New Insights Regarding the Use of Relevant Synthetic Compounds in Dentistry
Abstract
:1. Introduction
2. Synthetic Compounds Used in Dentistry
2.1. Chlorhexidine (CHX)
2.1.1. History
2.1.2. Chemical Structure
2.1.3. Antimicrobial Spectrum and Mechanism of Action
2.1.4. Formulations and Current Uses of CHX in Dentistry
2.1.5. Adverse Effects
2.1.6. Experimental Methods Used to Evaluate the Efficacy and Safety of CHX in Dental Applications
2.1.7. Clinical Trials
2.2. Octenidine (OCT)
2.2.1. History
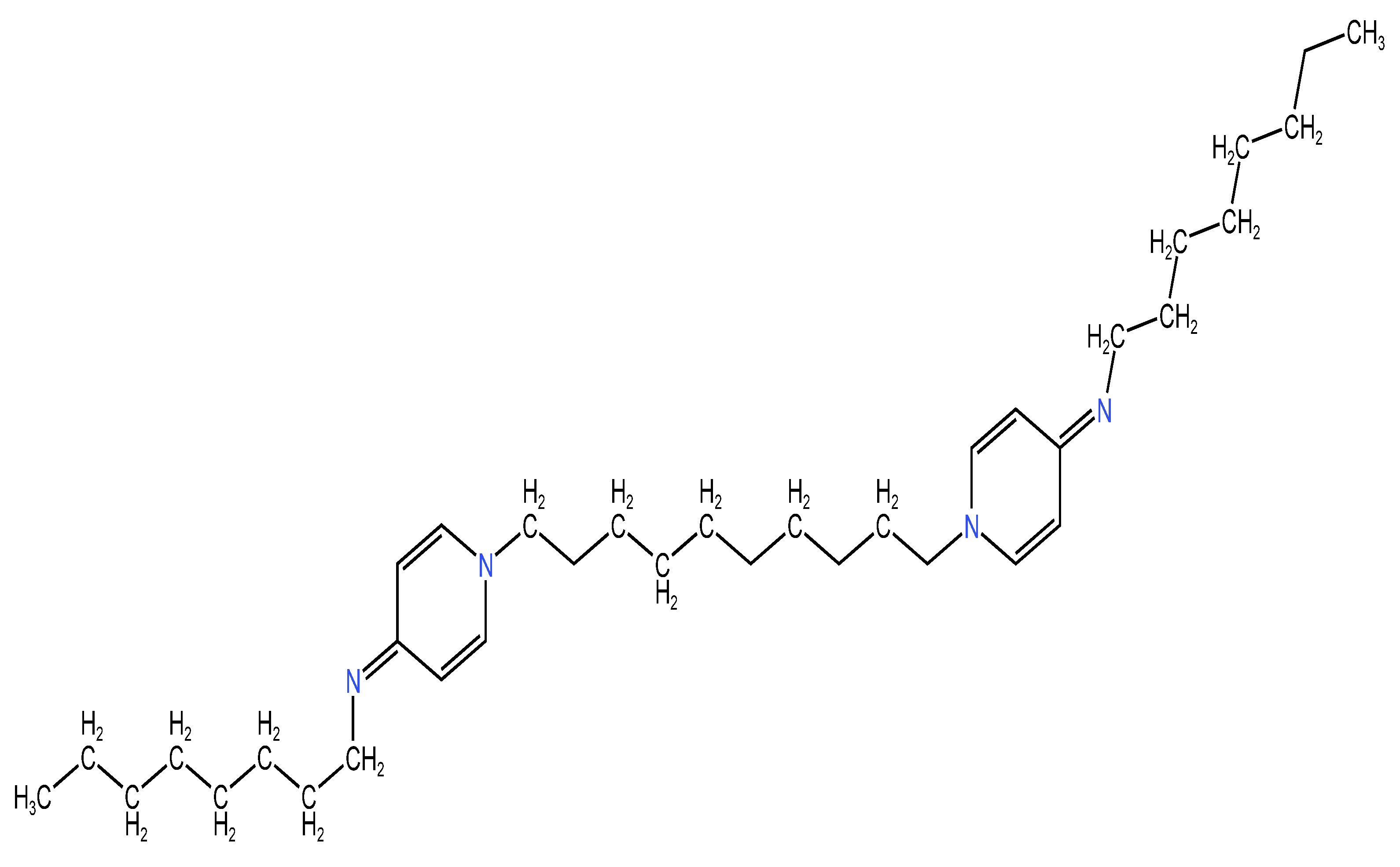
2.2.2. Chemical Structure
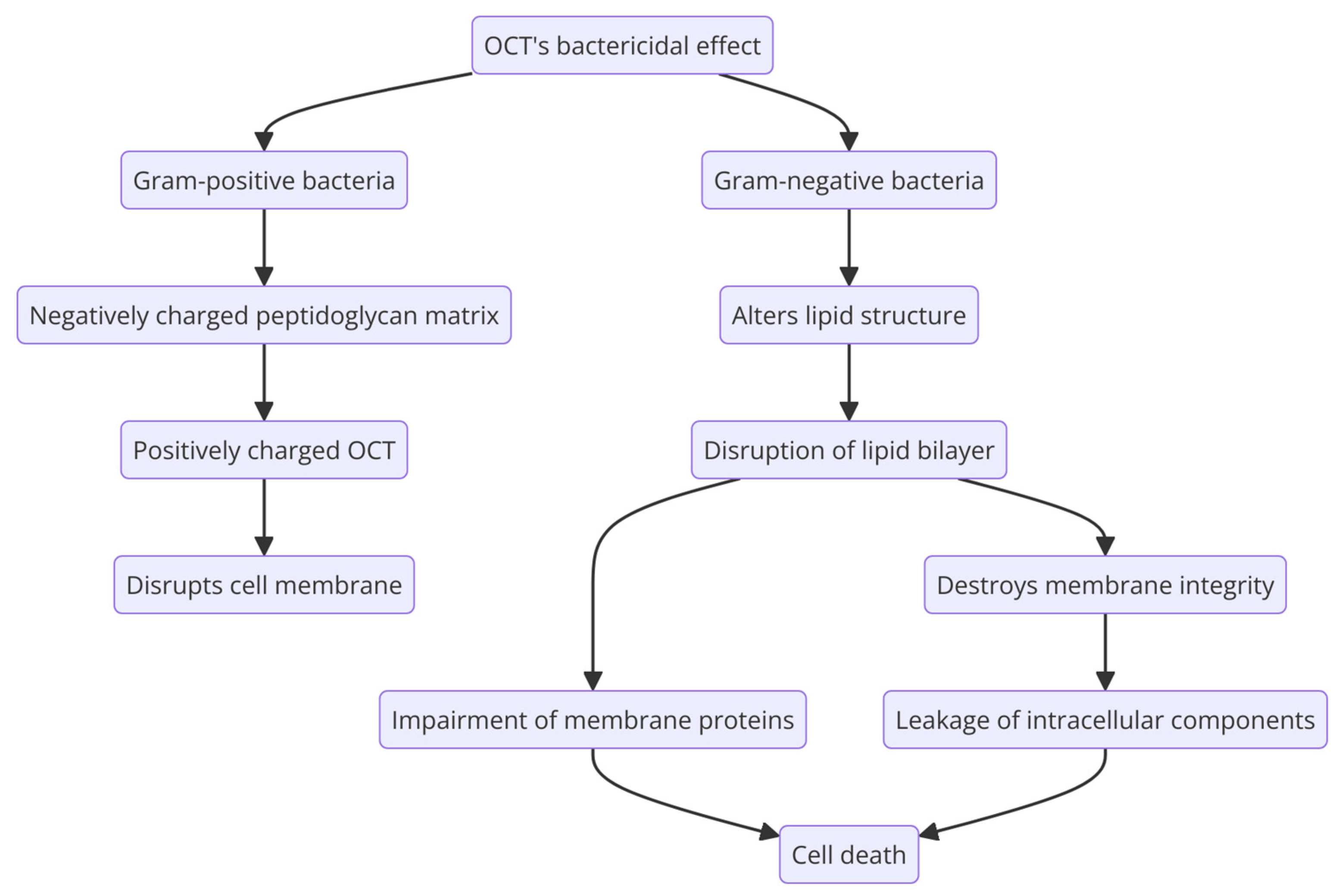
2.2.3. Antimicrobial Spectrum and Mechanism of Action
2.2.4. Formulations and Current Uses of OCT in Dentistry
2.2.5. Adverse Effects
2.2.6. Experimental Methods Used to Evaluate the Efficacy and Safety of OCT in Dental Applications
2.2.7. Clinical Trials
2.3. Povidone-Iodine (PVP-I)
2.3.1. History
2.3.2. Chemical Structure
2.3.3. Antimicrobial Spectrum and Mechanism of Action
2.3.4. Formulations and Current Use of PVP-I in Dentistry
2.3.5. Adverse Effects
2.3.6. Experimental Methods Used to Evaluate the Efficacy and Safety of PVP-I in Dental Applications
2.3.7. Clinical Trials
2.4. Sodium Hypochlorite (NaOCl)
2.4.1. History
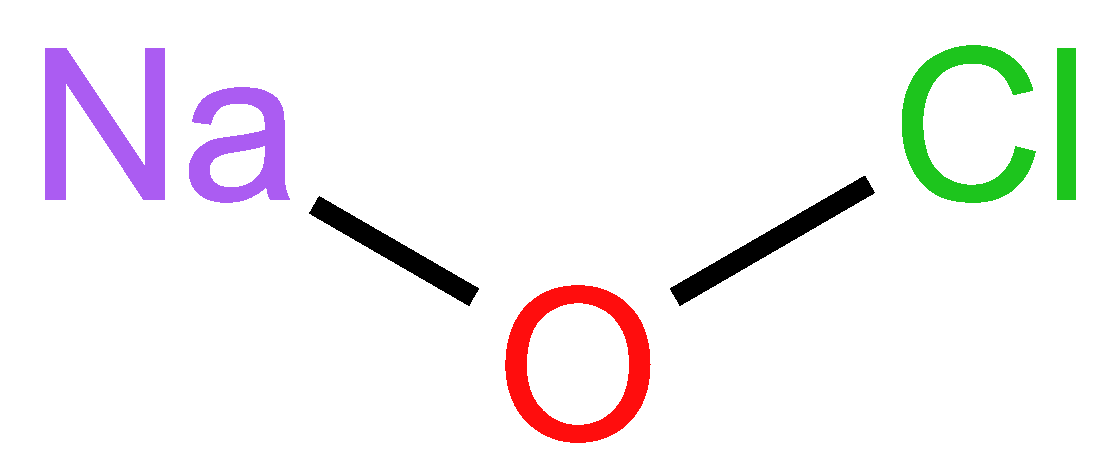
2.4.2. Chemical Structure
2.4.3. Antimicrobial Spectrum and Mechanism of Action
2.4.4. Formulations and Current Use of Sodium Hypochlorite in Dentistry
2.4.5. Adverse Effects
2.4.6. Experimental Methods Used to Evaluate the Efficacy and Safety of NaOCl in Dental Applications
2.4.7. Clinical Trials
2.5. Cetylpyridinium Chloride (CPC)
2.5.1. History
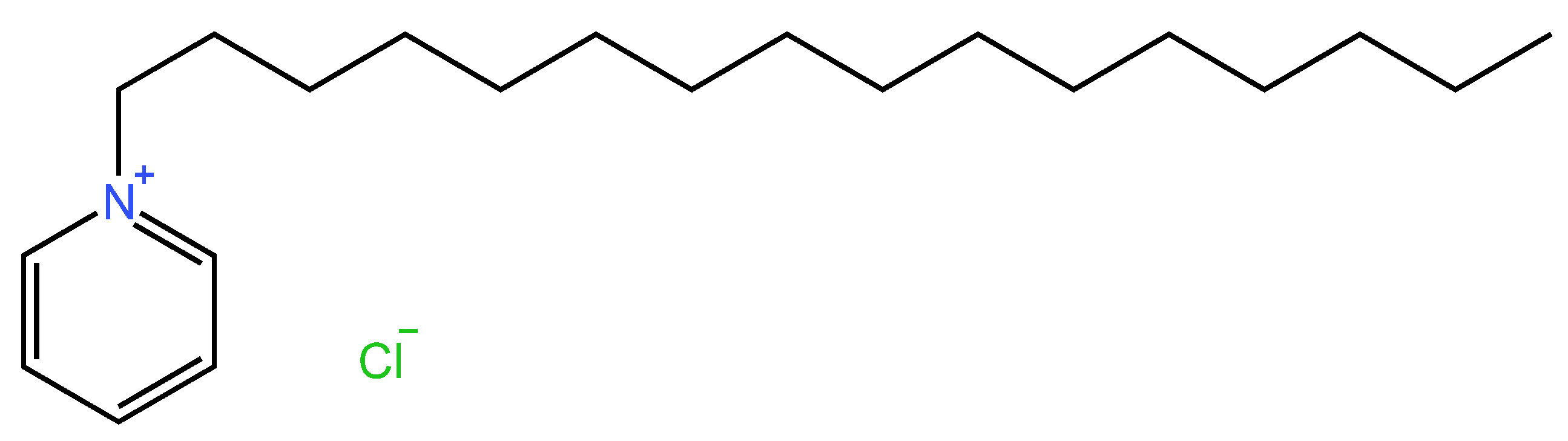
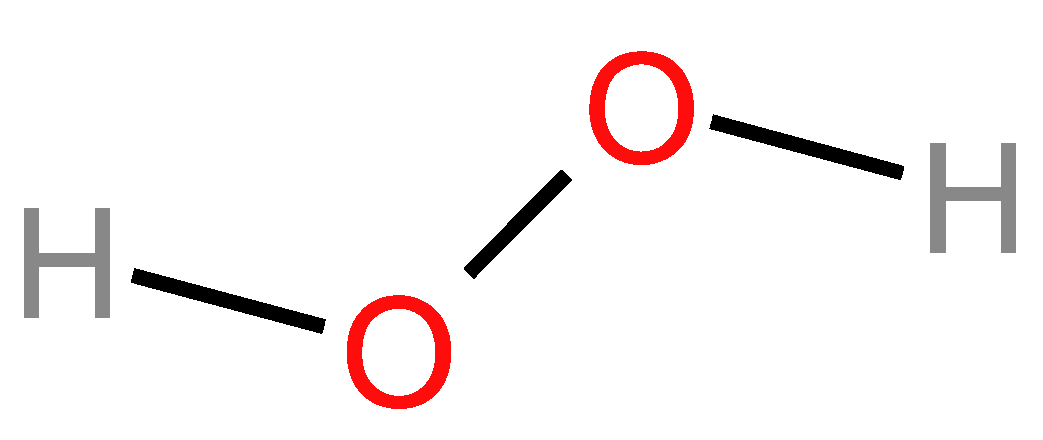
2.5.2. Chemical Structure
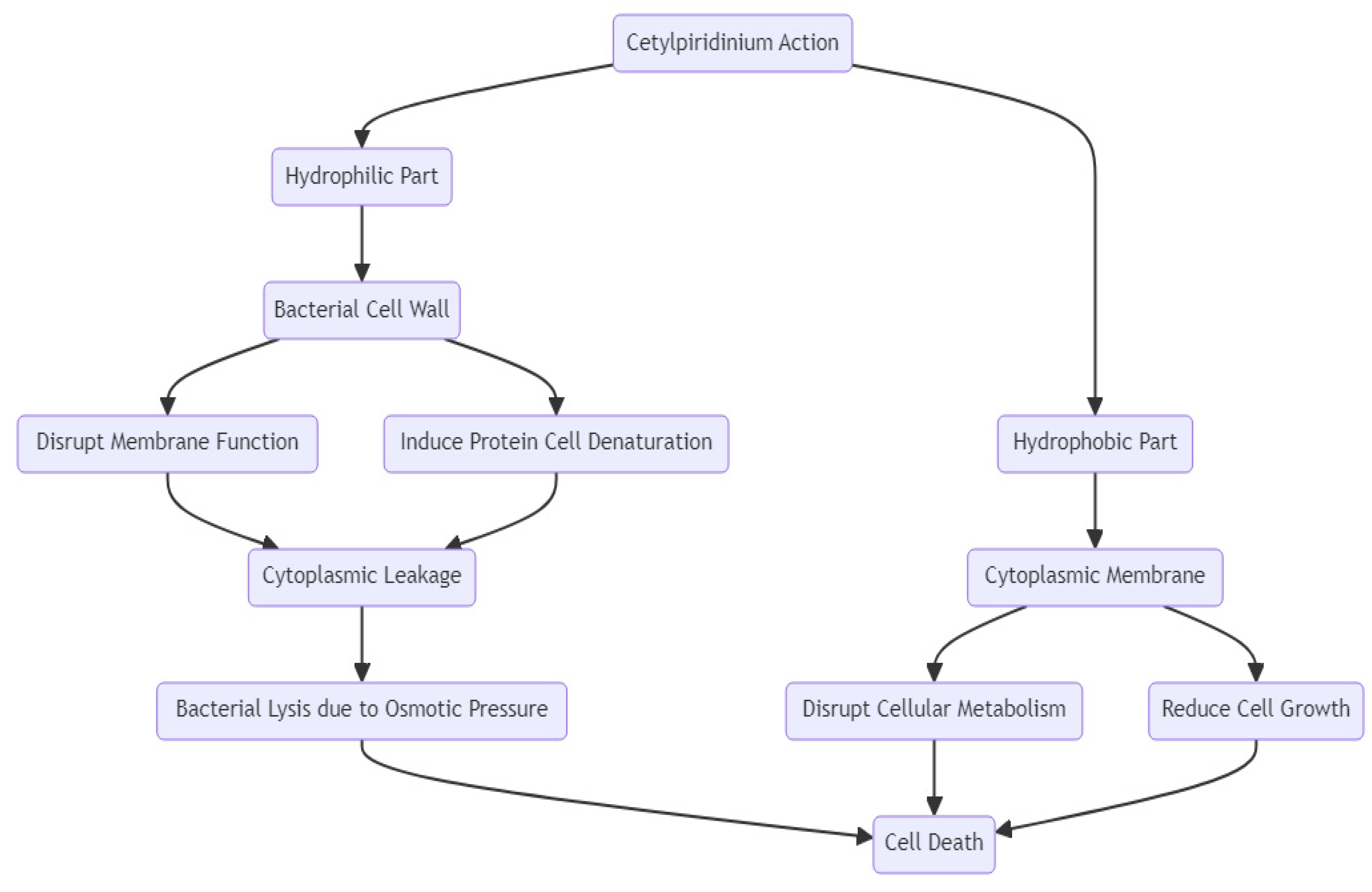
2.5.3. Antimicrobial Spectrum and Mechanism of Action
2.5.4. Formulations and Current Use of Cetylpyridinium Chloride in Dentistry
2.5.5. Adverse Effects
2.5.6. Experimental Methods Used to Evaluate the Efficacy and Safety of CPC in Dental Applications
2.5.7. Clinical Trials
2.6. Hydrogen Peroxide (HP)
2.6.1. History
2.6.2. Chemical Structure
2.6.3. Antimicrobial Spectrum and Mechanism of Action
2.6.4. Formulations and Current Uses of Hydrogen Peroxide in Dentistry
2.6.5. Adverse Effects
2.6.6. Experimental Methods Used to Evaluate the Efficacy and Safety of HP in Dental Applications
2.6.7. Clinical Trials
2.7. Safety Profiles of Synthetic Compounds
2.7.1. Chlorhexidine
2.7.2. Octenidine
2.7.3. Povidone-Iodine
2.7.4. Sodium Hypochlorite
2.7.5. Cetylpiridinium Chloride
2.7.6. Hydrogen Peroxide
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Gil-Montoya, J.A.; de Mello, A.L.F.; Barrios, R.; Gonzalez-Moles, M.A.; Bravo, M. Oral Health in the Elderly Patient and Its Impact on General Well-Being: A Nonsystematic Review. Clin. Interv. Aging 2015, 10, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Wang, G.; Hu, Z.; Wang, S.; Yan, Q.; Liu, X. Burden of Oral Disorders, 1990–2019: Estimates from the Global Burden of Disease Study 2019. Arch. Med. Sci. 2023, 19, 930. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.H.; Chen, X.; Daliri, E.B.M.; Kim, N.; Kim, J.R.; Yoo, D. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A.; Gershovich, E. The Prevention of Periodontal Disease—An Overview. Periodontology 2000 2020, 84, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Vulturar, R.; Buduru, S.; Cozma, A.; Fodor, A.; Chiș, A.; Lucaciu, O.; Damian, L.; Moldovan, M.L. Oral Microbiome: Getting to Know and Befriend Neighbors, a Biological Approach. Biomedicines 2022, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B. Present Status and Future Directions—Managing Discoloured Teeth. Int. Endod. J. 2022, 55, 922–950. [Google Scholar] [CrossRef] [PubMed]
- Wongchai, M.; Wongkaewkhiaw, S.; Kanthawong, S.; Roytrakul, S.; Aunpad, R. Dual-Function Antimicrobial-Antibiofilm Peptide Hybrid to Tackle Biofilm-Forming Staphylococcus epidermidis. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 44. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Chae, S.W.; Im, G.J.; Chung, J.W.; Song, J.J. Eugenol: A Phyto-Compound Effective against Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Clinical Strain Biofilms. PLoS ONE 2015, 10, e0119564. [Google Scholar] [CrossRef]
- Butler, J.; Handy, R.D.; Upton, M.; Besinis, A. Review of Antimicrobial Nanocoatings in Medicine and Dentistry: Mechanisms of Action, Biocompatibility Performance, Safety, and Benefits Compared to Antibiotics. ACS Nano 2023, 17, 7064–7092. [Google Scholar] [CrossRef]
- Wang, J.; Ju, T.; Guo, L.; Shan, W.; Wu, Q.; Zhang, H.; Zhang, J. Quorum-Quenching Enzyme Est816 Assisted Antibiotics against Periodontitis Induced by Aggregatibacter Actinomycetemcomitans in Rats. Front. Cell Infect. Microbiol. 2024, 14, 1368684. [Google Scholar] [CrossRef]
- Balagopal, S.; Arjunkumar, R. Chlorhexidine: The Gold Standard Antiplaque Agent. J. Pharm. Sci. Res. 2013, 5, 270–274. [Google Scholar]
- Chye, R.M.L.; Perrotti, V.; Piattelli, A.; Iaculli, F.; Quaranta, A. Effectiveness of Different Commercial Chlorhexidine-Based Mouthwashes After Periodontal and Implant Surgery: A Systematic Review. Implant. Dent. 2019, 28, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Makmuriana, L.; Wuriani; Lestari, L.; Usman, U.; Surtikanti, S.; Pradika, J.; Ramadhaniyati, R.; Haryanto, H. Chlorhexidine and Honey: Mouthwash Liquids in Reducing Halitosis of Stroke Patients. Enferm. Clin. 2021, 31, S677–S681. [Google Scholar] [CrossRef]
- Wassel, M.; Radwan, M.; Elghazawy, R. Direct and Residual Antimicrobial Effect of 2% Chlorhexidine Gel, Double Antibiotic Paste and Chitosan-Chlorhexidine Nanoparticles as Intracanal Medicaments against Enterococcus faecalis and Candida albicans in Primary Molars: An in-Vitro Study. BMC Oral Health 2023, 23, 296. [Google Scholar] [CrossRef] [PubMed]
- Sindhe, R.J.; Venkatesh, R.; Asha, V.; Arvind, M.; Manoj, G.K.; Pavithra, S. Comparison of Chlorhexidine and Benzydamine Mouth Rinses in the Management of Radiotherapy or Chemotherapy-Induced Oral Mucositis: A Systematic Review and Meta-Analysis. J. Indian Acad. Oral Med. Radiol. 2023, 35, 267–272. [Google Scholar] [CrossRef]
- Alzoman, H.; Alojaym, T.G.; Chalikkandy, S.N.; Mehmood, A.; Rashed, F.; Divakar, D.D. Comparison of an Herbal-and a 0.12% Chlorhexidine-Based Oral Rinse as Adjuncts to Nonsurgical Mechanical Debridement in the Management of Peri-Implant Mucositis: A Randomised Controlled Trial. Oral Health Prev. Dent. 2020, 18, 645–651. [Google Scholar] [CrossRef]
- Pałka, Ł.; Nowakowska-Toporowska, A.; Dalewski, B. Is Chlorhexidine in Dentistry an Ally or a Foe? A Narrative Review. Healthcare 2022, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Chatzigiannidou, I.; Teughels, W.; Van de Wiele, T.; Boon, N. Oral Biofilms Exposure to Chlorhexidine Results in Altered Microbial Composition and Metabolic Profile. npj Biofilms Microbiomes 2020, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance toward Chlorhexidine in Oral Bacteria-Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Mahant, R. A Contemporary Overview of Endodontic Irrigants—A Review. Austin J. Dent. Appl. 2014, 1, 105–115. [Google Scholar]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current Uses of Chlorhexidine for Management of Oral Disease: A Narrative Review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef] [PubMed]
- Mercan, U.; Gonen, Z.B.; Salkin, H.; Yalcin-Ulker, G.M.; Meral, D.G. Comparison of the Effect of Postoperative Care Agents on Human Gingival Fibroblasts: A Preliminary Study. Eur. Oral Res. 2019, 53, 67. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, A.; Kaspar, S.S.; Kathirvelu, R.P.; Srinivasan, B.; Srinivasan, S.; Sundram, R. Chlorhexidine: An Elixir for Periodontics. J. Pharm. Bioallied Sci. 2020, 12, S57–S59. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.C.; de Oliveira, R.B.; da Silva Mendonça, T.M. Should Oral Chlorhexidine Remain in Ventilator-Associated Pneumonia Prevention Bundles? Med. Intensiv. 2022, 46, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Giovannini, L.; Baccani, I.; Giuliani, V.; Pace, R.; Rossolini, G.M. In Vitro Antimicrobial Activity of the Decontaminant HybenX® Compared to Chlorhexidine and Sodium Hypochlorite against Common Bacterial and Yeast Pathogens. Antibiotics 2019, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.; Szkaradkiewicz, A.K. Chlorhexidine—Pharmaco-biological Activity and Application. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1321–1326. [Google Scholar] [PubMed]
- Solderer, A.; Kaufmann, M.; Hofer, D.; Wiedemeier, D.; Attin, T.; Schmidlin, P.R. Efficacy of Chlorhexidine Rinses after Periodontal or Implant Surgery: A Systematic Review. Clin. Oral Investig. 2019, 23, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Osso, D.; Kanani, N. Antiseptic Mouth Rinses: An Update on Comparative Effectiveness, Risks and Recommendations. J. Dent. Hyg. 2013, 87, 10–18. [Google Scholar] [PubMed]
- Deus, F.P.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269. [Google Scholar] [CrossRef]
- Opstrup, M.S.; Jemec, G.B.E.; Garvey, L.H. Chlorhexidine Allergy: On the Rise and Often Overlooked. Curr. Allergy Asthma Rep. 2019, 19, 23. [Google Scholar] [CrossRef]
- Silla, M.P.; Montiel Company, J.M.; Almerich Silla, J.M. Use of Chlorhexidine Varnishes in Preventing and Treating Periodontal Disease. A Review of the Literature. Med. Oral Patol. Oral Cir. Bucal 2008, 13, e257–e260. [Google Scholar]
- Rosa, C.D.D.R.D.; Gomes, J.M.d.L.; de Moraes, S.L.D.; Lemos, C.A.A.; da Fonte, T.P.; Limirio, J.P.J.d.O.; Pellizzer, E.P. Use of Chlorhexidine Chip after Scaling and Root Planning on Periodontal Disease: A Systematic Review and Meta-Analysis. Saudi Dent. J. 2021, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Deus, F.P.; Ouanounou, A. Mouthwashes and Their Use in Dentistry: A Review. Oral Health 2021, 1, 22–34. [Google Scholar]
- Richards, D. Chlorhexidine Mouthwash More Effective than Dentifrice or Gel. Evid. Based Dent. 2015, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Oliveira-Neto, J.M.; Moore, D. Chlorhexidine Treatment for the Prevention of Dental Caries in Children and Adolescents. Cochrane Database Syst. Rev. 2015, 2015, CD008457. [Google Scholar] [CrossRef] [PubMed]
- Supranoto, S.C.; Slot, D.E.; Addy, M.A.; Van der Weijden, G.A. The Effect of Chlorhexidine Dentifrice or Gel versus Chlorhexidine Mouthwash on Plaque, Gingivitis, Bleeding and Tooth Discoloration: A Systematic Review. Int. J. Dent. Hyg. 2015, 13, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Gartenmann, S.J.; Dörig, I.; Sahrmann, P.; Held, U.; Walter, C.; Schmidlin, P.R. Influence of Different Post-Interventional Maintenance Concepts on Periodontal Outcomes: An Evaluation of Three Systematic Reviews. BMC Oral Health 2017, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Bryce, G.; Bomfim, D.I.; Bassi, G.S. Pre- and Post-Operative Management of Dental Implant Placement. Part 2: Management of Early-Presenting Complications. Br. Dent. J. 2014, 217, 171–176. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of Stage I-III Periodontitis-The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Jhinger, N.; Kapoor, D.; Jain, R. Comparison of Periochip (Chlorhexidine Gluconate 2.5 Mg) and Arestin (Minocycline Hydrochloride 1 Mg) in the Management of Chronic Periodontitis. Indian J. Dent. 2015, 6, 20. [Google Scholar] [CrossRef]
- Sahrmann, P.; Bettschart, C.; Wiedemeier, D.B.; Al-Majid, A.; Attin, T.; Schmidlin, P.R. Treatment of Peri-Implant Mucositis with Repeated Application of Chlorhexidine Chips or Gel during Supportive Therapy—A Randomized Clinical Trial. Dent. J. 2019, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Frankenthal, S.; Levi, G.; Elimelech, R.; Shoshani, E.; Rosenfeld, O.; Tagger-Green, N.; Shlomi, B. Treatment of Peri-Implantitis Using Multiple Applications of Chlorhexidine Chips: A Double-Blind, Randomized Multi-Centre Clinical Trial. J. Clin. Periodontol. 2012, 39, 1198–1205. [Google Scholar] [CrossRef]
- Machtei, E.E.; Romanos, G.; Kang, P.; Travan, S.; Schmidt, S.; Papathanasiou, E.; Tatarakis, N.; Tandlich, M.; Liberman, L.H.; Horwitz, J.; et al. Repeated Delivery of Chlorhexidine Chips for the Treatment of Peri-Implantitis: A Multicenter, Randomized, Comparative Clinical Trial. J. Periodontol. 2021, 92, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, P.K.; Prasad, K.V.V. Effects of a Chlorhexidine Mouthwash on Clinical Parameters of Gingivitis, Dental Plaque and Oral Polymorphonuclear Leukocytes [PMN]. Contemp. Clin. Trials Commun. 2019, 19, 100473. [Google Scholar] [CrossRef] [PubMed]
- Göstemeyer, G.; Kohls, A.; Paris, S.; Schwendicke, F. Root Caries Prevention via Sodium Fluoride, Chlorhexidine and Silver Diamine Fluoride in Vitro. Odontology 2018, 106, 274–281. [Google Scholar] [CrossRef]
- Rathee, M.; Jain, P. Gingivitis. Aust. J. Pharm. 2023, 96, 64–67. [Google Scholar]
- InformedHealth. org [Internet]. Overview: Gingivitis and Periodontitis; Institute for Quality and Efficiency in Health Care (IQWiG): Cologne, Germany, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279593/ (accessed on 27 May 2024).
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine Mouthrinse as an Adjunctive Treatment for Gingival Health. Cochrane Database Syst. Rev. 2017, 2017, CD008676. [Google Scholar] [CrossRef]
- Krupa, N.C.; Thippeswamy, H.M.; Chandrashekar, B.R. Antimicrobial Efficacy of Xylitol, Probiotic and Chlorhexidine Mouth Rinses among Children and Elderly Population at High Risk for Dental Caries—A Randomized Controlled Trial. J. Prev. Med. Hyg. 2022, 63, e282–e287. [Google Scholar] [CrossRef]
- Abdulkareem, A.A.; Al Marah, Z.A.; Abdulbaqi, H.R.; Alshaeli, A.J.; Milward, M.R. A Randomized Double-Blind Clinical Trial to Evaluate the Efficacy of Chlorhexidine, Antioxidant, and Hyaluronic Acid Mouthwashes in the Management of Biofilm-Induced Gingivitis. Int. J. Dent. Hyg. 2020, 18, 268–277. [Google Scholar] [CrossRef]
- Kamath, D.G.; Nadimpalli, H.; Nayak, S.U.; Rajendran, V.; Natarajan, S. Comparison of Antiplaque and Anti-Gingivitis Effects of Aloe Vera Mouthwash with Chlorhexidine in Fixed Orthodontic Patients—A Randomized Controlled Trial. Int. J. Dent. Hyg. 2023, 21, 211–218. [Google Scholar] [CrossRef]
- Ahmad, B. The Efficacy of Chlorhexidine Gel as an Adjunctive Treatment for Patients with Chronic Periodontitis. Indian J. Forensic Med. Toxicol. 2020, 14, 544–550. [Google Scholar] [CrossRef]
- Bamashmous, S.; Kotsakis, G.A.; Kerns, K.A.; Leroux, B.G.; Zenobia, C.; Chen, D.; Trivedi, H.M.; McLean, J.S.; Darveau, R.P. Human Variation in Gingival Inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2012578118. [Google Scholar] [CrossRef] [PubMed]
- Kurt, S.M.; Demirci, G.K.; Serefoglu, B.; Kaval, M.E.; Çalışkan, M.K. Usage of chlorhexidine as a final irrigant in one-visit root canal treatment in comparison with conventional two-visit root canal treatment in mandibular molars: A randomized clinical trial. J. Evid. Based Dent. Pract. 2022, 22, 101759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, Y.; Haapasalo, M. Effectiveness of Endodontic Disinfecting Solutions against Young and Old Enterococcus faecalis Biofilms in Dentin Canals. J. Endod. 2012, 38, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, N.; Ejaj, M.; D’cruz, T.; Subbiah, G.; Rameezuddin, T.; Subbiah, K. An In Vitro Study to Determine the Antibacterial Activity of Chlorhexidine and Herbal Mouthrinses against Enterococcus faecalis. J. Pharm. Bioallied Sci. 2022, 14, 995. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Bargale, S.; Dave, B.H.; Deshpande, A.; Kariya, P.B.; Karri, A. Comparison of Antimicrobial Efficacy of (between) 0.2% Chlorhexidine and Herbal Mouthwash on Salivary Streptococcus mutans: A Randomized Controlled Pilot Study. Contemp. Clin. Dent. 2018, 9, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Al-Maweri, S.A.; Nassani, M.Z.; Alaizari, N.; Kalakonda, B.; Al-Shamiri, H.M.; Alhajj, M.N.; Al-Soneidar, W.A.; Alahmary, A.W. Efficacy of Aloe Vera Mouthwash versus Chlorhexidine on Plaque and Gingivitis: A Systematic Review. Int. J. Dent. Hyg. 2020, 18, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Yates, R.; Jenkins, S.; Newcombe, R.; Wade, W.; Moran, J.; Addy, M. A 6-Month Home Usage Trial of a 1% Chlorhexidine Toothpaste (1). Effects on Plaque, Gingivitis, Calculus and Toothstaining. J. Clin. Periodontol. 1993, 20, 130–138. [Google Scholar] [CrossRef]
- Chamsai, B.; Soodvilai, S.; Opanasopit, P.; Samprasit, W. Topical Film-Forming Chlorhexidine Gluconate Sprays for Antiseptic Application. Pharmaceutics 2022, 14, 1124. [Google Scholar] [CrossRef]
- Talha, B.; Swarnkar, S.A. Xerostomia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545287/ (accessed on 9 June 2024).
- Guggenheimer, J.; Moore, P.A. Xerostomia: Etiology, Recognition and Treatment. J. Am. Dent. Assoc. 2003, 134, 61–69. [Google Scholar] [CrossRef]
- Marcott, S.; Dewan, K.; Kwan, M.; Baik, F.; Lee, Y.-J.; Sirjani, D. Where Dysphagia Begins: Polypharmacy and Xerostomia. Fed. Pract. 2020, 37, 234. [Google Scholar] [PubMed]
- Fons-Badal, C.; Fons-Font, A.; Labaig-Rueda, C.; Solá-Ruiz, M.F.; Selva-Otaolaurruchi, E.; Agustín-Panadero, R. Analysis of Predisposing Factors for Rapid Dental Calculus Formation. J. Clin. Med. 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Microbiology of Dental Plaque Biofilms and Their Role in Oral Health and Caries. Dent. Clin. N. Am. 2010, 54, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, B.M.; Krishnegowda, S.C.; Rudranaik, S.; Beedubail, S.P. Assessment of Color Changes in Teeth and Composite Resins under the Influence of Chlorhexidine with and without Anti-Discoloration System: An in Vitro Study. J. Conserv. Dent. 2023, 26, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, S.; Bellei, E.; Generali, L.; Tomasi, A.; Bertoldi, C. A Proteomic Analysis of Discolored Tooth Surfaces after the Use of 0.12% Chlorhexidine (CHX) Mouthwash and CHX Provided with an Anti-Discoloration System (ADS). Materials 2021, 14, 4338. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, E.; Tetè, G.; Bova, F.; Pantaleo, G.; Gastaldi, G.; Capparè, P.; Gherlone, E. Antibacterial Properties and Side Effects of Chlorhexidine-Based Mouthwashes. A Prospective, Randomized Clinical Study. J. Osseointegr 2020, 12, 2–7. [Google Scholar] [CrossRef]
- Kamolnarumeth, K.; Thussananutiyakul, J.; Lertchwalitanon, P.; Rungtanakiat, P.; Mathurasai, W.; Sooampon, S.; Arunyanak, S.P. Effect of Mixed Chlorhexidine and Hydrogen Peroxide Mouthrinses on Developing Plaque and Stain in Gingivitis Patients: A Randomized Clinical Trial. Clin. Oral Investig. 2021, 25, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Mihajlo, P.; Olivera, T.P.; Tamara, T.; Kiro, P. Side Effects Associated with Chlorhexidine Mouthwashes Use. Maced. Pharm. Bull. 2022, 68, 377–378. [Google Scholar] [CrossRef]
- Rose, M.A.; Garcez, T.; Savic, S.; Garvey, L.H. Chlorhexidine Allergy in the Perioperative Setting: A Narrative Review. Br. J. Anaesth. 2019, 123, e95–e103. [Google Scholar] [CrossRef]
- Pemberton, M.N.; Gibson, J. Chlorhexidine and Hypersensitivity Reactions in Dentistry. Br. Dent. J. 2012, 213, 547–550. [Google Scholar] [CrossRef]
- Watts, T.J.; Thursfield, D.; Haque, R. Fixed Drug Eruption Due to Chlorhexidine Mouthwash Confirmed by Lesional Patch Testing. J. Allergy Clin. Immunol. Pract. 2019, 7, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Wanin, S.; Baron, M.; Carra, S.; Saf, S.; Bourgoin-Heck, M.; Chiriac, A.M. Chlorhexidine anaphylaxis in three children secondary to oral exposure without evidence of mucosal breach. Pediatr. Allergy Immunol. 2022, 33, e13897. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, H.; Meng, J. A rare case of chlorhexidine- and clindamycin-induced anaphylaxis. Asian Pac. J. Allergy Immunol. 2023, 41, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Xu, C.; Zhang, X.; Li, J.; Dong, G.; Cao, J.; Zhou, T. Chlorhexidine Exposure of Clinical Klebsiella Pneumoniae Strains Leads to Acquired Resistance to This Disinfectant and to Colistin. Int. J. Antimicrob. Agents 2019, 53, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Mensitieri, F.; Caggiano, M.; Gaudino, G.; Charlier, B.; Coglianese, A.; Amato, A.; Di Spirito, F.; Amato, M.; Dal Piaz, F.; Izzo, V. In Vitro Evaluation of Antibacterial and Antibiofilm Activity of Different Chlorhexidine-Containing Mouthwash Formulations against Streptococcus mutans. Appl. Sci. 2023, 13, 7531. [Google Scholar] [CrossRef]
- Kashiwazaki, J.; Nakamura, K.; Hara, Y.; Harada, R.; Wada, I.; Kanemitsu, K. Evaluation of the Cytotoxicity of Various Hand Disinfectants and Ozonated Water to Human Keratinocytes in a Cultured Epidermal Model. Adv. Skin. Wound Care 2020, 33, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Werner, J.; Kirsch, T.; Zuckerman, J.D.; Virk, M.S. Cytotoxicity Evaluation of Chlorhexidine Gluconate on Human Fibroblasts, Myoblasts, and Osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Fernández, M.; Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; de Luna-Bertos, E.; Ruiz, C.; Ramos-Torrecillas, J.; Illescas-Montes, R. Effect of the Most Common Wound Antiseptics on Human Skin Fibroblasts. Clin. Exp. Dermatol. 2022, 47, 1543. [Google Scholar] [CrossRef]
- Prietto, N.R.; Martins, T.M.; Santinoni, C.d.S.; Pola, N.M.; Ervolino, E.; Bielemann, A.M.; Leite, F.R.M. Treatment of Experimental Periodontitis with Chlorhexidine as Adjuvant to Scaling and Root Planing. Arch. Oral Biol. 2020, 110, 104600. [Google Scholar] [CrossRef]
- Ripari, F.; Cera, A.; Freda, M.; Zumbo, G.; Zara, F.; Vozza, I. Tea Tree Oil versus Chlorhexidine Mouthwash in Treatment of Gingivitis: A Pilot Randomized, Double Blinded Clinical Trial. Eur. J. Dent. 2020, 14, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Izzetti, R.; Perić, M.; Marhl, U.; Nisi, M.; Gennai, S. Early Periodontal Wound Healing after Chlorhexidine Rinsing: A Randomized Clinical Trial. Clin. Oral Investig. 2024, 28, 354. [Google Scholar] [CrossRef]
- Mauland, E.K.; Preus, H.R.; Aass, A.M. Comparison of Commercially Available 0.2% Chlorhexidine Mouthwash with and without Anti-Discoloration System: A Blinded, Crossover Clinical Trial. J. Clin. Periodontol. 2020, 47, 1522–1527. [Google Scholar] [CrossRef]
- Grover, V.; Mahendra, J.; Gopalakrishnan, D.; Jain, A. Effect of Octenidine Mouthwash on Plaque, Gingivitis, and Oral Microbial Growth: A Systematic Review. Clin. Exp. Dent. Res. 2021, 7, 450. [Google Scholar] [CrossRef] [PubMed]
- Köck, R.; Denkel, L.; Feßler, A.T.; Eicker, R.; Mellmann, A.; Schwarz, S.; Geffers, C.; Hübner, N.O.; Leistner, R. Clinical Evidence for the Use of Octenidine Dihydrochloride to Prevent Healthcare-Associated Infections and Decrease Staphylococcus aureus Carriage or Transmission—A Review. Pathogens 2023, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Seiser, S.; Janker, L.; Zila, N.; Mildner, M.; Rakita, A.; Matiasek, J.; Bileck, A.; Gerner, C.; Paulitschke, V.; Elbe-Bürger, A. Octenidine-Based Hydrogel Shows Anti-Inflammatory and Protease-Inhibitory Capacities in Wounded Human Skin. Sci. Rep. 2021, 11, 32. [Google Scholar] [CrossRef]
- Lu, D.; Li, F.; Zhao, C.; Ye, Y.; Zhang, X.; Yang, P.; Zhang, X. A Remineralizing and Antibacterial Coating for Arresting Caries. J. Dent. Res. 2023, 102, 1315–1325. [Google Scholar] [CrossRef]
- Vidović, B.; Gušić, I.; Tamaš, I.; Mihajlović, D.; Mitić, V.; Obradović, R.; Radovanović, M.; Brkić, S. The Effect of the Octenidine-Based Oral Antiseptic on the Structure of Microbial Communities and Periodontal Status in Patients with Fixed Orthodontic Treatments. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8598–8605. [Google Scholar] [CrossRef]
- Grocholewicz, K.; Mikłasz, P.; Zawiślak, A.; Sobolewska, E.; Janiszewska-Olszowska, J. Fluoride Varnish, Ozone and Octenidine Reduce the Incidence of White Spot Lesions and Caries during Orthodontic Treatment: Randomized Controlled Trial. Sci. Rep. 2022, 12, 13985. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, R.A.; Ajitha, P.; Subbaiyan, H. Comparative Evaluation of the Antimicrobial Efficacy of Octenidine Dihydrochloride with Contemporary Root Canal Disinfectants: A Systematic Review. J. Pharm. Res. Int. 2020, 32, 64–76. [Google Scholar] [CrossRef]
- Rath, A.; Wong, M.; Li, K.; Wong, A.; Tan, L.; Tan, K.; Pannuti, C.M. Efficacy of Adjunctive Octenidine Hydrochloride as Compared to Chlorhexidine and Placebo as Adjuncts to Instrumentation in Stage I–II Periodontitis: A Double-Blinded Randomized Controlled Trial. Int. J. Dent. Hyg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ariel, H.; Kahn, A.; Hila, Z.O.; Anton, S.; Natan, G.; Kolerman, R. A Thermosensitive Gel with an Active Hyaluronic Acid Ingredient That Contains an Octenidine Preservation System as an Adjunct to Scaling and Root Planning: A Randomized Prospective Clinical Study. Clin. Oral Investig. 2022, 26, 3721–3733. [Google Scholar] [CrossRef] [PubMed]
- Welk, A.; Zahedani, M.; Beyer, C.; Kramer, A.; Müller, G. Antibacterial and Antiplaque Efficacy of a Commercially Available Octenidine-Containing Mouthrinse. Clin. Oral Investig. 2016, 20, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.V.S.; Gibb, A.P. Use of Octenidine Dihydrochloride in Meticillin-Resistant Staphylococcus aureus Decolonisation Regimens: A Literature Review. J. Hosp. Infect. 2010, 74, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Ön, A.; Pabst, G.; Zellner, A.; Lohner, K. Octenidine: Novel Insights into the Detailed Killing Mechanism of Gram-Negative Bacteria at a Cellular and Molecular Level. Int. J. Antimicrob. Agents 2020, 56, 106146. [Google Scholar] [CrossRef] [PubMed]
- Jockel-Schneider, Y.; Schlagenhauf, U.; Petsos, H.; Rüttermann, S.; Schmidt, J.; Ziebolz, D.; Wehner, C.; Laky, M.; Rott, T.; Noack, M.; et al. Impact of 0.1% Octenidine Mouthwash on Plaque Re-Growth in Healthy Adults: A Multi-Center Phase 3 Randomized Clinical Trial. Clin. Oral Investig. 2021, 25, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, K.; Meister, T.L.; Todt, D.; Krawczyk, A.; Paßvogel, L.; Becker, B.; Paulmann, D.; Bischoff, B.; Pfaender, S.; Brill, F.H.H.; et al. Comparison of the In-Vitro Efficacy of Different Mouthwash Solutions Targeting SARS-CoV-2 Based on the European Standard EN 14476. J. Hosp. Infect. 2021, 111, 180–183. [Google Scholar] [CrossRef]
- Mateos-Moreno, M.V.; Mira, A.; Ausina-Márquez, V.; Ferrer, M.D. Oral Antiseptics against Coronavirus: In-Vitro and Clinical Evidence. J. Hosp. Infect. 2021, 113, 30. [Google Scholar] [CrossRef]
- Smeets, R.; Pfefferle, S.; Büttner, H.; Knobloch, J.K.; Lütgehetmann, M. Impact of Oral Rinsing with Octenidine Based Solution on SARS-CoV-2 Loads in Saliva of Infected Patients an Exploratory Study. Int. J. Environ. Res. Public. Health 2022, 19, 5582. [Google Scholar] [CrossRef]
- Kalbhenn, J.; Zieger, B.; Casetti, F. Severe herpes-simplex-virus-1-reactivation during severe SARS-CoV-2 infection. J. Clin. Images Med. Case Rep. 2021, 2, 1067. [Google Scholar] [CrossRef]
- Knox, P.P.; Lukashev, E.P.; Korvatovsky, B.N.; Mamedov, M.D.; Strakhovskaya, M.G.; Gvozdev, D.A.; Paschenko, V.Z.; Rubin, A.B. The Influence of Cationic Antiseptics on the Processes of Light Energy Conversion in Various Photosynthetic Pigment-Protein Complexes. Photosynth. Res. 2024, 161, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Buttress, J.A.; Vejzovic, D.; Ön, A.; Piller, P.; Kolb, D.; Lohner, K.; Strahl, H. Disruption of the Cytoplasmic Membrane Structure and Barrier Function Underlies the Potent Antiseptic Activity of Octenidine in Gram-Positive Bacteria. Appl. Environ. Microbiol. 2022, 88, e0018022. [Google Scholar] [CrossRef] [PubMed]
- Greener, M. Octenidine: Antimicrobial activity and clinical efficacy. Wounds UK 2011, 7, 74–78. [Google Scholar]
- Khabadze, Z.; Makeeva, I.; Makeeva, M.; Nazarova, D.; Shilyaeva, E.; Bakaev, Y.; Dashtieva, M.; Kozhevnikova, L.; Pilschikova, O.; Mordanov, O. The Use of the Antiseptic Solution “Octenisept” in Endodontic Practice: The Systematic Review. J. Int. Dent. Med. Res. 2022, 15, 1348–1351. [Google Scholar]
- Joon, A.; Khetarpal, A.; Dahiya, S. IP Indian Journal of Conservative and Endodontics Comparitive Evaluation of Antimicrobial Efficacy of 0.1% Octenidine Dihydrochloride, 2% Chlorhexidine and 2% Chitosan against E. faecalis within the Dentinal Tubules. IP Indian J. Conserv. Endod. 2020, 5, 192–199. [Google Scholar] [CrossRef]
- Kaur, K.; Singh, T.; Benipal, P.S. Antimicrobial Efficacy of Octenidine Dihydrochloride And Artemisia Annua Plant Extract as Root Canal Irrigants—An In Vivo Study. J. Dent. Oral Sci. 2021, 3, 1–12. [Google Scholar] [CrossRef]
- Chum, J.D.; Lim, D.J.Z.; Sheriff, S.O.; Pulikkotil, S.J.; Suresh, A.; Davamani, F. In Vitro Evaluation of Octenidine as an Antimicrobial Agent against Staphylococcus epidermidis in Disinfecting the Root Canal System. Restor. Dent. Endod. 2019, 44, e8. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.M.; Alharbi, T.M.; Alqahtani, M.S.; Elfasakhany, F.M.; Afifi, I.K.; Rajeh, M.T.; Fattouh, M.; Kenawi, L.M.M. A Comparative Evaluation of Antibacterial Efficacy of Moringa oleifera Leaf Extract, Octenidine Dihydrochloride, and Sodium Hypochlorite as Intracanal Irrigants against Enterococcus faecalis: An In Vitro Study. Int. J. Dent. 2023, 2023, 7690497. [Google Scholar] [CrossRef]
- Cherian, B.; Gehlot, P.M.; Manjunath, M.K. Comparison of the Antimicrobial Efficacy of Octenidine Dihydrochloride and Chlorhexidine with and without Passive Ultrasonic Irrigation—An Invitro Study. J. Clin. Diagn. Res. 2016, 10, ZC71–ZC77. [Google Scholar] [CrossRef]
- Amrita, A.; Agarwal, P.; Agarwal, M.C.; Agarwal, A.; Garg, J.; Mehra, P. Comparative Evaluation of Octenidine with Chlorhexidine Mouthwash in Gingivitis and Periodontitis Patients: A Randomized Clinical Trial. J. Pharm. Bioallied Sci. 2024, 16, S789. [Google Scholar] [CrossRef] [PubMed]
- Razi, M.A.; Ahuja, A.; Qamar, S.; Mahajan, A.; Mittal, P. Efficacy of 0.2% Chlorhexidine Gluconate and 0.1% Octenidine Dihydrochloride Mouth Rinses in Patients with Plaque Induced Gingivitis: Double Blinded Randomised Case Control Study. Univ. J. Dent. Sci. 2021, 7, 9–16. [Google Scholar] [CrossRef]
- Sadanandan, S.; Suhas, S.; Venugopal, S.; Karur, K. Comparative Evaluation of 0.1% Octenidine Mouthwash with 0.2% Chlorhexidine Mouthwash in Prevention of Plaque and Gingivitis—A Clinicomicrobiological Study. RGUHS J. Dent. Sci. 2021, 13, 202–210. [Google Scholar] [CrossRef]
- Babalska, Z.Ł.; Korbecka-Paczkowska, M.; Karpiński, T.M. Wound Antiseptics and European Guidelines for Antiseptic Application in Wound Treatment. Pharmaceuticals 2021, 14, 1253. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, N.; Kienzl, P.; Tajpara, P.; Vierhapper, M.; Matiasek, J.; Elbe-Bürger, A. The Antiseptic Octenidine Inhibits Langerhans Cell Activation and Modulates Cytokine Expression upon Superficial Wounding with Tape Stripping. J. Immunol. Res. 2019, 2019, 5143635. [Google Scholar] [CrossRef] [PubMed]
- Reda, B.; Dudek, J.; Martínez-Hernández, M.; Hannig, M. Effects of Octenidine on the Formation and Disruption of Dental Biofilms: An Exploratory In Situ Study in Healthy Subjects. J. Dent. Res. 2021, 100, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Salah, T.; Salem, H.N. The Effect of Octenidine Dihydrochloride on the Antibacterial Activity of a Formulated Resin Composite: An in Vitro Study. Bull. Natl. Res. Cent. 2024, 48, 1–6. [Google Scholar] [CrossRef]
- Spettel, K.; Bumberger, D.; Camp, I.; Kriz, R.; Willinger, B. Efficacy of Octenidine against Emerging Echinocandin-, Azole- and Multidrug-Resistant Candida albicans and Candida Glabrata. J. Glob. Antimicrob. Resist. 2022, 29, 23–28. [Google Scholar] [CrossRef]
- Mohan, M.; Muddappa, S.C.; Venkitachalam, R.; Prabath, S.V.P.; Rajan, R.R.; Kavitha, R. Comparison of Antimicrobial Efficacy of Octenidine Dihydrochloride and Chlorhexidine as Endodontic Irrigant: A Systematic Review. World J. Dent. 2023, 14, 373–381. [Google Scholar] [CrossRef]
- Lorenz, K.; Jockel-Schneider, Y.; Petersen, N.; Stölzel, P.; Petzold, M.; Vogel, U.; Hoffmann, T.; Schlagenhauf, U.; Noack, B. Impact of Different Concentrations of an Octenidine Dihydrochloride Mouthwash on Salivary Bacterial Counts: A Randomized, Placebo-Controlled Cross-over Trial. Clin. Oral Investig. 2018, 22, 2917–2925. [Google Scholar] [CrossRef]
- Cai, X.; Venkatesan, J.K.; Schmitt, G.; Reda, B.; Cucchiarini, M.; Hannig, M.; Madry, H. Cytotoxic Effects of Different Mouthwash Solutions on Primary Human Articular Chondrocytes and Normal Human Articular Cartilage—An in Vitro Study. Clin. Oral Investig. 2023, 27, 4987–5000. [Google Scholar] [CrossRef]
- Addy, M.; Moran, J. Mechanisms of Stain Formation on Teeth, in Particular Associated with Metal Ions and Antiseptics. Adv. Dent. Res. 1995, 9, 450–456. [Google Scholar] [CrossRef]
- Mueller-Wirth, N.; Buenter, A.; Jörg, L.; Ebo, D.G.; Glatz, M.; Fernando, S.L.; Spoerl, D.; Helbling, A.; Hausmann, O.; Gupta, N.; et al. IgE-Mediated Chlorhexidine Allergy—Cross-Reactivity with Other Biguanide Disinfectants. Allergy 2020, 75, 3237–3247. [Google Scholar] [CrossRef] [PubMed]
- Gugsch, F.; Tan, C.K.; Oh, D.Y.; Paßvogel, L.; Steinhauer, K. Efficacy of Octenidine- and Chlorhexidine-Based Wash-Mitts against Candida albicans and Candida Auris—A Comparative Study. J. Hosp. Infect. 2024, 143, 91–96. [Google Scholar] [CrossRef]
- Schmidt, J.; Zyba, V.; Jung, K.; Rinke, S.; Haak, R.; Mausberg, R.F.; Ziebolz, D. Cytotoxic Effects of Octenidine Mouth Rinse on Human Fibroblasts and Epithelial Cells—An in Vitro Study. Drug Chem. Toxicol. 2016, 39, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cabanilla, L.L. Periodontal Probing Depth Measurement: A Review. Compend. Contin. Educ. Dent. 2009, 30, 12–14, 16, 18–21. [Google Scholar]
- Corbet, E.F. Oral Diagnosis and Treatment Planning: Part 3. Periodontal Disease and Assessment of Risk. Br. Dent. J. 2012, 213, 111–121. [Google Scholar] [CrossRef]
- Azevedo, C.L.; Henriques, P.S.G.; Pannuti, C.M.; Michel-Crosato, E. Selfie Dental Plaque Index: A New Tool for Dental Plaque Assessment. J. Clin. Exp. Dent. 2022, 14, e926. [Google Scholar] [CrossRef]
- Durani, P.; Leaper, D. Povidone–Iodine: Use in Hand Disinfection, Skin Preparation and Antiseptic Irrigation. Int. Wound J. 2008, 5, 376. [Google Scholar] [CrossRef]
- Makhayeva, D.N.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Polymeric Iodophors: Preparation, Properties, and Biomedical Applications. Rev. J. Chem. 2020, 10, 2020. [Google Scholar] [CrossRef]
- Amtha, R.; Kanagalingam, J. Povidone-Iodine in Dental and Oral Health: A Narrative Review. J. Int. Oral Health 2020, 12, 407–412. [Google Scholar] [CrossRef]
- Jeronimo, L.P.; Choi, M.R.; Yeon, S.H.; Park, S.K.; Yoon, Y.H.; Choi, S.H.; Kim, H.J.; Jang, I.T.; Park, J.K.; Rha, K.S.; et al. Effects of Povidone-Iodine Composite on the Elimination of Bacterial Biofilm. Int. Forum Allergy Rhinol. 2020, 10, 884–892. [Google Scholar] [CrossRef] [PubMed]
- López-Álvarez, M.; Ulmer, H.; Klay, N.; van Dijl, J.M. Evaluation of New Polymer-Iodine Complexes for the Fabrication of Medical Devices. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bayer, G.; Grasselli, S.; Malchiodi, A.; Bayer, I.S. Antiseptic Povidone-Iodine Encapsulating Edible Phospholipid Gels. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126537. [Google Scholar] [CrossRef]
- Tatu, A.L.; Ardeleanu, V.; Elisei, A.M.; Buzia, O.D.; Miulescu, M.; Nwabudike, L.C. Undesirable Effects of Some Topical Antiseptics Chemical, Pharmacological and Dermatological Aspects. Rev. Chim. 2019, 70, 2276–2280. [Google Scholar] [CrossRef]
- Elzein, R.; Abdel-Sater, F.; Fakhreddine, S.; Hanna, P.A.; Feghali, R.; Hamad, H.; Ayoub, F. In Vivo Evaluation of the Virucidal Efficacy of Chlorhexidine and Povidone-Iodine Mouthwashes against Salivary SARS-CoV-2. A Randomized-Controlled Clinical Trial. J. Evid. Based Dent. Pract. 2021, 21, 101584. [Google Scholar] [CrossRef] [PubMed]
- Hasheminia, D.; Moaddabi, A.; Moradi, S.; Soltani, P.; Moannaei, M.; Issazadeh, M. The Efficacy of 1% Betadine Mouthwash on the Incidence of Dry Socket after Mandibular Third Molar Surgery. J. Clin. Exp. Dent. 2018, 10, e445. [Google Scholar] [CrossRef] [PubMed]
- Eggers, M. Infectious Disease Management and Control with Povidone Iodine. Infect. Dis. Ther. 2019, 8, 581–593. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.K.; Wa, C.T.C.; Villa, M.A. Povidone Iodine in Wound Healing: A Review of Current Concepts and Practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef]
- Arefin, M.K.; Khan, M.; Rumi, S.N.F.; Islam, M.N.; Talukder, D.C.; Osmani, H.Q.; Hossain, M.D.; Arafat, M.S.; Kaiser, A.; Samad, M.S.; et al. Post COVID Mucormycosis Prevention With Oro-Nasal Application of Povidone Iodine (Pvp-I). J. Dhaka Med. Coll. 2023, 30, 176–179. [Google Scholar] [CrossRef]
- Lepelletier, D.; Maillard, J.Y.; Pozzetto, B.; Simon, A. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob. Agents Chemother. 2020, 64, 1–13. [Google Scholar] [CrossRef]
- Anderson, D.E.; Sivalingam, V.; Kang, A.E.Z.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity against SARS-CoV-2, The Virus Causing COVID-19 Disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kirk-Bayley, J.; Challacombe, S.; Sunkaraneni, V.; Combes, J. The Use of Povidone Iodine Nasal Spray and Mouthwash during the Current COVID-19 Pandemic May Reduce Cross Infection and Protect Healthcare Workers. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Suzuki, R.; Suzuki, S. Povidone-Iodine-Induced Transient Triiodothyronine Thyrotoxicosis in a Japanese Patient with Prolonged Habitual Gargling: A Case Report and Literature Review. Medicine 2023, 102, e34631. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, G.; Venkat, M. The effect of iodine in patients using povidone-iodine mouth wash on thyroid function. Int. J. Otorhinolaryngol. Head. Neck Surg. 2019, 5, 1562. [Google Scholar] [CrossRef]
- Kanagalingam, J.; Feliciano, R.; Hah, J.H.; Labib, H.; Le, T.A.; Lin, J.C. Practical Use of Povidone-Iodine Antiseptic in the Maintenance of Oral Health and in the Prevention and Treatment of Common Oropharyngeal Infections. Int. J. Clin. Pract. 2015, 69, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Capriotti, J.; Brown, S.M.; Tessema, B. Povidone-Iodine Use in Sinonasal and Oral Cavities: A Review of Safety in the COVID-19 Era. Ear Nose Throat J. 2020, 99, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lo, E.; Leung, K.; Chu, C. Clinical Trial Combining PVP-I and NaF in Preventing Root Caries. Int. Assoc. Dent. Res. 2019, 98. [Google Scholar]
- Kaewiad, K.; Nakpheng, T.; Srichana, T. Dental Floss Impregnated with Povidone-Iodine Coated with Eudragit L-100 as an Antimicrobial Delivery System against Periodontal-Associated Pathogens. J. Med. Microbiol. 2020, 69, 298–308. [Google Scholar] [CrossRef]
- Thi, A.; Nguyen, M.; Phan, N.D.; Anh, T.; Pham, V. Povidone-Iodine as Subgingival Irrigation in Chronic Periodontitis Treatment. Adv. Health Sci. Res. 2018, 4, 19–29. [Google Scholar] [CrossRef]
- Kida, D.; Gladysz, O.; Szulc, M.; Zborowski, J.; Junka, A.; Janeczek, M.; Lipinska, A.; Skalec, A.; Karolewicz, B. Development and Evaluation of a Polyvinylalcohol-Cellulose Derivative-Based Film with Povidone-Iodine Predicted for Wound Treatment. Polymers 2020, 12, 1271. [Google Scholar] [CrossRef]
- Managutti, A.; Managutti, S.A.; Patel, J.; Puthanakar, N.Y. Evaluation of Post-Surgical Bacteremia with Use of Povidone-Iodine and Chlorhexidine During Mandibular Third Molar Surgery. J. Maxillofac. Oral Surg. 2017, 16, 485. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, N.; Das, B.; Hugar, S.I.; Sarangi, P.; Garg, G.; Kamatchi Subramani, S. Comparing the Biofilm Removal Capacity of NaOCl, Povidone-Iodine, Chlorhexidine, Curcumin, and Triphala as Endodontic Irrigants. Cureus 2024, 16, e52067. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.L.; Johari, N.H. Comparative in Vitro Evaluation of the Antimicrobial Activities of Povidone-Iodine and Other Commercially Available Antiseptics against Clinically Relevant Pathogens. GMS Hyg. Infect. Control 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Amin, M.S.; Harrison, R.L.; Benton, T.S.; Roberts, M.; Weinstein, P. Effect of Povidone-Iodine on Streptococcus mutans in Children with Extensive Dental Caries. Pediatr. Dent. 2004, 26, 5–10. [Google Scholar] [PubMed]
- Farebrother, J.; Zimmermann, M.B.; Andersson, M. Excess Iodine Intake: Sources, Assessment, and Effects on Thyroid Function. Ann. N. Y. Acad. Sci. 2019, 1446, 44–65. [Google Scholar] [CrossRef] [PubMed]
- Bílek, R.; Dvořáková, M.; Grimmichová, T.; Jiskra, J. Iodine, Thyroglobulin and Thyroid Gland. Physiol. Res. 2020, 69, S225–S236. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Sivaraman, K.; Radhakrishnan, R.; Balakrishnan, D.; Narayana, A. Can Povidone Iodine Gargle/Mouthrinse Inactivate SARS-CoV-2 and Decrease the Risk of Nosocomial and Community Transmission during the COVID-19 Pandemic? An Evidence-Based Update. Jpn. Dent. Sci. Rev. 2021, 57, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Zhao, R.; Ren, X.; Li, Y.; Ji, P. Oral Transmucosal Absorption of Iodine after Intraoral Preparation with Povidone-Iodine Prior to Oral Surgeries: A Randomized Controlled Study in 12 Male Patients. Clin. Oral Investig. 2022, 26, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, S.G.; Obuekwe, O.N.; Iyorzor, S.O.; Sani, M.I. Seizures Associated with Povidone-Iodine Impregnated Antral Pack in a Child: A Case Report and Review of Literature: Runing Title: Povidone-Iodine Induced Seizures. Niger. Dent. J. 2024, 32, 68–71. [Google Scholar] [CrossRef]
- Jeyapriya, M.V.; Milling Tania, S.D.; Rathore, S.; Missier, S.; Shaga, B. Comparative Evaluation of Antibacterial Activity of Chlorhexidine, Povidone Iodine and Glutaraldehyde for Disinfection of Orthodontic Appliances—An In-Vitro Study. Int. J. Orthod. Rehabil. 2023, 14, 44–54. [Google Scholar] [CrossRef]
- Hoekstra, M.J.; Westgate, S.J.; Mueller, S. Povidone-Iodine Ointment Demonstrates in Vitro Efficacy against Biofilm Formation. Int. Wound J. 2017, 14, 172–179. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, J.; Liu, Y.; Ben, C.; Li, H.; Cheng, D.; Sun, Y.; Guang-Yi, W.; Zhu, S. Analysis of Povidone Iodine, Chlorhexidine Acetate and Polyhexamethylene Biguanide as Wound Disinfectants: In Vitro Cytotoxicity and Antibacterial Activity. BMJ Nutr. Prev. Health 2023, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Khorolsuren, Z.; Lang, O.; Vag, J.; Kohidai, L. Effect of Dental Antiseptic Agents on the Viability of Human Periodontal Ligament Cells. Saudi Dent. J. 2021, 33, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Shreenidhi, S.; Rajasekar, A. Clinical Efficacy of Different Concentrations of Povidone Iodine in the Management of Peri-Implant Mucositis. J. Long-Term Eff. Med. Implant. 2024, 34, 79–83. [Google Scholar] [CrossRef]
- Tsuda, S.; Soutome, S.; Hayashida, S.; Funahara, M.; Yanamoto, S.; Umeda, M. Topical Povidone Iodine Inhibits Bacterial Growth in the Oral Cavity of Patients on Mechanical Ventilation: A Randomized Controlled Study. BMC Oral Health 2020, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Baliga, S.D.; Baliga, S.S.; Rathi, P.; Jha, G. Efficacy of Hydrocortisone, Povidone-Iodine, and Normal Saline as an Irrigating Solution During Surgical Removal of Impacted Mandibular Third Molars: A Randomized Controlled Trial. Cureus 2024, 16, e53370. [Google Scholar] [CrossRef]
- Fernandez-Riera, Y.; Gutmann, J. Historical Reflections on the Use of Internal Bleaching to Manage Discolored Teeth. J. Hist. Dent. 2022, 70, 119–127. [Google Scholar]
- Dixit, S. Bleaching Agents: Chemicals That Ensure Stain-Free Clothes. Chem. Wkly. 2002, 47, 183–202. [Google Scholar]
- Spencer, H.R.; Ike, V.; Brennan, P.A. Review: The Use of Sodium Hypochlorite in Endodontics-Potential Complications and Their Management. Br. Dent. J. 2007, 202, 555–559. [Google Scholar] [CrossRef]
- Baruwa, A.O.; Martins, J.N.R.; Maravic, T.; Mazzitelli, C.; Mazzoni, A.; Ginjeira, A. Effect of Endodontic Irrigating Solutions on Radicular Dentine Structure and Matrix Metalloproteinases—A Comprehensive Review. Dent. J. 2022, 10, 219. [Google Scholar] [CrossRef]
- Bukhari, S.; Babaeer, A. Irrigation in Endodontics: A Review. Curr. Oral Health Rep. 2019, 6, 367–376. [Google Scholar] [CrossRef]
- Qutieshat, A.; Al Harthy, N.; Al Busaidi, S.; Al Sadoon, A.; Al Sayahien, D.; Sedqi, M.; Al Rashdi, S.; Al Ghammari, S. Antimicrobial Irrigation Solutions in Root Canal Treatment: A Glance at the Past, the Present, and the Future. Open Dent. J. 2023, 17, 1–21. [Google Scholar] [CrossRef]
- Tong, C.; Hu, H.; Chen, G.; Li, Z.; Li, A.; Zhang, J. Chlorine Disinfectants Promote Microbial Resistance in Pseudomonas sp. Environ. Res. 2021, 199, 111296. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.M.; Al Nesser, S.F.; Bshara, N.G.; AlMidani, A.N.; Comisi, J.C. Comparing the Efficacies of Two Chemo-Mechanical Caries Removal Agents (2.25% Sodium Hypochlorite Gel and Brix 3000), in Caries Removal and Patient Cooperation: A Randomized Controlled Clinical Trial. J. Dent. 2020, 93, 103280. [Google Scholar] [CrossRef] [PubMed]
- Zandi, H.; Rodrigues, R.C.V.; Kristoffersen, A.K.; Enersen, M.; Mdala, I.; Ørstavik, D.; Rôças, I.N.; Siqueira, J.F. Antibacterial Effectiveness of 2 Root Canal Irrigants in Root-Filled Teeth with Infection: A Randomized Clinical Trial. J. Endod. 2016, 42, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Aslanimehr, M.; Gholami, F.; Torbati, S.; Aalaei, S. Effect of Different Disinfecting Agents on Dental Impressions Contaminated with Candida albicans. Dent. Hypotheses 2021, 12, 139–143. [Google Scholar] [CrossRef]
- Mishra, R.; Chandrashekar, K.; Tripathi, V.; Hazari, A.; Sabu, B.; Sahu, A. Comparative Evaluation of Efficacy of 0.2% Sodium Hypochlorite (Hi Wash) Mouthwash with 0.2% Chlorhexidine Mouthwash on Plaque-Induced Gingivitis: A Clinical Trial. J. Indian Soc. Periodontol. 2019, 23, 534. [Google Scholar] [CrossRef] [PubMed]
- Degrossoli, A.; Müller, A.; Xie, K.; Schneider, J.F.; Bader, V.; Winklhofer, K.F.; Meyer, A.J.; Leichert, L.I. Neutrophil-Generated HOCl Leads to Non-Specific Thiol Oxidation in Phagocytized Bacteria. eLife 2018, 7, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G. Sodium Hypochlorite. Antiseptic Steward 2018, 161–210. [Google Scholar] [CrossRef]
- Ruksakiet, K.; Hanák, L.; Farkas, N.; Hegyi, P.; Sadaeng, W.; Czumbel, L.M.; Sang-Ngoen, T.; Garami, A.; Mikó, A.; Varga, G.; et al. Antimicrobial Efficacy of Chlorhexidine and Sodium Hypochlorite in Root Canal Disinfection: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Endod. 2020, 46, 1032–1041. [Google Scholar] [CrossRef]
- Swanson, S.; Fu, T.J. Effect of Water Hardness on Efficacy of Sodium Hypochlorite Inactivation of Escherichia Coli O157:H7 in Water. J. Food Prot. 2017, 80, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A.T.; Rodloff, A.C.; Labahn, M.; Reinhardt, M.; Truyen, U.; Speck, S. Efficacy of Sodium Hypochlorite against Multidrug-Resistant Gram-Negative Bacteria. J. Hosp. Infect. 2018, 100, e40–e46. [Google Scholar] [CrossRef] [PubMed]
- Al-Yasiri, I.; Ali, N.; Al-Feron, M.; Al-Nasrawi, S. Antibacterial Activity of Calcium Hydroxide Combined with Chlorhexidine or Sodium Hypochlorite against Gram Positive and Gram Negative Bacteria. J. Nat. Sci. Res. 2014, 4., 55–61. [Google Scholar]
- Montagner, H.; Montagner, F.; Braun, K.O.; Peres, P.E.C.; Gomes, B.P.F.d.A. In Vitro Antifungal Action of Different Substances over Microwaved-Cured Acrylic Resins. J. Appl. Oral Sci. 2009, 17, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; Meto, A.; Cicciù, F.; De Stefano, R. An Eventual Sars-CoV-2 Infection Prevention Protocol in the Medical Setting and Dental Office. Int. J. Environ. Res. Public. Health 2021, 18, 2593. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Estrela, C.R.A.; Luis Barbin, E.; César, J.; Spanó, E.; Marchesan, M.A.; Pécora, J.D. Mechanism of Action of Sodium Hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Osinnikova, D.N.; Moroshkina, E.B.; Mokronosova, E.S. Effect of Sodium Hypochlorite on Nucleic Acids of Different Primary and Secondary Structures. J. Phys. Conf. Ser. 2019, 1400, 033001. [Google Scholar] [CrossRef]
- Jose, J.; Thamilselvan, A.; Teja, K.V.; Rossi–Fedele, G. Influence of Access Cavity Design, Sodium Hypochlorite Formulation and XP-Endo Shaper Usage on Apical Debris Extrusion—A Laboratory Investigation. Aust. Endod. J. 2023, 49, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Herrera, D.R.; Pecorari, V.G.A.; Gomes, B.P.F.A. Endotoxin Levels after Chemomechanical Preparation of Root Canals with Sodium Hypochlorite or Chlorhexidine: A Systematic Review of Clinical Trials and Meta-Analysis. Int. Endod. J. 2019, 52, 19–27. [Google Scholar] [CrossRef]
- Ballal, N.V.; Duncan, H.F.; Rai, N.; Jalan, P.; Zehnder, M. Clinical Medicine Sodium Hypochlorite Reduces Postoperative Discomfort and Painful Early Failure after Carious Exposure and Direct Pulp Capping-Initial Findings of a Randomized Controlled Trial. J. Clin. Med. 2020, 9, 2408. [Google Scholar] [CrossRef]
- Farzaneh, S.; Parirokh, M.; Nakhaee, N.; Abbott, P.V. Effect of Two Different Concentrations of Sodium Hypochlorite on Postoperative Pain Following Single-Visit Root Canal Treatment: A Triple-Blind Randomized Clinical Trial. Int. Endod. J. 2018, 51, e2–e11. [Google Scholar] [CrossRef] [PubMed]
- Valera, M.C.; da Silva, K.C.G.; Maekawa, L.E.; Carvalho, C.A.T.; Koga-Ito, C.Y.; Camargo, C.H.R.; e Lima, R.S. Antimicrobial activity of sodium hypochlorite associated with intracanal medication for Candida albicans and Enterococcus faecalis inoculated in root canals. J. Appl. Oral Sci. 2009, 17, 555. [Google Scholar] [CrossRef] [PubMed]
- Shanker, K.; Suragimath, G.; Zope, S.A.; Varma, S.A.; Ashwinirani, S.R. A Case-Control Study to Assess and Compare the Efficacy of 0.2% Chlorhexidine and 0.25% Sodium Hypochlorite Mouthwash in Treatment of Chronic Gingivitis. Asian J. Pharm. Clin. Res. 2018, 11, 313–315. [Google Scholar] [CrossRef]
- Jurczyk, K.; Nietzsche, S.; Ender, C.; Sculean, A.; Eick, S. In-Vitro Activity of Sodium-Hypochlorite Gel on Bacteria Associated with Periodontitis. Clin. Oral Investig. 2016, 20, 2165–2173. [Google Scholar] [CrossRef]
- Hussain, A.M.; van der Weijden, G.A.; Slot, D.E. Effect of a Sodium Hypochlorite Mouthwash on Plaque and Clinical Parameters of Periodontal Disease—A Systematic Review. Int. J. Dent. Hyg. 2022, 20, 40–52. [Google Scholar] [CrossRef]
- Kardaras, G.; Christodorescu, R.; Boariu, M.; Rusu, D.; Belova, A.; Chinnici, S.; Vela, O.; Radulescu, V.; Boia, S.; Stratul, S.I. A Low-Cost Protocol Using the Adjunctive Action of Povidone–Iodine Irrigations and Sodium Hypochlorite Rinsing Solution in Step 2 of Periodontal Therapy for Patients with Stage III–IV Periodontitis: A Single-Blind, Randomized Controlled Trial. Dent. J. 2024, 12, 144. [Google Scholar] [CrossRef]
- Liu, S.; Zhai, H.; Fu, S.; Cui, C.; Xu, J.; Jiang, J.; Pan, P.; Zhang, B. Evaluation of the Cytotoxic Effects of Sodium Hypochlorite on Human Dental Stem Cells. Trop. J. Pharm. Res. 2018, 17, 2375–2380. [Google Scholar] [CrossRef]
- Sawada, K.; Nakahara, K.; Haga-Tsujimura, M.; Fujioka-Kobayashi, M.; Iizuka, T.; Miron, R.J. Effect of Irrigation Time of Antiseptic Solutions on Bone Cell Viability and Growth Factor Release. J. Craniofac Surg. 2018, 29, 376–381. [Google Scholar] [CrossRef]
- Böhle, S.; Röhner, E.; Zippelius, T.; Jacob, B.; Matziolis, G.; Rohe, S. Cytotoxic Effect of Sodium Hypochlorite (Lavanox 0.08%) and Chlorhexidine Gluconate (Irrisept 0.05%) on Human Osteoblasts. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 81–89. [Google Scholar] [CrossRef]
- Uğur Aydin, Z.; Akpinar, K.E.; Hepokur, C.; Erdönmez, D. Assessment of Toxicity and Oxidative DNA Damage of Sodium Hypochlorite, Chitosan and Propolis on Fibroblast Cells. Braz. Oral Res. 2018, 32, e119. [Google Scholar] [CrossRef]
- Youssef, A.R.; Alturkistani, E.; Muharrij, I.; Alsrehi, L.; Shafei, N.; Alzahrani, N.; Alqahtani, M. Effects of Chlorhexidine, Ethylenediaminetetraacetic Acid, and Sodium Hypochlorite on Cell Viability of Human Gingival Fibroblasts in Vitro. Saudi Endod. J. 2020, 10, 234–239. [Google Scholar] [CrossRef]
- Ahmadi, M.; Govil, S. Conventional to EndoVac: A Comparative Evaluation of Two Irrigation Systems in Microbial Reduction of Primary Root Canals Using Chemical Irrigants: An In Vivo Study. Int. J. Clin. Pediatr. Dent. 2023, 16, 113. [Google Scholar] [CrossRef]
- Kotecha, N.; Shah, N.C.; Doshi, R.J.; Kishan, K.V.; Luke, A.M.; Shetty, K.P.; Mustafa, M.; Pawar, A.M. Microbiological Effectiveness of Sodium Hypochlorite Gel and Aqueous Solution When Implemented for Root Canal Disinfection in Multirooted Teeth: A Randomized Clinical Study. J. Funct. Biomater. 2023, 14, 240. [Google Scholar] [CrossRef]
- Ulin, C.; Magunacelaya-Barria, M.; Dahlén, G.; Kvist, T. Immediate Clinical and Microbiological Evaluation of the Effectiveness of 0.5% versus 3% Sodium Hypochlorite in Root Canal Treatment: A Quasi-Randomized Controlled Trial. Int. Endod. J. 2020, 53, 591–603. [Google Scholar] [CrossRef]
- Mao, X.; Aue, D.L.; Buchalla, W.; Hiller, K.A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob. Agents Chemother. 2020, 64, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Osimitz, T.G.; Droege, W. Quaternary Ammonium Compounds: Perspectives on Benefits, Hazards, and Risk. Toxicol. Res. Appl. 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Şenel, S.; Özdoğan, A.I.; Akca, G. Current Status and Future of Delivery Systems for Prevention and Treatment of Infections in the Oral Cavity. Drug Deliv. Transl. Res. 2021, 11, 1703–1734. [Google Scholar] [CrossRef] [PubMed]
- Brezhnev, A.; Tang, F.K.; Kwan, C.S.; Basabrain, M.S.; Tsoi, J.K.H.; Matinlinna, J.P.; Neelakantan, P.; Leung, K.C.F. One-Pot Preparation of Cetylpyridinium Chloride-Containing Nanoparticles for Biofilm Eradication. ACS Appl. Bio Mater. 2023, 6, 1221–1230. [Google Scholar] [CrossRef]
- Latimer, J.; Munday, J.L.; Buzza, K.M.; Forbes, S.; Sreenivasan, P.K.; McBain, A.J. Antibacterial and Anti-Biofilm Activity of Mouthrinses Containing Cetylpyridinium Chloride and Sodium Fluoride. BMC Microbiol. 2015, 15, 169. [Google Scholar] [CrossRef]
- Langa, G.; Muniz, F.; Costa, R.; da Silveira, T.; Rösing, C. The Effect of Cetylpyridinium Chloride Mouthrinse as Adjunct to Toothbrushing Compared to Placebo on Interproximal Plaque and Gingival Inflammation—A Systematic Review with Meta-Analyses. Clin. Oral Investig. 2021, 25, 745–757. [Google Scholar] [CrossRef]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent Development of Active Ingredients in Mouthwashes and Toothpastes for Periodontal Diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef] [PubMed]
- Rizwana, N. The Role of Cetylpyridinium Chloride Mouthwash In The Treatment of Periodontitis. Int. J. Pharm. Sci. Invent. 2013, 2, 36–37. [Google Scholar]
- Stawarz-Janeczek, M.; Kryczyk-Poprawa, A.; Muszyńska, B.; Opoka, W.; Pytko-Polończyk, J. Disinfectants Used in Stomatology and SARS-CoV-2 Infection. Eur. J. Dent. 2020, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Al-Sada, H.A.; Al-Gharrawi, H.A. Effect of Addition of Cetylpyridinium Chloride Cationic Surfactant on the Antimicrobial Activity of Chlorhexidine Endodontic Irrigant. Int. J. Dent. 2024, 2024, 2449447. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Sawa, H.; Sasaki, M.; Orba, Y.; Maishi, N.; Tsumita, T.; Ushijima, N.; Hida, Y.; Sano, H.; Kitagawa, Y.; et al. Antiviral Effect of Cetylpyridinium Chloride in Mouthwash on SARS-CoV-2. Sci. Rep. 2022, 12, 14050. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, P.K.; Haraszthy, V.I.; Zambon, J.J. Antimicrobial Efficacy of 0·05% Cetylpyridinium Chloride Mouthrinses. Lett. Appl. Microbiol. 2013, 56, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Riveira-Muñoz, E.; Garcia-Vidal, E.; Bañó-Polo, M.; León, R.; Blanc, V.; Clotet, B.; Ballana, E. Cetylpyridinium Chloride-Containing Mouthwashes Show Virucidal Activity against Herpes Simplex Virus Type 1. Viruses 2023, 15, 1433. [Google Scholar] [CrossRef] [PubMed]
- Oo, M.M.T.; Oo, P.H.; Saddki, N. Efficacy of 0.05% Cetylpyridinium Chloride Mouthwash as an Adjunct to Toothbrushing Compared with 0.12% Chlorhexidine Gluconate Mouthwash in Reducing Dental Plaque and Gingival Inflammation: A Randomized Control Trial. Int. J. Dent. Hyg. 2023, 21, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Yoshihara, K.; Nagaoka, N.; Makita, Y.; Obika, H.; Okihara, T.; Matsukawa, A.; Yoshida, Y.; Van Meerbeek, B. Rechargeable Anti-Microbial Adhesive Formulation Containing Cetylpyridinium Chloride Montmorillonite. Acta Biomater. 2019, 100, 388–397. [Google Scholar] [CrossRef]
- Cardot, J.M.; Savania, N.; Targett, D.; Freeman, B.; Gray, H.; Stahl, T.; Kästner, U.; Kulasekaran, A. Validated Correlation of Mass Loss and Drug Release in Vitro and in Healthy Subjects for Sugared and Sugar-Free Cetylpyridinium Chloride (CPC) and Benzocaine (1.4 Mg/10 Mg) Lozenges Supports in Vitro Mass Loss and Corresponding Drug Release as a Surrogate for Local Bioequivalence. J. Drug Deliv. Sci. Technol. 2022, 77, 103822. [Google Scholar] [CrossRef]
- Fujimoto, A.; Fujii, K.; Suido, H.; Fukuike, H.; Miyake, N.; Suzuki, H.; Eguchi, T.; Tobata, H. Changes in Oral Microflora Following 0.3% Cetylpyridinium Chloride-Containing Mouth Spray Intervention in Adult Volunteers after Professional Oral Care: Randomized Clinical Study. Clin. Exp. Dent. Res. 2023, 9, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Karikalan, V.; Panneerselvam, A.; Vallalperuman, K. Physico—Chemical analysis on Cetylpyridinium Chloride (CPC) with alcohol solution at different temperatures—Ultrasonic, UV and FTIR Analysis. Dig. J. Nanomater. Biostruct. 2018, 13, 115–128. [Google Scholar]
- Husain, A. Effects of Cetylpyridinium Chloride-Based Chewing Gum Plus Tooth Brushing on Plaque Formation and Gingivitis: A Randomized Triple-Blind, Crossover, Placebo-Controlled Clinical Trial. Master’s Thesis, The University of Texas Health Science Center at Houston School of Dentistry, Houston, TX, USA, 2020. [Google Scholar]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.H.; Cho, J.W.; Yoo, H.J.; Shin, K.H.; Shin, G.H.; Jeon, Y.M.; Lee, J.C. Antibacterial Effect of Mouthwash Containing CPC against Dental Caries Caused Bacteria. J. Korean Acad. Oral Health 2021, 45, 87–91. [Google Scholar] [CrossRef]
- Lv, S.; Fan, W.; Fan, B. Enhanced in Vitro Antibacterial Effect against Enterococcus faecalis by Using Both Low-Dose Cetylpyridinium Chloride and Silver Ions. BMC Oral Health 2023, 23, 299. [Google Scholar] [CrossRef]
- Thirunarayanan, S.; Hegde, M. Value Addition Property of a Cationic Surfactant on Endodontic Irrigant: A Confocal Laser Scanning Microscope Study. J. Conserv. Dent. 2022, 25, 380. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, Z.; Falsafi, P.; Ghanizadeh, M.; Mardani, Z.; Sarvcharandabi, M.; Aghazadeh, M. Comparison of Cytotoxicity of Cetylpyridinium Chloride with Sodium Hypochlorite, Chlorhexidine and Halita as an Endodontic Irrigant Using MTT Assay. Avicenna J. Dent. Res. 2018, 10, 126–132. [Google Scholar] [CrossRef]
- Okamoto, N.; Saito, A.; Okabayashi, T.; Komine, A. Virucidal Activity and Mechanism of Action of Cetylpyridinium Chloride against SARS-CoV-2. J. Oral Maxillofac. Surg. Med. Pathol. 2022, 34, 800–804. [Google Scholar] [CrossRef]
- de Miranda, S.L.F.; Damaceno, J.T.; Faveri, M.; Figueiredo, L.C.; Soares, G.M.S.; Bueno-Silva, B. In Vitro Antimicrobial Effect of Cetylpyridinium Chloride on Complex Multispecies Subgingival Biofilm. Braz. Dent. J. 2020, 31, 103–108. [Google Scholar] [CrossRef]
- Yaghmoor, W.; Ruiz-Torruella, M.; Ogata, Y.; Natto, Z.S.; Finkelman, M.; Kawai, T.; Hur, Y. Effect of Preoperative Chlorhexidine, Essential Oil, and Cetylpyridinium Chloride Mouthwashes on Bacterial Contamination during Dental Implant Surgery: A Randomized Controlled Clinical Trial. Saudi Dent. J. 2024, 36, 492–497. [Google Scholar] [CrossRef]
- Olejnik, E.; Szymanska, J. Active Ingredients of Mouthwashes. Acta Pol. Pharm. Drug Res. 2021, 77, 825–832. [Google Scholar] [CrossRef]
- Tadakamadla, S.K.; Bharathwaj, V.V.; Duraiswamy, P.; Sforza, C.; Tartaglia, G.M. Clinical Efficacy of a New Cetylpyridinium Chloride-Hyaluronic Acid–Based Mouthrinse Compared to Chlorhexidine and Placebo Mouthrinses—A 21-Day Randomized Clinical Trial. Int. J. Dent. Hyg. 2020, 18, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Rösing, C.K.; Cavagni, J.; Gaio, E.J.; Muniz, F.W.M.G.; Ranzan, N.; Oballe, H.J.R.; Friedricht, S.A.; Severo, R.M.; Stewart, B.; Zhang, Y.P. Efficacy of Two Mouthwashes with Cetylpyridinium Chloride: A Controlled Randomized Clinical Trial. Braz. Oral Res. 2017, 31, e47. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Romero, C.; Hernández-Delgadillo, R.; Nakagoshi-Cepeda, S.E.; Sánchez-Najéra, R.I.; Escamilla-García, E.; Solís-Soto, J.M.; García-Cuellar, C.M.; Sánchez-Pérez, Y.; Flores-Treviño, S.M.; Pineda-Aguilar, N.; et al. Antimicrobial and Antitumor Activities of an Alginate-Based Membrane Loaded with Bismuth Nanoparticles and Cetylpyridinium Chloride. J. Appl. Biomater. Funct. Mater. 2024, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kőhidai, Z.; Takács, A.; Lajkó, E.; Géczi, Z.; Pállinger, É.; Láng, O.; Kőhidai, L. The Effects of Mouthwashes in Human Gingiva Epithelial Progenitor (HGEPp) Cells. Clin. Oral Investig. 2022, 26, 4559–4574. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.D.; Eick, S.; Moritz, A.; Lussi, A.; Gruber, R. Cytotoxicity and Antimicrobial Activity of Oral Rinses In Vitro. BioMed Res. Int. 2017, 2017, 4019723. [Google Scholar] [CrossRef]
- Ülker, M.; Çelik, A.C.T.; Yavuz, E.; Kahvecioğlu, F.; Esra Ülker, H. Real-Time Analysis of Antiproliferative Effects of Mouthwashes Containing Alcohol, Sodium Fluoride, Cetylpyridinium Chloride, and Chlorhexidine In Vitro. BioMed Res. Int. 2021, 2021, 2610122. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Antonio, J.M.; Lee, Y.S.; Fadriquela, A.; Kim, S.M.; Han, S.Y.; Lee, Y.; You, J.; Kim, C.S.; Lee, K.J. Oral Health Effect of an Oral Rinse Containing Cetylpyridinium Chloride: A Randomized Clinical Trial. Mol. Cell Toxicol. 2023, 20, 671–678. [Google Scholar] [CrossRef]
- Otagiri, H.; Kurita, H.; Yamada, S.-I.; Sakai, H.; Tobata, H.; Yanai, K.; Matsubara, K.; Eguchi, T. Efficacy of Cetylpridium Chloride Mouthwash Compared to Povidone Iodine on Oral Flora for Perioperative Patient Care: A Randomized Controlled Feasibility Study. J. Oral Maxillofac. Surg. Med. Pathol. 2023, 35, 473–479. [Google Scholar] [CrossRef]
- Brewer, T.F.; Garcia, F.J.; Onak, C.S.; Carroll, K.S.; Chang, C.J. Chemical Approaches to Discovery and Study of Sources and Targets of Hydrogen Peroxide Redox Signaling Through NADPH Oxidase Proteins HHS Public Access. Annu. Rev. Biochem. 2015, 84, 765–790. [Google Scholar] [CrossRef]
- Abdollahi, M.; Hosseini, A. Hydrogen Peroxide. Ency Toxicol. 2014, 2, 967–970. [Google Scholar] [CrossRef]
- Marshall, M.V.; Cancro, L.P.; Fischman, S.L. Hydrogen Peroxide: A Review of Its Use in Dentistry. J. Periodontol. 1995, 66, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Katalinić, I.; Smojver, I.; Morelato, L.; Vuletić, M.; Budimir, A.; Gabrić, D. Evaluation of the Photoactivation Effect of 3% Hydrogen Peroxide in the Disinfection of Dental Implants: In Vitro Study. Biomedicines 2023, 11, 1002. [Google Scholar] [CrossRef] [PubMed]
- Odor, A.A.; Bechir, E.S.; Forna, D.A. Effect of Hydrogen Peroxide Photoactivated Decontamination Using 940 Nm Diode Laser in Periodontal Treatment: A Pilot Study. Photobiomodul Photomed. Laser Surg. 2020, 38, 614–624. [Google Scholar] [CrossRef]
- Matys, J.; Gedrange, T.; Dominiak, M.; Grzech-Leśniak, K. The Impact of Hydrogen Peroxide (H2O2) Fumigation on Bacterial Levels in Dental Office Environments: A Randomized Clinical Trial Investigation. J. Clin. Med. 2023, 12, 7551. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Mata, A.; Silveira, J.; Pereira, R.; Putignano, A.; Orsini, G.; Monterubbianesi, R.; Marques, D. Hydrogen Peroxide Release Kinetics of Four Tooth Whitening Products—In Vitro Study. Materials 2021, 14, 7597. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khaleefa, S.A.; Basheer, M.I. Photolysis of methylene blue dye using an advanced oxidation process (ultraviolet light and hydrogen peroxide). J. Eng. Sustain. Dev. 2021, 25, 59–67. [Google Scholar] [CrossRef]
- Duca, G.; Travin, S. Reactions’ Mechanisms and Applications of Hydrogen Peroxide. Am. J. Phys. Chem. 2020, 9, 36. [Google Scholar] [CrossRef]
- Atabaki, M.S.; Haghgoo, J.M.; Khoshhal, M.; Arabi, R.; Khodadoostan, A.; Gholami, L. Clinical Effect of Periodontal Pocket Irrigation with H2O2. Avicenna J. Dent. Res. 2018, 3, 55–61. [Google Scholar]
- Yaneva, B.K.; Dermendzhieva, Y.B.; Mutafchieva, M.Z.; Stamenov, N.V.; Kavlakova, L.B.; Tanev, M.Z.; Karaslavova, E.; Tomov, G.T. Randomised Controlled Trial Comparing the Clinical Effectiveness of Mouthwashes Based on Essential Oils, Chlorhexidine, Hydrogen Peroxide and Prebiotic in Gingivitis Treatment. Folia Med. 2022, 64, 588–595. [Google Scholar] [CrossRef]
- Li, Y.; Greenwall, L. Safety Issues of Tooth Whitening Using Peroxide-Based Materials. Br. Dent. J. 2013, 215, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Funahara, M.; Soutome, S.; Nakamura, A.; Soh, I.; Honda, H.; Hikiji, H. Comparison of the Efficacy of Three Types of Disinfectants Approved for Oral Use in Japan in Reducing the Bacterial Count of Tongue Coating: A Randomised-Controlled Study. Oral Health Prev. Dent. 2021, 19, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.V.; Rath, T.; Radtke, C. Hydrogen Peroxide (H2O2): A Review of Its Use in Surgery. Wien. Med. Wochenschr. 2019, 169, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.A.; Del Prete, A.; Lazzarino, A.I. Hydrogen Peroxide and Viral Infections: A Literature Review with Research Hypothesis Definition in Relation to the Current COVID-19 Pandemic. Med. Hypotheses 2020, 144. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Liu, J.; Cao, B.; Li, B.; Tian, S. Hydrogen Peroxide Acts on Sensitive Mitochondrial Proteins to Induce Death of a Fungal Pathogen Revealed by Proteomic Analysis. PLoS ONE 2011, 6, e21945. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Silveira, J.; Dias, S.; Cardoso, A.; Mata, A.; Marques, D. Bleaching Efficacy and Quality of Life of Different Bleaching Techniques—Randomized Controlled Trial. Clin. Oral Investig. 2022, 26, 7167. [Google Scholar] [CrossRef] [PubMed]
- Maran, B.M.; Matos, T.d.P.; Castro, A.d.S.d.; Vochikovski, L.; Amadori, A.L.; Loguercio, A.D.; Reis, A.; Berger, S.B. In-Office Bleaching with Low/Medium vs. High Concentrate Hydrogen Peroxide: A Systematic Review and Meta-Analysis. J. Dent. 2020, 103, 103499. [Google Scholar] [CrossRef] [PubMed]
- Grootveld, M.; Lynch, E.; Page, G.; Chan, W.; Percival, B.; Anagnostaki, E.; Mylona, V.; Bordin-Aykroyd, S.; Grootveld, K.L. Potential Advantages of Peroxoborates and Their Ester Adducts Over Hydrogen Peroxide as Therapeutic Agents in Oral Healthcare Products: Chemical/Biochemical Reactivity Considerations In Vitro, Ex Vivo And In Vivo. Dent. J. 2020, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Gousalya, V.; Dhamodhar, D.; Prabu, D.; Mohan, R.M.; Bharathwaj, V.V.; Sindhu, R.; Elakiya, S. Effectiveness of Hydrogen Peroxide Concentrations on Bleaching and Tooth Sensitivity—A Systematic Review. Indian J. Contemp. Dent. 2022, 11, 1–8. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.; Garcia-Godoy, F.; Park, Y.S. Dentin Abrasion Using Whitening Toothpaste with Various Hydrogen Peroxide Concentrations. Am. J. Dent. 2023, 36, 55–61. [Google Scholar]
- Müller-Heupt, L.K.; Wiesmann-Imilowski, N.; Kaya, S.; Schumann, S.; Steiger, M.; Bjelopavlovic, M.; Deschner, J.; Al-Nawas, B.; Lehmann, K.M. Effectiveness and Safety of Over-the-Counter Tooth-Whitening Agents Compared to Hydrogen Peroxide In Vitro. Int. J. Mol. Sci. 2023, 24, 1956. [Google Scholar] [CrossRef]
- Kwon, S.R.; Oyoyo, U.; Li, Y. Effect of Light Activation on Tooth Whitening Efficacy and Hydrogen Peroxide Penetration: An in Vitro Study. J. Dent. 2013, 41, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Žekonis, G.; Žekonis, J.; Gleiznys, A.; Noreikienė, V.; Balnytė, I.; Šadzevičienė, R.; Narbutaitė, J. Effect of Supragingival Irrigation with Aerosolized 0.5% Hydrogen Peroxide on Clinical Periodontal Parameters, Markers of Systemic Inflammation, and Morphology of Gingival Tissues in Patients with Periodontitis. Med. Sci. Monit. 2016, 22, 3713–3721. [Google Scholar] [CrossRef]
- El Mobadder, M.; Nammour, S.; Namour, M.; Namour, A.; Grzech-Leśniak, K. Disinfection Potential of 980 Nm Diode Laser and Hydrogen Peroxide (3%) in “Critical Probing Depths” Periodontal Pockets: Retrospective Study. Life 2022, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Ramos, E.; Urbieta, I.R.; Rodríguez, D. Is Hydrogen Peroxide an Effective Mouthwash for Reducing the Viral Load of SARS-CoV-2 in Dental Clinics? Saudi Dent. J. 2022, 34, 237. [Google Scholar] [CrossRef]
- Greenwall-Cohen, J.; Francois, P.; Silikas, N.; Greenwall, L.; Le Goff, S.; Attal, J.P. The Safety and Efficacy of “over the Counter” Bleaching Products in the UK. Br. Dent. J. 2019, 226, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.M.M.; Carneiro, T.S.; Favoreto, M.W.; Borges, C.P.F.; Reis, A.; Loguercio, A.D.; Meireles, S.S. Effect of Whitening Toothpastes with Different Hydrogen Peroxide Concentrations: Penetration into the Pulp Chamber and Color Change. J. Dent. 2024, 144, 104951. [Google Scholar] [CrossRef]
- Silveira, F.M.; Schuch, L.F.; Schimidt, T.R.; Lopes, M.P.; Wagner, V.P.; Só, B.B.; Palo, R.M.; Martins, M.D. Potentially Carcinogenic Effects of Hydrogen Peroxide for Tooth Bleaching on the Oral Mucosa: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2024, 131, 375–383. [Google Scholar] [CrossRef]
- Terra, R.; da Silva, K.L.; Vochikovski, L.; Sutil, E.; Rezende, M.; Loguercio, A.D.; Reis, A. Effect of Daily Usage Time of 4% Hydrogen Peroxide on the Efficacy and Bleaching-Induced Tooth Sensitivity: A Single-Blind Randomized Clinical Trial. Oper. Dent. 2021, 46, 395–405. [Google Scholar] [CrossRef]
- Ferraz, N.K.L.; Nogueira, L.C.; Neiva, I.M.; Ferreira, R.C.; Moreira, A.N.; Magalhães, C.S. Longevity, Effectiveness, Safety, and Impact on Quality of Life of Low-Concentration Hydrogen Peroxides in-Office Bleaching: A Randomized Clinical Trial. Clin. Oral Investig. 2019, 23, 2061–2070. [Google Scholar] [CrossRef]
- Murphy, E.C.; Friedman, A.J. Hydrogen Peroxide and Cutaneous Biology: Translational Applications, Benefits, and Risks. J. Am. Acad. Dermatol. 2019, 81, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Madhusudanan, S.; Ranjith, A. Comparative Evaluation of Cytotoxicity of 0.12% and 0.2% Chlorhexidine, 2% Povidone Iodine, 3% Hydrogen Peroxide and 0.9% Normal Saline Solutions on Fibroblasts—An In Vitro Study. J. Clin. Diagn. Res. 2020, 14, 21–25. [Google Scholar]
- Gutiérrez-Venegas, G.; Guadarrama-Solís, A.; Muñoz-Seca, C.; Arreguín-Cano, J.A. Hydrogen Peroxide-Induced Apoptosis in Human Gingival Fibroblasts. Int. J. Clin. Exp. Pathol. 2015, 8, 15563. [Google Scholar] [PubMed]
- Vieira, A.; Marques, J.; Cruz, M.; Mendonça, C.; Marques, D.; Mata, A. Cytotoxic Effects of Hydrogen Peroxide on Periodontal Cells. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2020, 60, 111–117. [Google Scholar] [CrossRef]
- Kugel, G.; Gerlach, R.W.; Aboushala, A.; Ferreira, S.; Magnuson, B. Long-Term Use of 6.5% Hydrogen Peroxide Bleaching Strips on Tetracycline Stain: A Clinical Study. Compend. Contin. Educ. Dent. 2011, 32, 50–56. [Google Scholar]
- Bortolatto, J.F.; Pretel, H.; Floros, M.C.; Luizzi, A.C.C.; Dantas, A.A.R.; Fernandez, E.; Moncada, G.; de Oliveira, O.B. Low Concentration H2O2/TiO_N in Office Bleaching. J. Dent. Res. 2014, 93, 66S–71S. [Google Scholar] [CrossRef] [PubMed]
- Cherry, M.; Daly, C.G.; Mitchell, D.; Highfield, J. Effect of Rinsing with Povidone–Iodine on Bacteraemia Due to Scaling: A Randomized-Controlled Trial. J. Clin. Periodontol. 2007, 34, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Lachapelle, J.M.; Castel, O.; Casado, A.F.; Leroy, B.; Micali, G.; Tennstedt, D.; Lambert, J. Therapeutic Perspective Antiseptics in the Era of Bacterial Resistance: A Focus on Povidone Iodine. Clin. Pract. 2013, 10, 579–592. [Google Scholar] [CrossRef]
- Miller, K.; Monto, A.; Keipp Talbot, H.; Gaglani, M.; McNeal, T.; Silveira, F.; Zimmerman, R.; Middleton, D.; Ghamande, S.; Murthy, K.; et al. Feasibility and Acceptability of Intranasal Povidone Iodine Decolonization among Orthopedic Trauma Surgery Patients. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, s62–s63. [Google Scholar] [CrossRef]
- Cardoso, A.B.; Pereira, R.; Silveira, J.; Dias, S.; Mata, A.; Marques, D. In-Office Tooth Bleaching Effectiveness with Different Soft-Tissue Barriers-Randomized Controlled Trial. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2021, 62, 141–149. [Google Scholar] [CrossRef]






| Uses | Formulations | Type of Study | Spectrum | Results | Ref. |
|---|---|---|---|---|---|
| Dental caries | CHX 35% varnishes, CHX 1% gel | In vitro | Lactobacillus rhamnosus | Bacterial numbers showed little to no change on the 40 bovine root dentin samples (p > 0.05). It is unlikely to prevent root caries from gaining prevalence. | [45] |
| Dental caries | CHX 0.2% mouthrinse | Controlled trial | Streptococcus mutans | It significantly ↓ the amount of S. mutans in saliva after using 2.5 mL of the mouthwash for 1 min, twice a day. It may be beneficial for dental caries prevention in children. However, the herbal mouthwash presented better results with fewer side effects at the end of the 15-day trial (p = 0.002). | [57] |
| Gingivitis | 0.12%, 0.2% CHX mouthwashes | Systematic review | - | It effectively ↓ dental plaque formation, which can further lead to gingival inflammation. | [58] |
| Gingivitis | 1% CHX toothpaste | Home usage trial | - | The prevalence of gingivitis was significantly ↓ by the end of the 6-month trial compared to the control (p < 0.001) | [59] |
| Periodontal disease | CHX chip (2.5 mg) | Randomized clinical trial | - | It ↓ the probing depth and plaque index. The CHX chip could be an adjunctive therapy for scaling and root planing in periodontitis. | [32] |
| Root canal treatment | Chitosan CHX-nanoparticles (CS-CHX NPs), 2% CHX gel | In vitro | Enterococcus faecalis, Candida albicans | Both formulations significantly ↓ the pathogens. In a shorter period, the CS-CHX NPs formulation was comparable to the gel one, but the antimicrobial activity was significantly higher compared to the other medicament groups (p < 0.001). They proved to be beneficial in decreasing the most commonly encountered pathogens in root canal infections. | [14] |
| Peri-implant mucositis | 0.12% CHX mouthwash | Randomized controlled trial | - | It ↓ plaque formation, bleeding on probing, and probing depth (p = 0.01). It was demonstrated to be a useful adjunct treatment for mechanical debridement for peri-implant mucositis. | [16] |
| Biofilm formation | 0.5% w/w CHX spray | In vitro and in vivo | Staphylococcus aureus | The inhibition of S. aureus was observed in vitro and antiseptic activity was observed in vivo (p < 0.05). This confirms that CHX spray can decrease biofilm formation, thereby reducing the incidence of other dental problems. | [60] |
| Uses | Formulation | Type of Study | Spectrum | Results | Ref. |
|---|---|---|---|---|---|
| Biofilm formation | 0.1% OCT mouthwash | Crossover study | - | The biofilm thickness appears to be significantly ↓ on the 60 enamel samples when the mouthwash is used once every 12 h for 30 s, making OCT a great candidate for combating several dental problems. The biofilm thickness was 12.32 ± 6.58 µm in control compared to 0.54 ± 0.09 µm after 48 h post-treatment. | [118] |
| Dental caries | 1%, 1.5% OCT added to a resin composite | In vitro | Streptococcus mutans | The composite resin increased OCT’s antibacterial action at both concentrations against S. mutans after 30 days (p < 0.05), demonstrates its beneficial role in caries prevention. | [119] |
| Gingivitis, periodontitis in patients following orthodontic treatment | 1% OCT mouthwash | Systematic review | Streptococcus mutans, Lactobacilus spp. | It ↓ microbial growth, helping with plaque-induced gingivitis and periodontal damage. It also prevents white spot lesions in patients following orthodontic treatment. | [86] |
| Root canal treatment | 0.0125%, 0.025%, 0.05%, 0.1% OCT solution | In vitro | Staphylococcus epidermidis | OCT significantly ↓ the pathogen levels at all concentrations in a dose-dependent manner compared to control (p < 0.05). Notably, at the concentration of 0.1%, it exhibited the most potent antibacterial activity, completely eradicating S. epidermidis. | [110] |
| Yeast infection | 0.001%, 0.05%, 0.1% OCT mouthwashes | In vitro | Candida albicans, Candida glabrata | The 0.001% concentration was not sufficiently effective against the strains. OCT at 0.05% and 0.1% successfully inhibited C. albicans, even after 30 s of exposure. Only the 0.1% concentration was yeasticidal against C. glabrata within 2 min of contact time. | [120] |
| Uses | Formulation | Type of Study | Spectrum | Results | Ref. |
|---|---|---|---|---|---|
| Biofilm reduction | 10% PVP-I solution | In vitro | α-hemolytic Streptoccocus sp., Hafnia alvei, Bacteroides ureolyticus, Actinomyces odontolyticus, Actinomyces meyeri, Finegoldia magna, Parvimonas micra | Ten samples were collected from patients with pulpitis who had necrotic pulp. The samples were divided into two groups (A and B). In sample A, α-hemolytic Streptococcus sp., H. alvei, B. ureolyticus, A. odontolyticus, and A. meyeri were present, and in sample B, α-hemolytic Streptococcus sp. strains, F. magna, A. meyeri, and P. micra were present. After 15 min, both samples had a significantly ↓ CFU count (sample A—p = 0.000152; sample B—p = 0.000311). In both samples, pathogen levels were fully eliminated after 40 min. These results support PVP-I’s beneficial action in endodontics. | [155] |
| Biofilm reduction | 1%, 7.5% gargle, 0.45% throat spray | In vitro | MRSA, Escherichia coli, Enterococcus faecium, Streptococcus pyogenes, Streprococcus mutans, Streptococcus sanguinis, Streptococcus pneumoniae, Streptococcus agalactiae, Pseudomonas aeruginosa, Haemophilus influenzae, Klebsiella pneumoniae, Staphylococcus epidermidis, Candida albicans, Candida glabrata, Coxsackievirus A16, Enterovirus 71 | It showed antibacterial (>5 lg reduction) and antifungal (>4 lg reduction) activity after 30–60 s of exposure, as well as antiviral (>4 lg reduction) effects after 0.5–30 min at all concentrations against all pathogens. | [156] |
| Dental caries | 10% PVP-I solution | Controlled trial | Streptococcus mutans | The study included 25 children who were divided into 2 groups: Group 1–13 experimental children and Group 2–11 children as a control. They received treatment with PVP-I 3 times at 2-month intervals for 4 months. PVP-I significantly ↓ S. mutans levels at 6 months in both groups (p = 0.003), with p = 0.0005 in Group 1 and p = 0.004 in Group 2. At 1-year follow-up, 2 experimental children and 5 from the control presented caries, however, the difference was not statistically significant (p = 0.06). This suggests PVP-I’s potential role in dental caries prevention, however, more clinical studies are needed to confirm this hypothesis. | [157] |
| Pre-procedural rinse | 1% PVP-I mouthwash | In vivo | SARS-CoV-2 | Twenty-five patients gargled with PVP-I for 30 s. After 5 min, the saliva was collected, and it was observed that the mouthwash significantly ↓ the viral load in the mouth cavity compared to 0 subjects from the control (p < 0.0001). This demonstrated that PVP-I mouthwash can decrease the spread of SARS-CoV-2 during dental treatments. | [138] |
| Alveolar osteitis (dry socket) | 1% Betadine mouthwash | Controlled trial | - | Ninety-seven patients rinsed preoperatively with 1% betadine mouthwash before the surgical extraction of mandibular third molars, while ninety-two subjects were the controls. There was a significant difference in the ↓ incidence of dry socket in the experimental group compared to the control (p = 0.036), with 5 subjects who received treatment experiencing alveolar osteitis compared to 13 in the control. Additionally, it was demonstrated that the incidence of alveolar osteitis was correlated with an increase in age (p = 0.003). People older than 30 years presented a higher incidence of dry socket. | [139] |
| Uses | Formulation | Type of Study | Spectrum | Results | Ref |
|---|---|---|---|---|---|
| Dental caries | 2.25% NaOCl gel | Controlled clinical trial | - | Ten children received NaOCl gel for 2 min. The application was repeated until caries-free. It was effective in removing carious dentine from primary teeth, although conventional methods such as drilling are considered to be faster (p < 0.001). Additionally, the treatment lowered the pain score compared to conventional treatment (p = 0.005). | [177] |
| Gingivitis | 0.2% NaOCl mouthwash | Controlled clinical trial | Streptococcus spp., Enterococcus faecalis | Sixty patients were divided into two equal groups and received either 0.2% CHX mouthwash or 0.2% NaOCl mouthwash. In both groups, there was a significant difference from baseline and by day 21 in the plaque index, gingival index, and gingival bleeding index. It can be an alternative to 0.2% CHX mouthwash for removing debris and reducing the incidence of plaque-induced gingivitis due to better tolerability. | [180] |
| Biofilm reduction | Mixture of an aminoacid (AA) and 0.475%, 0.2%, 0.3%, 0.4% NaOCl gel | In vitro | Porphyromonas gingivalis, Tannerella forsythia, Fusobacterium nucleatum, Streptococcus gordonii, Actinomyces naeslundii, Parvimonas micra, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, Campylobacter rectus, Eikenella corrodens, Filifactor alocis, Capnocytophaga gingivalis, Eubacterium nodatum | All mixtures significantly ↓ CFU counts compared to the control (p < 0.001). However, the gel containing 0.3% NaOCl was the most active. Biofilm quantity was significantly lower at 0.2% (p = 0.004) and 0.3% (p = 0.012) concentrations of NaOCl vs control. Only the 0.4% concentration ↓ biofilm metabolic activity (p = 0.040). The gel was more effective against Gram-negative bacteria associated with periodontitis. While it did not eliminate multiple bacterial biofilms, it significantly ↓ the number of pathogens. | [197] |
| Root canal treatment | 1% NaOCl solution | Controlled clinical trial | Streptococcus spp., Enterococcus faecalis | After irrigation with NaOCl in 20 patients, it was observed that 65% of the canals were free of bacteria. This confirms the solution is a beneficial irrigator for treating teeth with apical periodontitis. | [178] |
| Yeast infection | 0.525%, 5.25% NaOCl solution | In vitro | Candida albicans | Both formulations eliminated almost all C. albicans colonies from the 20 samples of silicone impressions at different time stamps. The mean ± SD: NaOCl at 0.525% after 5 min—67.55 ± 35.55; NaOCl at 0.525% after 10 min— 0 ± 0.000; 5.25% NaOCl after 5 min—0 ± 0.000; 5.25% NaOCl after 10 min—0 ± 0.000. The most potent concentration was NaOCl at 5.25%, as it ↓ C. albicans completely at both intervals. | [179] |
| Uses | Formulation | Type of Study | Spectrum | Results | Ref. |
|---|---|---|---|---|---|
| Dental plaque reduction and gingival inflammation | 0.05% CPC mouthwash | Randomized controlled trial | - | After 6 weeks of treatment, CPC mouthwash ↓ plaque and gingivitis, with fewer adverse effects, such as teeth staining, taste alteration, and numbness, compared to 0.12% CHX mouthwash. | [221] |
| Subgingival biofilm reduction | 0.075% CPC mouthwash | In vitro | Actinomyces naeslundii, Actinomyces oris, Actinomyces gerencseriae, Actinomyces israelii, Actinomyces odontolyticus, Aggregatibacter actinomycetemcomitans, Veillonella parvula, Streptococcus sanguinis, Streptococcus oralis, Staphylococcus intermedius, Streptococcus gordonii, Streptococcus mitis, Streptococcus constellatus, Streptococcus anginosus, Selenomonas noxia, Capnocytophaga ochracea, Capnocytophaga gingivalis, Eikenella corrodens, Capnocytophaga, Fusobacterium nucleatum vicentii, Fusobacterium nucleatum polymorphum, Eubacterium nodatum, Parvimonas micra, Campylobacter showae, Fusobacterium periodonticum, Prevotella intermedia, Porphyromonas gingivalis, Tannerella forsythia, Exiguobacterium saburrum, Propionibacterium acnes, Gemella marbillorum | There were 8 treatments in total with a twice-daily application, with 1 min exposure per application. It decreased the biofilm’s metabolic activity by 60% and ↓ the bacterial total counts by 96%. The only remaining pathogens from the multispecies biofilm were F.n. vincentii, and P. gingivalis. This attests to the potential benefits of CPC as an adjuvant periodontal treatment. | [233] |
| Dental implant surgery | 0.07% CPC mouthwash | Randomized controlled clinical trial | Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Treponema denticola, Aggregatibacter actinomycetemcomitans | Patients rinsed with 15 mL of the CPC mouthwash for 60 s preoperatively. This treatment ↓ all pathogen levels, except for A. actinomycetemcomitans. | [234] |
| Viral infections | 0.05%, 0.07% CPC mouthwashes | In vitro | Herpes simplex virus Type 1(HSV-1), Human papillomavirus (HPV) | CPC mouthwashes had virucidal activity on HSV-1 after 1–2 min of application. | [220] |
| Uses | Formulation | Type of Study | Spectrum | Results | Ref. |
|---|---|---|---|---|---|
| Biofilm reduction on dental implants | 3% H2O2 solution | In vitro | Candida albicans, Staphylococcus aureus | It ↓ both fungal and bacterial numbers, making it a potential disinfectant agent for peri-implantitis. | [247] |
| Periodontal therapy | 3% H2O2 solution | Clinical trial | - | It ↓ gingival bleeding, lowered potential inflammation, and ↓ probing depths, making it a subgingival irrigant suitable for periodontal treatment. | [253] |
| Gingivitis | 0.8% H2O2 mouthwash | Clinical trial | - | It ↓ the plaque index that can contribute to or aggravate gingivitis. | [254] |
| Halitosis | H2O2 solution | Randomized controlled study | - | It ↓ bacterial counts from the tongue’s surface, which is the primary cause of halitosis. | [256] |
| COVID-19 | 1% H2O2 mouthrinse | SARS-CoV-2 | It ↓ the viral load in 1 min, effectively preventing potential infection from spreading in dental cabinets. | [269] | |
| Teeth whitening | 6% H2O2 paint-on varnish, 6% H2O2 tray | Randomized double-blinded clinical trial | - | Both formulations properly bleached the teeth of the 40 patients and maintained a whiter appearance up to the 6-month follow-up with significant differences in color from the baseline (p < 0.05) | [260] |
| CHX [21,68,69,70,71,72,73,74,75] | OCT [86,99,125] | PVP-I [133,134,146,147,162,281,282,283] | NaOCl [198,199] | CPC [213,236,237] | H2O2 [273,274,275,284] | |
|---|---|---|---|---|---|---|
| Frequent | Teeth and tongue staining, unpleasant taste, xerostomia, teeth, and tongue discoloration | Teeth and tongue discoloration and dysgeusia | - | Unpleasant taste | Mouth ulcerations | Tooth sensitivity, gingival irritation, and oral lesions |
| Not frequent | Burning sensation, taste alteration, irritation, tongue numbness, and changes in oral mucosa | Teeth and tongue staining, burning sensations, ulcerations, irritation, nausea, and headaches | Staining of teeth and tongue, unpleasant taste, and rise in TSH levels | Tooth staining, redness of tongue, burning sensation, and xerostomia | Teeth staining, tongue numbness, taste disturbances, gingival bleeding, and sublingual swelling | - |
| Rare | Allergic reactions | Anaphylaxis | Thyrotoxicosis, seizures, and irritation | Changes in taste, mucosal irritation | Tooth sensitivity, oral discomfort, glossodynia | - |
| Severe | Anaphylaxis | Anaphylaxis | Thyrotoxicosis and seizures | Unpleasant taste | - | Tooth sensitivity |
| Moderate | Skin rashes, itching, and swelling | Dysgeusia, tongue discoloration, and headache | - | Teeth staining, xerostomia | - | - |
| Mild | Skin rashes, itching, and swelling | Tongue staining, dysgeusia, and tingling of the tongue | Staining of teeth and tongue, and rise in TSH levels | Teeth staining and xerostomia | Teeth and tongue staining | Tooth sensitivity and oral lesions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrel, S.-I.; Matichescu, A.; Dinu, S.; Buzatu, R.; Popovici, R.; Dinu, D.C.; Bratu, D.C. New Insights Regarding the Use of Relevant Synthetic Compounds in Dentistry. Molecules 2024, 29, 3802. https://doi.org/10.3390/molecules29163802
Dumitrel S-I, Matichescu A, Dinu S, Buzatu R, Popovici R, Dinu DC, Bratu DC. New Insights Regarding the Use of Relevant Synthetic Compounds in Dentistry. Molecules. 2024; 29(16):3802. https://doi.org/10.3390/molecules29163802
Chicago/Turabian StyleDumitrel, Stefania-Irina, Anamaria Matichescu, Stefania Dinu, Roxana Buzatu, Ramona Popovici, Dorin Cristian Dinu, and Dana Cristina Bratu. 2024. "New Insights Regarding the Use of Relevant Synthetic Compounds in Dentistry" Molecules 29, no. 16: 3802. https://doi.org/10.3390/molecules29163802






