Boosting the Activation of Molecular Oxygen and the Degradation of Rhodamine B in Polar-Functional-Group-Modified g-C3N4
Abstract
1. Introduction
2. Results and Discussion
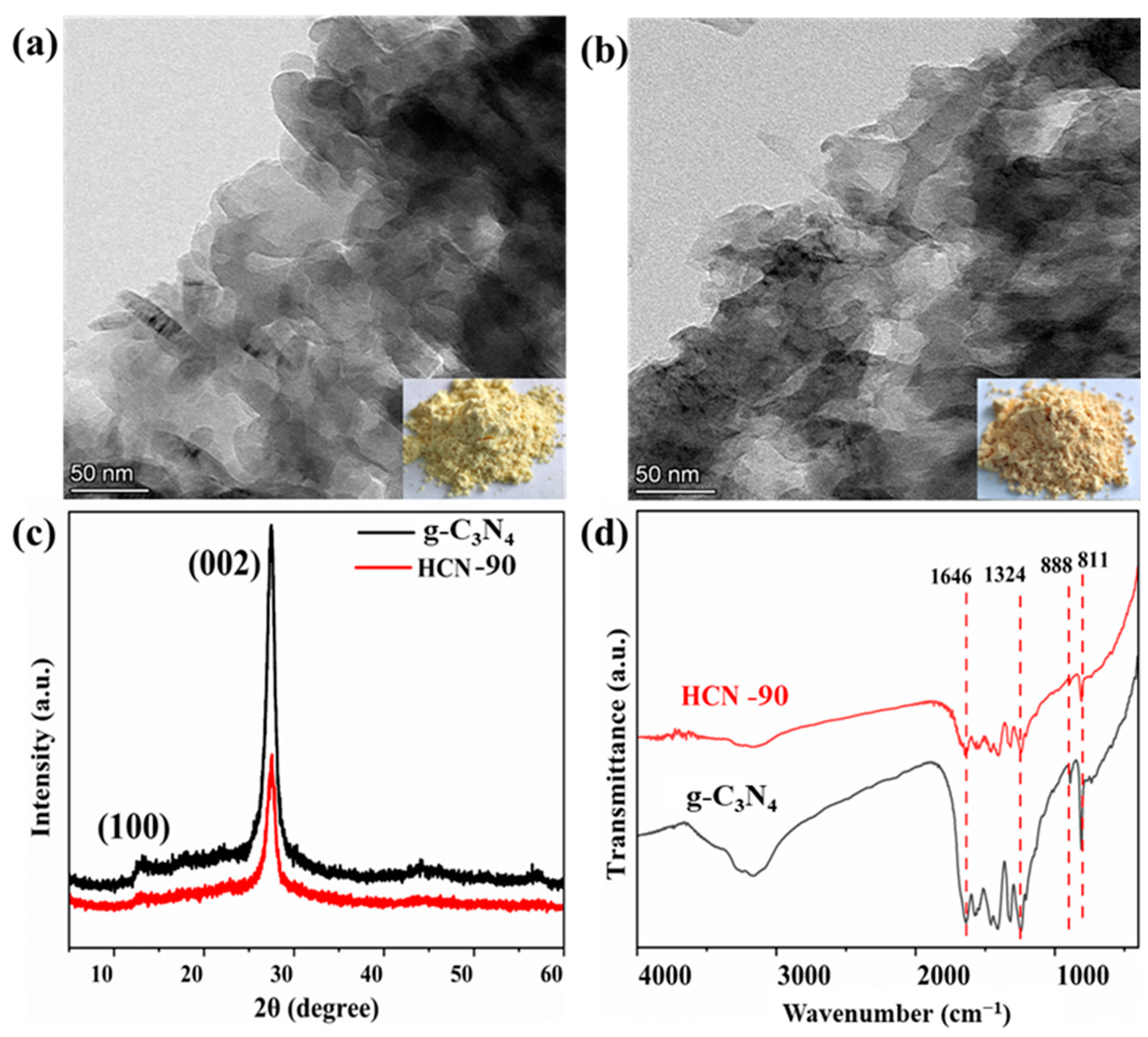
2.1. Characterization of g-C3N4 and HCN
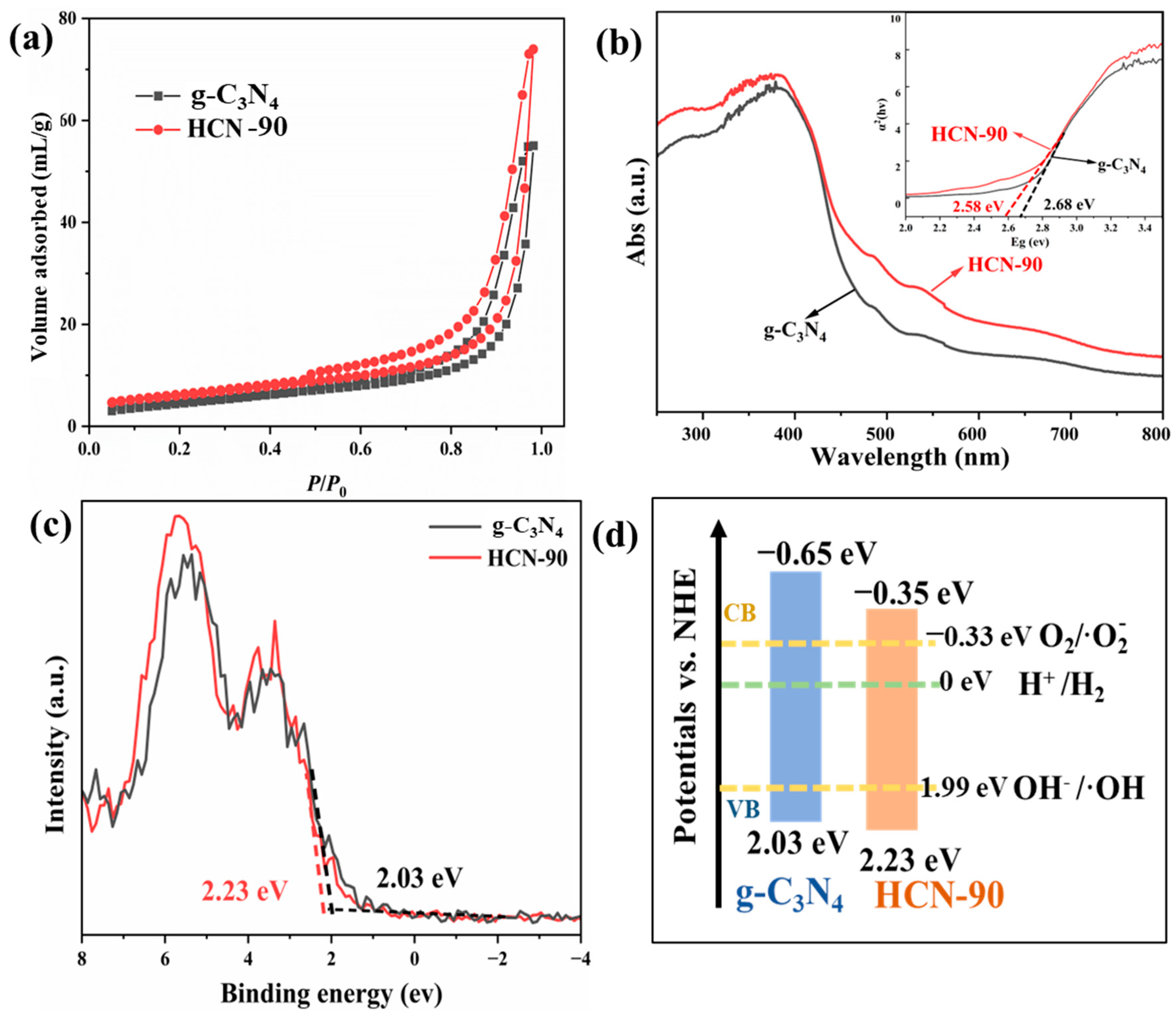
2.2. Photoelectric Property
2.3. Visible-Light Photocatalytic Activity Measurements
3. Materials and Methods
3.1. Materials
3.2. Preparation of HCN
3.3. Characterization
3.4. Evaluation of Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohod, A.V.; Momotko, M.; Shah, N.S.; Marchel, M.; Imran, M.; Kong, L.; Boczkaj, G. Degradation of Rhodamine dyes by Advanced Oxidation Processes (AOPs)—Focus on cavitation and photocatalysis—A critical review. Water Resour. Ind. 2023, 30, 100220. [Google Scholar] [CrossRef]
- Teo, S.H.; Ng, C.H.; Islam, A.; Abdulkareem-Alsultan, G.; Joseph, C.G.; Janaun, J.; Taufiq-Yap, Y.H.; Khandaker, S.; Islam, G.J.; Znad, H.; et al. Sustainable toxic dyes removal with advanced materials for clean water production: A comprehensive review. J. Clean. Prod. 2022, 332, 130039. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Adsorption and photocatalytic removal of Rhodamine B from wastewater using carbon-based materials. FlatChem 2021, 29, 100277. [Google Scholar] [CrossRef]
- Lara-Ramos, J.A.; Diaz-Angulo, J.; Machuca-Martínez, F. Use of modified flotation cell as ozonation reactor to minimize mass transfer limitations. Chem. Eng. J. 2021, 405, 126978. [Google Scholar] [CrossRef]
- Sharmoukh, W.; Abdelhamid, H.N. Fenton-like Cerium Metal—Organic Frameworks (Ce-MOFs) for Catalytic Oxidation of Olefins, Alcohol, and Dyes Degradation. J. Clust. Sci. 2023, 34, 2509–2519. [Google Scholar] [CrossRef]
- Wang, C.; Shi, P.; Guo, C.; Guo, R.; Qiu, J. CuCO2O4/CF cathode with bifunctional and dual reaction centers exhibits high RhB degradation in electro-Fenton systems. J. Electroanal. Chem. 2024, 956, 118072. [Google Scholar] [CrossRef]
- Zanaty, M.; Zaki, A.H.; El-Dek, S.I.; Abdelhamid, H.N. Zeolitic imidazolate framework@hydrogen titanate nanotubes for efficient adsorption and catalytic oxidation of organic dyes and microplastics. J. Environ. Chem. Eng. 2024, 12, 112547. [Google Scholar] [CrossRef]
- Li, T.; Zhao, L.; He, Y.; Cai, J.; Luo, M.; Lin, J. Synthesis of g-C3N4/SmVO4 composite photocatalyst with improved visible light photocatalytic activities in RhB degradation. Appl. Catal. B Environ. 2013, 129, 255–263. [Google Scholar] [CrossRef]
- Yu, J.; Bao, P.; Liu, J.; Jin, Y.; Li, J.; Lv, Y. Cu and Ni dual-doped ZnO nanostructures templated by cellulose nanofibrils for the boosted visible-light photocatalytic degradation of wastewater pollutants. Green Chem. 2023, 25, 10530–10537. [Google Scholar] [CrossRef]
- Tang, C.; Qiu, X.; Cheng, Z.; Jiao, N. Molecular oxygen-mediated oxygenation reactions involving radicals. Chem. Soc. Rev. 2021, 50, 8067–8101. [Google Scholar] [CrossRef]
- Zhan, H.; Zhou, Q.; Li, M.; Zhou, R.; Mao, Y.; Wang, P. Photocatalytic O2 activation and reactive oxygen species evolution by surface B-N bond for organic pollutants degradation. Appl. Catal. B Environ. 2022, 310, 121329. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, L.; Wang, J.; Li, Q.; He, W.; Yin, J.J. Surface Structure-Dependent Molecular Oxygen Activation of BiOCl Single-Crystalline Nanosheets. J. Am. Chem. Soc. 2013, 135, 15750–15753. [Google Scholar] [CrossRef] [PubMed]
- Anglada, J.M.; Martins-Costa, M.; Francisco, J.S.; Ruiz-Lopez, M.F. Interconnection of reactive oxygen species chemistry across the interfaces of atmospheric, environmental, and biological processes. Acc. Chem. Res. 2015, 48, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Ben, H.; Liu, Y.; Liu, X.; Liu, X.; Ling, C.; Liang, C.; Zhang, L. Diffusion-Controlled Z-Scheme-Steered Charge Separation across PDI/BiOI Heterointerface for Ultraviolet, Visible, and Infrared Light-Driven Photocatalysis. Adv. Funct. Mater. 2021, 31, 202102315. [Google Scholar] [CrossRef]
- Li, Q.; Li, F.T. Recent advances in molecular oxygen activation via photocatalysis and its application in oxidation reactions. Chem. Eng. J. 2021, 421, 129915. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Dai, J.; Songsiriritthigul, P.; Oo, T.Z.; Zaw, M.; Lwin, N.W.; Aung, S.H.; Chen, F. Defect engineering in 0D/2D TiO2/g-C3N4 heterojunction for boosting photocatalytic degradation of tetracycline in a tetracycline/Cu2+ combined system. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132624. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, P.R.; Sharma, G.; Naushad, M.; Rana, A.; Mola, G.T.; Stadler, F.J. Carbon nitride, metal nitrides, phosphides, chalcogenides, perovskites and carbides nanophotocatalysts for environmental applications. Environ. Chem. Lett. 2018, 17, 655–682. [Google Scholar] [CrossRef]
- Che, H.; Wang, P.; Chen, J.; Gao, X.; Liu, B.; Ao, Y. Rational design of donor-acceptor conjugated polymers with high performance on peroxydisulfate activation for pollutants degradation. Appl. Catal. B Environ. 2022, 316, 121611. [Google Scholar] [CrossRef]
- Li, G.; Xie, Z.; Chai, S.; Chen, X.; Wang, X. A facile one-step fabrication of holey carbon nitride nanosheets for visible-light-driven hydrogen evolution. Appl. Catal. B Environ. 2021, 283, 119637. [Google Scholar] [CrossRef]
- Sun, S.; Peng, B.; Song, Y.; Wang, R.; Song, H.; Lin, W. Engineering Z-Scheme FeOOH/PCN with Fast Photoelectron Transfer and Surface Redox Kinetics for Efficient Solar-Driven CO2 Reduction. ACS Appl. Mater. Interfaces 2023, 15, 12957–12966. [Google Scholar] [CrossRef]
- Wang, X.; Tang, W.; Jiang, L.; Feng, J.; Yang, J.; Zhou, S.; Li, W.; Yuan, X.; Wang, H.; Wang, J.; et al. Mechanism insights into visible light-induced crystalline carbon nitride activating periodate for highly efficient ciprofloxacin removal. Chem. Eng. J. 2023, 471, 144521. [Google Scholar] [CrossRef]
- Tan, J.; Li, Z.; Li, J.; Wu, J.; Yao, X.; Zhang, T. Graphitic carbon nitride-based materials in activating persulfate for aqueous organic pollutants degradation: A review on materials design and mechanisms. Chemosphere 2021, 262, 127675. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wen, Y.; Ma, J.; Dong, D.; Shen, Y.; Liu, S.; Ma, H.; Zhang, Y. Unraveling fundamental active units in carbon nitride for photocatalytic oxidation reactions. Nat. Commun. 2021, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Cheng, L.; Liao, Y.; Xiang, Q. Effect of Functional Group Modifications on the Photocatalytic Performance of g-C3N4. Small 2023, 19, e2300109. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Gao, X.; Chen, J.; Hou, J.; Ao, Y.; Wang, P. Iodide-Induced Fragmentation of Polymerized Hydrophilic Carbon Nitride for High-Performance Quasi-Homogeneous Photocatalytic H2O2 Production. Angew. Chem. Int. Ed. 2021, 60, 25546–25550. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.; Ouyang, S.; Lu, D.; Wang, X.; Wang, D.; Ye, J. In situ surface alkalinized g-C3N4 toward enhancement of photocatalytic H2 evolution under visible-light irradiation. J. Mater. Chem. A 2016, 4, 2943–2950. [Google Scholar] [CrossRef]
- Yu, S.; Li, J.; Zhang, Y.; Li, M.; Dong, F.; Zhang, T.; Huang, H. Local spatial charge separation and proton activation induced by surface hydroxylation promoting photocatalytic hydrogen evolution of polymeric carbon nitride. Nano Energy 2018, 50, 383–392. [Google Scholar] [CrossRef]
- Li, J.; He, C.; Xu, N.; Wu, K.; Huang, Z.; Zhao, X.; Nan, J.; Xiao, X. Interfacial bonding of hydroxyl-modified g-C3N4 and Bi2O2CO3 toward boosted CO2 photoreduction: Insights into the key role of OH groups. Chem. Eng. J. 2023, 452, 139191. [Google Scholar] [CrossRef]
- She, X.; Zhu, X.; Yang, J.; Song, Y.; She, Y.; Liu, D.; Wu, J.; Yu, Q.; Li, H.; Liu, Z.; et al. Grain-boundary surface terminations incorporating oxygen vacancies for selectively boosting CO2 photoreduction activity. Nano Energy 2021, 84, 105869. [Google Scholar] [CrossRef]
- Bu, X.; Li, J.; Yang, S.; Sun, J.; Deng, Y.; Yang, Y.; Wang, G.; Peng, Z.; He, P.; Wang, X.; et al. Surface Modification of C3N4 through Oxygen-Plasma Treatment: A Simple Way toward Excellent Hydrophilicity. ACS Appl. Mater. Interfaces 2016, 8, 31419–31425. [Google Scholar] [CrossRef]
- Li, H.J.; Sun, B.W.; Sui, L.; Qian, D.J.; Chen, M. Preparation of water-dispersible porous g-C3N4 with improved photocatalytic activity by chemical oxidation. Phys. Chem. Chem. Phys. 2015, 17, 3309–3315. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Ma, H.; Wu, D.; Zhang, Y.; Du, B.; Wei, Q. Cathodic electrochemiluminescence immunosensor based on nanocomposites of semiconductor carboxylated g-C3N4 and graphene for the ultrasensitive detection of squamous cell carcinoma antigen. Biosens. Bioelectron. 2014, 55, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhong, X.; Chai, Y.; Yuan, R. Ultrasensitive electrochemiluminescence biosensor for organophosphate pesticides detection based on carboxylated graphitic carbon nitride-poly(ethylenimine) and acetylcholinesterase. Electrochim. Acta 2017, 224, 194–200. [Google Scholar] [CrossRef]
- Ming, L.; Yue, H.; Xu, L.; Chen, F. Hydrothermal synthesis of oxidized g-C3N4 and its regulation of photocatalytic activity. J. Mater. Chem. A 2014, 2, 19145–19149. [Google Scholar] [CrossRef]
- Moon, G.-h.; Fujitsuka, M.; Kim, S.; Majima, T.; Wang, X.; Choi, W. Eco-Friendly Photochemical Production of H2O2 through O2 Reduction over Carbon Nitride Frameworks Incorporated with Multiple Heteroelements. ACS Catal. 2017, 7, 2886–2895. [Google Scholar] [CrossRef]
- You, Q.; Zhang, Q.; Gu, M.; Du, R.; Chen, P.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Self-assembled graphitic carbon nitride regulated by carbon quantum dots with optimized electronic band structure for enhanced photocatalytic degradation of diclofenac. Chem. Eng. J. 2022, 431, 133927. [Google Scholar] [CrossRef]
- Luo, L.; Gong, Z.; Ma, J.; Wang, K.; Zhu, H.; Li, K.; Xiong, L.; Guo, X.; Tang, J. Ultrathin sulfur-doped holey carbon nitride nanosheets with superior photocatalytic hydrogen production from water. Appl. Catal. B Environ. 2021, 284, 119742. [Google Scholar] [CrossRef]
- Merschjann, C.; Tschierlei, S.; Tyborski, T.; Kailasam, K.; Orthmann, S.; Hollmann, D.; Schedel-Niedrig, T.; Thomas, A.; Lochbrunner, S. Complementing Graphenes: 1D Interplanar Charge Transport in Polymeric Graphitic Carbon Nitrides. Adv. Mater. 2015, 27, 7993–7999. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation Performance of g-C3N4 Fabricated by Directly Heating Melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Wang, Z.; Dawson, G.; Chen, W. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity. J. Mater. Chem. 2011, 21, 14398–14401. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Z.; Sun, Y.; Ho, W.-K.; Zhang, H. Engineering the nanoarchitecture and texture of polymeric carbon nitride semiconductor for enhanced visible light photocatalytic activity. J. Colloid Interface Sci. 2013, 401, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Preparation and Enhanced Visible-Light Photocatalytic H2-Production Activity of Graphene/C3N4 Composites. J. Phys. Chem. C 2011, 115, 7355–7363. [Google Scholar] [CrossRef]
- Feng, J.; Li, M. Large-Scale Synthesis of a New Polymeric Carbon Nitride—C3N3 with Good Photoelectrochemical Performance. Adv. Funct. Mater. 2020, 30, 2001502. [Google Scholar] [CrossRef]
- Xie, Y.; Li, Y.; Huang, Z.; Zhang, J.; Jia, X.; Wang, X.-S.; Ye, J. Two types of cooperative nitrogen vacancies in polymeric carbon nitride for efficient solar-driven H2O2 evolution. Appl. Catal. B Environ. 2020, 265, 118581. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, B.; Jiang, C.; Cheng, B.; You, W.; Yu, J. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Li, Z.; Huang, Z.H.; Kang, F.; Yang, Q.H. Holey Graphitic Carbon Nitride Nanosheets with Carbon Vacancies for Highly Improved Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-Scheme Photocatalyst. Adv. Mater. 2022, 34, e2107668. [Google Scholar] [CrossRef]
- Yang, H.; Xu, B.; Yuan, S.; Zhang, Q.; Zhang, M.; Ohno, T. Synthesis of Y-doped CeO2/PCN nanocomposited photocatalyst with promoted photoredox performance. Appl. Catal. B Environ. 2019, 243, 513–521. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Li, R.; Zhang, Q.; Chen, T.; Liu, H.; Liu, Y.; Lv, W.; Liu, G. An efficient metal-free phosphorus and oxygen co-doped g-C3N4 photocatalyst with enhanced visible light photocatalytic activity for the degradation of fluoroquinolone antibiotics. Chem. Eng. J. 2019, 374, 242–253. [Google Scholar] [CrossRef]
- Yi-Zhu, P.; Wan-Hong, M.; Man-Ke, J.; Xiao-Rong, Z.; Johnson, D.M.; Ying-Ping, H. Comparing the degradation of acetochlor to RhB using BiOBr under visible light: A significantly different rate-catalyst dose relationship. Appl. Catal. B Environ. 2016, 181, 517–523. [Google Scholar] [CrossRef]
- Shi, W.; Fang, W.X.; Wang, J.C.; Qiao, X.; Wang, B.; Guo, X. pH-controlled mechanism of photocatalytic RhB degradation over g-C3N4 under sunlight irradiation. Photochem. Photobiol. Sci. 2021, 20, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liao, M.; Zhang, Z.; Wang, H.; Chen, D.; Feng, Y. Enhanced photodegradation performance of Rhodamine B with g-C3N4 modified by carbon nanotubes. Sep. Purif. Technol. 2020, 244, 116618. [Google Scholar] [CrossRef]
- Meng, X.; Li, Z.; Zeng, H.; Chen, J.; Zhang, Z. MoS2 quantum dots-interspersed Bi2WO6 heterostructures for visible light-induced detoxification and disinfection. Appl. Catal. B Environ. 2017, 210, 160–172. [Google Scholar] [CrossRef]
- Ma, L.; Wang, G.; Jiang, C.; Bao, H.; Xu, Q. Synthesis of core-shell TiO2@g-C3N4 hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl. Surf. Sci. 2018, 430, 263–272. [Google Scholar] [CrossRef]
- Tran, D.A.; Nguyen Pham, C.T.; Nguyen Ngoc, T.; Nguyen Phi, H.; Hoai Ta, Q.T.; Truong, D.H.; Nguyen, V.T.; Luc, H.H.; Nguyen, L.T.; Dao, N.N.; et al. One-step synthesis of oxygen doped g-C3N4 for enhanced visible-light photodegradation of Rhodamine B. J. Phys. Chem. Solids 2021, 151, 109900. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.-X.; Wang, H.-L.; Jiang, W.-F. In situ fabrication of ultrathin-g-C3N4/AgI heterojunctions with improved catalytic performance for photodegrading rhodamine B solution. Appl. Surf. Sci. 2021, 538, 148132. [Google Scholar] [CrossRef]
- Nguyen Van, M.; Mai, O.L.T.; Pham Do, C.; Lam Thi, H.; Pham Manh, C.; Nguyen Manh, H.; Pham Thi, D.; Do Danh, B. Fe-Doped g-C3N4: High-Performance Photocatalysts in Rhodamine B Decomposition. Polymers 2020, 12, 1963. [Google Scholar] [CrossRef]
- Dharani, S.; Gnanasekaran, L.; Arunachalam, S.; Zielinska-Jure, A.; Almoallim, H.S.; Soto-Moscoso, M. Photodegrading rhodamine B dye with cobalt ferrite-graphitic carbon nitride (CoFe2O4/g-C3N4) composite. Environ. Res. 2024, 258, 119484. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Y.; Yan, Z.; Li, T.; Jing, Q.; Liu, P. Montmorillonite induced assembly of multi-element doped g-C3N4 nanosheets with enhanced activity for Rhodamine B photodegradation. Appl. Clay Sci. 2022, 218, 106432. [Google Scholar] [CrossRef]
- Ali, G.; Jazib Abbas Zaidi, S.; Abdul Basit, M.; Park, T.J. Synergetic performance of systematically designed g-C3N4/rGO/SnO2 nanocomposite for photodegradation of Rhodamine-B dye. Appl. Surf. Sci. 2021, 570, 151140. [Google Scholar] [CrossRef]
- Reza Saadati-Gullojeh, M.; Ghanbari, M.; Salavati-Niasari, M. Facile preparation and characterization of Zn2Ti3O8/g-C3N4 nanocomposites for degradation of rhodamine B under simulated sunlight. Sol. Energy 2024, 268, 112316. [Google Scholar] [CrossRef]




| Catalyst | Irradiation Source | Irradiation Time (min) | Degradation Rate (%) | RhB Concentration (mg/L) | Catalyst Dosage (g/L) | Reusability | K (min−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| HCN-90 | 50 W LED | 30 | 98.8 | 5 | 0.47 | After five cycles: 93% | 0.125 | This work |
| g-C3N4/SmVO4 | 500 W Xe arc lamp | 120 | >90 | 10 | 1 | After ten cycles: 93% | 0.0345 | [8] |
| TiO2@g-C3N4 | 300 W Xe arc lamp | 100 | 93.3 | 4.79 | 1.33 | After five cycles: 90% | 0.021 | [55] |
| 40-OCN | 30 W LED lamp | 140 | >90 | 30 | 1 | After five cycles: >80% | 0.0193 | [56] |
| g-C3N4/CNTs | 300 W xenon lamp | 60 | 98.1 | 10 | 0.2 | After five cycles: 90% | 0.051 | [53] |
| g-C3N4/AgI | 500 W Xe lamp | 100 | 73.86 | 20 | 0.67 | After four cycles: >70% | 0.0723 | [57] |
| FeCN-7 | 300 W xenon lamp | 60 | - | 20 | 2 | After three cycles: 95% | 0.117 | [58] |
| CoFe2O4/g-C3N4 | 500 W Halogen lamp | 120 | 57 | - | 0.6 | After five cycles: 55% | - | [59] |
| CN-UM/Mt | 300 W Xe lamp | 60 | almost 99 | 10 | 0.5 | - | 0.053 | [60] |
| g-C3N4/5-rGO/SnO2 | 100 W halogen lamp | 120 | 83.2 | 7.2 | 0.15 | - | 0.0285 | [61] |
| Zn2Ti3O8/g-C3N4 | 400 W visible lamp | 90 | 89.0 | 10 | 0.5 | After five cycles: 83% | 0.0211 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yang, M.; Zhang, H.; Chen, Y.; Ji, Y.; Yu, R.; Liu, Z. Boosting the Activation of Molecular Oxygen and the Degradation of Rhodamine B in Polar-Functional-Group-Modified g-C3N4. Molecules 2024, 29, 3836. https://doi.org/10.3390/molecules29163836
Chen J, Yang M, Zhang H, Chen Y, Ji Y, Yu R, Liu Z. Boosting the Activation of Molecular Oxygen and the Degradation of Rhodamine B in Polar-Functional-Group-Modified g-C3N4. Molecules. 2024; 29(16):3836. https://doi.org/10.3390/molecules29163836
Chicago/Turabian StyleChen, Jing, Minghua Yang, Hongjiao Zhang, Yuxin Chen, Yujie Ji, Ruohan Yu, and Zhenguo Liu. 2024. "Boosting the Activation of Molecular Oxygen and the Degradation of Rhodamine B in Polar-Functional-Group-Modified g-C3N4" Molecules 29, no. 16: 3836. https://doi.org/10.3390/molecules29163836
APA StyleChen, J., Yang, M., Zhang, H., Chen, Y., Ji, Y., Yu, R., & Liu, Z. (2024). Boosting the Activation of Molecular Oxygen and the Degradation of Rhodamine B in Polar-Functional-Group-Modified g-C3N4. Molecules, 29(16), 3836. https://doi.org/10.3390/molecules29163836






