Abstract
Inflammatory bowel disease (IBD) is a chronic, lifelong disorder characterized by inflammation of the gastrointestinal (GI) tract. The exact etiology of IBD remains incompletely understood due to its multifaceted nature, which includes genetic predisposition, environmental factors, and host immune response dysfunction. Currently, there is no cure for IBD. This review discusses the available treatment options and the challenges they present. Importantly, we examine emerging therapeutics, such as biologics and immunomodulators, that offer targeted treatment strategies for IBD. While many IBD patients do not respond adequately to most biologics, recent clinical trials combining biologics with small-molecule drugs (SMDs) have provided new insights into improving the IBD treatment landscape. Furthermore, numerous novel and specific therapeutic targets have been identified. The high cost of IBD drugs poses a significant barrier to treatment, but this challenge may be alleviated with the development of more affordable biosimilars. Additionally, emerging point-of-care protein biomarkers from serum and plasma are showing potential for enhancing the precision of IBD diagnosis and prognosis. Several natural products (NPs), including crude extracts, small molecules, and peptides, have demonstrated promising anti-inflammatory activity in high-throughput screening (HTS) systems and advanced artificial intelligence (AI)-assisted platforms, such as molecular docking and ADMET prediction. These platforms are advancing the search for alternative IBD therapies derived from natural sources, potentially leading to more affordable and safer treatment options with fewer side effects.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic, recurring inflammatory condition of the gastrointestinal (GI) tract with a multifaceted cause, typically manifesting in adolescence or early adulthood [1]. Inflammatory bowel diseases have significantly impacted global health over the past three decades, with a 47% increase in incidence and a 69% rise in mortality worldwide [2]. Inflammatory bowel diseases comprise two subtypes, ulcerative colitis (UC) and Crohn’s disease (CD), with UC specifically affecting the mucosal layer of the colon and rectum. In contrast, CD is more complex, affecting the entire GI tract, most commonly the ileum, and involves full-thickness inflammation [3]. The extent of inflammation in UC varies, ranging from proctitis (inflammation of the rectum) to left-sided or distal colitis or extensive colitis (pancolitis) (3). In CD, inflammation is non-contiguous, affecting the colon, the ileum, or both (ileocolonic). Ulcerative colitis is a T-helper cell type 2 (Th2)-driven disease mediated by interleukin (IL)-5 and IL-13, whereas CD involves a Th1 response with interferon (IFN)-γ and IL-2 cytokines [4].
Clinically, IBD is a heterogeneous group of diseases with varying pathological symptoms. Thus, differentiating UC from CD is difficult in almost 5–15% of IBD patients [3], suggesting that the pathophysiology of IBD is multifaceted. The common IBD symptoms include loss of appetite and weight, diarrhoea, abdominal pain, fatigue, anaemia, fever, or night sweats.
The exact aetiology of IBD is still yet to be ascertained, and as a result, there is currently no cure for IBD. Several studies have attributed the cause of IBD to a combination of multiple factors, including genetic susceptibility, immune response dysfunction, gut microbial dysbiosis, and environmental factors [5]. Recently, Boaz et al. (2022) [6] investigated the potential risk of developing IBD due to family history by comparing 35 familial and 88 sporadic IBD patients. The study determined that familial IBD has a stronger association with the early onset of IBD with more adverse phenotypes. Similarly, patients with familial CD showed more adverse clinical outcomes than those with sporadic CD [7]. Additionally, altered gut microbiota and resultant metabolites may also be implicated in IBD pathogenesis, including colorectal cancer. For instance, a genetically susceptible individual has a dysregulated mucosal immune response to commensal gut flora (microbial dysbiosis) [8]. On the other hand, Brand et al. [9] showed that IBD patients share gut microbiome signatures with their healthy co-twins.
Altered gut microbiota and their metabolites play a vital role in IBD pathogenesis. Liu et al. (2022) [10] analysed the gut bacterial diversity between the genetic variant mice carrying Atg16L1T300A and their wild type; genetic variant mice had more abundance of bacteria associated with IBD (e.g., Tyzzerella, Mucispirillum, Ruminococcaceae, and Cyanobacteria). Moreover, there were reduced mucin secretion and bacteria associated with mucin production (Akkermansia) compared to the wild type. The result suggests that altered microbiota may increase the risk of developing CD among carriers of this genetic variant.
Due to the complexity of the disease, currently available treatments can only induce remission among the patients, and unfortunately, many patients relapse at some time point [11]. Therefore, there is an urgent need for better treatment options for IBD patients or, otherwise, a cure. Given the numerous side effects of current therapies, alternative treatments from natural products, such as medicinal herbs and helminths, are highly sought after due to their reduced side effects. This review explores potential natural product solutions compared to existing treatments to improve the lives of IBD patients. Information on IBD and natural products was retrieved through a comprehensive literature search using PubMed, Scopus, Google Scholar, Web of Science, and MEDLINE Ovid online databases, with suitable keywords, including ‘BD’, ‘ulcerative colitis’, ‘Crohn’s disease’, ‘IBD therapies’, ‘challenges’, ‘side effects’, ‘biologics’, ‘immunomodulators’, ‘helminths’, ‘microbes’, ‘small molecules’, ‘emerging therapeutics for IBD’, ‘traditional treatments’, ‘IBD drugs’, ‘clinical trial’, ‘natural products’, ‘anti-inflammatory’, ‘artificial intelligence (AI)’, and ‘AI in drug discovery’.
2. Existing IBD Treatments and Challenges
IBD treatment initially relied on corticosteroids, aminosalicylates (ASA), and immunosuppressants for many years. Olsalazine, balsalazide, and sulfasalazine are common oral aminosalicylates for treating mild to moderately active UC (Table 1). However, they are associated with side effects, including cardio- and hepatorenal toxicity and sexual dysfunction [12]. Corticosteroids can reduce colonic inflammation via downregulating the nuclear factor kappa B (NF-κB) pathway [13]. Budesonide (corticosteroid) can treat both UC and CD, but it is unsuitable for short-term treatment due to its low bioavailability and first-pass effect when taken orally [12]. Since prolonged dependence on corticosteroids has numerous side effects (Table 1), IBD patients who are refractory to or rely on corticosteroids use immunosuppressant drugs, such as methotrexate (MTX) and 6-mercaptopurine (6-MP). They are cheaper and accessible to take orally [12], but MTX is toxic to bone marrow and liver, and it is not suitable for pregnant patients as they are also toxic to embryos [12,14]. Cyclosporine is considered less effective than tacrolimus, especially in oral form, due to its lower absorption in the colon [12,15]. These conventional therapies have numerous side effects and limited efficacy; new effective drugs with fewer side effects are required.

Table 1.
Existing approved drugs for IBD, their brand names, delivery route, and associated side effects.
The advent of biologics, such as anti-TNF agents, has improved the treatment strategy for IBD, as they are more specific to the disease target than conventional therapies. Four TNF inhibitors are currently available for treating IBD: infliximab and adalimumab (for UC and CD), certolizumab (CD only), and golimumab (UC only) (Table 1). Anti-TNF antibodies neutralise secretory TNF (s-TNF) and transmembrane TNF (tm-TNF) from binding to their receptors, thus alleviating inflammation [12]. They either induce apoptosis of TNF-producing cells or block leucocyte infiltrations by downregulating cell adhesion proteins (such as e-selectin, ICAM-1, and VCAM-1) [17]. Although TNF inhibitors are one of the preferred therapies for IBD, their repeated use may induce immunogenicity [18]. Moreover, during their initial treatment, up to 30% of patients do not respond adequately (primary non-responder), and 40% relapse during treatment (secondary non-response) [19]. Thus, stratification of subjects at risk of developing immunogenicity and identifying non-responders is essential before giving/choosing anti-TNF therapies.
The treatment option for IBD has further widened with the approvals of anti-integrins (vedolizumab and natalizumab), Janus kinase (JAK) inhibitors (tofacitinib, filgotinib, and upadacitinib), and anti-p19 antibodies (ustekinumab and risankizumab) (Table 1). Chu et al. (2023) [20] conducted a network meta-analysis on the efficacy and safety of anti-integrin antibodies against UC. They found that vedolizumab had the highest efficacy in achieving and maintaining clinical remission. While infliximab showed the highest efficacy for endoscopic improvement, guselkumab and ustekinumab exhibited the lowest risks for recurrence and adverse events for UC, respectively [20].
Could combining multiple biologics be an alternative therapy to maximise efficacy with fewer side effects? Several studies have tried combination therapies (CoT) of biologics or biologics with SMD (e.g., anti-TNF + anti-integrins) against IBD. A recent phase 2a VEGA study by Sands et al. (2022) [21] compared a CoT using guselkumab plus golimumab over their monotherapy in adults with moderate to severe active UC. Patients who received CoT showed a significantly higher clinical response (83.1%) than those who received monotherapy with guselkumab (74.6%) or golimumab (61.1%). Kwaspisz et al. (2021) [22] and Ahmed et al. (2022) [23] also showed similar results with an anti-TNF or vedolizumab with ustekinumab as an ideal combination therapy for IBD besides minor adverse events such as Salmonella gastroenteritis and Clostridium difficile infections. Although more studies will be required, promising results such as mucosal healing [24,25] and safety profiles from CoT [26,27] have brought new hope for IBD patients. Biosimilars, for example, CT-P13 and exemption for infliximab and adalimumab, respectively, are already in the market, which has eased the affordability of treatment for many IBD patients. A study conducted by Schreiber et al. (2021) [28] obtained similar efficacy between infliximab and its biosimilar, CT-P13. Despite similar effectiveness and safety, the exemption cost is one-fifth of adalimumab [12].
3. Therapeutic Drugs for IBD in the Pipeline
The advent of new biological and small-molecule therapies has made significant progress in the treatment landscape of IBD, and many more are in the pipeline (Table 2). Janus kinase (JAK) inhibitors, immunosuppressants, and anti-trafficking molecules are a few examples. Compared to biologics, SMD is cheaper and has a shorter half-life and low immunogenicity. JAK is a non-receptor tyrosine-protein kinase that mediates cytokine signalling, and there are four types: JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2) [12]. As intracellular signal mediators interact and work in pairs, JAK interacts with signal transducers and activators of transcription [29], forming the JAK-STAT signalling pathway, which transmits inflammatory signals to the nucleus. Blocking this pathway cuts the inflammatory signals reaching the nucleus, thereby reducing the synthesis of downstream inflammatory cytokines and inflammation [30]. Tofacitinib, upadacitinib, and filgotinib are a few examples of recently approved JAK inhibitors for UC.
Tofacitinib, a JAK inhibitor, has demonstrated good CD tolerance in phase II trials [31]. Upadacitinib, a second-generation JAK inhibitor, has demonstrated better selectivity for JAK1 and JAK2 than tofacitinib for UC and is now in phase III trials for CD [32]. It has shown superior endoscopic improvements but is associated with adverse effects like pneumonia, nasopharyngitis, gastroenteritis, and malignancies [33,34]. Filgotinib, another JAK1 inhibitor, is in phase III trials for CD [35]. Deucravacitinib, a TYK2 inhibitor, is in phase II trials for both UC and CD [31]. Mongersen, a Smad7 antisense oligonucleotide, can restore TGF-β1-Smad signalling and is in phase II trials for UC [31].
Sphingosine-1-phosphate (SI1P) modulators are another new therapeutic drug for IBD. S1P is a lysophospholipid signalling metabolite which binds to G-protein-coupled receptors (S1PR1-5) on T cells [12], promoting differentiation, migration and proliferation of lymphocytes. S1P modulators block the S1P pathway, as both UC and CD are due to lymphocyte recruitment into the GI tract. Ozanimod, an oral S1P/S1P5 receptor agonist, is in phase III trials for CD [36]. Estrasimod, another S1P inhibitor, is under development and has shown better clinical remission rates in phase III trials for UC compared to placebo, with no reported deaths or malignancies [37].
Cytokine inhibitors such as anti-IL12/23 agents block the p35 and p40 subunits of IL-12 and the p19 and p40 subunits of IL-23, essential for differentiating CD4+ T cells [38]. Interleukin (IL)-12/IL-23 inhibitors (e.g., ustekinumab) prevent the interaction of these cytokines with their receptors, subsequently blocking the IL-12/IL-23 signalling to prevent further activation of Th1/Th17 cells involved in the pathogenesis of CD [12,38].
More IL-12/IL-23 inhibitors and anti-integrin/anti-adhesion agents are undergoing clinical assessment for their efficacy and safety in treating IBD (Table 2) [39,40,41,42,43]. Generally, biologics are considered better than SMD as biologics are targeted treatment and could reduce the hospitalisation rate and produce improved long-term effects [44]. However, they are expensive, can produce life-threatening side effects, and not all patients can afford these biologics. Thus, a significant proportion of patients still require surgical treatment.
One of the challenges in IBD drug clinical trials is the need for standardised endpoints. For instance, determining appropriate clinical endpoints (e.g., mucosal healing, clinical remission) that are universally accepted and meaningful is challenging. Additionally, maintaining patient participation over long trial periods can be difficult due to the chronic nature of the disease and the potential side effects of the treatment, as mentioned above. IBD trials often show high placebo response rates [45], which can obscure the actual effectiveness of the investigational drug. Despite these challenges, engaging patients and advocacy groups to ensure trial designs meet patient needs may help improve recruitment and retention. Thus, a collaborative and multifaceted approach, combining scientific, regulatory, and patient-centred strategies, should be adopted to expedite the IBD drug development process.

Table 2.
Therapeutic drugs and targets for treating IBD in the pipeline.
Table 2.
Therapeutic drugs and targets for treating IBD in the pipeline.
| Types of Treatment | Drugs | Route of Administration | Drug Target | Clinical Trial Phase | References |
|---|---|---|---|---|---|
| Anti-adhesion/anti-trafficking molecules | Abrilumab (AMG181) | SC | α4β7-integrin | CD: II; UC: II | [31] |
| AJM 347 | Oral | α4β7-integrin | UC: I/II | [31] | |
| Alicaforsen | Oral | ICAM-1 mRNA | CD: III; UC: II | [31] | |
| Carotegrast methyl (AJM 300) | Oral | α4-integrin | UC: III | [46] | |
| Etrolizumab | IV, SC | α4β7, αEβ7, and β7-integrins | CD: III; UC: III | [46,47,48] | |
| GSK1605786A | Oral | CCR9 | CD: III | [31] | |
| Natalizumab | IV | α4-integrin | CD: III | [31] | |
| Ontamalimab (PF-00547659) | SC | MAdCAM | CD: II; UC: II | [31] | |
| Ontamalimab (SHP647) | SC | MAdCAM-1 | CD: III; UC: III | [31] | |
| PN-943 | Oral | α4β7-integrin (gut restricted) | UC: II | [31] | |
| PTG-100 | Oral | α4β7-integrin | UC: 11a | [31] | |
| Vedolizumab SC | SC | α4β7-integrin | CD: III; UC: III | [49] | |
| Anti-TNF | CT-P13 | SC | TNF | CD: III; UC: III | [31] |
| OPRX-106 | Oral | TNF | UC: II | [31] | |
| IL-10 fusion biologic | AAMT-101 | Oral | IL-10 | UC: Ia | [31] |
| IL-12/IL-23 inhibitors | Brazikumab | IV, SC | p19 subunit of IL-23 | CD: I; UC: I | [42] |
| Guselkumab | SC | p19 subunit of IL-23 | CD: III; UC: III | [43] | |
| Mirikizumab | IV, SC | p19 subunit of IL-23 | CD: III; UC: III | [40,41] | |
| Risankizumab | IV | Cytochrome p450 | CD: I; UC: I | [31] | |
| Risankizumab | IV, SC | p19 subunit of IL-23 | UC: III | [39,41] | |
| IL-36 inhibitor | Spesolimab | IV | IL-36R | CD: II; UC: III | [31] |
| Immunosuppressants | GSK2831781 | IV | LAG3 | UC: II | [31] |
| Ravagalimab (ABBV-323) | IV, SC | CD40 | UC: IIa | [31] | |
| JAK inhibitors | Brepocitinib (PF-06700841) | Oral | TYK2/JAK1 | CD: IIa; UC: IIb | [50] |
| Deucravacitinib (BMS-986165) | Oral | TYK2 | CD: II; UC: II | [31] | |
| Filgotinib | Oral | JAK1 | CD: III | [35] | |
| Ivarmacitinib | Oral | JAK1 | UC: II | [31] | |
| Izencitinib (TD-1473) | Oral | Gut-selective pan-JAK | UC: III | [31] | |
| Peficitinib | Oral | JAK3 | UC: IIb | [31] | |
| Ritlecitinib (PF-06651600) | Oral | JAK3/TEC kinase | CD: II; UC: II | [50] | |
| SHR-0302 | Oral | JAK1 | CD: II; UC: II | [31] | |
| Tofacitinib | Oral | JAK1/JAK3 | CD: II | [31] | |
| Upadacitinib | Oral | JAK1 | CD: III | [33,34] | |
| PDE4 inhibitor | Apremilast | Oral | PDE4 | UC: II | [51] |
| S1P receptor modulators | Amiselimod (MT-1303) | Oral | S1PR1,5 | CD: II; UC: II | [31] |
| CBP-307 | Oral | S1PR1 | UC: II | [31] | |
| Etrasimod | Oral | S1PR1/S1PR4/S1PR5 | CD: III; UC: III | [31] | |
| Ozanimod | Oral | S1PR1/S1PR5 | CD: III | [36] | |
| Smad7 antisense oligonucleotide | Laquinimod | Oral | NF-κB | CD: IIa | [31] |
| Mongersen (GED-0301) | Oral | Smad7 | UC: II | [31] | |
| Thalidomide | Oral | CRBN | CD: II; Pediatric IBD: III | [31] | |
| Spore-based microbiome | SER-287 | Oral | Firmicutes | UC: Ib | [52] |
| TLI1A agonist | PF-06480605 | SC | TL1A/TNFSF15 | UC: II | [31] |
| TLR9 agonist | Cobitolimod | Topical (enema) | TLR9 | UC: III | [31] |
| NP-derived | Curcumin and artesunate | Oral | NA | CD: IIa | [31] |
| Mastiha | Oral | NA | UC: II | [31] | |
| Saffron extract | Oral | NA | UC: II | [31] | |
| Trichuris suis ova (TSO) | Oral | NA | UC: II | [31] |
CD: Crohn’s disease; CD: cluster of differentiation; CCR9: chemokine receptor-9; CRBN: cereblon; IL: interleukin; IV: intravenous; SC: sub-cutaneous; ICAM-1: intercellular adhesion molecule-1; JAK: Janus kinase; LAG3: lymphocyte activation gene 3; MAdCAM-1: mucosal addressin cell adhesion molecule 1; mRNA: messenger RNA; NA: not available; NF-κB: nuclear factor kappa B; PDE4: phosphodiesterase 4 inhibitor; S1P: sphingosine-1-phosphate; Smad: mothers against decapentaplegic homolog; TEC: Tec kinase; TLRs: toll-like receptors; TNFSF: TNF receptor superfamily; TYK: tyrosine kinase; UC: ulcerative colitis. All details regarding the clinical trials of IBD drugs currently in development were obtained from www.clinicaltrials.gov (accessed on 30 June 2024) [31].
4. Natural Products as Potential Anti-Inflammatories for Treating IBD
4.1. Plants—Higher Plants, Fungi, and Medicinal Plants
Natural products (NPs) and their derivatives have been a promising pool for discovering therapeutic leads, including anti-inflammatories. Medicinal plants and their traditional formularies have shown protection against colonic inflammation by restoring epithelial tight junctions, increasing mucin secretion, preventing luminal microbial dysbiosis, and reducing oxidative stress in the gut [53]. More than 79781 NP-derived small molecules have been registered in the anti-inflammatory compound database (AICD), freely accessible at https://956023.ichengyun.net/AICD/index.php (accessed on 22 July 2024). Moreover, as many as 28 randomised clinical trials (RCTs) on 18 herbs, including Curcuma longa (e.g., curcumin), have shown promising results against IBD [54]. For instance, curcumin isolated from C. longa in combination with artesunate (a derivative of artemisinin isolated from Artemisia annua) is currently under phase II clinical trial for CD [31]. Berberine, the main bioactive component in many plants, including the Chinese medicinal herb Coptis chinensis, reduced the recurrence rate of UC remission; however, it was withdrawn from phase IV clinical trials due to lack of funding [31]. Other plant-derived IBD clinical therapeutic leads are epigallocatechin-3-gallate (isolated from Camellia sinensis) and triptolide (isolated from Tripterygium wilfordii), but their status for further clinical trials after preliminary results remains unknown. The structure of some commonly isolated natural products from plants that showed potent anti-inflammatory activities are shown in Figure 1.
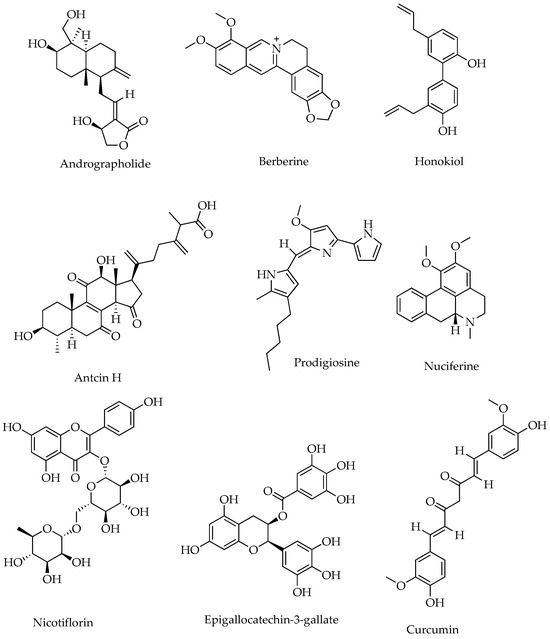
Figure 1.
Representative phytochemical structures of anti-inflammatory SMs.
4.2. Animals—Helminths
Numerous studies using helminths and their products have ameliorated inflammation in various IBD animal models. For instance, in the trinitrobenzene sulfonic acid (TNBS)-induced colitis model, mice infected with Schistosoma mansoni or its eggs showed reduced colonic inflammation [55]. Similarly, low-molecular-weight metabolite fractions from somatic extracts and excretory–secretory products (ESPs) of Ancylostoma caninum also protect against TNBS-induced colitis in mice by significantly reducing IL-23, TNF, and IL-1β cytokines [56]. Several clinical studies using ESPs from helminths were conducted, among which Trichuris suis ova (TSO) stood out as most promising. Recently, the probiotic treatment of UC patients with TSO has completed a phase II clinical trial (NCT03565939); however, the result from this trial is not yet accessible or published [31].
Many studies have also examined the possible additive effects of combined therapy using various natural products to identify better therapeutic agents for IBD with fewer side effects. For instance, a study examined the anti-inflammatory potential of Leiurus quinquestriatus (LO venom) venom in an acetic acid-induced colitis mice model. In colitic mice, LO venom showed reduced COX-2, IL-22, and TLR-9 expression (Table 3) [57]. The study further assessed the anti-colitic property of LO venom in combination with the IBD drug mesalazine, whereby the combined treatment more significantly protected the colonic tissues of mice [57]. The study did not identify anti-inflammatory components of the crude venom, which is worthwhile to pursue based on promising results. SjDX5-53, a peptide identified from S. japonicum eggs, enhanced Treg function, suppressing inflammation in colitis and psoriasis-like models [58]. It mainly induced tolerogenic dendritic cells (tolDCs) via TLR2 signalling, promoting Treg generation and peripheral tolerance [58], suggesting the potential of parasite-derived peptides in treating autoimmune conditions, including IBD (Table 3).

Table 3.
Natural product-derived anti-inflammatory agents investigated for treating inflammations and inflammatory disorders.
4.3. Microbial Sources
Recent research underscores the bidirectional relationship between IBD progression and gut microbiota changes, highlighting the gut microbiome’s dual role in IBD [87,88]. When balanced, the microbiome supports immune regulation, barrier integrity, and overall gut health [89]. For example, gut microbes convert primary bile acids into secondary bile acids, regulate RORγ-expressing Tregs, and metabolize tryptophan, crucial for immune activation and anti-inflammatory responses [90,91,92]. However, in IBD, dysbiosis, an imbalance in the gut microbiota can exacerbate disease progression. The study had shown that microbial species like Fusobacterium nucleatum and Ruminococcus gnavus were significantly increased in CD compared to controls, while the presence of beneficial microbes such as Eubacterium rectale and Ruminococcus albus, known for their anti-inflammatory effects, were decreased [93]. This dysbiosis contributes to worsening gut inflammation and the exacerbation of IBD symptoms. In this view, it is essential to understand the connection between IBD and the microbiome to develop novel microbiome-targeted therapies for IBD.
Microbiota, chiefly probiotic strains, have shown promising anti-inflammatory benefits. Their anti-inflammatory effect is mainly due to their ability to produce short-chain fatty acids (SCFAs), which can restore the population of beneficial gut microbiota while suppressing harmful strains by the protective mucosal layer [84,94]. They also produce anti-inflammatory molecules, chiefly polysaccharides [95]. There are also several studies on the probiotic treatment of IBD using various microbes and their genetically modified species, such as Escherichia coli Nissle 1917 [84,94]. Specific probiotic strains like Lactobacillus plantarum CKCC1312 and L. fermentum CKCC1858 have proven beneficial for UC via promoting mucosal integrity [84]. Bacteroides thetaiotaomicron alleviated clinical symptoms of dextran sulfate sodium (DSS)-induced colitis by promoting the differentiation of Treg/Th2 cells and suppressing Th1/Th17 cell development [81]. It significantly boosted FoxP3 expression, demethylated multiple CpG sites in the FoxP3 promoter, and activated AHR, which may have contributed to colitis protection [81]. However, epigenetic FoxP3 regulation by B. thetaiotaomicron is implicated with uncertain long-term immune changes; thus, it should be considered cautiously (Table 3).
Reactive oxygen species (ROS) contribute to intestinal inflammation, implicating antioxidant enzymes like catalase and superoxide dismutase (SOD) in treating IBD. Engineered Escherichia coli Nissle 1917 (ECN-pE) overexpressing catalase and SOD, coated with chitosan and sodium alginate via electrostatic assembly, demonstrated enhanced bioavailability in the gastrointestinal tract. In a mouse IBD model, this coated ECN-pE effectively reduced inflammation, repaired epithelial barriers, and positively modulated intestinal microbial communities. These findings suggest a promising approach for using probiotic bacteria to develop living therapeutic proteins for inflammatory intestinal disorders [94]. Minas Frescal cheese containing Lactococcus lactis NCDO 2118 probiotic effectively ameliorated DSS-induced colitis in mice by enhancing tight junction, protein gene expression, and modulating cytokine production [84]. Further, it prevented goblet cell damage and reduced inflammatory cell infiltration into the colon mucosa [84]. These findings suggest probiotic functional foods as an adjunct therapy in UC management along with conventional treatments.
Fungi species like Auricularia polytricha and Flammulina velutipes also showed potential in treating IBD by controlling key signaling pathways, including NF-κB and Keap1/Nrf2, and altering the gut microbiota [96], indicating A. polytricha and F. velutipes as potential probiotics for gut health. Marine-derived fungi produced unique indole-terpenoids with significant anti-inflammatory activity. Dai et al. [78] isolated 27 compounds from Penicillium sp. ZYX-Z-143, including new indole-diterpenoids, penpaxilloids E, schipenindolene A, and paxilline D that inhibited NO production, showing these molecules as novel chemical scaffolds for developing new anti-inflammatory drugs (Table 3).
5. Advances in Artificial Intelligence-Guided Drug Discovery for IBD Treatments
Artificial intelligence (AI) and AI-assisted tools have recently played a vital role in drug discovery. They have leveraged the drug discovery process by increasing efficiency, lowering costs, and improving precision by enabling the prediction of structure–activity and drug–target interactions [97]. AI-driven computer-aided drug design [98] and high-performance algorithms, such as machine learning (ML) and deep learning (DL), quantitative structure–activity relationship (QSAR) modelling, pharmacophore modelling, and de novo drug design have streamlined the drug screening process and helped select promising/hit compounds precisely [99]. For instance, AI was used for developing a protein kinase C (PKC) theta inhibitor (currently in phase 1 clinical trials), and machine learning (ML) was used to integrate many pharmacological characteristics and compute the dosages for the first-in-human trial (FIHT) [100].
Artificial intelligence is also applied in analytical chemistry, particularly for phase and baseline corrections and nuclear magnetic resonance (NMR) spectrum analysis while isolating lead compounds from NPs [101]. For instance, NMR machine software such as Bruker’s DL approach and TopSpin software version 4.1.3 achieved human-level accuracy and superior phase and baseline correction for 1D 1H NMR spectra. A DL algorithm can automatically recognise the signal area from NMR spectra, enhancing full automation in analytical chemistry [101].
Further, the integration of AI in bioinformatic tools, such as MetaWIBELE (Metagenomic Workflow for Identification of Biologically Enhanced Lysine Export), has assisted in identifying over 340,000 potentially bioactive protein families in active phases of IBD from metagenomic data [102]. The analysis identified possibly contributing targets involving Enterobacteriaceae pilins and VWF-like exoproteins. It also uncovered several other proposed mechanisms of cell–cell communication, such as molybdoproteins and extracellular metabolic chaperones [102]. Other AI-driven tools that help predict and identify metabolites from NPs through clustering analysis include XenoSite’s neural network, DP4-AI, and MS2DeepScore [103,104] (Table 3). Artificial intelligence approaches should be considered cautiously, as many operate as black boxes that do not connect predictions to underlying mechanisms or offer functional explanations for discovered associations, correlations, and recommended decisions. Understanding causal mechanistic insights is essential for clinical applicability in complex and heterogeneous diseases like IBD. Moreover, due to the potential harm of poorly validated models, thorough experimental and clinical validation is crucial before implementing machine learning-based models in clinical practice. From an analytics perspective, it is imperative to prioritize the development of interpretable machine learning models.
AI-based in silico approaches, such as molecular docking and protein–protein interaction studies, have demonstrated that curcumin and epigallocatechin gallate (EGCG) exhibit high binding affinities for the NLRP3 protein within the inflammasome complex, surpassing even that of the selective inhibitor MCC950 [105]. These findings suggest curcumin and EGCG could be promising lead compounds for inflammatory conditions involving NLRP3 inflammasome activation, including IBD [105]. Despite numerous studies revealing the potential anti-inflammatory properties of phenolic acids from plants, the precise anti-inflammatory mechanisms remain unclear. When a study examined the anti-inflammatory properties of chlorogenic acid, rosmarinic acid, and ellagic acid through comprehensive network pharmacology, molecular docking, and dynamic simulations [106], selected phenolic acids suppressed TNF convertase, preventing TNF generation (Table 3).
Molecular docking and ADMET prediction have expedited the drug discovery process by studying the pharmacokinetic properties of drug candidates to identify potentially suitable protein binding sites faster. For instance, the molecular docking investigation of the phytochemical constituents of methanol extract of Nyctanthes arbortristis leaves revealed their significant potential for inhibiting both COX-1 and COX-2 enzymes, a finding corroborated by the ADMET analysis. This led to the isolation of abortitristoside A and abortitristoside B, which inhibited COX-2 and COX-1 enzymes [107]. Although NPs remain the primary source of therapeutic leads or scaffolds, the overall drug development process, including clinical trials, continues to be challenged by high attrition rates because of limited funding support due to a lack of patent protection (e.g., for crude NPs) and rigor involved in designing a clinical trial [108]. Additional hurdles that may continue to challenge NP-based drug discovery are lack of accessibility, sustainability, and difficulty synthesising identified drug leads in bulk as required for repeated trials.
6. Limitations and Challenges of Using AI in Drug Discovery from Natural Products
Exploring NPs and their bioactive compounds offers promising drug prospects due to unique mechanisms, low toxicity, and fewer side effects [109]. However, NPs are acknowledged for their multifaceted and varied chemical structures, exhibiting challenges for AI algorithms to model and predict precisely [110]. The distinctive nature of many NPs often results in limited data for AI training, obscuring model simplification. Imprecise or biased data can lead to flawed predictions, impeding the identification of drug candidates [111]. Similarly, AI models, particularly deep learning, often function as black boxes, suggesting predictions without clear justifications, which is challenging in drug discovery, where understanding the mechanism of action is critical [112].
Further, AI predictions necessitate authentication through experimental methods, which can be resource-intensive and time-consuming, limiting their practical application in natural product drug discovery [113]. Additionally, using AI to discover drugs from natural products raises concerns about biopiracy and the equitable distribution of benefits, especially when involving traditional knowledge from indigenous communities [114]. Ensuring ethical AI use in drug discovery, including evading bias in training data and decision-making, is fundamental for fairness and transparency [115]. Hence, interdisciplinary research is crucial for unlocking these natural compounds’ therapeutic potential.
7. Conclusions and Future Directions
Conventional treatments using corticosteroids, aminosalicylates, and immunosuppressants have been fundamental options for IBD patients despite associated side effects. The advent of biologics, including anti-TNFs, JAK inhibitors, and, recently, anti-integrins, has significantly improved IBD treatment outcomes. However, variable efficacy, side effects, and high costs have been major constraints in daily clinical practice. Many new IBD therapeutics are in the pipeline, but they require more clinical validations and safety assessments using more extensive IBD cohort studies.
Natural products from plants, helminths, and microbes exhibit considerable promise as anti-inflammatory agents for treating IBD, including a few already in early clinical trial phases. (e.g., curcumin and berberine). Helminth excretory/secretory products and probiotic microbial strains, including Lactobacillus species, have also shown efficacy in preclinical and clinical studies. However, they have to pass through more validations for their safety and efficacy before their appearance in the clinical application. The role of the gut microbiome in IBD is rapidly advancing, with probiotics and prebiotics exhibiting potential. However, the exact mechanisms of action remain unclear. Hence, further intensive research is crucial to ascertain specific microbial strains with therapeutic potential, clarify their mechanisms of action, and address the current limited knowledge regarding long-term safety and efficacy.
Artificial intelligence (AI)-assisted technologies, including machine learning and deep learning, have streamlined chemical structure forecasting, synthesis pathway proposals, and drug–target interaction elucidation processes. AI-assisted tools, such as molecular docking and protein–protein interaction studies, have identified promising anti-inflammatory leads such as curcumin and epigallocatechin gallate. However, AI-assisted approaches often need more transparency in their predictions and elucidation of underlying pharmacological mechanisms. Therefore, it is crucial to develop interpretable machine learning models to enhance clinical applicability and ensure the safety and reliability of AI-driven predictions. Thorough experimental and clinical validation of these models is essential before their implementation in clinical practice. While current treatment options have significantly improved IBD patient care, applying AI platforms and interdisciplinary collaborations may further speed up the search for better treatments for IBD. In doing so, we may be able to see at least a few new IBD drugs in the next couple of decades.
Author Contributions
Conceptualization, P.W., T.J. and K.Y.; writing—original draft preparation, K.Y. and T.J.; writing—review and editing, P.W., funding acquisition, P.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a James Cook University Postgraduate Research Scholarship (JCUPRS) to T.J. and an NHMRC Ideas Grant (APP1183323 and APP 2029349) to P.W.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, X.; Yuan, Z.; Wu, J.; He, Y.; Lu, G.; Zhang, D.; Zhao, Y.; Wu, R.; Lv, Y.; Cai, K.; et al. An Orally-Administered Nanotherapeutics with Carbon Monoxide Supplying for Inflammatory Bowel Disease Therapy by Scavenging Oxidative Stress and Restoring Gut Immune Homeostasis. ACS Nano 2023, 17, 21116–21133. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, D.; Haritunians, T.; Ruan, Y.; Daly, M.J.; Huang, H.; McGovern, D.P.B. Profiling the inflammatory bowel diseases using genetics, serum biomarkers, and smoking information. iScience 2023, 26, 108053. [Google Scholar] [CrossRef]
- Piotrowska, M.; Binienda, A.; Fichna, J. The role of fatty acids in Crohn’s disease pathophysiology—An overview. Mol. Cell. Endocrinol. 2021, 538, 111448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, M.; Yan, X.; Zhang, L.; Lu, N.; Ma, Y.; Zhang, Y.; Cui, M.; Zhang, M.; Zhang, M. Oral Nanotherapeutics of Andrographolide/Carbon Monoxide Donor for Synergistically Anti-inflammatory and Pro-resolving Treatment of Ulcerative Colitis. ACS Appl. Mater. Interfaces 2023, 15, 36061–36075. [Google Scholar] [CrossRef]
- Boaz, E.; Bar-Gil Shitrit, A.; Schechter, M.; Goldin, E.; Reissman, P.; Yellinek, S.; Koslowsky, B. Inflammatory bowel disease in families with four or more affected first-degree relatives. Scand. J. Gastroenterol. 2023, 58, 20–24. [Google Scholar] [CrossRef]
- Dong, S.; Xiang, X.; Zhang, Y.; Liu, R.; Ye, L.; Cao, Q. Differences of clinical phenotype between familial and sporadic Crohn’s disease in East China. Int. J. Color. Dis. 2024, 39, 107. [Google Scholar] [CrossRef]
- Hu, S.; Uniken Venema, W.T.; Westra, H.J.; Vich Vila, A.; Barbieri, R.; Voskuil, M.D.; Blokzijl, T.; Jansen, B.H.; Li, Y.; Daly, M.J.; et al. Inflammation status modulates the effect of host genetic variation on intestinal gene expression in inflammatory bowel disease. Nat. Commun. 2021, 12, 1122. [Google Scholar] [CrossRef]
- Brand, E.C.; Klaassen, M.A.Y.; Gacesa, R.; Vich Vila, A.; Ghosh, H.; de Zoete, M.R.; Boomsma, D.I.; Hoentjen, F.; Horjus Talabur Horje, C.S.; van de Meeberg, P.C.; et al. Healthy Cotwins Share Gut Microbiome Signatures With Their Inflammatory Bowel Disease Twins and Unrelated Patients. Gastroenterology 2021, 160, 1970–1985. [Google Scholar] [CrossRef]
- Liu, H.; Gao, P.; Jia, B.; Lu, N.; Zhu, B.; Zhang, F. IBD-Associated Atg16L1T300A Polymorphism Regulates Commensal Microbiota of the Intestine. Front. Immunol. 2021, 12, 772189. [Google Scholar] [CrossRef]
- Zhang, B.; Gulati, A.; Alipour, O.; Shao, L. Relapse From Deep Remission After Therapeutic De-escalation in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohns Colitis 2020, 14, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Di, B.; Xu, L.L. Recent advances in the treatment of IBD: Targets, mechanisms and related therapies. Cytokine Growth Factor. Rev. 2023, 71–72, 1–12. [Google Scholar] [CrossRef]
- Hussenbux, A.; Silva, A.D. Steroids in inflammatory bowel disease: A clinical review. J. Prescr. Pract. 2021, 3, 107–111. [Google Scholar] [CrossRef]
- Cao, R.H.; Grimm, M.C. Pregnancy and medications in inflammatory bowel disease. Obstet. Med. 2021, 14, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Fujii, T.; Kinoshita, K.; Kawamoto, A.; Hibiya, S.; Takenaka, K.; Saito, E.; Nagahori, M.; Ohtsuka, K.; Watanabe, M.; et al. Intravenous tacrolimus is a superior induction therapy for acute severe ulcerative colitis compared to oral tacrolimus. BMC Gastroenterol. 2021, 21, 494. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef]
- Ward, D.; Nyboe Andersen, N.; Gørtz, S.; Thorn Iversen, A.; Højgaard Allin, K.; Beaugerie, L.; Kirchgesner, J.; Jess, T. Tumor Necrosis Factor Inhibitors in Inflammatory Bowel Disease and Risk of Immune Mediated Inflammatory Diseases. Clin. Gastroenterol. Hepatol. 2024, 22, 135–143.e8. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Sandborn, W.J.; Panaccione, R.; Domènech, E.; Pouillon, L.; Siegmund, B.; Danese, S.; Ghosh, S. Tumour necrosis factor inhibitors in inflammatory bowel disease: The story continues. Ther. Adv. Gastroenterol. 2021, 14, 17562848211059954. [Google Scholar] [CrossRef]
- Goll, R.; Moe, Ø.K.; Johnsen, K.-M.; Meyer, R.; Friestad, J.; Gundersen, M.D.; Kileng, H.; Johnsen, K.; Florholmen, J.R. Pharmacodynamic mechanisms behind a refractory state in inflammatory bowel disease. BMC Gastroenterol. 2022, 22, 464. [Google Scholar] [CrossRef]
- Chu, X.; Biao, Y.; Liu, C.; Zhang, Y.; Liu, C.; Ma, J.-Z.; Guo, Y.; Gu, Y. Network meta-analysis on efficacy and safety of different biologics for ulcerative colitis. BMC Gastroenterol. 2023, 23, 346. [Google Scholar] [CrossRef]
- Sands, B.E.; Feagan, B.G.; Sandborn, W.J.; Shipitofsky, N.; Marko, M.; Sheng, S.; Johanns, J.; Germinaro, M.; Vetter, M.; Panés, J.; et al. OP36 Efficacy and safety of combination induction therapy with guselkumab and golimumab in participants with moderately-to-severely active Ulcerative Colitis: Results through week 12 of a phase 2a randomized, double-blind, active-controlled, parallel-group, multicenter, proof-of-concept study. J. Crohn’s Colitis 2022, 16, i042–i043. [Google Scholar] [CrossRef]
- Kwapisz, L.; Raffals, L.E.; Bruining, D.H.; Pardi, D.S.; Tremaine, W.J.; Kane, S.V.; Papadakis, K.A.; Coelho-Prabhu, N.; Kisiel, J.B.; Heron, V.; et al. Combination Biologic Therapy in Inflammatory Bowel Disease: Experience From a Tertiary Care Center. Clin. Gastroenterol. Hepatol. 2021, 19, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Galati, J.; Kumar, A.; Christos, P.J.; Longman, R.; Lukin, D.J.; Scherl, E.; Battat, R. Dual Biologic or Small Molecule Therapy for Treatment of Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, e361–e379. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Zhao, S.S.; Hyrich, K.; Yiu, Z.; Barton, A.; Bowes, J. Pos1604 Il-13 Inhibition Used for Atopic Diseases Is Associated with Risk of Psoriatic Arthritis. In Proceedings of the European Congress of Rheumatology, Milan, Italy, 31 May–3 June 2023; pp. 1146–1147. [Google Scholar]
- Wlazło, M.; Meglicka, M.; Wiernicka, A.; Osiecki, M.; Kierkuś, J. Dual Biologic Therapy in Moderate to Severe Pediatric Inflammatory Bowel Disease: A Retrospective Study. Children 2022, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Ibrahim, N.; Elawad, M.; Al-Mudahka, F.; Abdelrhman, H.; Akobeng, A.K. P617 Dual biologic therapy in pediatric Inflammatory Bowel Disease. J. Crohn’s Colitis 2023, 17, i747. [Google Scholar] [CrossRef]
- Schreiber, S.; Ben-Horin, S.; Leszczyszyn, J.; Dudkowiak, R.; Lahat, A.; Gawdis-Wojnarska, B.; Pukitis, A.; Horynski, M.; Farkas, K.; Kierkus, J.; et al. Randomized Controlled Trial: Subcutaneous vs Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology 2021, 160, 2340–2353. [Google Scholar] [CrossRef]
- Antoniou, E.; Margonis, G.A.; Angelou, A.; Pikouli, A.; Argiri, P.; Karavokyros, I.; Papalois, A.; Pikoulis, E. The TNBS-induced colitis animal model: An overview. Ann. Med. Surg. 2016, 11, 9–15. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Song, B.; Xiong, Y.; Wang, J.; Chen, D. Targeting JAK/STAT signaling pathways in treatment of inflammatory bowel disease. Inflamm. Res. 2021, 70, 753–764. [Google Scholar] [CrossRef]
- National Library of Medicine. Clinicaltirals.gov [Internet]; National Library of Medicine (US): Bethesda, MD, USA, 2024. [Google Scholar]
- Ananthakrishnan, A.N. Upadacitinib for ulcerative colitis. Lancet 2022, 399, 2077–2078. [Google Scholar] [CrossRef]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.; Siffledeen, J.; Hébuterne, X.; Nakase, H.; Higgins, P.; Chen, M.H.; Sanchez-Gonzalez, Y.; et al. OP24 Efficacy and safety of upadacitinib induction therapy in patients with Moderately to Severely Active Ulcerative Colitis: Results from the phase 3 U-ACHIEVE study. J. Crohn’s Colitis 2021, 15, S022–S024. [Google Scholar] [CrossRef]
- Vermeire, S.; Danese, S.; Zhou, W.; Pangan, A.; Greenbloom, S.; D’Haens, G.; Panes, J.; Juillerat, P.; Lindsay, J.O.; Loftus, E.V., Jr.; et al. OP23 Efficacy and safety of upadacitinib as induction therapy in patients with Moderately to Severely Active Ulcerative Colitis: Results from phase 3 U-ACCOMPLISH study. J. Crohn’s Colitis 2021, 15, S021–S022. [Google Scholar] [CrossRef]
- Feagan, B.G.; Danese, S.; Loftus, E.V., Jr.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempiński, R.; et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; D’Haens, G.; Wolf, D.C.; Jovanovic, I.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2021, 385, 1280–1291. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Vermeire, S.; Peyrin-Biroulet, L.; Dubinsky, M.C.; Panes, J.; Yarur, A.; Ritter, T.; Baert, F.; Schreiber, S.; Sloan, S.; et al. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): Two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet 2023, 401, 1159–1171. [Google Scholar] [CrossRef]
- Rezazadeh, F.; Ramos, N.; Saliganan, A.D.; Al-Hallak, N.; Chen, K.; Mohamad, B.; Wiesend, W.N.; Viola, N.T. Detection of IL12/23p40 via PET Visualizes Inflammatory Bowel Disease. J. Nucl. Med. 2023, 64, 1806–1814. [Google Scholar] [CrossRef]
- Bossuyt, P.; Bresso, F.; Dubinsky, M.; Ha, C.; Siegel, C.; Zambrano, J.; Kligys, K.; Kalabic, J.; Zhang, Y.; Panaccione, R. OP40 Efficacy of risankizumab induction and maintenance therapy by baseline Crohn’s Disease location: Post hoc analysis of the phase 3 ADVANCE, MOTIVATE, and FORTIFY studies. J. Crohn’s Colitis 2022, 16, i048. [Google Scholar] [CrossRef]
- Sands, B.E.; Peyrin-Biroulet, L.; Kierkus, J.; Higgins, P.D.R.; Fischer, M.; Jairath, V.; Hirai, F.; D’Haens, G.; Belin, R.M.; Miller, D.; et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients With Crohn’s disease. Gastroenterology 2022, 162, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Feagan, B.G.; Panés, J.; Baert, F.; Louis, E.; Dewit, O.; Kaser, A.; Duan, W.R.; Pang, Y.; Lee, W.-J.; et al. Long-Term Safety and Efficacy of Risankizumab Treatment in Patients with Crohn’s Disease: Results from the Phase 2 Open-Label Extension Study. J. Crohn’s Colitis 2021, 15, 2001–2010. [Google Scholar] [CrossRef]
- Wang, J.; Goren, I.; Yang, B.; Lin, S.; Li, J.; Elias, M.; Fiocchi, C.; Rieder, F. Review article: The sphingosine 1 phosphate/sphingosine 1 phosphate receptor axis—A unique therapeutic target in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2022, 55, 277–291. [Google Scholar] [CrossRef]
- Sandborn, W.J.; D’Haens, G.R.; Reinisch, W.; Panés, J.; Chan, D.; Gonzalez, S.; Weisel, K.; Germinaro, M.; Frustaci, M.E.; Yang, Z.; et al. Guselkumab for the Treatment of Crohn’s disease: Induction Results From the Phase 2 GALAXI-1 Study. Gastroenterology 2022, 162, 1650–1664.e8. [Google Scholar] [CrossRef]
- D’Amico, F.; Tasopoulou, O.; Fiorino, G.; Zilli, A.; Furfaro, F.; Allocca, M.; Sileri, P.; Spinelli, A.; Peyrin-Biroulet, L.; Danese, S. Early Biological Therapy in Operated Crohn’s Disease Patients Is Associated With a Lower Rate of Endoscopic Recurrence and Improved Long-term Outcomes: A Single-center Experience. Inflamm. Bowel Dis. 2022, 29, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Klosterhalfen, S. The Placebo and Nocebo Responses in Clinical Trials in Inflammatory Bowel Diseases. Front. Pharmacol. 2021, 12, 641436. [Google Scholar] [CrossRef]
- Danese, S.; Colombel, J.-F.; Lukas, M.; Gisbert, J.P.; D’Haens, G.; Hayee, B.h.; Panaccione, R.; Kim, H.-S.; Reinisch, W.; Tyrrell, H.; et al. Etrolizumab versus infliximab for the treatment of moderately to severely active ulcerative colitis (GARDENIA): A randomised, double-blind, double-dummy, phase 3 study. Lancet Gastroenterol. Hepatol. 2022, 7, 118–127. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Hart, A.; Bossuyt, P.; Long, M.; Allez, M.; Juillerat, P.; Armuzzi, A.; Loftus, E.V., Jr.; Ostad-Saffari, E.; Scalori, A.; et al. Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (HICKORY): A phase 3, randomised, controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Lakatos, P.L.; Ritter, T.; Hanauer, S.; Bressler, B.; Khanna, R.; Isaacs, K.; Shah, S.; Kadva, A.; Tyrrell, H.; et al. Etrolizumab for maintenance therapy in patients with moderately to severely active ulcerative colitis (LAUREL): A randomised, placebo-controlled, double-blind, phase 3 study. Lancet Gastroenterol. Hepatol. 2022, 7, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; D’Haens, G.; Baert, F.; Danese, S.; Kobayashi, T.; Loftus, E.V., Jr.; Bhatia, S.; Agboton, C.; Rosario, M.; Chen, C.; et al. Efficacy and Safety of Subcutaneous Vedolizumab in Patients With Moderately to Severely Active Crohn’s Disease: Results From the VISIBLE 2 Randomised Trial. J. Crohn’s Colitis 2021, 16, 27–38. [Google Scholar] [CrossRef]
- Sandborn, W.; Danese, S.; Leszczyszyn, J.; Romatowski, J.; Altintas, E.; Peeva, E.; Vincent, M.; Reddy, P.; Banfield, C.; Banerjee, A.; et al. OP33 Oral ritlecitinib and brepocitinib in patients with Moderate to Severe Active Ulcerative Colitis: Data from the VIBRATO umbrella study. J. Crohn’s Colitis 2021, 15, S030–S031. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Liu, M.; Fan, C.; Feng, C.; Lu, Q.; Xiang, C.; Lu, H.; Yang, X.; Wu, B.; et al. Targeting PDE4 as a promising therapeutic strategy in chronic ulcerative colitis through modulating mucosal homeostasis. Acta Pharm. Sin. B 2022, 12, 228–245. [Google Scholar] [CrossRef]
- Henn, M.R.; O’Brien, E.J.; Diao, L.; Feagan, B.G.; Sandborn, W.J.; Huttenhower, C.; Wortman, J.R.; McGovern, B.H.; Wang-Weigand, S.; Lichter, D.I.; et al. A Phase 1b Safety Study of SER-287, a Spore-Based Microbiome Therapeutic, for Active Mild to Moderate Ulcerative Colitis. Gastroenterology 2021, 160, 115–127.e30. [Google Scholar] [CrossRef]
- Xu, Q.; Yao, Y.; Liu, Y.; Zhang, J.; Mao, L. The mechanism of traditional medicine in alleviating ulcerative colitis: Regulating intestinal barrier function. Front. Pharmacol. 2023, 14, 1228969. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, P.; Godoy-Brewer, G.; Maniyar, I.; White, J.; Maas, L.; Parian, A.M.; Limketkai, B. Herbal Medicines for the Treatment of Active Ulcerative Colitis: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 934. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Xu, N.; Wang, X.; Vallée, I.; Liu, M.; Liu, X. Helminth therapy for immune-mediated inflammatory diseases: Current and future perspectives. J. Inflamm. Res. 2022, 15, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Jamtsho, T.; Yeshi, K.; Perry, M.J.; Loukas, A.; Wangchuk, P. Approaches, Strategies and Procedures for Identifying Anti-Inflammatory Drug Lead Molecules from Natural Products. Pharmaceuticals 2024, 17, 283. [Google Scholar] [CrossRef]
- Mahmoud, H.A.; Salama, W.M.; Mariah, R.A.; Eid, A.M. Ameliorative effect of Leiurus quinquestriatus venom on acetic acid-induced colitis in mice. Sci. Afr. 2021, 14, e01009. [Google Scholar] [CrossRef]
- Ni, Y.; Xiong, R.; Zhu, Y.; Luan, N.; Yu, C.; Yang, K.; Wang, H.; Xu, X.; Yang, Y.; Sun, S. A target-based discovery from a parasitic helminth as a novel therapeutic approach for autoimmune diseases. EBioMedicine 2023, 95, 104751. [Google Scholar] [CrossRef]
- Song, J.; Chen, Y.; Lv, Z.; Taoerdahong, H.; Li, G.; Li, J.; Zhao, X.; Jin, X.; Chang, J. Structural characterization of a polysaccharide from Alhagi honey and its protective effect against inflammatory bowel disease by modulating gut microbiota dysbiosis. Int. J. Biol. Macromol. 2024, 259, 128937. [Google Scholar] [CrossRef]
- Hou, J.; Gong, H.; Gong, Z.; Tan, X.; Qin, X.; Nie, J.; Zhu, H.; Zhong, S. Structural characterization and anti-inflammatory activities of a purified polysaccharide from fruits remnants of Alpinia zerumbet (Pers.) Burtt. et Smith. Int. J. Biol. Macromol. 2024, 267, 131534. [Google Scholar] [CrossRef]
- Tran, Q.T.; Gan, P.X.; Liao, W.; Mok, Y.K.; Chai, C.L.; Wong, W.F. Degradation of MK2 with natural compound andrographolide: A new modality for anti-inflammatory therapy. Pharmacol. Res. 2023, 194, 106861. [Google Scholar] [CrossRef]
- Zhong, Y.-B.; Kang, Z.-P.; Wang, M.-X.; Long, J.; Wang, H.-Y.; Huang, J.-Q.; Wei, S.-Y.; Zhou, W.; Zhao, H.-M.; Liu, D.-Y. Curcumin ameliorated dextran sulfate sodium-induced colitis via regulating the homeostasis of DCs and Treg and improving the composition of the gut microbiota. J. Funct. Foods. 2021, 86, 104716. [Google Scholar] [CrossRef]
- Jing, W.; Dong, S.; Luo, X.; Liu, J.; Wei, B.; Du, W.; Yang, L.; Luo, H.; Wang, Y.; Wang, S. Berberine improves colitis by triggering AhR activation by microbial tryptophan catabolites. Pharmacol. Res. 2021, 164, 105358. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.; Su, J.; Zhong, R.; Yin, S.; Zhao, Z.; Sun, Z. Hypersampsonone H attenuates ulcerative colitis via inhibition of PDE4 and regulation of cAMP/PKA/CREB signaling pathway. Int. Immunopharmacol. 2024, 128, 111490. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Kong, R.; Han, W.; Bao, W.; Shi, Y.; Ye, L.; Lu, J. Honokiol alleviates ulcerative colitis by targeting PPAR-γ–TLR4–NF-κB signaling and suppressing gasdermin-D-mediated pyroptosis in vivo and in vitro. Int. Immunopharmacol. 2022, 111, 109058. [Google Scholar] [CrossRef]
- Kulhari, U.; Kundu, S.; Mugale, M.N.; Sahu, B.D. Nuciferine alleviates intestinal inflammation by inhibiting MAPK/NF-κB and NLRP3/Caspase 1 pathways in vivo and in vitro. Int. Immunopharmacol. 2023, 115, 109613. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, T.; Zhang, M.; Hong, B.; Jin, B.; Hu, C.; Wang, J.; Chen, Y.; Zhang, L.; Wang, Y. Flavokawain B inhibits NF-κB inflammatory signaling pathway activation in inflammatory bowel disease by targeting TLR2. Toxicol. Appl. Pharmacol. 2024, 486, 116922. [Google Scholar] [CrossRef] [PubMed]
- Ekhtiar, M.; Ghasemi-Dehnoo, M.; Mirzaei, Y.; Azadegan-Dehkordi, F.; Amini-Khoei, H.; Lorigooini, Z.; Samiei-Sefat, A.; Bagheri, N. The coumaric acid and syringic acid ameliorate acetic acid-induced ulcerative colitis in rats via modulator of Nrf2/HO-1 and pro-inflammatory cytokines. Int. Immunopharmacol. 2023, 120, 110309. [Google Scholar] [CrossRef]
- He, X.; Liu, J.; Long, G.; Xia, X.-H.; Liu, M. 2, 3, 5, 4′-Tetrahydroxystilbene-2-O-β-D-glucoside, a major bioactive component from Polygoni multiflori Radix (Heshouwu) suppresses DSS induced acute colitis in BALb/c mice by modulating gut microbiota. Biomed. Pharmacother. 2021, 137, 111420. [Google Scholar] [CrossRef]
- Lamichhane, G.; Pandeya, P.R.; Lamichhane, R.; Yun, H.D.; Shrivastava, A.K.; Cheon, J.-y.; Sapkota, B.; Devkota, H.P.; Jung, H.-J. Evaluation of anti-inflammatory potential of extract, fractions and major compounds of Ponciri Fructus in LPS-induced RAW 264.7 cells. Curr. Res. Biotechnol. 2023, 6, 100138. [Google Scholar] [CrossRef]
- Zhou, M.; Zhi, J.; Zhi, J.; Xiong, Z.; Wu, F.; Lu, Y.; Hu, Q. Polysaccharide from Strongylocentrotus nudus eggs regulates intestinal epithelial autophagy through CD36/PI3K-Akt pathway to ameliorate inflammatory bowel disease. Int. J. Biol. Macromol. 2023, 244, 125373. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhu, X.; Shen, J.; Chen, H.; Zhou, G. Mechanism of Nicotiflorin in San-Ye-Qing rhizome for anti-inflammatory effect in ulcerative colitis. Phytomedicine 2024, 129, 155564. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Gong, L.; Zhu, T.; Zhou, W.; Kong, L.; Luo, J. Identification of Tubocapsanolide A as a novel NLRP3 inhibitor for potential treatment of colitis. Biochem. Pharmacol. 2021, 190, 114645. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huo, J.; Zhong, S.; Zhu, J.; Li, Y.; Li, X. Chemical structure and anti-inflammatory activity of a branched polysaccharide isolated from Phellinus baumii. Carbohydr. Polym. 2021, 268, 118214. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-T.; Li, L.-H.; Chiu, H.-W.; Menon, M.P.; Hsu, H.-T.; Lin, W.-Y.; Wu, C.-H.; Ho, C.-L.; Hua, K.-F. Antcin-H, a natural triterpene derived from Antrodia cinnamomea, ameliorates dextran sulfate sodium-induced colitis in mice by inhibiting the NLRP3 inflammasome. J. Tradit. Complement. Med. 2024. [Google Scholar] [CrossRef]
- Cai, D.; Liu, Y.-Y.; Tang, X.-P.; Zhang, M.; Cheng, Y.-X. Minor ergosteroids and a 19-nor labdane-type diterpenoid with anti-inflammatory effects from Ganoderma lucidum. Phytochemistry 2024, 222, 114052. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.-M.; Veeraperumal, S.; Tan, K.; Zhong, S.; Cheong, K.-L. The in vitro anti-inflammatory mechanism of Porphyra haitanensis oligosaccharides on lipopolysaccharide-induced injury in IEC-6 cells. J. Funct. Foods 2024, 112, 106005. [Google Scholar] [CrossRef]
- Dai, L.-T.; Yang, L.; Guo, J.-C.; Ma, Q.-Y.; Xie, Q.-Y.; Jiang, L.; Yu, Z.-F.; Dai, H.-F.; Zhao, Y.-X. Anti-diabetic and anti-inflammatory indole diterpenes from the marine-derived fungus Penicillium sp. ZYX-Z-143. Bioorg. Chem. 2024, 145, 107205. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Peng, W.-R.; Zhang, D.; Sun, H.-X.; Li, L.; Sun, F.; Gu, Z.-C.; Lin, H.-W. Marine sponge-derived alkaloid ameliorates DSS-induced IBD via inhibiting IL-6 expression through modulating JAK2-STAT3-SOCS3 pathway. Int. Immunopharmacol. 2024, 129, 111576. [Google Scholar] [CrossRef]
- Ihekweazu, F.D.; Engevik, M.A.; Ruan, W.; Shi, Z.; Fultz, R.; Engevik, K.A.; Chang-Graham, A.L.; Freeborn, J.; Park, E.S.; Venable, S. Bacteroides ovatus promotes IL-22 production and reduces trinitrobenzene sulfonic acid–driven colonic inflammation. Am. J. Pathol. 2021, 191, 704–719. [Google Scholar] [CrossRef]
- Li, K.; Hao, Z.; Du, J.; Gao, Y.; Yang, S.; Zhou, Y. Bacteroides thetaiotaomicron relieves colon inflammation by activating aryl hydrocarbon receptor and modulating CD4+ T cell homeostasis. Int. Immunopharmacol. 2021, 90, 107183. [Google Scholar] [CrossRef]
- Cordeiro, B.F.; Alves, J.L.; Belo, G.A.; Oliveira, E.R.; Braga, M.P.; da Silva, S.H.; Lemos, L.; Guimarães, J.T.; Silva, R.; Rocha, R.S.; et al. Therapeutic Effects of Probiotic Minas Frescal Cheese on the Attenuation of Ulcerative Colitis in a Murine Model. Front. Microbiol. 2021, 12, 623920. [Google Scholar] [CrossRef]
- Chae, S.A.; Ramakrishnan, S.R.; Kim, T.; Kim, S.-R.; Bang, W.Y.; Jeong, C.-R.; Yang, J.; Kim, S.-J. Anti-inflammatory and anti-pathogenic potential of Lacticaseibacillus rhamnosus IDCC 3201 isolated from feces of breast-fed infants. Microb. Pathog 2022, 173, 105857. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, L.; Chen, L.; Wang, J.; Liu, A.; Luo, P.; Zhan, M.; Zhou, X.; Chen, L.; Zhang, J. Lactobacillus fermentum CKCC1858 and Lactobacillus plantarum CKCC1312 ameliorate the symptoms of ulcerative colitis in mouse model induced by dextran sulfate sodium. J. Funct. Foods. 2024, 112, 105995. [Google Scholar] [CrossRef]
- Nie, H.; Li, Y.; Lu, X.-L.; Yan, J.; Liu, X.-R.; Yin, Q. Prodigiosin derived from chromium-resistant Serratia sp. prevents inflammation and modulates gut microbiota homeostasis in DSS-induced colitis mice. Int. Immunopharmacol. 2023, 116, 109800. [Google Scholar] [CrossRef]
- Cao, Y.; Gao, J.; Zhang, L.; Qin, N.; Zhu, B.; Xia, X. Jellyfish skin polysaccharides enhance intestinal barrier function and modulate the gut microbiota in mice with DSS-induced colitis. Food Funct. 2021, 12, 10121–10135. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lee, M.; Chang, E.B. The gut microbiome and inflammatory bowel diseases. Annu. Rev. Med. 2022, 73, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Vindigni, S.M.; Zisman, T.L.; Suskind, D.L.; Damman, C.J. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: A tripartite pathophysiological circuit with implications for new therapeutic directions. Ther. Adv. Gastroenterol. 2016, 9, 606–625. [Google Scholar] [CrossRef]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L. Bile acid metabolites control TH17 and Treg cell differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Song, X.; Sun, X.; Oh, S.F.; Wu, M.; Zhang, Y.; Zheng, W.; Geva-Zatorsky, N.; Jupp, R.; Mathis, D.; Benoist, C. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 2020, 577, 410–415. [Google Scholar] [CrossRef]
- Clooney, A.G.; Eckenberger, J.; Laserna-Mendieta, E.; Sexton, K.A.; Bernstein, M.T.; Vagianos, K.; Sargent, M.; Ryan, F.J.; Moran, C.; Sheehan, D. Ranking microbiome variance in inflammatory bowel disease: A large longitudinal intercontinental study. Gut 2021, 70, 499–510. [Google Scholar] [CrossRef]
- Zhou, J.; Li, M.; Chen, Q.; Li, X.; Chen, L.; Dong, Z.; Zhu, W.; Yang, Y.; Liu, Z.; Chen, Q. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat. Commun. 2022, 13, 3432. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, Y.; Zhang, R.; Chen, Y.; Wang, F.; Zhang, M. Gut microbial fermentation promotes the intestinal anti-inflammatory activity of Chinese yam polysaccharides. Food Chem. 2023, 402, 134003. [Google Scholar] [CrossRef]
- Tsopmejio, I.S.N.; Ding, M.; Wei, J.; Zhao, C.; Jiang, Y.; Li, Y.; Song, H. Auricularia polytricha and Flammulina velutipes ameliorate inflammation and modulate the gut microbiota via regulation of NF-κB and Keap1/Nrf2 signaling pathways on DSS-induced inflammatory bowel disease. Food Biosci. 2022, 47, 101426. [Google Scholar] [CrossRef]
- Mak, K.-K.; Wong, Y.-H.; Pichika, M.R. Artificial Intelligence in Drug Discovery and Development; Springer Nature: Cham, Switzerland, 2023. [Google Scholar]
- Powrie, F.; Leach, M.W.; Mauze, S.; Menon, S.; Caddle, L.B.; Coffman, R.L. Inhibition of Thl responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1994, 1, 553–562. [Google Scholar] [CrossRef]
- Jukič, M.; Bren, U. Machine learning in antibacterial drug design. Front. Pharmacol. 2022, 13, 864412. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P. Impact of artificial intelligence on prognosis, shared decision-making, and precision medicine for patients with inflammatory bowel disease: A perspective and expert opinion. Ann. Med. 2023, 55, 2300670. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, S.; Paruzzo, F.; Bolliger, C. Deep Learning-Based Phase and Baseline Correction of 1D 1H NMR Spectra. Public Bruker White Paper. 2021. Available online: https://www.bruker.com/en/products-and-solutions/mr/nmr-software/topspin.html (accessed on 8 June 2024).
- Zhang, Y.; Bhosle, A.; Bae, S.; McIver, L.J.; Pishchany, G.; Accorsi, E.K.; Thompson, K.N.; Arze, C.; Wang, Y.; Subramanian, A.; et al. Discovery of bioactive microbial gene products in inflammatory bowel disease. Nature 2022, 606, 754–760. [Google Scholar] [CrossRef]
- Howarth, A.; Ermanis, K.; Goodman, J.M. DP4-AI automated NMR data analysis: Straight from spectrometer to structure. Chem. Sci. 2020, 11, 4351–4359. [Google Scholar] [CrossRef]
- Huber, F.; van der Burg, S.; van der Hooft, J.J.; Ridder, L. MS2DeepScore: A novel deep learning similarity measure to compare tandem mass spectra. J. Cheminform. 2021, 13, 84. [Google Scholar] [CrossRef]
- Jena, A.B.; Dash, U.C.; Duttaroy, A.K. An in silico investigation on the interactions of curcumin and epigallocatechin-3-gallate with NLRP3 inflammasome complex. Biomed. Pharmacother. 2022, 156, 113890. [Google Scholar] [CrossRef] [PubMed]
- Ekowati, J.; Tejo, B.A.; Maulana, S.; Kusuma, W.A.; Fatriani, R.; Ramadhanti, N.S.; Norhayati, N.; Nofianti, K.A.; Sulistyowaty, M.I.; Zubair, M.S. Potential Utilization of Phenolic Acid Compounds as Anti-Inflammatory Agents through TNF-α Convertase Inhibition Mechanisms: A Network Pharmacology, Docking, and Molecular Dynamics Approach. ACS Omega 2023, 8, 46851–46868. [Google Scholar] [CrossRef]
- Vishwakarma, R.K.; Negi, A.; Negi, D.S. Abortitristoside A and desrhamnosylverbanscoside: The potential COX-2 inhibitor from the leaves of Nyctanthes arbor-tristis as Anti-inflammatory agents based on the in-vitro assay, molecular docking and ADMET prediction. Chem. Pap. 2022, 77, 3035–3049. [Google Scholar] [CrossRef]
- Simoben, C.V.; Babiaka, S.B.; Moumbock, A.F.A.; Namba-Nzanguim, C.T.; Eni, D.B.; Medina-Franco, J.L.; Günther, S.; Ntie-Kang, F.; Sippl, W. Challenges in natural product-based drug discovery assisted with in silico-based methods. RSC Adv. 2023, 13, 31578–31594. [Google Scholar] [CrossRef] [PubMed]
- Jamtsho, T.; Loukas, A.; Wangchuk, P. Pharmaceutical Potential of Remedial Plants and Helminths for Treating Inflammatory Bowel Disease. Pharmaceuticals 2024, 17, 819. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar]
- Schneider, G. Automating drug discovery. Nat. Rev. Drug Discov. 2018, 17, 97–113. [Google Scholar]
- Samek, W.; Wiegand, T.; Müller, K.-R. Explainable artificial intelligence: Understanding, visualizing and interpreting deep learning models. arXiv 2017, arXiv:1708.08296. [Google Scholar]
- Ekins, S.; Puhl, A.C.; Zorn, K.M.; Lane, T.R.; Russo, D.P.; Klein, J.J.; Hickey, A.J.; Clark, A.M. Exploiting machine learning for end-to-end drug discovery and development. Nat. Mater. 2019, 18, 435–441. [Google Scholar] [CrossRef]
- Robinson, D. Confronting Biopiracy: Challenges, Cases and International Debates; Routledge: Abingdon, UK, 2010. [Google Scholar]
- Mittelstadt, B.D.; Allo, P.; Taddeo, M.; Wachter, S.; Floridi, L. The ethics of algorithms: Mapping the debate. Big Data Soc. 2016, 3, 2053951716679679. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).