Vicia faba L. Pod Valves: A By-Product with High Potential as an Adjuvant in the Treatment of Parkinson’s Disease
Abstract
:1. Introduction
2. Results and Discussion
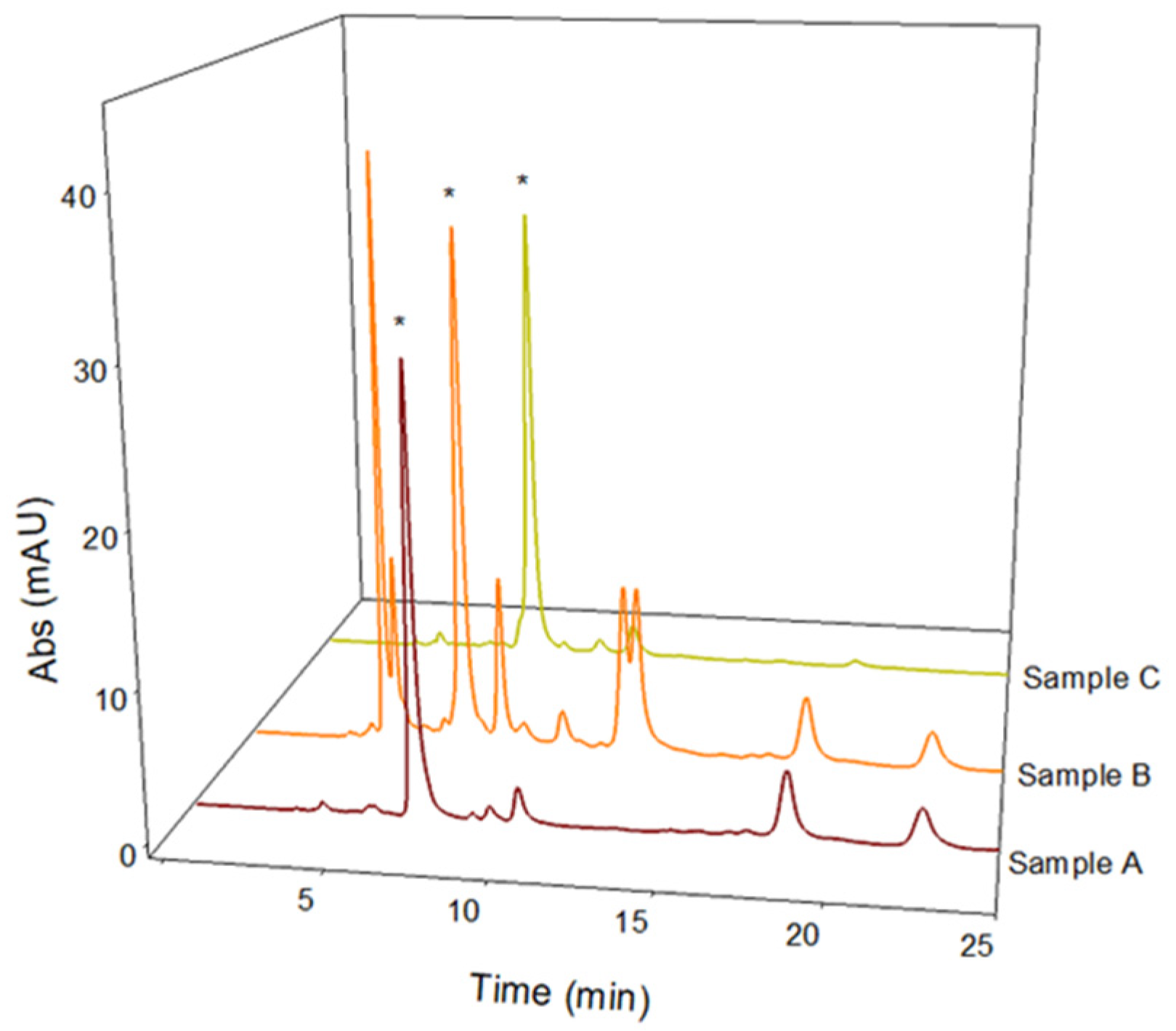
2.1. LC/UV Quantification of L-Dopa in Lucan Broad Bean Pod Valves
2.2. LC-MS/MS Phenolic Compounds Characterisation in Lucan Broad Bean Pods
2.2.1. Phenolic Acids and Derivatives
2.2.2. Flavonoid Compounds
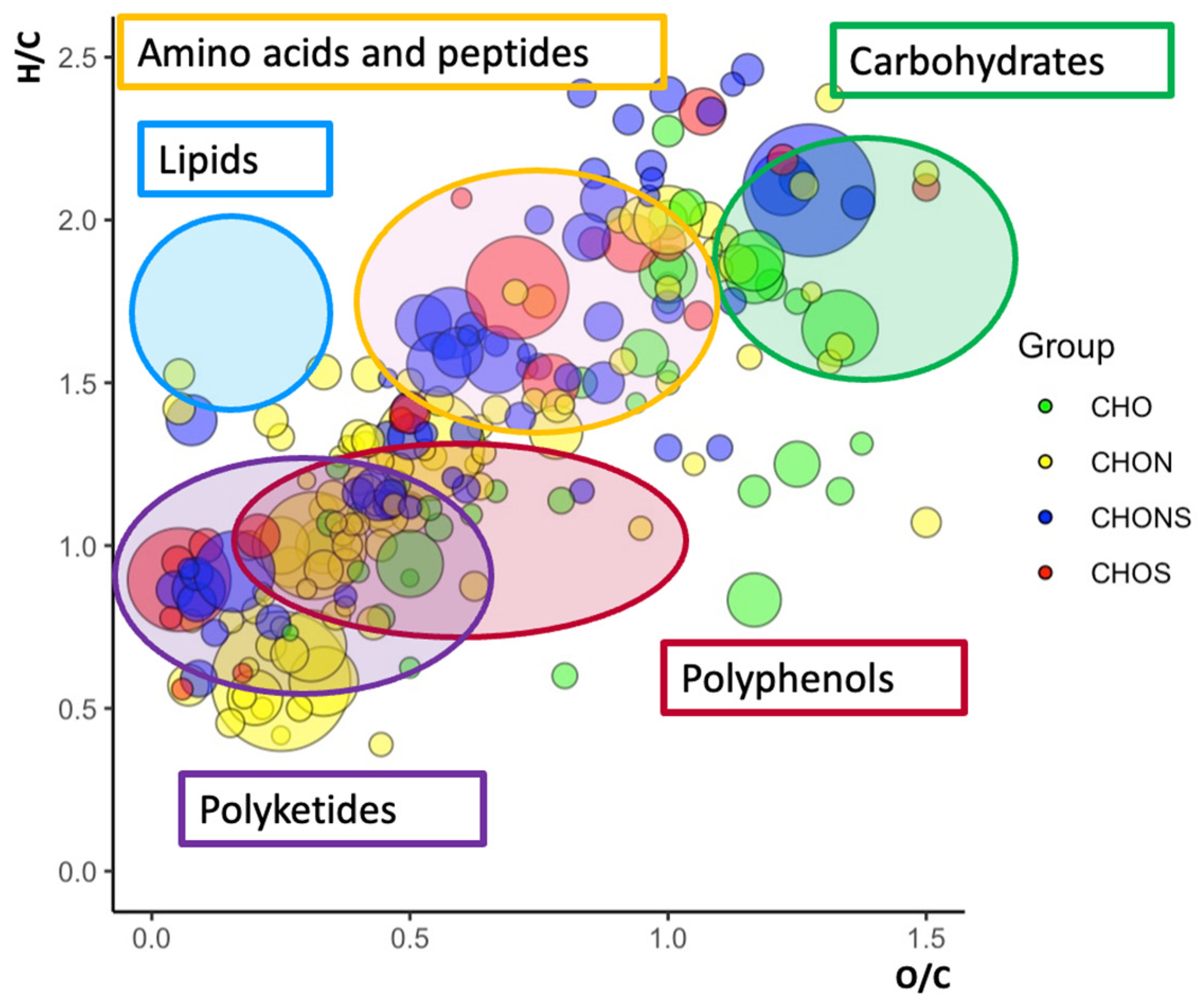
2.3. Van Krevelen Plots
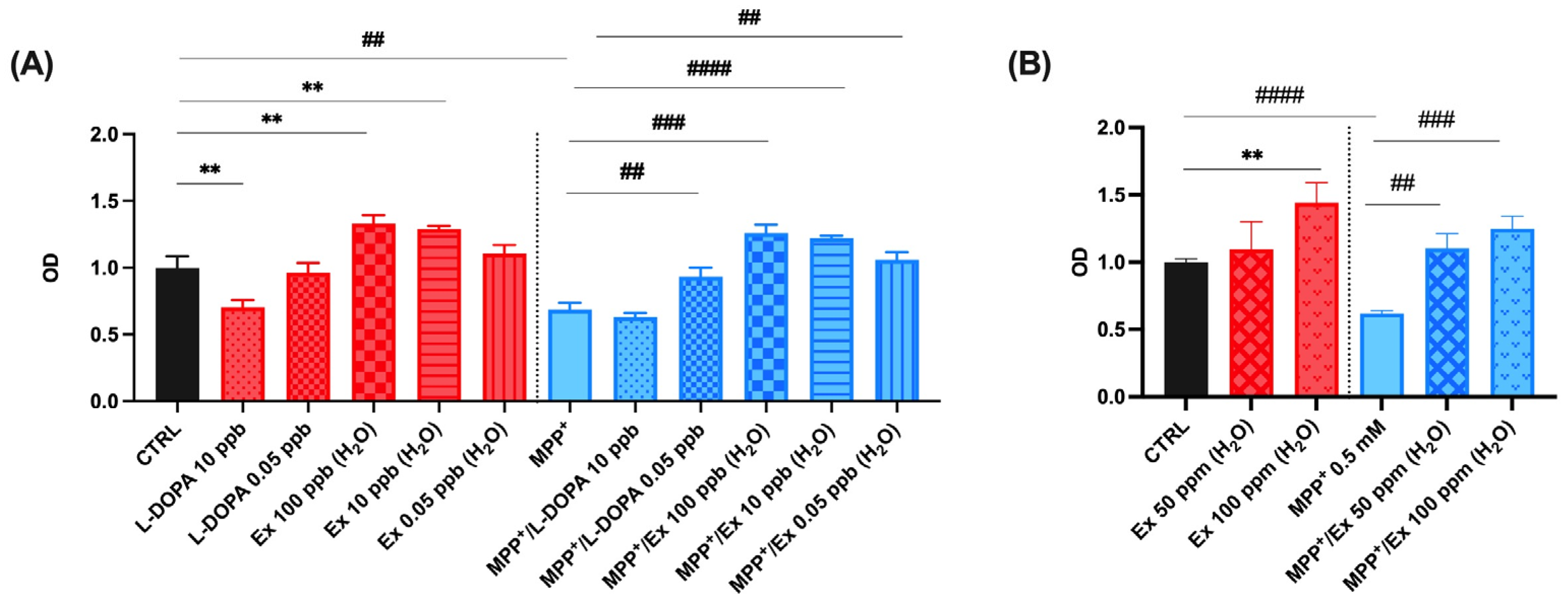
2.4. Vicia faba L. Pod Aqueous Extracts Increase Cell Viability in SHSY-5Y Cells after MPP+-Induced Neurotoxicity
2.5. L-Dopa Quantification and LC-MS/MS Antioxidant Phenolic Compound Characterization in Naturally Acidic Solutions
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. LC-UV Conditions
3.4. LC-ESI/LTQ-Orbitrap/MS2 Conditions
3.5. Van Krevelen Plots Analysis
3.6. Cell Culture, Treatment, and Cell Viability Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renna, M.; De Cillis, F.; Leoni, B.; Acciardi, E.; Santamaria, P. From by-Product to Unconventional Vegetable: Preliminary Evaluation of Fresh Fava Hulls Highlights Richness in L-Dopa and Low Content of Anti-Nutritional Factor. Foods 2020, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Arráez-Román, D.; Warad, I.; Fernández-Gutiérrez, A.; Segura-Carretero, A. UHPLC/MS2-based approach for the comprehensive metabolite profiling of bean (Vicia faba L.) by-products: A promising source of bioactive constituents. Food Res. Int. 2017, 93, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Izzo, L.; Lombardi, S.; Gaspari, A.; De Pascale, S.; Grosso, M.; Ritieni, A. Analysis of Polyphenolic Compounds in Water-Based Extracts of Vicia faba L.: A Potential Innovative Source of Nutraceutical Ingredients. Antioxidants 2022, 11, 2453. [Google Scholar] [CrossRef]
- FAO, FAOSTAT. Food and Agriculture Organization of the United Nations. Rome, 2018. Available online: http://www.fao.org (accessed on 1 February 2024).
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Romani, A.; Mulinacci, N.; Zarini, S.; Conte, D.; Vincieri, F.F.; Galli, C. Antioxidant and other biological activities of olive mill waste waters. J. Agric. Food Chem. 1999, 47, 3397–3401. [Google Scholar] [CrossRef] [PubMed]
- Azizah, A.H.; Nik Ruslawati, N.M.; Sweetee, T. Extraction and characterization of antioxidant from cocoa by-products. Food Chem. 1999, 64, 199–202. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Antioxidant Dietary Fiber Product: A New Concept and a Potential Food Ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef]
- Sotillo, D.R.; Hadley, M.; Holm, E.T. Potato peel waste: Stability and antioxidant activity of a freeze-dried extract. J. Food Sci. 1994, 59, 1031–1033. [Google Scholar] [CrossRef]
- Valente, I.M.; Maia, M.R.G.; Malushi, N.; Oliveira, H.M.; Papa, L.; Rodrigues, J.A.; Fonseca, A.J.M.; Cabrita, A.R.J. Profiling of phenolic compounds and antioxidant properties of European varieties and cultivars of Vicia faba L. pods. Phytochemistry 2018, 152, 223–229. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20 (Suppl. 2), 1700–1741. [Google Scholar] [CrossRef]
- Stagos, D. Antioxidant Activity of Polyphenolic Plant Extracts. Antioxidants 2020, 9, 19. [Google Scholar] [CrossRef]
- Tesoro, C.; Ciriello, R.; Lelario, F.; Di Capua, A.; Pascale, R.; Bianco, G.; Dell’Agli, M.; Piazza, S.; Guerrieri, A.; Scrano, L.; et al. Development and Validation of a Reversed-Phase HPLC Method with UV Detection for the Determination of L-Dopa in Vicia faba L. Broad Beans. Molecules 2022, 27, 7468. [Google Scholar] [CrossRef]
- Tesoro, C.; Cembalo, G.; Guerrieri, A.; Bianco, G.; Acquavia, M.A.; Di Capua, A.; Lelario, F.; Ciriello, R. A Critical Overview of Enzyme-Based Electrochemical Biosensors for L-Dopa Detection in Biological Samples. Chemosensors 2023, 11, 523. [Google Scholar] [CrossRef]
- Cembalo, G.; Ciriello, R.; Tesoro, C.; Guerrieri, A.; Bianco, G.; Lelario, F.; Acquavia, M.A.; Di Capua, A. An Amperometric Biosensor Based on a Bilayer of Electrodeposited Graphene Oxide and Co-Crosslinked Tyrosinase for L-Dopa Detection in Untreated Human Plasma. Molecules 2023, 28, 5239. [Google Scholar] [CrossRef]
- Apaydin, H.; Ertan, S.; Özekmekçi, S. Broad bean (Vicia faba)—A natural source of L-dopa—Prolongs “on” periods in patients with Parkinson’s disease who have “on-off” fluctuations. Mov. Disord. 2000, 15, 164–166. [Google Scholar] [CrossRef]
- Tesoro, C.; Lelario, F.; Ciriello, R.; Bianco, G.; Di Capua, A.; Acquavia, M.A. An Overview of Methods for L-Dopa Extraction and Analytical Determination in Plant Matrices. Separations 2022, 9, 224. [Google Scholar] [CrossRef]
- Burbano, C.; Cuadrado, C.; Muzquiz, M.; Cubero, J.I. Variation of favism-inducing factors (vicine, convicine and L-DOPA) during pod development in Vicia faba L. Plant Foods Hum. Nutr. 1995, 47, 265–275. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Gavrilova, V.; Kajdzanoska, M.; Gjamovski, V.; Stefova, M. Separation, characterization and quantification of phenolic compounds in blueberries and red and black currants by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2011, 59, 4009–4018. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Hafeez, N.; Rauf, A.; Bashir, S.; Linfang, H.; Rehman, M.U.; Mubarak, M.S.; Uddin, M.S.; Bawazeer, S.; Shariati, M.A.; et al. Rengasamy, Phyllanthus emblica: A comprehensive review of its therapeutic benefits. S. Afr. J. Bot. 2021, 138, 278–310. [Google Scholar] [CrossRef]
- Gülmez, G.; Şen, A.; Şekerler, T.; Algül, F.K.; Çilingir-Kaya, Ö.T.; Şener, A. The antioxidant, anti-inflammatory, and antiplatelet effects of Ribes rubrum L. fruit extract in the diabetic rats. J. Food Biochem. 2022, 46, e14124. [Google Scholar] [CrossRef]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a source of bioactive constituents: A review on their characterization, properties and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 982–999. [Google Scholar] [CrossRef]
- Vilairat, C.; Kobtrakul, K.; Vimolmangkang, S. Enhanced Physicochemical Stability of the L-DOPA Extract of Mucuna pruriens Seeds by Adding Phyllanthus emblica. Molecules 2023, 28, 1573. [Google Scholar] [CrossRef]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Pittaluga, A.; Boggia, R. Pulsed Ultrasound-Assisted Extraction as an Alternative Method to Conventional Maceration for the Extraction of the Polyphenolic Fraction of Ribes nigrum Buds: A New Category of Food Supplements Proposed by The FINNOVER Project. Foods 2019, 8, 466. [Google Scholar] [CrossRef]
- Martin, K.R.; Appel, C.L. Polyphenols as Dietary Supplements: A Double-Edged Sword. Nutr. Diet Suppl. 2009, 2, 6403722. [Google Scholar] [CrossRef]
- Madrigal-Carballo, S.; Rodriguez, G.; Krueger, C.G.; Dreher, M.; Reed, J.D. Pomegranate (Punica granatum) supplements: Authenticity, antioxidant and polyphenol composition. J. Funct. Foods 2009, 1, 324–329. [Google Scholar] [CrossRef]
- Otsuka, F.A.M.; Santos, R.B.; Chaves, L.F.; Santos, R.S.; Chaves Filho, A.B.; Miyamoto, S.; Matos, H.R. Identification of caffeic acid and rutin by UHPLC MS/MS and antioxidant activity of Commelina erecta Lineu. in cell culture. An. Da Acad. Bras. De Cienc. 2020, 92, e20190491. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; del Mar Contreras, M.; Arráez-Román, D.; Fernández-Gutiérrez, A.; Segura-Carretero, A. UHPLC-ESI-QTOF-MS-based metabolic profiling of Vicia faba L. (Fabaceae) seeds as a key strategy for characterization in foodomics. Electrophoresis 2014, 35, 1571–1581. [Google Scholar] [CrossRef]
- Regos, I.; Urbanella, A.; Treutter, D. Identification and quantification of phenolic compounds from the forage legume sainfoin (Onobrychis viciifolia). J. Agric. Food Chem. 2009, 57, 5843–5852. [Google Scholar] [CrossRef]
- Kite, G.C.; Rowe, E.R.; Lewis, G.P.; Veitch, N.C. Acylated flavonol tri- and tetraglycosides in the flavonoid metabolome of Cladrastis kentukea (Leguminosae). Phytochemistry 2011, 72, 372–384. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Garcia-Grau, M.M.; Tomas-Lorente, F. Flavonoid concentration changes in maturing broad bean pods. J. Agric. Food Chem. 1991, 39, 255–258. [Google Scholar] [CrossRef]
- Spanou, C.; Bourou, G.; Dervishi, A.; Aligiannis, N.; Angelis, A.; Komiotis, D.; Skaltsounis, A.L.; Kouretas, D. Antioxidant and chemopreventive properties of polyphenolic compounds derived from Greek legume plant extracts. J. Agric. Food Chem. 2008, 56, 6967–6976. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Dueñas, M.; Estrella, I.; Hernández, T. Occurrence of phenolic compounds in the seed coat and the cotyledon of peas (Pisum sativum L.). Eur. Food Res. Technol. 2004, 219, 116–123. [Google Scholar]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef]
- Hodson, T.O. Root-mean-square error (RMSE) or mean absolute error (MAE): When to use them or not. Geosci. Model Dev. 2022, 15, 5481–5487. [Google Scholar]
- Onzo, A.; Pascale, R.; Acquavia, M.A.; Cosma, P.; Gubitosa, J.; Gaeta, C.; Iannece, P.; Tsybin, Y.; Rizzi, V.; Guerrieri, A.; et al. Untargeted analysis of pure snail slime and snail slime-induced Au nanoparticles metabolome with MALDI FT-ICR MS. J. Mass Spectrom. 2021, 56, e4722. [Google Scholar] [CrossRef] [PubMed]
- Onzo, A.; Acquavia, M.A.; Pascale, R.; Iannece, P.; Gaeta, C.; Lelario, F.; Ciriello, R.; Tesoro, C.; Bianco, G.; Di Capua, A. Untargeted metabolomic analysis by ultra-high-resolution mass spectrometry for the profiling of new Italian wine varieties. Anal. Bioanal. Chem. 2022, 414, 7805–7812. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, A.; Cipollone, I.; Verde, R.; Kalkan, H.; Moriello, C.; Iannotti, F.A.; Di Marzo, V.; Piscitelli, F. The endocannabinoidome mediator N-oleoylglycine is a novel protective agent against 1-methyl-4-phenyl-pyridinium-induced neurotoxicity. Front. Aging Neurosci. 2022, 14, 926634. [Google Scholar] [CrossRef]
- Susa, F.; Pisano, R. Advances in Ascorbic Acid (Vitamin C) Manufacturing: Green Extraction Techniques from Natural Sources. Processes 2023, 11, 3167. [Google Scholar] [CrossRef]
- Shi, J.; Gao, X.; Zhang, A.; Qin, X.; Du, G. Characterization of multiple chemical components of GuiLingJi by UHPLC-MS and 1H NMR analysis. J. Pharm. Anal. 2022, 12, 460–469. [Google Scholar] [CrossRef]
- Di Stefano, V.; Pitonzo, R.; Novara, M.E.; Bongiorno, D.; Indelicato, S.; Gentile, C.; Avellone, G.; Bognanni, R.; Scandurra, S.; Melilli, M.G. Antioxidant activity and phenolic composition in pomegranate (Punica granatum L.) genotypes from south Italy by UHPLC-Orbitrap-MS approach. J. Sci. Food Agric. 2019, 99, 1038–1045. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Flavonoids and Ellagitannins Characterization, Antioxidant and Cytotoxic Activities of Phyllanthus acuminatus Vahl. Plants 2017, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. High-Throughput Screening and Characterization of Phenolic Compounds in Stone Fruits Waste by LC-ESI-QTOF-MS/MS and Their Potential Antioxidant Activities. Antioxidants 2021, 10, 234. [Google Scholar] [CrossRef]
- Yan, L.; Yin, P.; Ma, C.; Liu, Y. Method Development and Validation for Pharmacokinetic and Tissue Distributions of Ellagic Acid Using Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Molecules 2014, 19, 18923–18935. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumara, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar]
- Tahir, N.I.; Shaari, K.; Abas, F.; Ahmad Parveez, G.K.; Ahmad Tarmizi, H.; Ramli, U.S. Identification of oil palm (Elaeis guineensis) spear leaf metabolites using mass spectrometry and neutral loss analysis. J. Oil Palm Res. 2013, 25, 72–83. [Google Scholar]
- Gil-Martínez, L.; Mut-Salud, N.; Ruiz-García, J.A.; Falcón-Piñeiro, A.; Maijó-Ferré, M.; Baños, A.; De la Torre-Ramírez, J.M.; Guillamón, E.; Verardo, V.; Gómez-Caravaca, A.M. Phytochemicals Determination, and Antioxidant, Antimicrobial, Anti-Inflammatory and Anticancer Activities of Blackberry Fruits. Foods 2023, 12, 1505. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Mumtaz, M.W.; Danish, M.; Rashid, U.; Mukhtar, H.; Irfan, A.; Anwar, F.; Saari, N. UHPLC-QTOF-MS/MS metabolites profiling and antioxidant/antidiabetic attributes of Cuscuta reflexa grown on Casearia tomentosa: Exploring phytochemicals role via molecular docking. Int. J. Food Prop. 2020, 23, 918–940. [Google Scholar] [CrossRef]

| Peak # | Retention Time (min) | Molecular Formula | a m/z Exp. [M-H]− | b m/z Calc. [M-H]− | c Mass Accuracy (RMS) | Major Fragments | Assignment | Reference |
|---|---|---|---|---|---|---|---|---|
| Phenolic acid derivatives | ||||||||
| 1 | 0.60 | C9H8O4 | 179.0343 | 179.0349 | 0.44 | 134.9868 | Caffeic acid | [28] |
| 2 | 0.90 | C15H20O10 | 359.0990 | 359.0972 | 1.84 | 197.0450 | Syringic acid hexoside | [2] |
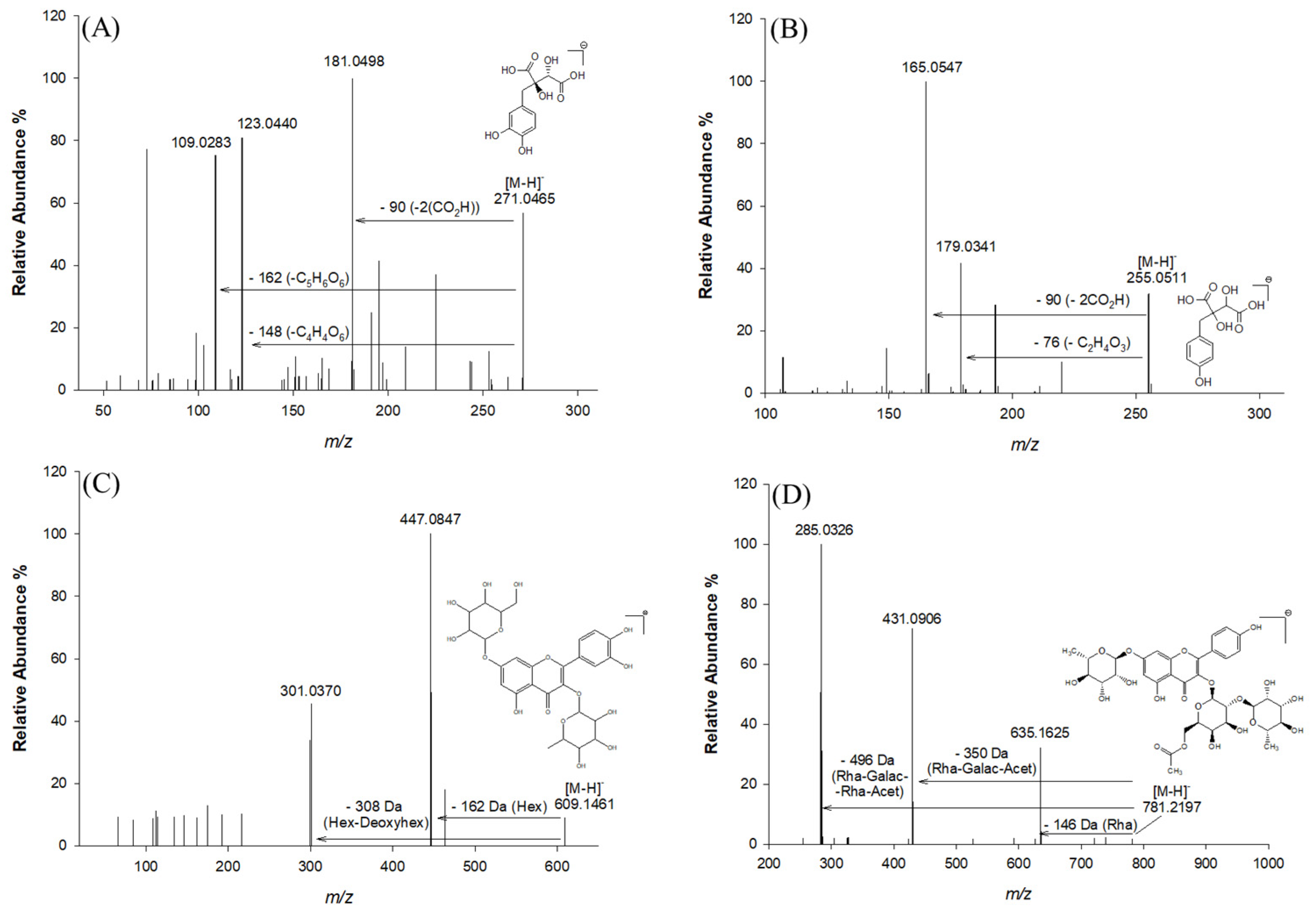
| 3 | 0.91 | C11H12O8 | 271.0465 | 271.0459 | 1.99 | 181.0498; 123.0440; 109.0283; 165.0546; 151.0392 | Fukiic acid | [2] |
| 4 | 1.24 | C13H16O9 | 315.0727 | 315.0722 | 1.60 | 152.0105; 153.0185; 108.0208; 109.0282 | Protocatechuic acid hexoside | [29] |
| 5 | 1.56 | C11H12O7 | 255.0511 | 255.0510 | 0.17 | 193.0499; 179.0341; 165.0547 | Piscidic acid | [2] |
| 6 | 3.47 | C12H14O8 | 285.0620 | 285.0616 | 1.47 | 209.0458; 195.0655; | Methylfukiic acid | [2] |
| 7 | 3.80 | C15H18O8 | 325.0930 | 325.0929 | 0.31 | 163.0389; 119.0489 | Coumaroylhexose | [29] |
| 8 | 4.57 | C11H12O6 | 239.0560 | 239.0561 | 0.38 | 178.9771 | Eucomic acid | [29] |
| 9 | 6.29 | C15H18O9 | 341.0881 | 341.0878 | 0.95 | 179.0350 | Caffeoylhexose | [2] |
| 10 | 6.48 | C19H22O13 | 457.0991 | 457.0988 | 0.85 | 179.0340 | Cutaric acid hexoside | [2] |
| 11 | 6.61 | C16H20O9 | 355.1042 | 355.1035 | 1.98 | 193.0499 | Ferulic acid hexoside | [2] |
| 12 | 9.11 | C13H12O8 | 295.0458 | 295.0459 | 0.65 | 133.0131; 115.0023; 135.0440 | Coutaric acid or Phaseolic acid | [2] |
| 13 | 12.07 | C13H12O7 | 279.0513 | 279.0510 | 0.98 | 163.0390; 119.0492 | p-Coumaroyl-malic acid | [30] |
| Flavonoid derivatives | ||||||||
| 14 | 13.41 | C33H40O20 | 755.2040 | 755.2049 | 1.25 | 301.0449; 447.0854 | Quercetin rhamnosyl rutinoside | [31] |
| 15 | 15.55 | C33H40O19 | 739.2112 | 739.2091 | 2.89 | 431.0901; 593.1484 | Rhoifolin glucoside | [32] |
| 16 | 17.58 | C27H30O16 | 609.1461 | 609.1474 | 2.12 | 447.0847; 301.0370 | Quercetin hexose deoxyhexose | [29] |
| 17 | 18.77 | C33H40O20 | 755.2058 | 755.2040 | 2.30 | 301.0349 | Quercetin rhamnosylrutinoside | [2] |
| 18 | 20.02 | C27H30O15 | 593.1512 | 593.1522 | 1.73 | 447.0945; 285.0406; | Kaempferol-hexoside-rhamnoside | [32] |
| 19 | 20.06 | C35H42O21 | 797.2146 | 797.2166 | 2.47 | 651.1550; 447.0854 | Quercetin-rhamnosyl acetyl-hexoside-rhamnoside | [33] |
| 20 | 20.08 | C27H30O15 | 593.1512 | 593.1525 | 2.15 | 447.0945; 300.9995 | Quercetin di-rhamnoside | [2] |
| 21 | 20.11 | C27H30O15 | 593.1524 | 593.1512 | 2.03 | 285.0406; 284.0306 | Kaempferol 3-O-rutinoside | [34] |
| 22 | 20.97 | C33H40O19 | 739.2091 | 739.2112 | 2.89 | 285.0406; 593.1508 | Kaempferol-rhamnosyl-galactoside-rhamnoside | [2] |
| 23 | 21.58 | C35H42O20 | 781.2197 | 781.2219 | 2.81 | 635.1625; 431.0906; 285.0426 | Kaempferol-(rhamnosyl-acetyl-galactoside)-rhamnoside | [32] |
| 24 | 22.09 | C29H32O17 | 651.1567 | 651.1582 | 2.35 | 301.0361; 447.0853 | Quercetin-acetyl-rutinoside | [2] |
| 25 | 24.84 | C29H32O16 | 635.1618 | 635.1621 | 0.55 | 285.0405; 489.1048; 431.0906 | Kaempferol-acetyl-glucoside-rhamnoside | [32] |
| L-Dopa (mg/g dw) in 2% w/v Phyllanthus emblica L. BP Extract | L-Dopa (mg/g dw) in 5% w/v Punica granatum L. BP Extract | L-Dopa (mg/g dw) in 2% w/v Ribes rubrum L. BP Extract | L-Dopa (mg/g dw) in HCl 0.1 M BP Extract | % L-Dopa (mg/g dw) in Naturally Acidic Aqueous Solutions BP Extracts/L-Dopa (mg/g dw) in HCl 0.1 M BPs Extract | pH Value of 2% w/v Phyllanthus emblica L. Solution | pH Value of 5% w/v Punica granatum L. Solution | pH Value of 2% w/v Ribes rubrum L. Solution |
|---|---|---|---|---|---|---|---|
| 21.87 ± 0.76 (a) | 21.84 ± 0.75 (a) | 16.65 ± 0.80 (b) | 22.95 ± 0.74 (a) |
| 3.30 | 3.79 | 3.37 |
| Phyllanthus emblica L. | Ribes rubrum L. | Punica granatum L. | Ion Form | m/z Calc. a | m/z Exp. b | Molecular Formula | Mass Accuracy (RMS) c | Major Fragments | Assignment | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| yes | no | yes | [M-H]− | 169.0142 | 169.0140 | C7H6O5 | 1.3 | 125.0246; 169.0142 | Gallic Acid | [45] |
| yes | yes | yes | [M+H]+ | 449.1078 | 449.1066 | C21H20O11 | 2.8 | 287.0543 | Kaempferol-3-O-glucoside | [46] |
| yes | yes | yes | [M-H]− | 463.0882 | 463.0871 | C21H20O12 | 2.4 | 300.9982 | Quercetin 3-O-hexoside | [46,47] |
| no | yes | yes | [M+H]+ | 611.1607 | 611.1585 | C27H30O16 | 3.3 | 449.1068; 287.0544 | Rutin | [46] |
| yes | no | yes | [M-H]− | 355.1035 | 355.1021 | C16H20O9 | 3.7 | 179.0558; 193.0350; 161.0453 | Ferulic acid 4-O-glucoside | [48] |
| yes | no | yes | [M-H]− | 447.0569 | 447.0556 | C20H16O12 | 2.8 | 300.9986 | Ellagic acid deoxyhexoside | [46] |
| yes | no | yes | [M-H]− | 633.0733 | 633.0706 | C27H22O18 | 4.4 | 300.9990; 275.0197; 615.0624 | Phyllanemblinin B | [47] |
| yes | no | yes | [M-H]− | 300.9989 | 300.9982 | C14H6O8 | 2.7 | 257.0089; 229.0140; 185.0243 | Ellagic acid | [49] |
| yes | no | yes | [M-H]− | 447.0569 | 447.0556 | C20H16O12 | 2.8 | 299.9912; 300.9986 | Ellagic acid-rhamnopyranoside | [50] |
| yes | yes | yes | [M-H]− | 191.0197 | 191.0196 | C6H8O7 | 0.76 | 111.0090 | Citric acid | [51] |
| yes | yes | yes | [M-H]− | 331.0671 | 331.0664 | C13H16O10 | 2.17 | 169.0143; 271.0459; 211.0248 | Galloylglucose isomer I/II | [50] |
| no | yes | no | [M-H]− | 477.1038 | nd d | C22H22O12 | 301.0345; 112.9855; 174.9559; 85.0769 | Hesperetin 30-O-glucuronide | [48] | |
| yes | yes | yes | [M-H]− | 515.1195 | 515.1222 | C25H24O12 | 5.22 | 353.0717; 191.0195; 179.0559 | 1,5-Dicaffeoylquinic acid | [48] |
| no | yes | yes | [M-H]− | 353.0725 | 353.0718 | C12H18O12 | 2.26 | 173.0092;111.0090 | 6-O-(beta-D-glucopyranosyloxy)-L-ascorbic acid | [52] |
| no | yes | yes | [M-H]− | 387.1144 | 387.1126 | C13H24O13 | 4.7 | 225.0612;179.0559;89.0247;161.0454 | 7-(αD-glucopyranosyloxy)-2,3,4,5,6-pentahydroxyheptanoic acid. | [53] |
| yes | no | yes | [M-H]− | 633.0727 | 633.0706 | C27H21O18 | 4.39 | 300.9990;275.0197;463.0515 | Phyllanemblinin B | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesoro, C.; Lelario, F.; Piscitelli, F.; Di Capua, A.; Della Sala, P.; Montoro, P.; Bianco, G.; Acquavia, M.A.; Dell’Agli, M.; Piazza, S.; et al. Vicia faba L. Pod Valves: A By-Product with High Potential as an Adjuvant in the Treatment of Parkinson’s Disease. Molecules 2024, 29, 3943. https://doi.org/10.3390/molecules29163943
Tesoro C, Lelario F, Piscitelli F, Di Capua A, Della Sala P, Montoro P, Bianco G, Acquavia MA, Dell’Agli M, Piazza S, et al. Vicia faba L. Pod Valves: A By-Product with High Potential as an Adjuvant in the Treatment of Parkinson’s Disease. Molecules. 2024; 29(16):3943. https://doi.org/10.3390/molecules29163943
Chicago/Turabian StyleTesoro, Carmen, Filomena Lelario, Fabiana Piscitelli, Angela Di Capua, Paolo Della Sala, Paola Montoro, Giuliana Bianco, Maria Assunta Acquavia, Mario Dell’Agli, Stefano Piazza, and et al. 2024. "Vicia faba L. Pod Valves: A By-Product with High Potential as an Adjuvant in the Treatment of Parkinson’s Disease" Molecules 29, no. 16: 3943. https://doi.org/10.3390/molecules29163943







