band Assignment |
|---|---|
| 3450 | Stretching of the aliphatic O–H bond |
| 2934, 2831 | C–H stretch in methyl and methylene groups |
| 1600 | Aromatic skeleton vibrations plus C–O stretching |
| 1510 | Aromatic vibrations of G units |
| 1574 | Aromatic skeleton vibrations plus C–O stretching |
| 1458 | C–H deformations (asymmetric in –CH3 and –CH2–) |
| 1423 | Aromatic skeleton vibrations combined with C–H in plane deformations |
| 1383 | Aliphatic C–H stretching in CH3 and phenolic OH |
| 1363 | Aliphatic C–H stretching in CH3 and phenolic OH |
| 1299 | G ring plus C+O stretching |
| 1245 | C–C, C–O, and C=O stretching |
| 1183 | Typical for HGS lignin samples; C–O in ester groups |
| 1099 | Aromatic C–H deformation of S units |
| 1038 | Aromatic C–H in plane deformation plus C–O deformation in primary alcohols plus C–H stretching (unconjugated) |
| 911 | C–H out of plane (aromatic rings) |
| 827 | C–H out of plane in positions 2, 5, and 6 (G units) |
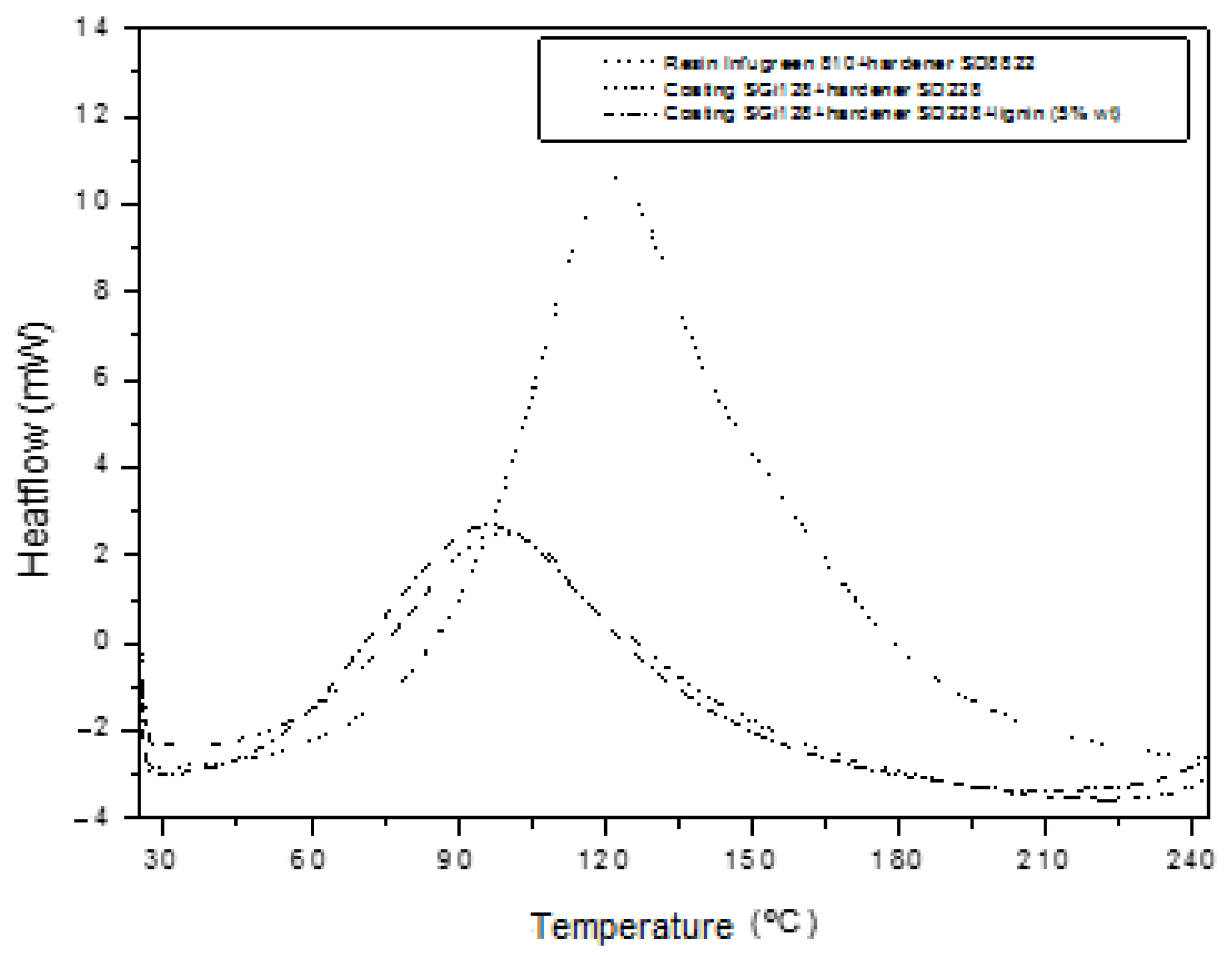
| Formulation | Tm (°C) | ΔH (J/g) |
|---|---|---|
| Infugreen 810 resin + SD8822 hardener | 122 | 496 |
| SGi128 coating + SD228 hardener | 99 | 225 |
| SGi128 coating + SD228 hardener + lignin (5% wt.) | 97 | 210 |
| Sa (μm) | |||
|---|---|---|---|
| Reference | KL (2.5% wt.) | KL (5% wt.) | |
| Initial | 1.6 ± 0.1 | 1.8 ± 0.2 | 1.4 ± 0.1 |
| 500 h UV | 4.4 ± 0.3 | 4.6 ± 0.3 | 3.4 ± 0.2 |
| Property | Units | Value |
|---|---|---|
| Viscosity at 20 °C | mPas | 215.0 |
| Density at 20 °C | g/cm3 | 1.2 |
| Pot life at 20 °C 500 g. | h | 4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seoane-Rivero, R.; Ares-Elejoste, P.; Gondra, K.; Amini, S.; de Hoyos, P.-L.; Gonzalez-Alriols, M. Sustainable Epoxy Composites with UV Resistance Based on New Kraft Lignin Coatings. Molecules 2024, 29, 3697. https://doi.org/10.3390/molecules29153697
Seoane-Rivero R, Ares-Elejoste P, Gondra K, Amini S, de Hoyos P-L, Gonzalez-Alriols M. Sustainable Epoxy Composites with UV Resistance Based on New Kraft Lignin Coatings. Molecules. 2024; 29(15):3697. https://doi.org/10.3390/molecules29153697
Chicago/Turabian StyleSeoane-Rivero, Rubén, Patricia Ares-Elejoste, Koldo Gondra, Sara Amini, Pedro-Luis de Hoyos, and Maria Gonzalez-Alriols. 2024. "Sustainable Epoxy Composites with UV Resistance Based on New Kraft Lignin Coatings" Molecules 29, no. 15: 3697. https://doi.org/10.3390/molecules29153697
APA StyleSeoane-Rivero, R., Ares-Elejoste, P., Gondra, K., Amini, S., de Hoyos, P.-L., & Gonzalez-Alriols, M. (2024). Sustainable Epoxy Composites with UV Resistance Based on New Kraft Lignin Coatings. Molecules, 29(15), 3697. https://doi.org/10.3390/molecules29153697







