Comparison of Eco-Friendly Ionic Liquids and Commercial Bio-Derived Lubricant Additives in Terms of Tribological Performance and Aquatic Toxicity
Abstract
:1. Introduction
2. Materials and Methods
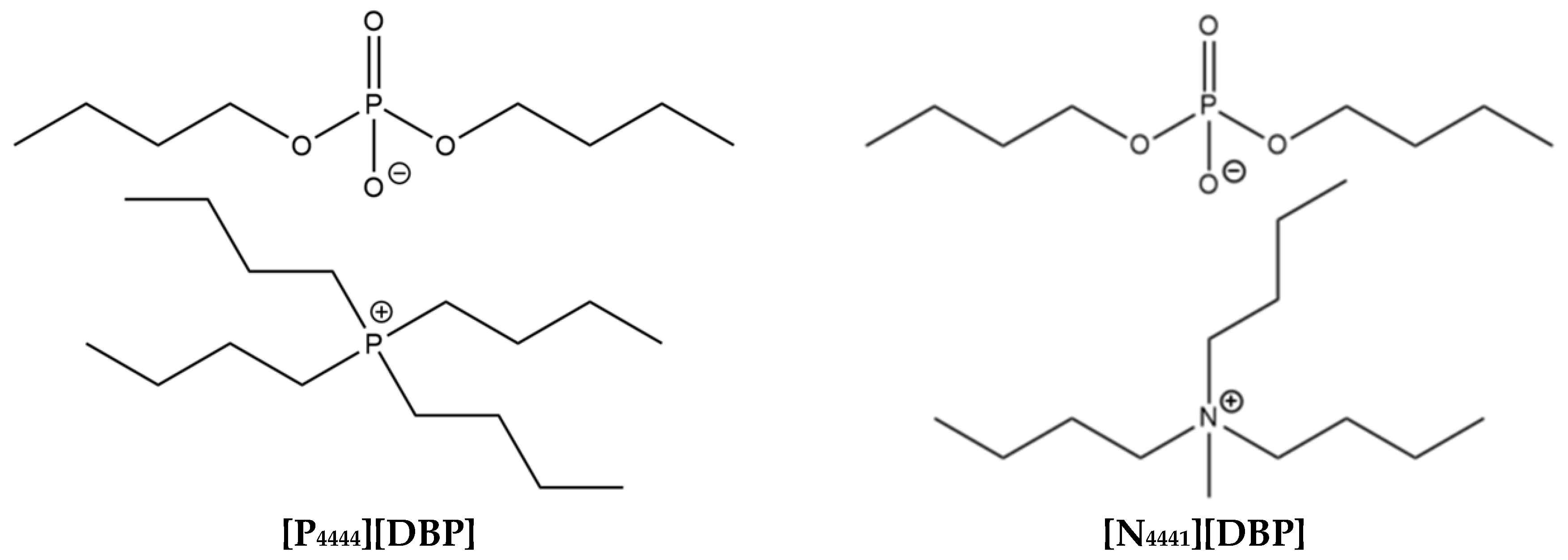
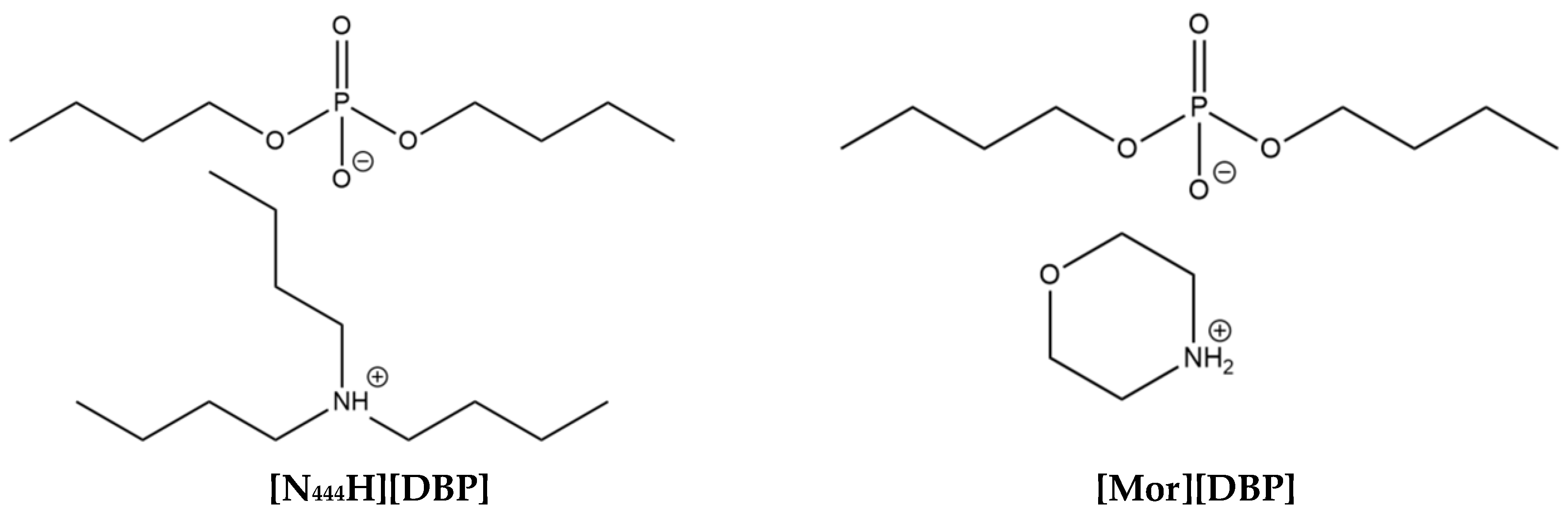
2.1. Selection and Synthesis of ILs
2.2. Commercial Baseline Lubricant and Anti-Wear Additives
2.3. Base Oils
2.4. Tribological Evaluation
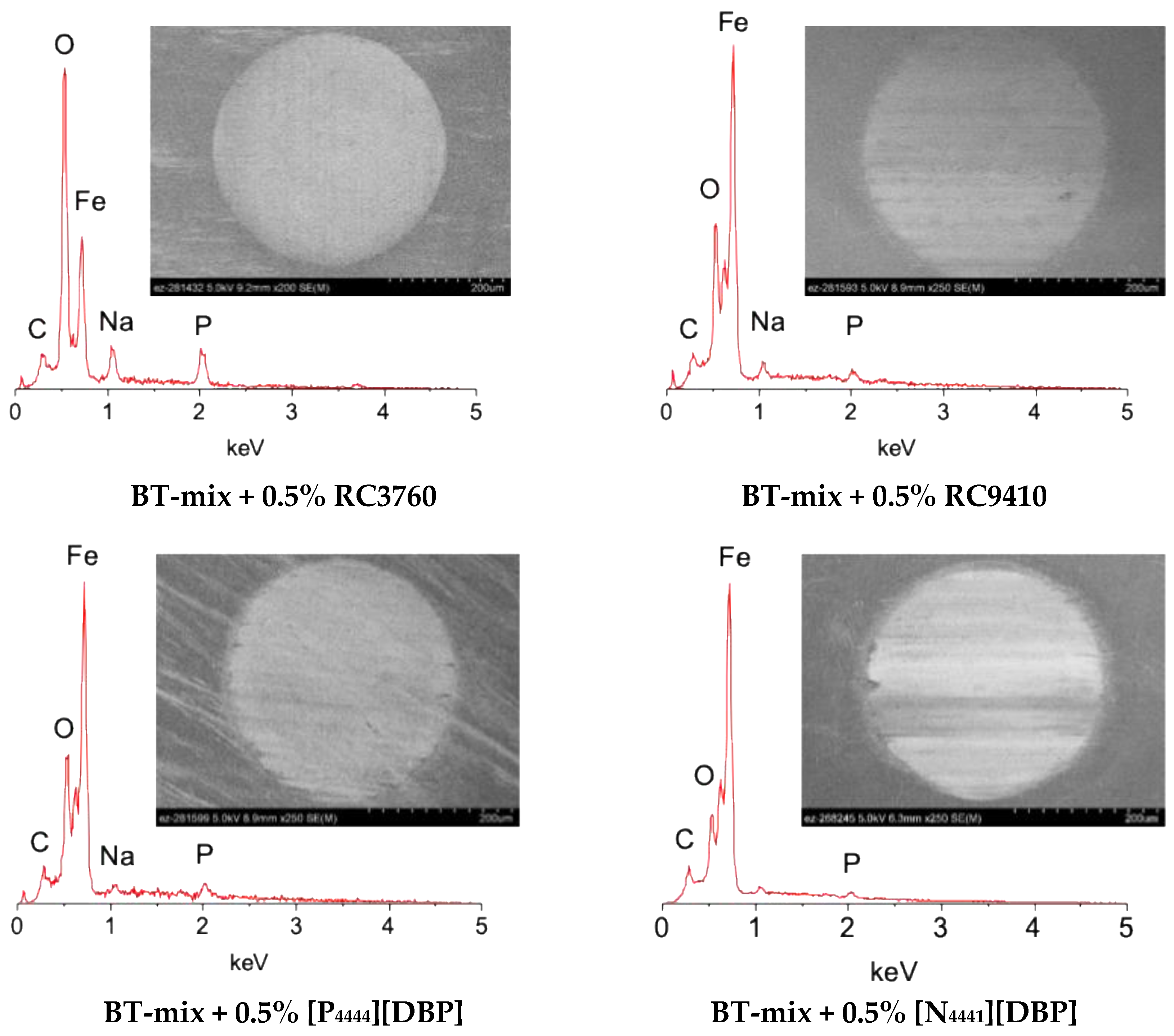
2.5. Surface Characterization
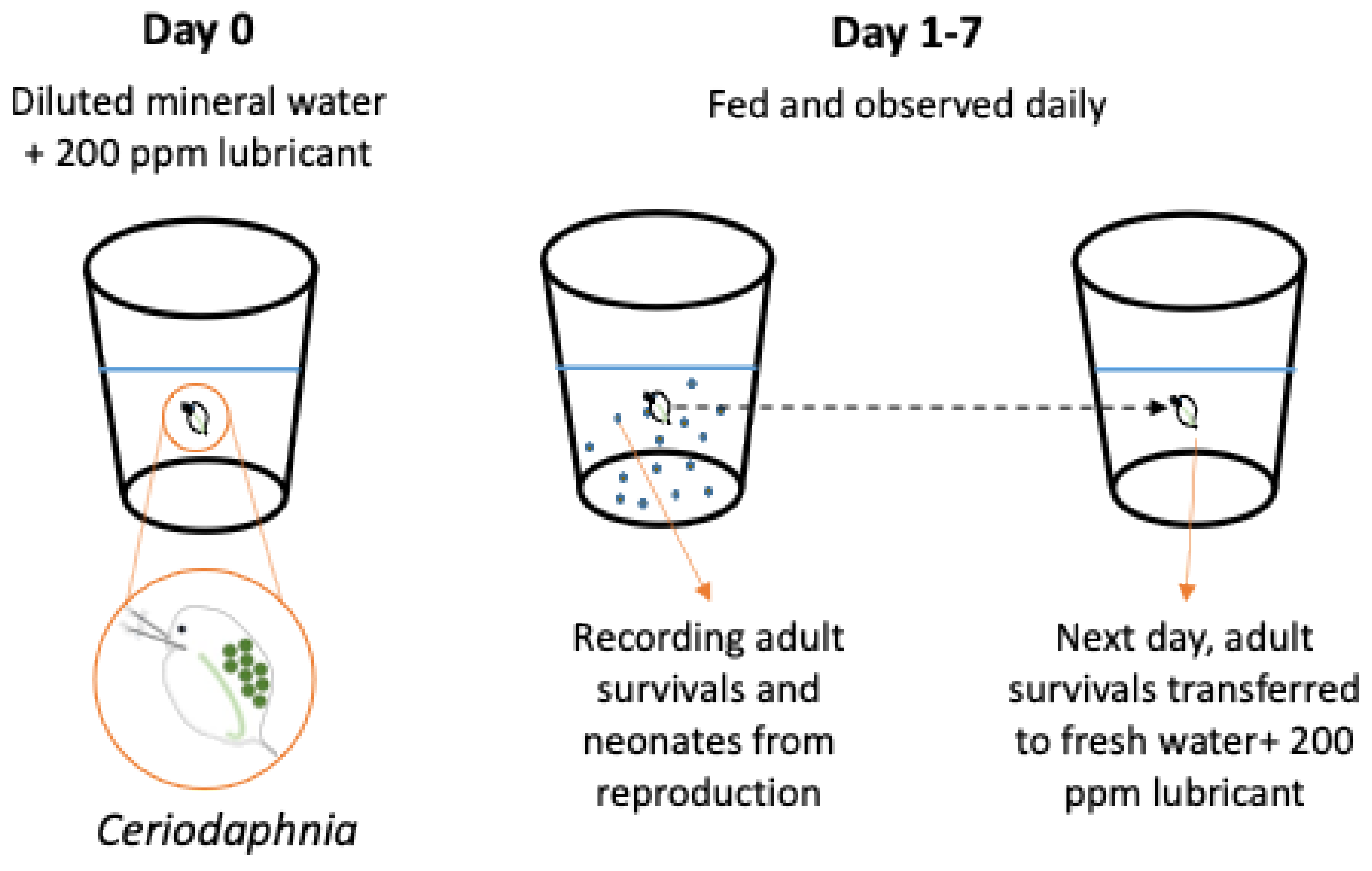
2.6. Toxicity Test
3. Result and Discussion
3.1. Molecular Structures, Thermal Stability, and Oil Solubility of the ILs
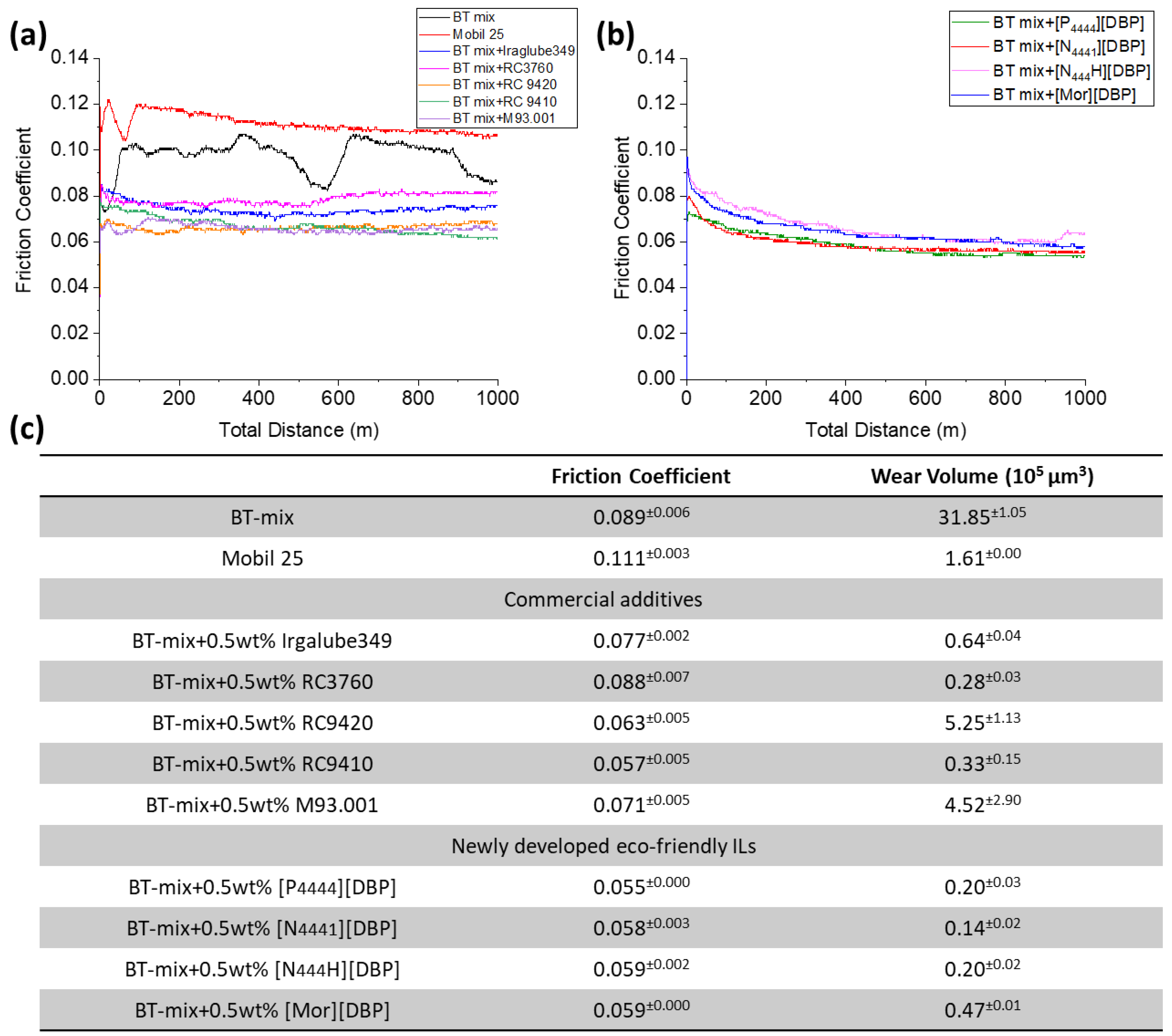
3.2. Tribological Behavior
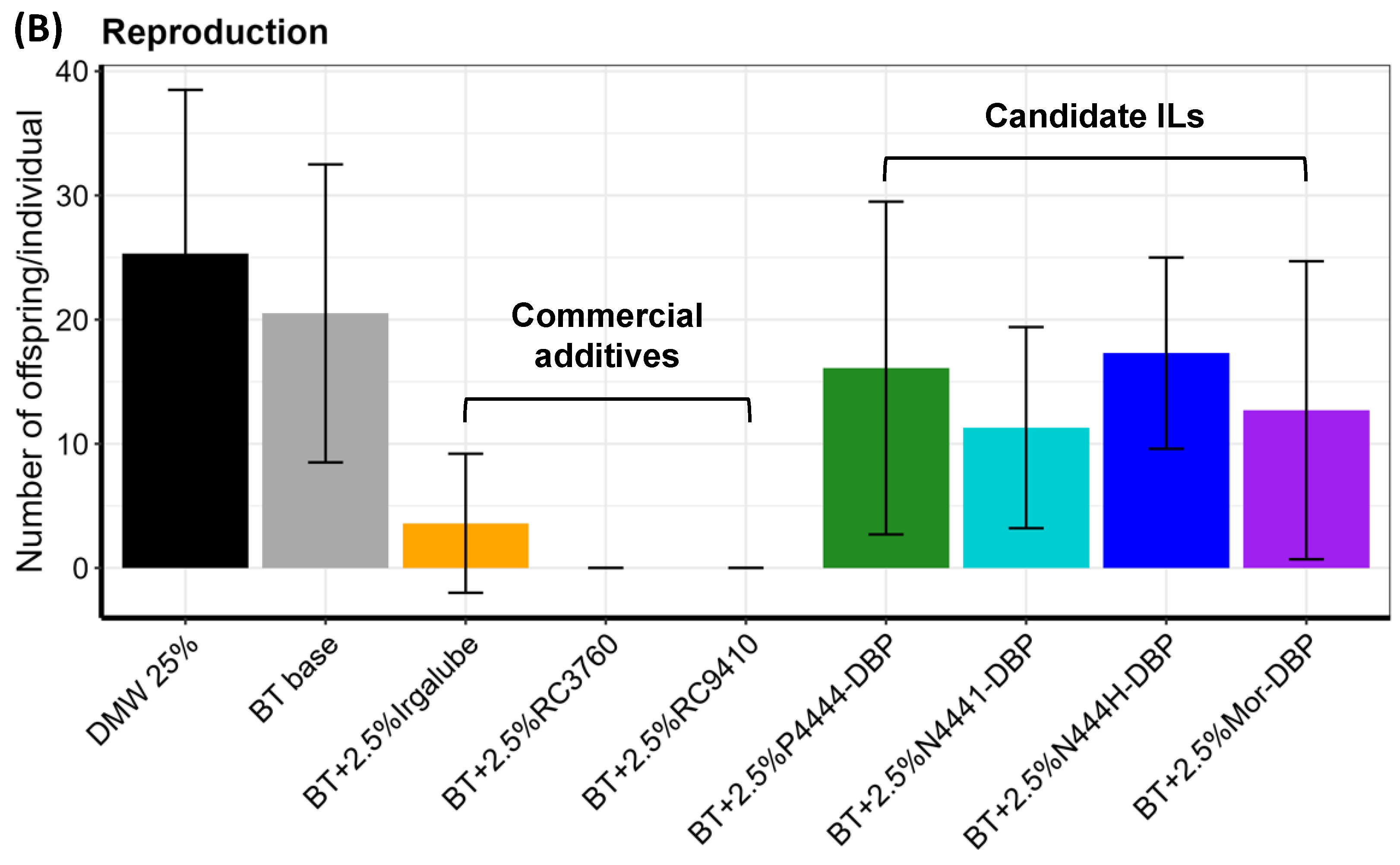
3.3. Aquatic Toxicity
3.4. ILs vs. Commercial Anti-Wear Additives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demand for Lubricants Worldwide from 2000 to 2022. Available online: https://www.statista.com/statistics/411616/lubricants-demand-worldwide/#:~:text=The%20total%20global%20demand%20for,to%2035.6%20million%20metric%20tons (accessed on 1 July 2024).
- Bau, A.; Bruni, G.; Hussin, L.; Kiewell, D.; Kohler, B.; Verity, R. Lubes Growth Opportunities Remain Despite Switch to Electric Vehicles; McKinsey & Company: Hong Kong, China, 2018. [Google Scholar]
- Mang, T.; Gosalia, A. Lubricants and Their Market. In Lubricants and Lubrication; Wiley: New York, NY, USA, 2017; pp. 1–10. [Google Scholar]
- Etkin, D.S. Worldwide analysis of in-port vessel operational lubricant discharges and leakages. In Proceedings of the 33 AMOP Technical Seminar on Environmental Contamination and Response, Halifax, NS, Canada, 7–9 June 2010. [Google Scholar]
- Bartz, W.J. Lubricants and the environment. Tribol. Int. 1998, 31, 35–47. [Google Scholar] [CrossRef]
- Global Environmentally Acceptable Lubricants Market By Type (Mineral Oils, Fixed Oils), By Application (Deep-sea, In-land/Coastal), By Geographic Scope And Forecast; Report ID: 633476, 2024. Available online: https://www.verifiedmarketreports.com/product/environmentally-acceptable-lubricants-market/ (accessed on 1 July 2024).
- Nagendramma, P.; Kaul, S. Development of ecofriendly/biodegradable lubricants: An overview. Renew. Sustain. Energy Rev. 2012, 16, 764–774. [Google Scholar] [CrossRef]
- Rudnick, L.R. Lubricant Additives: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- He, X.; Luo, H.; Mathews, T.J.; Stevenson, L.; Geeza, T.J.; Kumara, C.; Meyer, H.M., III; Qu, J. Minimizing Toxicity and Optimizing Lubricity of Ionic Liquids for Eco-Friendly Lubrication. ACS Sustain. Chem. Eng. 2024, 12, 4344–4355. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Seddon, K.R. Ionic Liquids. Clean Prod. Process. 1999, 1, 223–236. [Google Scholar] [CrossRef]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef] [PubMed]
- Somers, A.E.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic Liquids as Lubricant Additives: A Review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zou, K.; Wang, R.; Wang, X.; Liang, Y.; Yu, Q.; Cai, M.; Zhou, F. Tribological properties of quaternary ammonium salt polymeric ionic liquids as water-based lubricant additive. Tribology 2022, 42, 1246–1257. [Google Scholar]
- Zheng, D.; Su, T.; Ju, C. Influence of ecofriendly protic ionic liquids on the corrosion and lubricating properties of water-glycol. Tribol. Int. 2022, 165, 107283. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Zhang, E.; Chen, Y.; Li, W.; Cheng, S. Tribological performance of fatty acid ammonium ionic liquids as anti-wear additives in water-based fluids. Tribol. Int. 2024, 194, 109565. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, P.; Saran, S.; Khatri, O.P. Halogen-Free Bis(imidazolium)/Bis(ammonium)-Di[bis(salicylato)borate] Ionic Liquids as Energy-Efficient and Environmentally Friendly Lubricant Additives. ACS Appl. Mater. Interfaces 2014, 6, 15318–15328. [Google Scholar] [CrossRef] [PubMed]
- Reeves, C.J.; Siddaiah, A.; Menezes, P.L. Friction and Wear Behavior of Environmentally Friendly Ionic Liquids for Sustainability of Biolubricants. J. Tribol. 2019, 141, 051604. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, T.; Shang, W.; Sun, L.; Liu, D.; Tong, D.; Liu, S. Environmental friendly polyisobutylene-based ionic liquid containing chelated orthoborate as lubricant additive: Synthesis, tribological properties and synergistic interactions with ZDDP in hydrocarbon oils. Tribol. Int. 2017, 115, 297–306. [Google Scholar] [CrossRef]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Silva Pereira, C. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Gathergood, N.; Scammells, P.J. Design and preparation of room-temperature ionic liquids containing biodegradable side chains. Aust. J. Chem. 2002, 55, 557–560. [Google Scholar] [CrossRef]
- Yu, Y.; Nie, Y. Toxicity and antimicrobial activities of ionic liquids with halogen anion. J. Environ. Prot. 2011, 2, 298–303. [Google Scholar] [CrossRef]
- Garcia, M.T.; Gathergood, N.; Scammells, P.J. Biodegradable ionic liquids Part II. Effect of the anion and toxicology. Green Chem. 2005, 7, 9–14. [Google Scholar] [CrossRef]
- Qu, J.; Luo, H.; He, X. Ionic Liquids Containing Quaternary Ammonium and Phosphonium Cations, and Their Use as Environmentally Friendly Lubricant Additives. U.S. Patent #11,760,766, 19 September 2023. [Google Scholar]
- Yu, B.; Bansal, D.G.; Qu, J.; Sun, X.; Luo, H.; Dai, S.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G.; Smolenski, D.J. Oil-miscible and non-corrosive phosphonium-based ionic liquids as candidate lubricant additives. Wear 2012, 289, 58–64. [Google Scholar] [CrossRef]
- Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear (ASTM G0133-05R16). Available online: https://www.astm.org/g0133-05r16.html (accessed on 1 July 2024).
- Hamrock, B.J.; Dowson, D. Ball Bearing Lubrication: The Elastohydrodynamics of Elliptical Contacts; NASA: Washington, DC, USA, 1981.
- EPA. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Freshwater Organisms, 4th ed.; EPA: Washington, DC, USA, 2002.
- Anderson, B.G. The toxicity thresholds of various substances found in industrial wastes as determined by the use of Daphnia magna. Sew. Work. J. 1944, 16, 1156–1165. [Google Scholar]
- Ebert, D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia; National Library of Medicine: Bethesda, MD, USA, 2005.
- Tatarazako, N.; Oda, S. The water flea Daphnia magna (Crustacea, Cladocera) as a test species for screening and evaluation of chemicals with endocrine disrupting effects on crustaceans. Ecotoxicology 2007, 16, 197–203. [Google Scholar] [CrossRef]
- Landauer, A.K.; Barnhill, W.C.; Qu, J. Correlating mechanical properties and anti-wear performance of tribofilms formed by ionic liquids, ZDDP and their combinations. Wear 2016, 354–355, 78–82. [Google Scholar] [CrossRef]
- Zhou, Y.; Leonard, D.N.; Guo, W.; Qu, J. Understanding Tribofilm Formation Mechanisms in Ionic Liquid Lubrication. Sci. Rep. 2017, 7, 8426. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhou, Y.; Sang, X.; Leonard, D.N.; Qu, J.; Poplawsky, J.D. Atom probe tomography unveils growth mechanisms of wear-protective tribofilms formed by ZDDP, ionic liquid, and their combination. ACS Appl. Mater. Interfaces 2017, 9, 23152–23163. [Google Scholar] [CrossRef]
- Zhou, Y.; Leonard, D.N.; Meyer, H.M.; Luo, H.M.; Qu, J. Using diamond-like carbon coating and organophosphate lubricant additive together causes excessive tribochemical material removal? Adv. Mater. Interfaces 2015, 2, 1500213. [Google Scholar] [CrossRef]
- Barnhill, W.C.; Qu, J.; Luo, H.M.; Meyer, H.M., III; Ma, C.; Chi, M.; Papke, B.L. Phosphonium-organophosphate ionic liquids as lubricant additives: Effects of cation structure on physicochemical and tribological characteristics. ACS Appl. Mater. Interfaces 2014, 6, 22585–22593. [Google Scholar] [CrossRef]
- Zhou, Y.; Weber, J.; Viola, M.B.; Qu, J. Is more always better? Tribofilm evolution and tribological behavior impacted by the concentration of ZDDP, ionic liquid, and ZDDP-ionic liquid combination. Wear 2019, 432–433, 202951. [Google Scholar] [CrossRef]




| Mobil 25 Fully Formulated Hydraulic Oil | BT-4 Base Oil | BT-22 Base Oil | BT-Mix (Blend of BT-4 and BT-22 @ 0.593:0.407 Mass Ratio) | |
|---|---|---|---|---|
| Viscosity @ 40 °C (cSt) | 46 | 23.2 | 159.5 | 44.8 |
| Viscosity @ 100 °C (cSt) | 7.1 | 4.4~4.9 | 23.9 | 6.7 |
| Biodegradability (OECD 301B) | - | 88% | 79% | - |
| EcoToxicity (OECD 201/202/203) | - | >1000 mg/L (SDS) | >1000 mg/L (SDS) | - |
| Ionic Liquid | Solubility in BT-Mix | Commercial Additive | Solubility in BT-Mix |
|---|---|---|---|
| [P4444][DBP] | 1–2 wt% | Irgalube-349 | >5 wt% |
| [N4441][DBP] | >5 wt% | RC3760 | >5 wt% |
| [N444H][DBP] | >5 wt% | RC9420 | >5 wt% |
| [Mor][DBP] | >5 wt% | RC9410 | >5 wt% |
| M93.001 | >5 wt% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Stevenson, L.M.; Kumara, C.; Mathews, T.J.; Luo, H.; Qu, J. Comparison of Eco-Friendly Ionic Liquids and Commercial Bio-Derived Lubricant Additives in Terms of Tribological Performance and Aquatic Toxicity. Molecules 2024, 29, 3851. https://doi.org/10.3390/molecules29163851
He X, Stevenson LM, Kumara C, Mathews TJ, Luo H, Qu J. Comparison of Eco-Friendly Ionic Liquids and Commercial Bio-Derived Lubricant Additives in Terms of Tribological Performance and Aquatic Toxicity. Molecules. 2024; 29(16):3851. https://doi.org/10.3390/molecules29163851
Chicago/Turabian StyleHe, Xin, Louise M. Stevenson, Chanaka Kumara, Teresa J. Mathews, Huimin Luo, and Jun Qu. 2024. "Comparison of Eco-Friendly Ionic Liquids and Commercial Bio-Derived Lubricant Additives in Terms of Tribological Performance and Aquatic Toxicity" Molecules 29, no. 16: 3851. https://doi.org/10.3390/molecules29163851
APA StyleHe, X., Stevenson, L. M., Kumara, C., Mathews, T. J., Luo, H., & Qu, J. (2024). Comparison of Eco-Friendly Ionic Liquids and Commercial Bio-Derived Lubricant Additives in Terms of Tribological Performance and Aquatic Toxicity. Molecules, 29(16), 3851. https://doi.org/10.3390/molecules29163851








