Antibacterial Activity of Novel Agent N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan against Streptococcus mutans
Abstract
:1. Introduction
2. Results
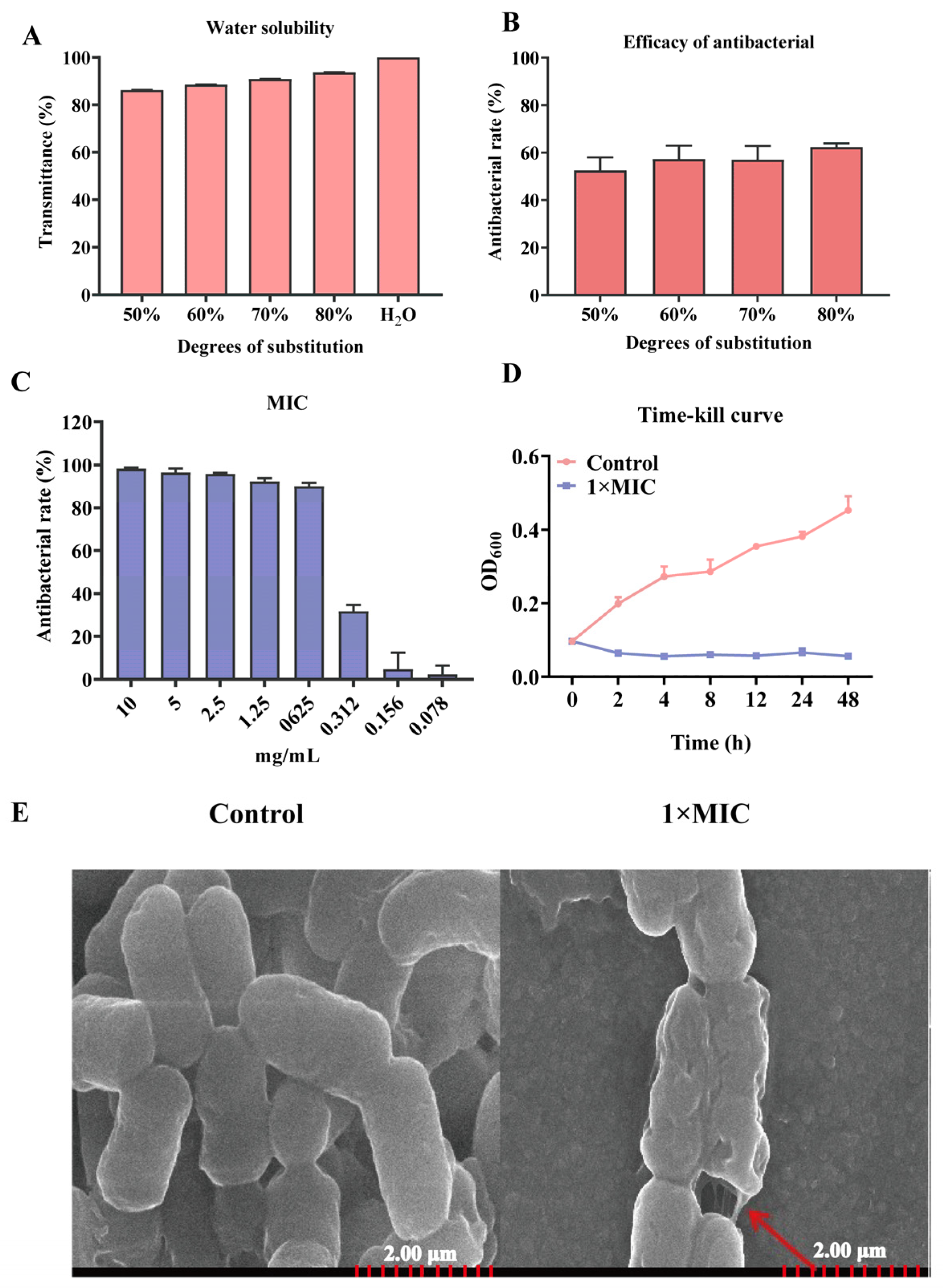
2.1. Characteristics of Antibacterial of N-2-HACC
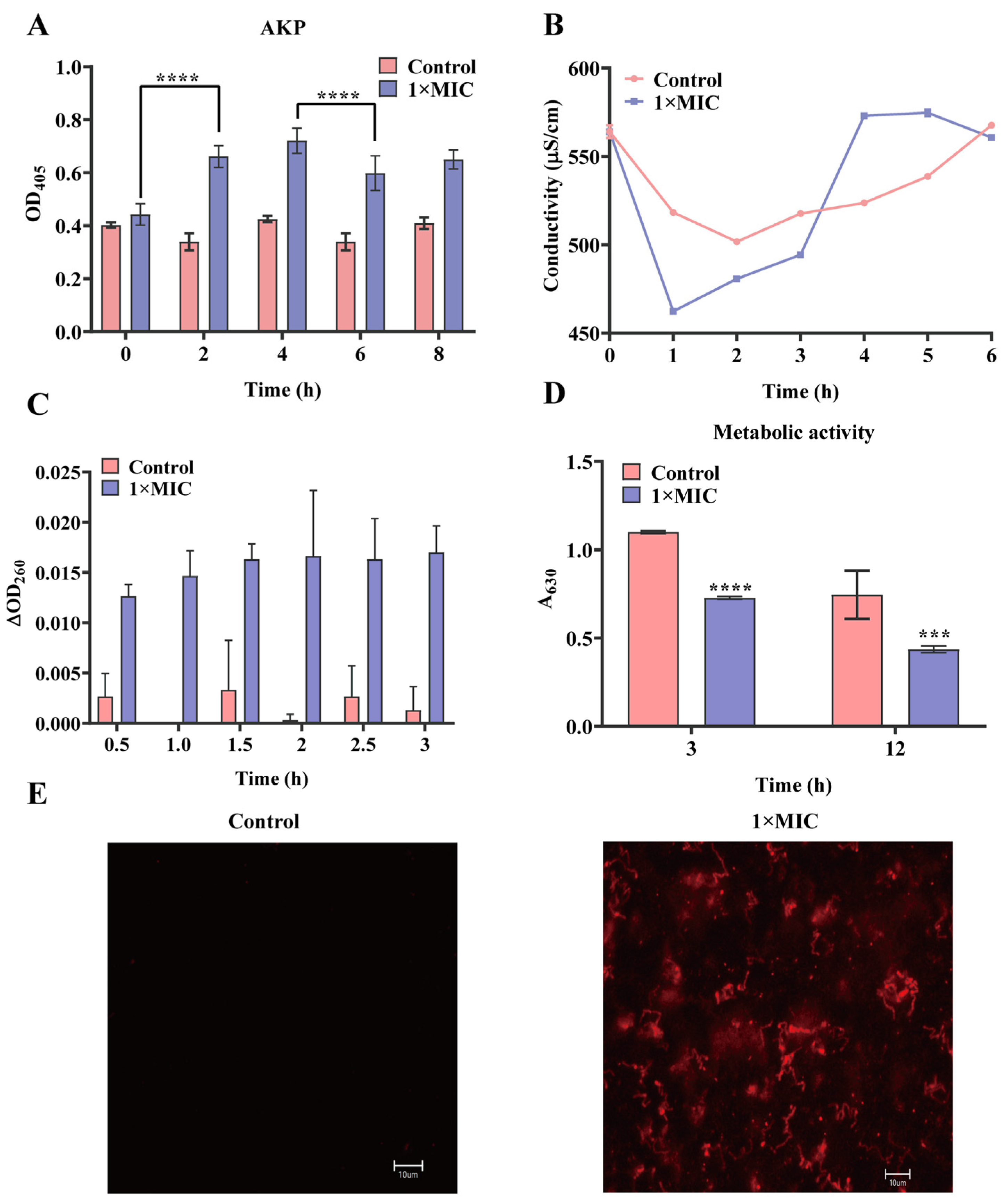
2.2. Effect of N-2-HACC on the Bacterial Cell Membrane and Cell Wall
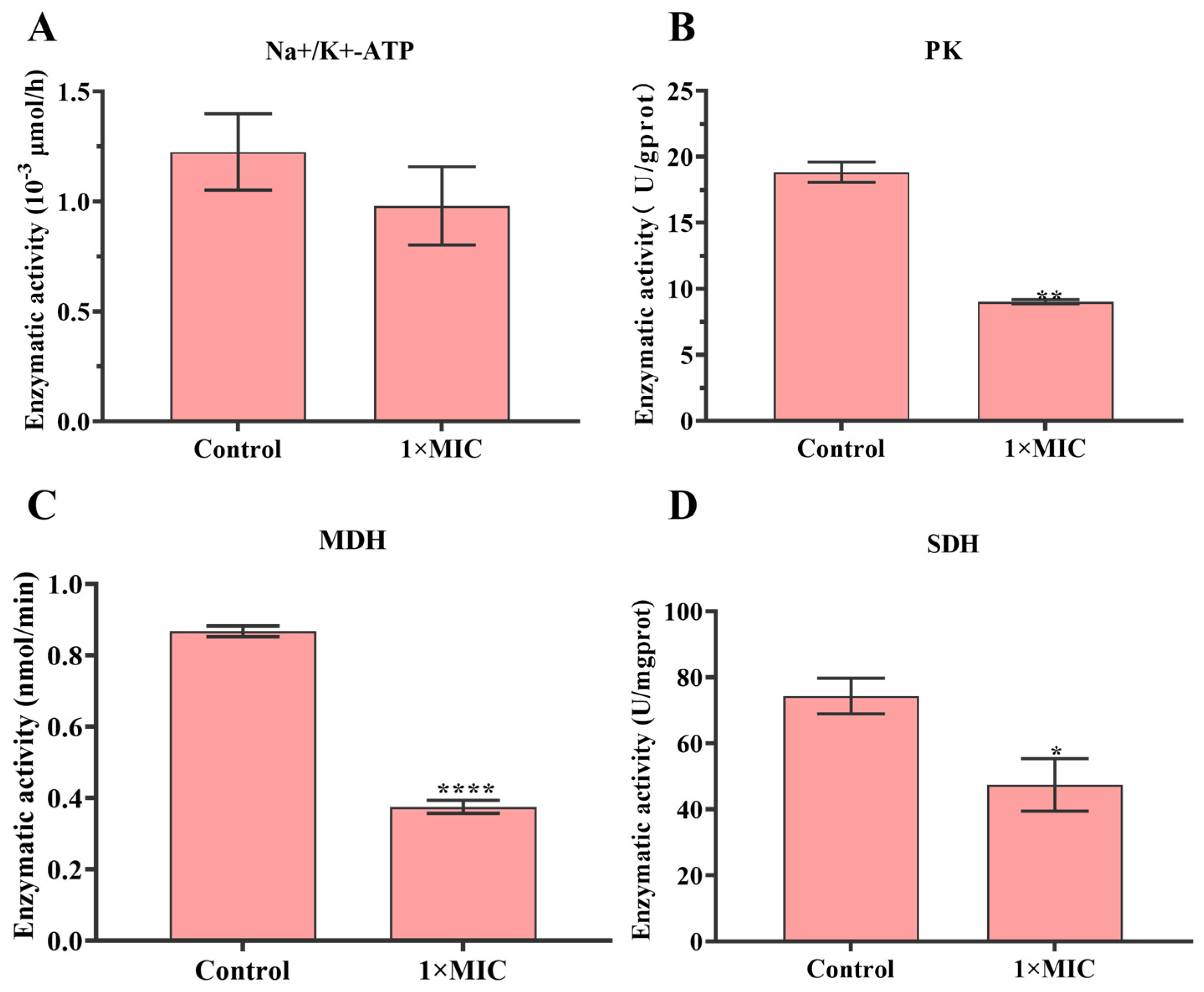
2.3. Effect of N-2-HACC on Bacterial Respiratory Metabolism
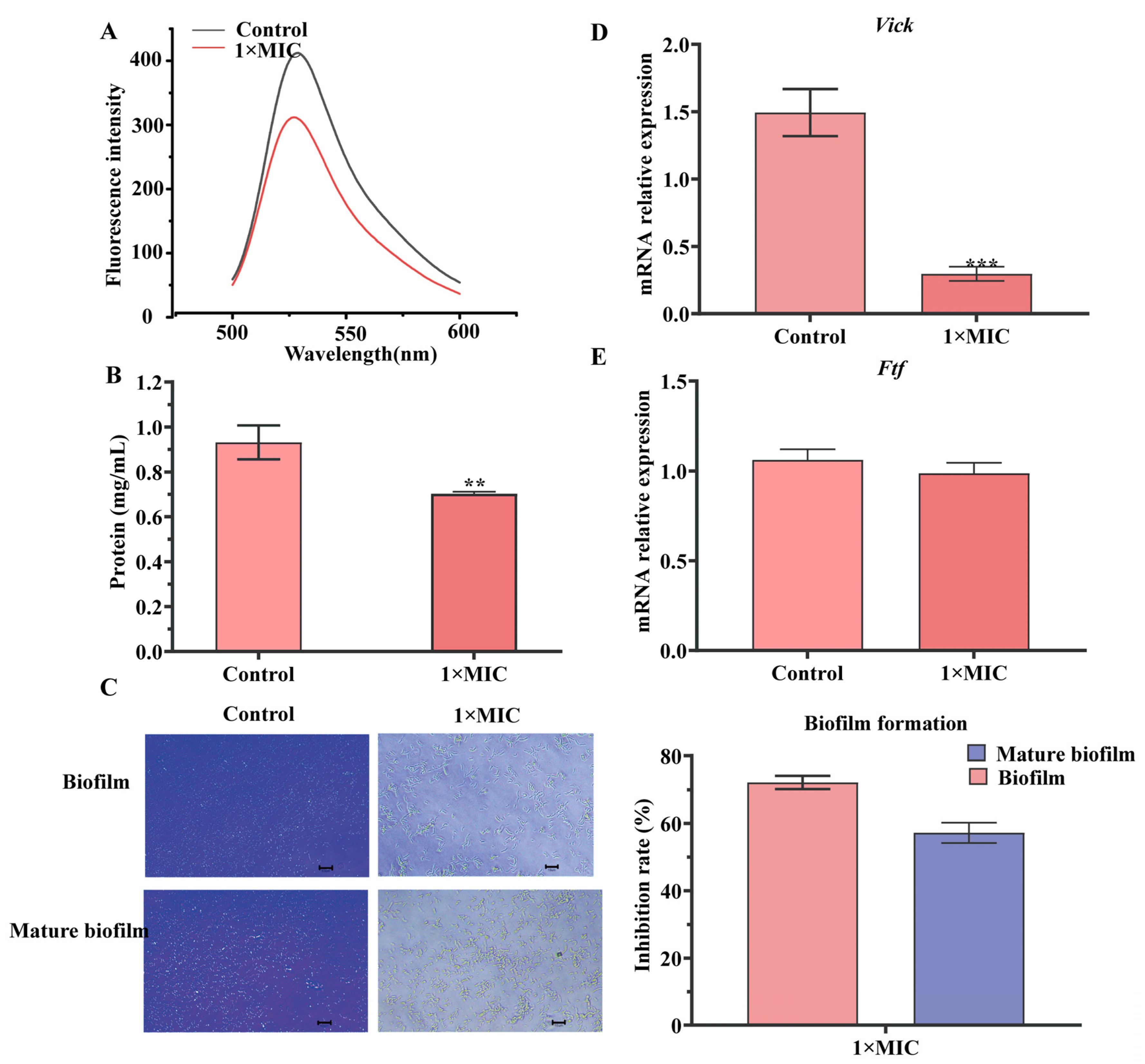
2.4. Effect of N-2-HACC on Bacterial DNA and Solution Protein
2.5. Effect of N-2-HACC on Biofilm Formation
3. Discussion
4. Materials and Methods
4.1. Synthesis of N-2-HACC with Different Degrees of Substitution
4.2. Water Solubility and Antibacterial Activity of Different Degrees of Substitution of N-2-HACC
4.3. Determination of Minimum Inhibitory Concentration (MIC)
4.4. N-2-HACC Time-Kill Curves
4.5. Scanning Electron Microscopy (SEM) Observation
4.6. Detection of Permeability of Cell Wall
4.7. Effect of N-2-HACC on the Cell Membrane of S. mutans
4.7.1. Propidium Iodide (PI) Staining
4.7.2. Conductivity Measurement
4.7.3. Detection of Nucleic Acid in BHI of S. mutans
4.7.4. Detection of Metabolic Activity of S. mutans
4.8. Effect of N-2-HACC on the Respiratory Metabolism of S. mutans
4.9. Effect of N-2-HACC on Contents of DNA and Soluble Protein of S. mutans
4.10. Detection of S. mutans Biofilm Formation
4.11. Effect of N-HACC on the Virulence Genes of S. mutans
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, C.S.; Choi, Y.K. Survey of adults’ perceptions of the association between chronic diseases and oral health. J. Dent. Hyg. Sci. 2017, 17, 12–19. [Google Scholar] [CrossRef]
- Nam, S.H. Antimicrobial activity of Crataegi fructus extract used for potential application in the prevention and treatment of oral diseases. Medicina 2023, 60, 13. [Google Scholar] [CrossRef] [PubMed]
- Antoniadou, M.; Rozos, G.; Vaiou, N.; Zaralis, K.; Ersanli, C.; Alexopoulos, A.; Tzora, A.; Varzakas, T.; Voidarou, C.C. The in vitro assessment of antibacterial and antioxidant efficacy in Rosa damascena and Hypericum perforatum extracts against pathogenic strains in the interplay of dental caries, oral health, and food microbiota. Microorganisms 2023, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Krzy’sciak, W.; Jurczak, A.; Ko´scielniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Ji, M.K.; Kim, H.; Jeong, G.; Kim, W.J.; Ryu, J.H.; Cho, H.; Lim, H.P. Effects of TiO2 nanotubes and reduced graphene oxide on Streptococcus mutans and Preosteoblastic cells at an early stage. Inter. J. Mol. Sci. 2024, 25, 1351. [Google Scholar] [CrossRef]
- Zhang, S. Dental caries and vaccination strategy against the major cariogenic pathogen, Streptococcus mutans. Curr. Pharm. Biotechnol. 2013, 14, 960–966. [Google Scholar] [CrossRef]
- Gao, Y.; Gong, X.; Yu, S.; Jin, Z.; Ruan, Q.; Zhang, C.; Zhao, K. Immune enhancement of N-2-Hydroxypropyl trimethyl ammonium chloride chitosan/carboxymethyl chitosan nanoparticles vaccine. Inter. J. Biol. Macromol. 2022, 220, 183–192. [Google Scholar] [CrossRef]
- Huang, K.X.; Zhou, L.Y.; Chen, J.Q.; Peng, N.; Chen, H.X.; Gu, H.Z.; Zou, T. Applications and perspectives of quaternized cellulose, chitin and chitosan: A review. Inter. J. Biol. Macromol. 2023, 242 Pt 3, 124990. [Google Scholar] [CrossRef]
- Qiu, Y.L.; Li, Y.; Zhang, G.L.; Hao, H.; Hou, H.M.; Bi, J. Quaternary-ammonium chitosan, a promising packaging material in the food industry. Carbohydr. Polym. 2024, 323, 121384. [Google Scholar] [CrossRef]
- Kritchenkov, A.S.; Egorov, A.R.; Volkova, O.V.; Artemjev, A.A.; Kurliuk, A.V.; Anh Le, T.; Hieu Truong, H.; Le-Nhat-Thuy, G.; Van Tran Thi, T.; Van Tuyen, N.; et al. Novel biopolymer-based nanocomposite food coatings that exhibit active and smart properties due to a single type of nanoparticles. Food Chem. 2021, 343, 128676. [Google Scholar] [CrossRef]
- Wang, L.; Xin, M.; Li, M.; Liu, W.; Mao, Y. Effect of the structure of chitosan quaternary phosphonium salt and chitosan quaternary ammonium salt on the antibacterial and antibiofilm activity. Int. J. Biol. Macromol. 2023, 242 Pt 2, 124877. [Google Scholar] [CrossRef]
- Wang, L.; Xin, M.; Li, M.; Zhang, T.; Pang, Y.; Mao, Y. Preparation of biguanidine quaternary ammonium salts grafted chitosan with enhanced antibacterial and antibiofilm activities. Carbohydr. Res. 2024, 538, 109078. [Google Scholar] [CrossRef]
- Dediu Botezatu, A.V.; Apetrei, R.M.; Costea Nour, I.F.; Barbu, V.; Grigore-Gurgu, L.; Botez, F.; Dinica, R.M.; Furdui, B.; Cârâc, G. Synthesis and characterization of novel chitosan derivatives (containing dipyridinium quaternary salts) with antimicrobial potential. Carbohydr. Res. 2023, 534, 108964. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gong, X.; Ruan, Q.; Xu, W.; Zhang, C.; Zhao, K. Antimicrobial application of chitosan derivatives and their nanocomposites. Curr. Med. Chem. 2023, 30, 1736–1755. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Li, W.; Cao, H.; Zhang, X.; Chen, G.; Wu, H.; Guo, C.; Zhang, Y.; Kang, H.; Wang, Y.; et al. Antimicrobial activity and cytotoxicity of N-2-HACC and characterization of nanoparticles with N-2-HACC and CMC as a vaccine carrier. Chem. Eng. J. 2013, 221, 331–341. [Google Scholar] [CrossRef]
- Cui, J.; Ji, X.; Mi, Y.; Miao, Q.; Dong, F.; Tan, W.; Guo, Z. Antimicrobial and antioxidant activities of N-2-Hydroxypropyltrimethyl ammonium chitosan derivatives bearing amino acid schiff bases. Mar. Drugs 2022, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Phuangkaew, T.; Booranabunyat, N.; Kiatkamjornwong, S.; Thanyasrisung, P.; Hoven, V.P. Amphiphilic quaternized chitosan: Synthesis, characterization, and anti-cariogenic biofilm property. Carbohydr. Polym. 2022, 277, 118882. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Pei, H.; Hu, W.; Zhu, Y.; Xu, H.; Ma, C.; Sun, J.; Li, H. A promising application of chitosan quaternary ammonium salt to removal of Microcystis aeruginosa cells from drinking water. Sci. Total Environ. 2017, 583, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jin, Z.; Ma, D.; Yin, T.H.; Zhao, K. Palmitic acid- and cysteine-functionalized nanoparticles overcome mucus and epithelial barrier for oral delivery of drug. Bioeng. Transl. Med. 2023, 8, e10510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Peng, Y.; Shi, X.; Zhao, K. Chitosan derivative composite nanoparticles as adjuvants enhance the cellular immune response via activation of the cGAS-STING pathway. Inter. J. Pharm. 2023, 636, 122847. [Google Scholar] [CrossRef]
- Khubiev, O.M.; Egorov, A.R.; Kirichuk, A.A.; Khrustalev, V.N.; Tskhovrebov, A.G.; Kritchenkov, A.S. Chitosan-Based Antibacterial Films for Biomedical and Food Applications. Int. J. Mol. Sci. 2023, 24, 10738. [Google Scholar] [CrossRef] [PubMed]
- Bloch, S.; Hager-Mair, F.F.; Andrukhov, O.; Schäffer, C. Oral streptococci: Modulators of health and disease. Front. Cell Infect. Microbiol. 2024, 14, 1357631. [Google Scholar] [CrossRef] [PubMed]
- Róna, V.; Bencze, B.; Kelemen, K.; Végh, D.; Tóth, R.; Kói, T.; Hegyi, P.; Varga, G.; Rózsa, N.K.; Géczi, Z. Effect of chitosan on the number of Streptococcus mutans in saliva: A meta-analysis and systematic review. Int. J. Mol. Sci. 2023, 24, 15270. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.G.; Xie, M.; Dong, Y.; Wu, H.; He, D.D.; Hu, G.Z.; Xu, E.P. Antimicrobial mechanisms of traditional Chinese medicine and reversal of drug resistance: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 5553–5561. [Google Scholar]
- Baquero, F.; Levin, B.R. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat. Rev. Microbiol. 2021, 19, 123–132. [Google Scholar] [CrossRef]
- Xie, M.; Gao, M.; Yun, Y.; Malmsten, M.; Rotello, V.M.; Zboril, R.; Akhavan, O.; Kraskouski, A.; Amalraj, J.; Cai, X.; et al. Antibacterial nanomaterials: Mechanisms, impacts on antimicrobial resistance and design principles. Angew Chem. Int. Ed. Engl. 2023, 62, e202217345. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.H.; Chitra, E.; Ramamurthy, S.; Ling, C.C.S.; Ambu, S.P.; Davamani, F. Cationic chitosan-propolis nanoparticles alter the zeta potential of S. epidermidis, inhibit biofilm formation by modulating gene expression and exhibit synergism with antibiotics. PLoS ONE 2019, 14, e0213079. [Google Scholar] [CrossRef]
- Czerkas, K.; Olchowik-Grabarek, E.; Łomanowska, M.; Abdulladjanova, N.; Sękowski, S. Antibacterial activity of plant polyphenols belonging to the tannins against Streptococcus mutans-potential against dental caries. Molecules 2024, 29, 879. [Google Scholar] [CrossRef]
- Macambira, D.V.C.; Almeida Júnior, J.S.; Silveira, C.F.M.; Sarrazin, S.L.F.; Moraes, T.M.P.; da Silva, B.A.; Minervino, A.H.H.; Moraes, W.P.; Barata, L.E.S. Antimicrobial activity on Streptococcus mutans and Enterococcus faecalis of Cyperus articulatus ethanolic extracts. Plants 2024, 13, 689. [Google Scholar] [CrossRef]
- Picolo, M.; Stephen, A.; Baysan, A. The antimicrobial effect of different vitamin D compounds on Streptococcus mutans and their impact on glycosyltransferase expression. J. Oral Microbiol. 2024, 16, 2327758. [Google Scholar] [CrossRef]
- Li, Z.; He, Q.; Xu, F.; Yin, X.; Guan, Z.; Song, J.; He, Z.; Yang, X.; Situ, C. Exploring the antibacterial potential and underlying mechanisms of Prunella vulgaris L. on methicillin-resistant Staphylococcus aureus. Foods 2024, 13, 660. [Google Scholar] [CrossRef]
- Meng, X.; Li, D.; Zhou, D.; Wang, D.; Liu, Q.; Fan, S. Chemical composition, antibacterial activity and related mechanism of the essential oil from the leaves of Juniperus rigida Sieb. et Zucc against Klebsiella pneumoniae. J. Ethnopharmacol. 2016, 194, 698–705. [Google Scholar] [CrossRef]
- Li, L.; Liang, F.; Li, C.; Hou, T.; Xu, D.A. Antibacterial mechanism of chitosan-gentamicin and its effect on the intestinal flora of litopenaeus vannamei infected with vibrio parahaemolyticus. Mar. Drugs 2022, 20, 702. [Google Scholar] [CrossRef] [PubMed]
- Kovács, R.; Erdélyi, L.; Fenyvesi, F.; Balla, N.; Kovács, F.; Vámosi, G.; Klusóczki, Á.; Gyöngyösi, A.; Bácskay, I.; Vecsernyés, M.; et al. Concentration-dependent antibacterial activity of chitosan on Lactobacillus plantarum. Pharmaceutics 2022, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Kamjumphol, W.; Chareonsudjai, P.; Chareonsudjai, S. Antibacterial activity of chitosan against Burkholderia pseudomallei. Microbiologyopen 2018, 7, e00534. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial activity and proposed action mechanism of linalool against Pseudomonas fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Zhang, N.; Liu, S.; Chen, M.; Xie, J. Epsilon-Polylysine inhibits Shewanella putrefaciens with membrane disruption and cell damage. Molecules 2019, 24, 727. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Chen, X.; Li, W.; Wang, L.; Li, W.; Du, J.; Zhang, S. Effects of cinnamon essential oil on the physiological metabolism of Salmonella enteritidis. Front. Microbiol. 2022, 13, 1035894. [Google Scholar] [CrossRef]
- Guo, Y.N.; He, K.R.; Liang, S.S.; Mou, R.W.; Lu, M.H.; He, Y.M.; Tang, L.P. The effect and mechanism of volatile oil emulsion from leaves of Clausena lansium (Lour.) skeels on Staphylococcus aureus in vitro. Front. Microbiol. 2024, 15, 1376819. [Google Scholar] [CrossRef] [PubMed]
| Genes | Primers Sequence (5′-3′) | |
|---|---|---|
| ftf | ftf-F | AAATATGAAGGCGGCTACAACG |
| ftf-R | CTTCACCAGTCTTAGCATCCTGAA | |
| vick | vick-F | ATGGCGGTAAGGTGACTG |
| vick-R | GACCCTTCGCCTTCCTCACTA | |
| 16S | 16S-F | CCTACGGGAGGCAGCAGTAG |
| 16S-R | CAACAGAGCTTTACGATCCGAAA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Gong, X.; Ruan, Q.; Zhang, C.; Zhao, K. Antibacterial Activity of Novel Agent N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan against Streptococcus mutans. Molecules 2024, 29, 4126. https://doi.org/10.3390/molecules29174126
Gao Y, Gong X, Ruan Q, Zhang C, Zhao K. Antibacterial Activity of Novel Agent N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan against Streptococcus mutans. Molecules. 2024; 29(17):4126. https://doi.org/10.3390/molecules29174126
Chicago/Turabian StyleGao, Yuan, Xiaochen Gong, Qicheng Ruan, Chunjing Zhang, and Kai Zhao. 2024. "Antibacterial Activity of Novel Agent N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan against Streptococcus mutans" Molecules 29, no. 17: 4126. https://doi.org/10.3390/molecules29174126
APA StyleGao, Y., Gong, X., Ruan, Q., Zhang, C., & Zhao, K. (2024). Antibacterial Activity of Novel Agent N-2-Hydroxypropyl Trimethyl Ammonium Chloride Chitosan against Streptococcus mutans. Molecules, 29(17), 4126. https://doi.org/10.3390/molecules29174126







