Inhibition of Cutaneous TRPV3 Channels by Natural Caffeic Acid for the Alleviation of Skin Inflammation
Abstract
:1. Introduction
2. Results
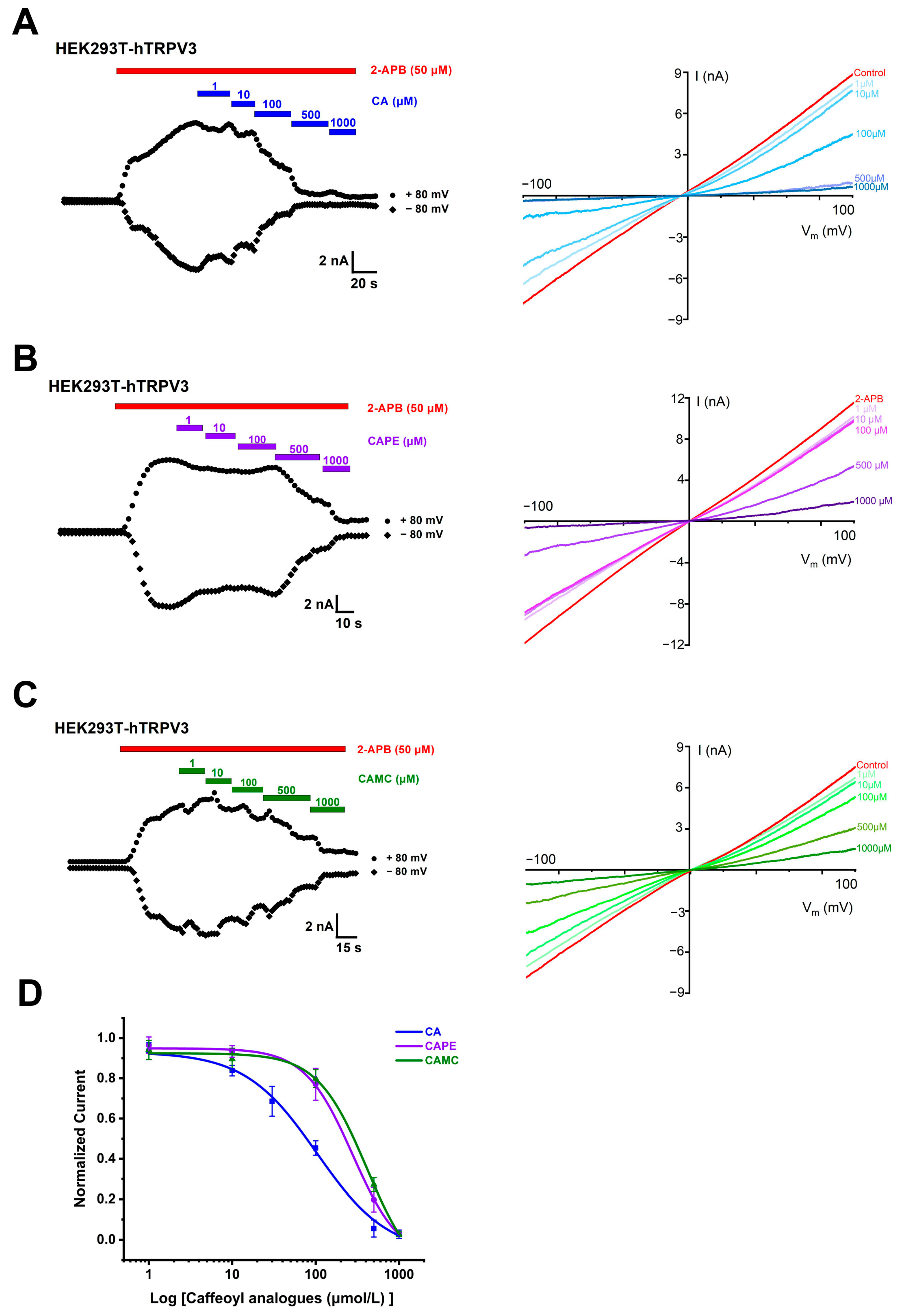
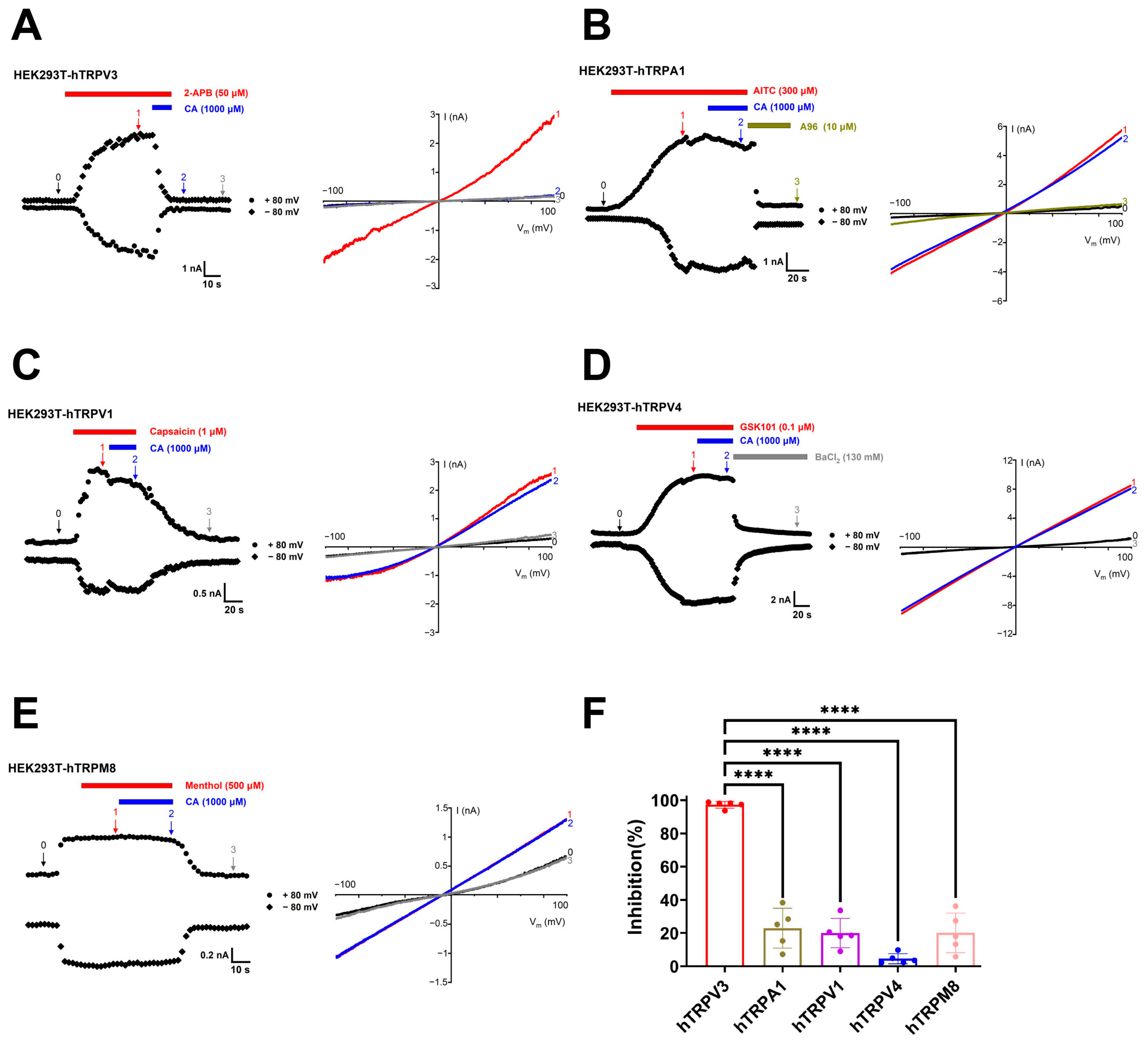
2.1. Concentration- and Structure-Dependent Inhibition of hTRPV3 Currents by Caffeoyl Analogues
2.2. Inhibition of Single TRPV3 Channel by Caffeic Acid
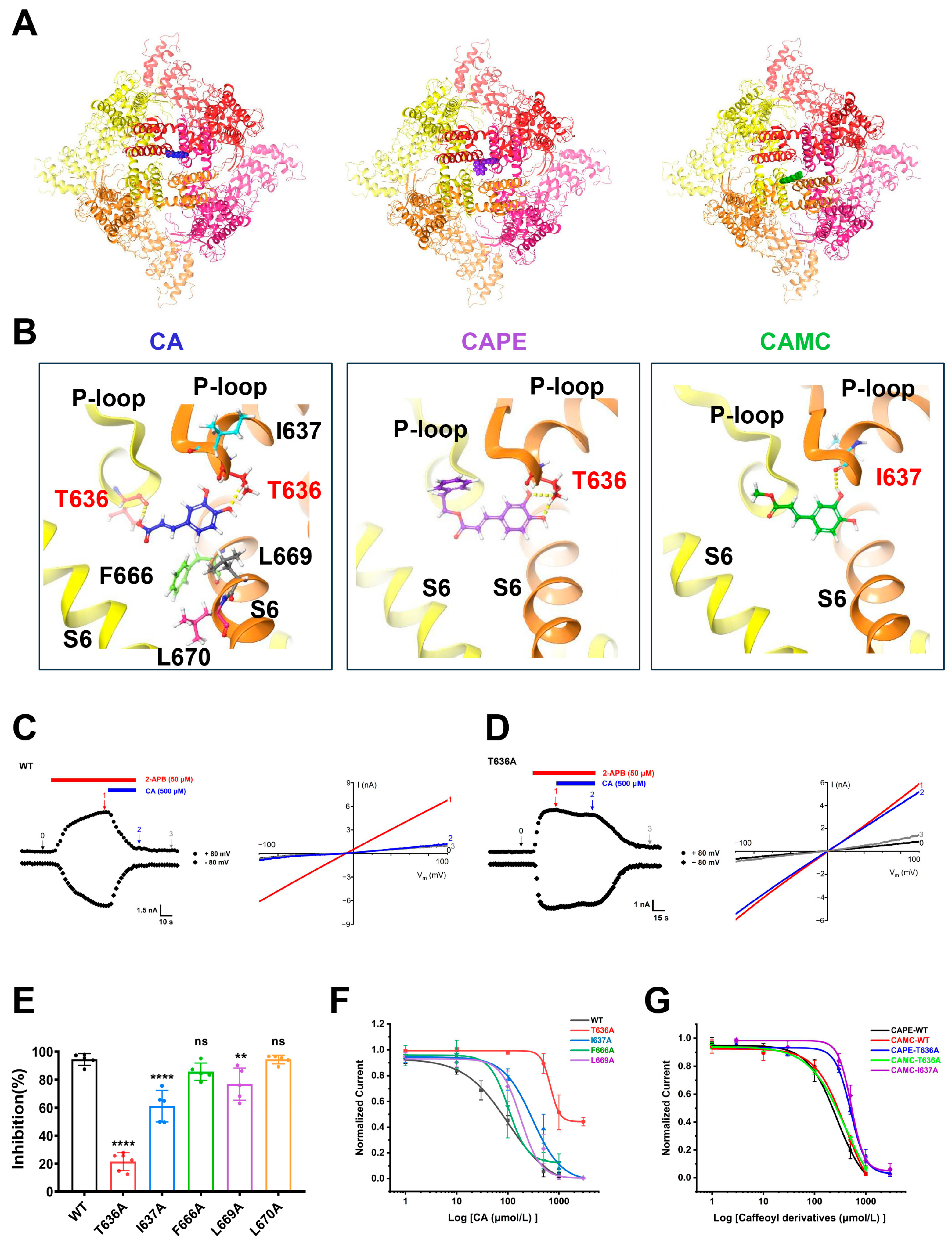
2.3. Identification of TRPV3 Residues Crucial for Caffeic Acid Binding
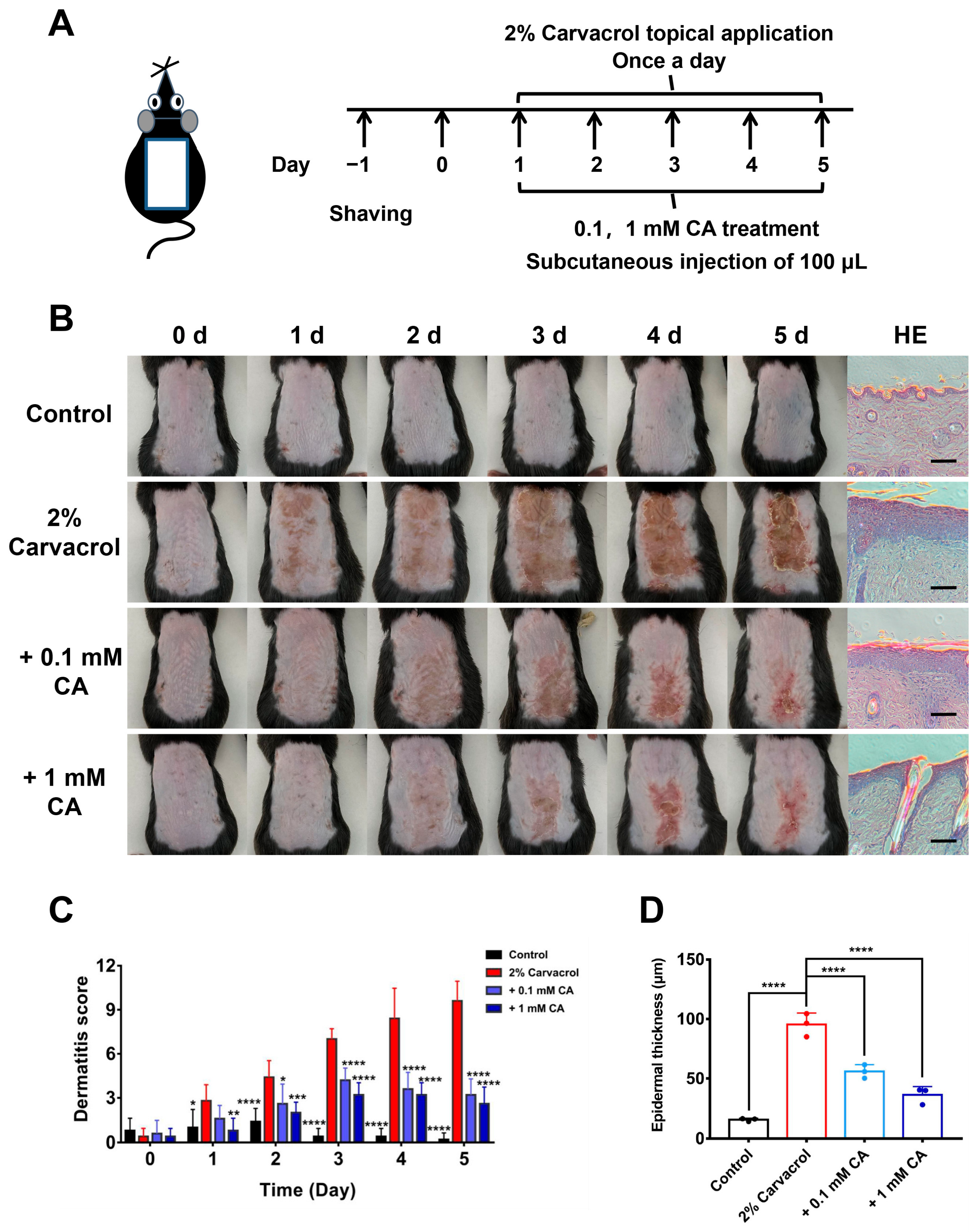
2.4. Caffeic Acid Alleviates Skin Inflammation Induced by Skin Sensitizer Carvacrol
3. Materials and Methods
3.1. Animals
3.2. Compounds
3.3. Cell Cultures
3.4. Electrophysiological Recordings
3.5. Molecular Docking and Site-Directed Mutagenesis
3.6. Mouse Models of Dermatitis Induced by Skin Sensitizer Carvacrol
3.7. Evaluation of Skin Lesions
3.8. Histological Sections of Skin Tissues
3.9. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kalinovskii, A.P.; Utkina, L.L.; Korolkova, Y.V.; Andreev, Y.A. TRPV3 Ion Channel: From Gene to Pharmacology. Int. J. Mol. Sci. 2023, 24, 8601. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, K. The Ca2+-Permeable Cation Transient Receptor Potential TRPV3 Channel: An Emerging Pivotal Target for Itch and Skin Diseases. Mol. Pharmacol. 2017, 92, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Qiao, X.; Wang, W.; He, S.; Liang, K.; Hong, X. TRPV3: Structure, Diseases and Modulators. Molecules 2023, 28, 774. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, M.; Yoshioka, T.; Matsutani, T.; Hikita, I.; Suzuki, M.; Oshima, I.; Tsukahara, K.; Arimura, A.; Horikawa, T.; Hirasawa, T.; et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J. Investig. Dermatol. 2006, 126, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, Q.; Lee, M.; Cao, X.; Zhang, J.; Ma, D.; Chen, L.; Hu, X.; Wang, H.; Wang, X.; et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am. J. Hum. Genet. 2012, 90, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Hu, L.; Cao, X.; Zhao, J.; Zhang, X.; Lee, M.; Wang, H.; Zhang, J.; Chen, Q.; Feng, C.; et al. GenotypePhenotype Correlation of TRPV3-Related Olmsted Syndrome. J. Investig. Dermatol. 2021, 141, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Vasas, N.; Penzes, Z.; Kistamas, K.; Nanasi, P.P.; Molnar, S.; Szegedi, A.; Szollosi, A.G.; Biro, T. Transient receptor potential vanilloid 3 expression is increased in non-lesional skin of atopic dermatitis patients. Exp. Dermatol. 2022, 31, 807–813. [Google Scholar] [CrossRef]
- Kim, H.O.; Cho, Y.S.; Park, S.Y.; Kwak, I.S.; Choi, M.G.; Chung, B.Y.; Park, C.W.; Lee, J.Y. Increased activity of TRPV3 in keratinocytes in hypertrophic burn scars with postburn pruritus. Wound Repair Regen. 2016, 24, 841–850. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, H.J.; Choi, Y.W.; Chung, B.Y.; Woo, S.Y.; Song, D.K.; Kim, H.O. TRPV3 Channel in Keratinocytes in Scars with Post-Burn Pruritus. Int. J. Mol. Sci. 2017, 18, 2425. [Google Scholar] [CrossRef]
- Cui, T.T.; Wang, G.X.; Wei, N.N.; Wang, K. A pivotal role for the activation of TRPV3 channel in itch sensations induced by the natural skin sensitizer carvacrol. Acta Pharmacol. Sin. 2018, 39, 331–335. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, G.; Sun, X.; Wang, K. Inhibition of the Warm Temperature-Activated Ca2+-Permeable Transient Receptor Potential Vanilloid TRPV3 Channel Attenuates Atopic Dermatitis. Mol. Pharmacol. 2019, 96, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hu, L.; Yue, Z.; Liao, D.; Guo, F.; Ke, H.; Jiang, D.; Yang, Y.; Lei, X. Structural basis of TRPV3 inhibition by an antagonist. Nat. Chem. Biol. 2022, 19, 81–90. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, J.; Wei, X.; Hu, J.; Ping, C.; Gao, Y.; Xie, C.; Wang, P.; Cao, P.; Cao, Z.; et al. Therapeutic inhibition of keratinocyte TRPV3 sensory channel by local anesthetic dyclonine. Elife 2021, 10, e68128. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qu, Y.; Zhang, C.; Niu, C.; Tang, X.; Sun, X.; Wang, K. Selective inhibition of overactive warmth-sensitive Ca2+-permeable TRPV3 channels by antispasmodic agent flopropione for alleviation of skin inflammation. J. Biol. Chem. 2024, 300, 105595. [Google Scholar] [CrossRef] [PubMed]
- Korolkova, Y.; Makarieva, T.; Tabakmakher, K.; Shubina, L.; Kudryashova, E.; Andreev, Y.; Mosharova, I.; Lee, H.S.; Lee, Y.J.; Kozlov, S. Marine Cyclic Guanidine Alkaloids Monanchomycalin B and Urupocidin A Act as Inhibitors of TRPV1, TRPV2 and TRPV3, but not TRPA1 Receptors. Mar. Drugs 2017, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Sun, L.-L.; Qi, H.; Gao, Q.; Wang, G.-X.; Wei, N.-N.; Wang, K. Antipruritic effect of natural coumarin osthole through selective inhibition of thermosensitive TRPV3 channel in the skin. Mol. Pharmacol. 2018, 94, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, A.; Nadezhdin, K.D.; Zakharian, E.; Sobolevsky, A.I. Structural mechanism of TRPV3 channel inhibition by the plant-derived coumarin osthole. EMBO Rep. 2021, 22, e53233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.; Qi, H.; Ma, Q.; Zhou, Q.; Wang, W.; Wang, K. Pharmacological Inhibition of the Temperature-Sensitive and Ca2+-Permeable Transient Receptor Potential Vanilloid TRPV3 Channel by Natural Forsythoside B Attenuates Pruritus and Cytotoxicity of Keratinocytes. J. Pharmacol. Exp. Ther. 2019, 368, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qi, H.; Wu, H.; Qu, Y.; Wang, K. Anti-pruritic and anti-inflammatory effects of natural verbascoside through selective inhibition of temperature-sensitive Ca2+-permeable TRPV3 channel. J. Dermatol. Sci. 2020, 97, 229–231. [Google Scholar] [CrossRef]
- Han, Y.L.; Luo, A.N.; Kamau, P.M.; Takomthong, P.; Hu, J.M.; Boonyarat, C.; Luo, L.; Lai, R. A plant-derived TRPV3 inhibitor suppresses pain and itch. Br. J. Pharmacol. 2021, 178, 1669–1683. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, L.; Jiao, K.; Xue, C.; Tang, Q.; Jiang, S.; Ren, Y.; Chen, H.; El-Aziz, T.M.A.; Abdelazeem, K.N.M.; et al. Scutellarein attenuates atopic dermatitis by selectively inhibiting transient receptor potential vanilloid 3 channels. Br. J. Pharmacol. 2022, 179, 4792–4808. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Shi, Y.; Wu, H.; Niu, C.; Sun, X.; Wang, K. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm. Sin. B 2022, 12, 723–734. [Google Scholar] [CrossRef]
- Dang, T.H.; Kim, J.Y.; Kim, H.J.; Kim, B.J.; Kim, W.K.; Nam, J.H. Alpha-Mangostin: A Potent Inhibitor of TRPV3 and Pro-Inflammatory Cytokine Secretion in Keratinocytes. Int. J. Mol. Sci. 2023, 24, 12930. [Google Scholar] [CrossRef] [PubMed]
- Thi, H.D.; Kim, J.Y.; Kim, H.J.; Kim, W.K.; Kim, S.J.; Nam, J.H. Inhibition of Ca2+-permeable TRPV3 and inflammatory cytokine release by honokiol and magnolol in human epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2024, 692, 149332. [Google Scholar] [CrossRef]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Olgierd, B.; Kamila, Z.; Anna, B.; Emilia, M. The Pluripotent Activities of Caffeic Acid Phenethyl Ester. Molecules 2021, 26, 1335. [Google Scholar] [CrossRef] [PubMed]
- Pradhananga, S.; Shim, W.S. Caffeic acid exhibits anti-pruritic effects by inhibition of multiple itch transmission pathways in mice. Eur. J. Pharmacol. 2015, 762, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Jo, S.; Choi, H.K.; Choi, S.; Byun, S.; Lim, T.G. Caffeic Acid Phenethyl Ester Inhibits UV-Induced MMP-1 Expression by Targeting Histone Acetyltransferases in Human Skin. Int. J. Mol. Sci. 2019, 20, 3055. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Sun, X.; Wang, G.; Liu, Y.; Wang, K. Pharmacological Activation of Thermo-Transient Receptor Potential Vanilloid 3 Channels Inhibits Hair Growth by Inducing Cell Death of Hair Follicle Outer Root Sheath. J. Pharmacol. Exp. Ther. 2019, 370, 299–307. [Google Scholar] [CrossRef]
- Tochtrop, G.P.; Genaro-Mattos, T.C.; Maurício, Â.Q.; Rettori, D.; Alonso, A.; Hermes-Lima, M. Antioxidant Activity of Caffeic Acid against Iron-Induced Free Radical Generation—A Chemical Approach. PLoS ONE 2015, 10, e0129963. [Google Scholar]
- Yang, W.S.; Jeong, D.; Yi, Y.S.; Park, J.G.; Seo, H.; Moh, S.H.; Hong, S.; Cho, J.Y. IRAK1/4-targeted anti-inflammatory action of caffeic acid. Mediat. Inflamm. 2013, 2013, 518183. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, D.; Zielinski, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Gimenez-Bastida, J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, Y.; Liu, W.; Wang, T.; Ma, X.; Yu, Z. Novel Insights into the Role of Keratinocytes-Expressed TRPV3 in the Skin. Biomolecules 2023, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Kasai, E.; Imura, K.; Yasui, K.; Shichijou, M.; Oshima, I.; Hirasawa, T.; Sakata, T.; Yoshioka, T. TRPV3 as a therapeutic target for itch. J. Investig. Dermatol. 2012, 132, 2109–2112. [Google Scholar] [CrossRef] [PubMed]
- Larkin, C.; Chen, W.; Szabo, I.L.; Shan, C.; Dajnoki, Z.; Szegedi, A.; Buhl, T.; Fan, Y.; O’Neill, S.; Walls, D.; et al. Novel insights into the TRPV3-mediated itch in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 1110–1114.e5. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fu, Y.; Malakhova, M.; Kurinov, I.; Zhu, F.; Yao, K.; Li, H.; Chen, H.; Li, W.; Lim, D.Y.; et al. Caffeic acid directly targets ERK1/2 to attenuate solar UV-induced skin carcinogenesis. Cancer Prev. Res. 2014, 7, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Balupillai, A.; Prasad, R.N.; Ramasamy, K.; Muthusamy, G.; Shanmugham, M.; Govindasamy, K.; Gunaseelan, S. Caffeic Acid Inhibits UVB-induced Inflammation and Photocarcinogenesis Through Activation of Peroxisome Proliferator-activated Receptor-gamma in Mouse Skin. Photochem. Photobiol. 2015, 91, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Balupillai, A.; Nagarajan, R.P.; Ramasamy, K.; Govindasamy, K.; Muthusamy, G. Caffeic acid prevents UVB radiation induced photocarcinogenesis through regulation of PTEN signaling in human dermal fibroblasts and mouse skin. Toxicol. Appl. Pharmacol. 2018, 352, 87–96. [Google Scholar] [CrossRef]
- Hong, J.; Mu, T.; Sun, H.; Blecker, C.; Richel, A. Photoprotective effects of sweet potato leaf polyphenols and caffeic acid against UV-induced skin-damage in BALB/C nude mice. Food Funct. 2022, 13, 7075–7087. [Google Scholar] [CrossRef]
- Qu, Y.; Sun, X.; Wei, N.; Wang, K. Inhibition of cutaneous heat-sensitive Ca2+-permeable transient receptor potential vanilloid 3 channels alleviates UVB-induced skin lesions in mice. FASEB J. 2023, 37, e23309. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, L.; Qu, Y.; Mo, S.; Sun, X.; Wang, K. Inhibition of Cutaneous TRPV3 Channels by Natural Caffeic Acid for the Alleviation of Skin Inflammation. Molecules 2024, 29, 3728. https://doi.org/10.3390/molecules29163728
Zhang G, Wang L, Qu Y, Mo S, Sun X, Wang K. Inhibition of Cutaneous TRPV3 Channels by Natural Caffeic Acid for the Alleviation of Skin Inflammation. Molecules. 2024; 29(16):3728. https://doi.org/10.3390/molecules29163728
Chicago/Turabian StyleZhang, Guoji, Liqin Wang, Yaxuan Qu, Shilun Mo, Xiaoying Sun, and Kewei Wang. 2024. "Inhibition of Cutaneous TRPV3 Channels by Natural Caffeic Acid for the Alleviation of Skin Inflammation" Molecules 29, no. 16: 3728. https://doi.org/10.3390/molecules29163728




