Immobilization of Lipase from Thermomyces Lanuginosus and Its Glycerolysis Ability in Diacylglycerol Preparation
Abstract
:1. Introduction
2. Results and Discussion
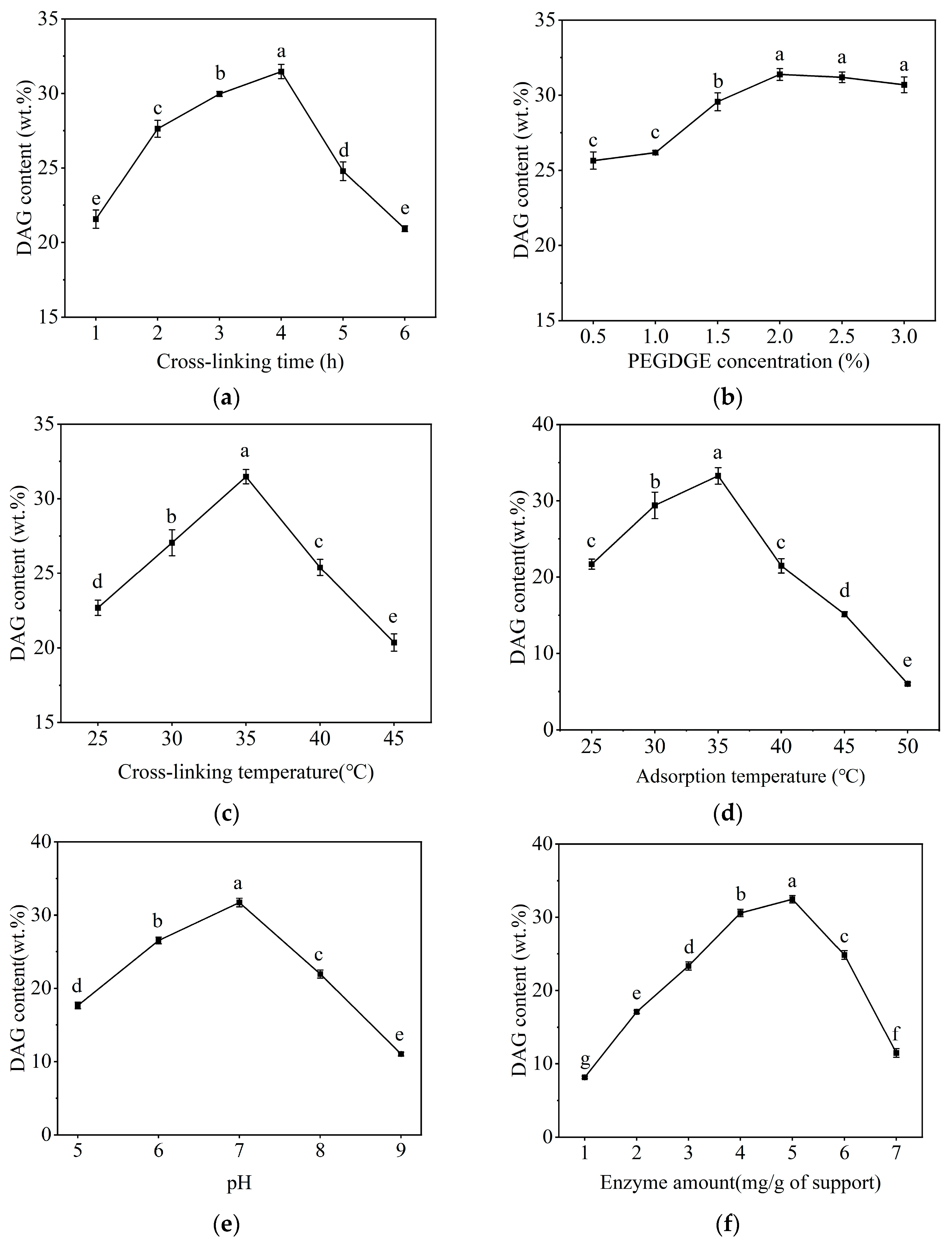
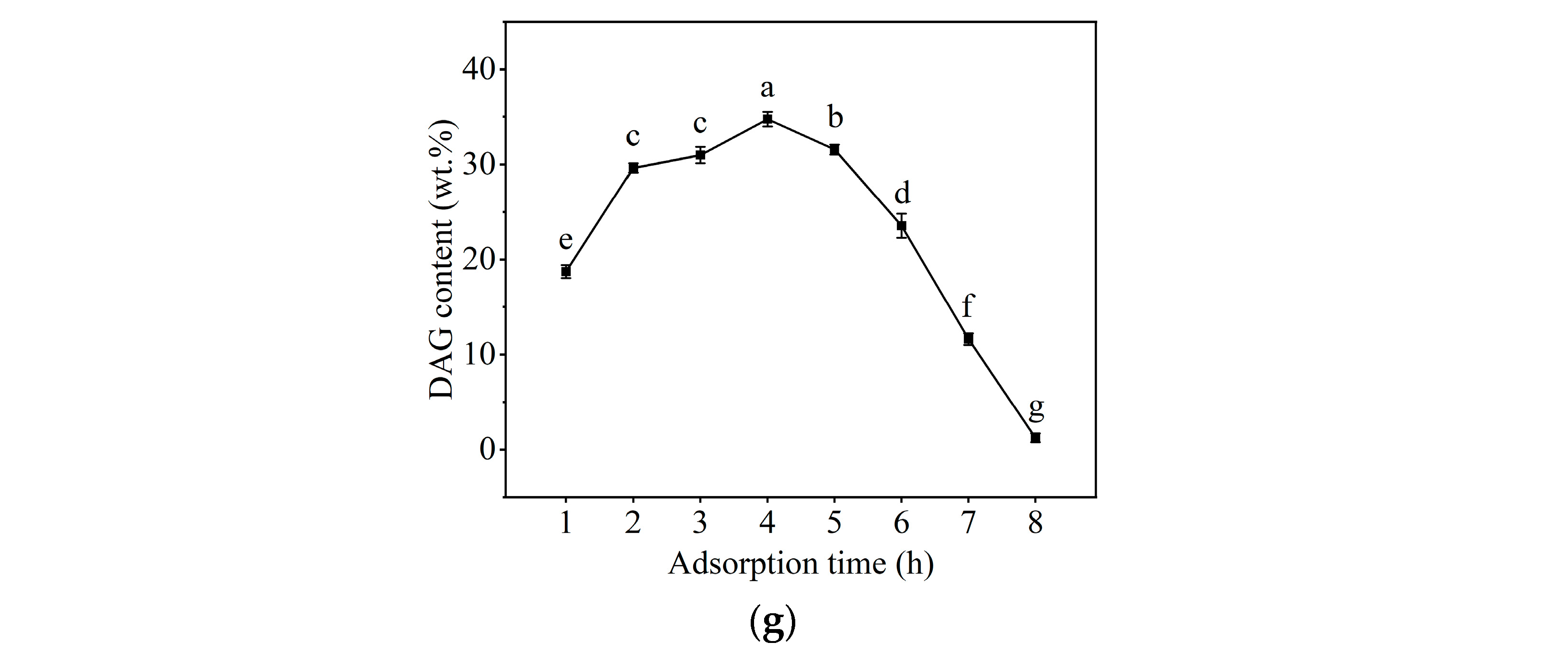
2.1. Selection of Immobilization Conditions
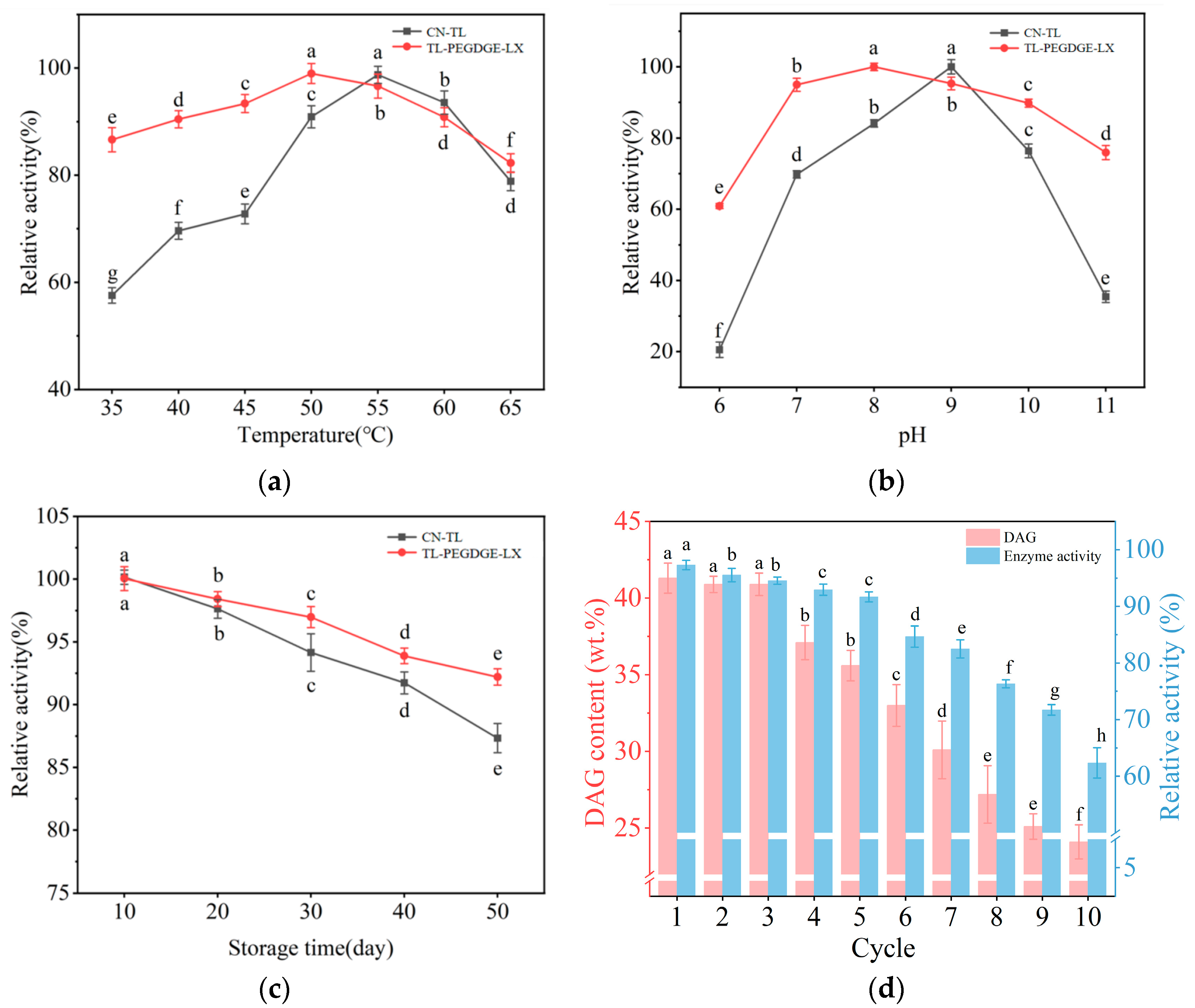
2.2. Stability of Immobilized Lipase
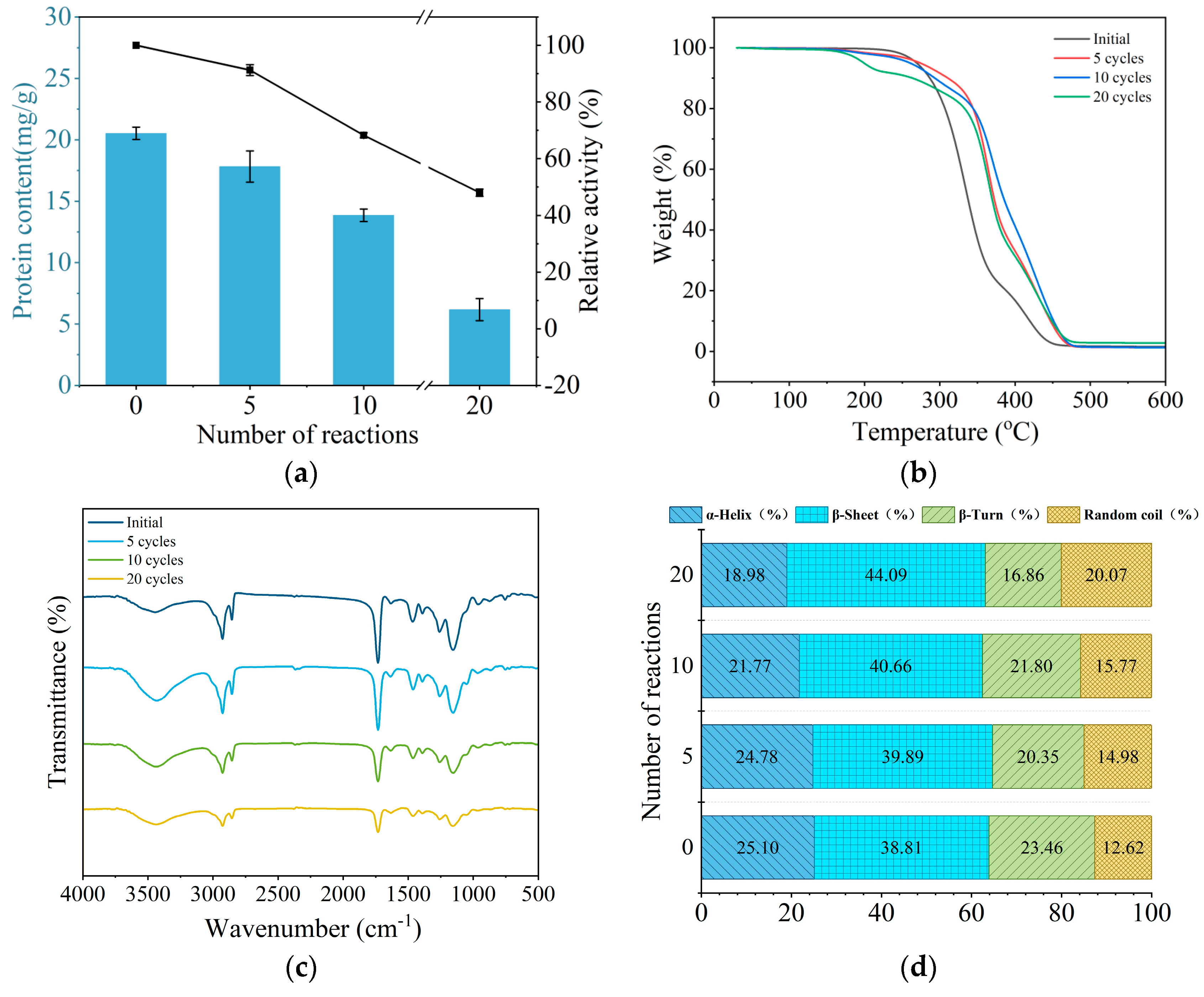
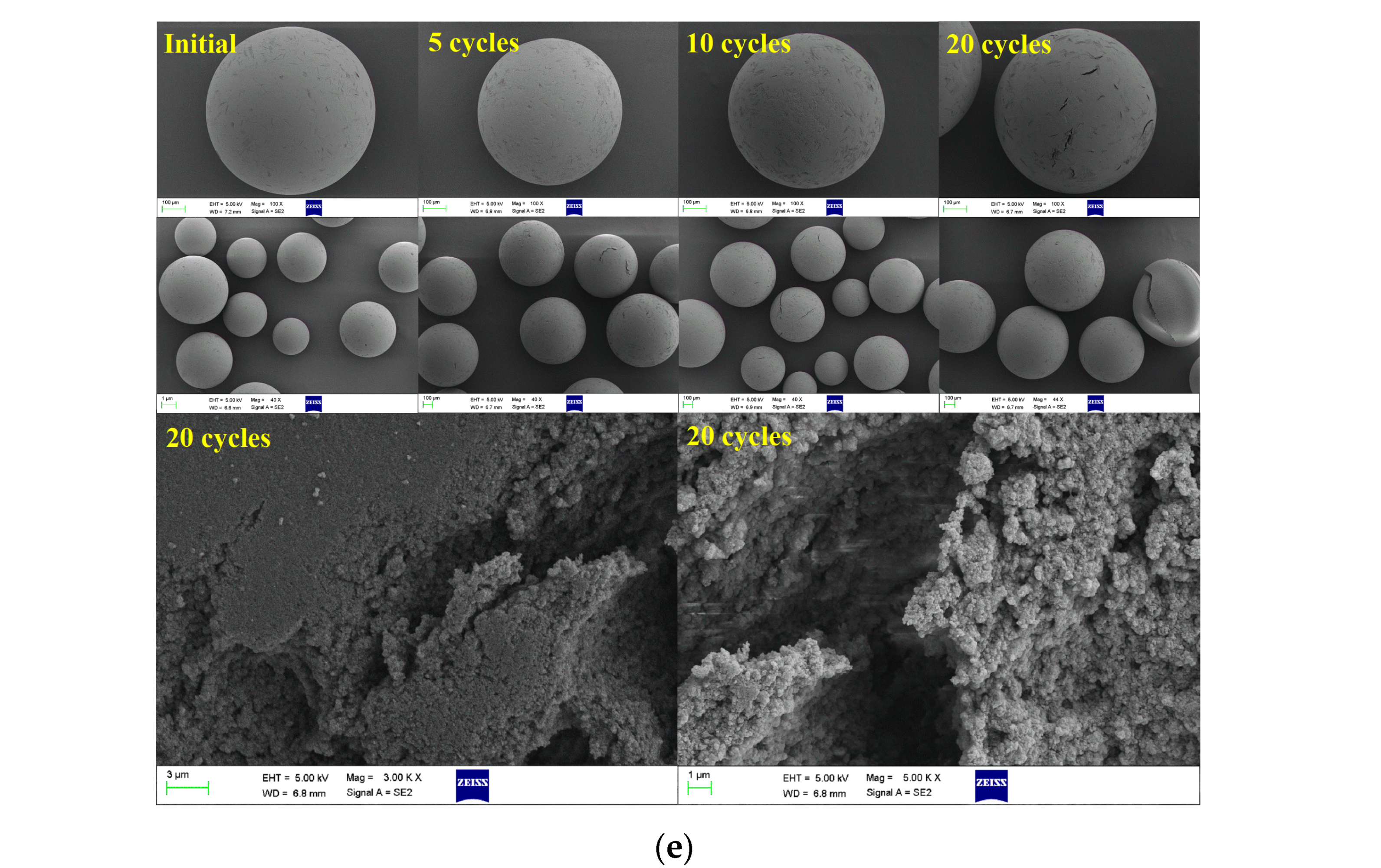
2.3. Characterization of Immobilized Enzyme During Repeated Use
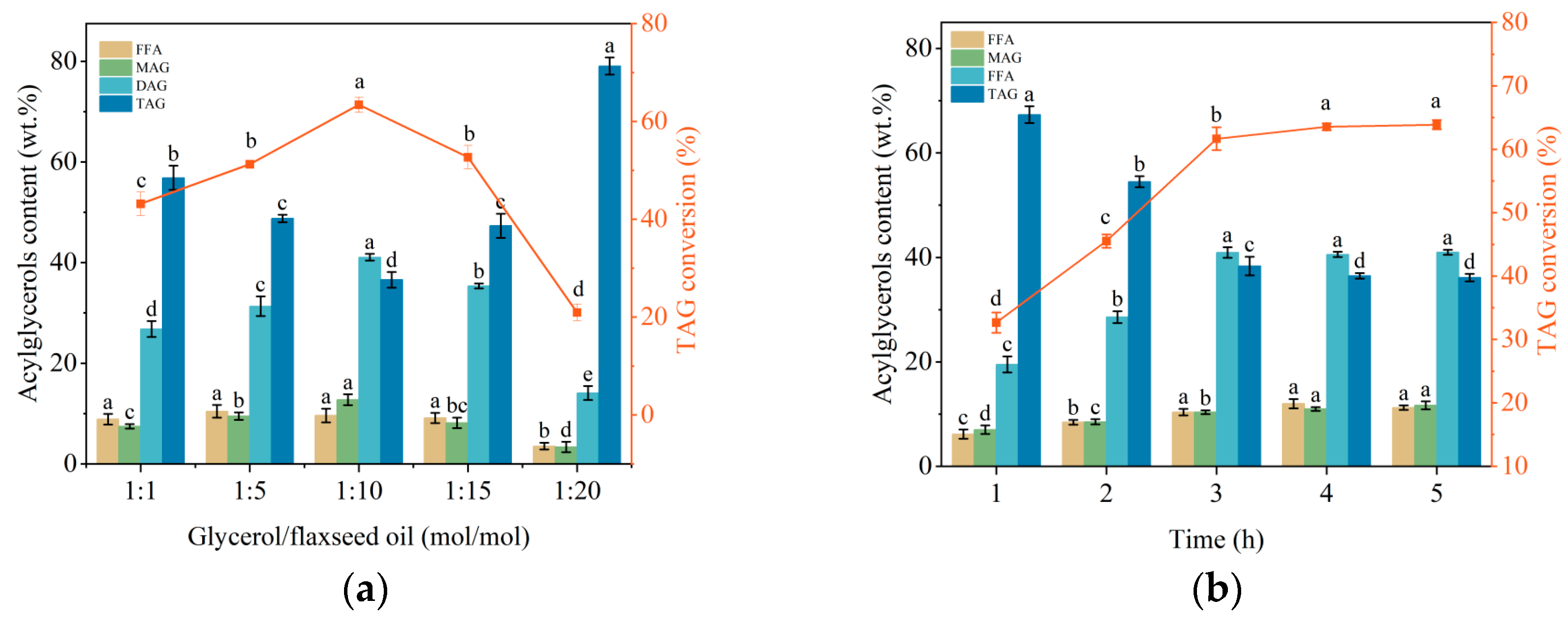
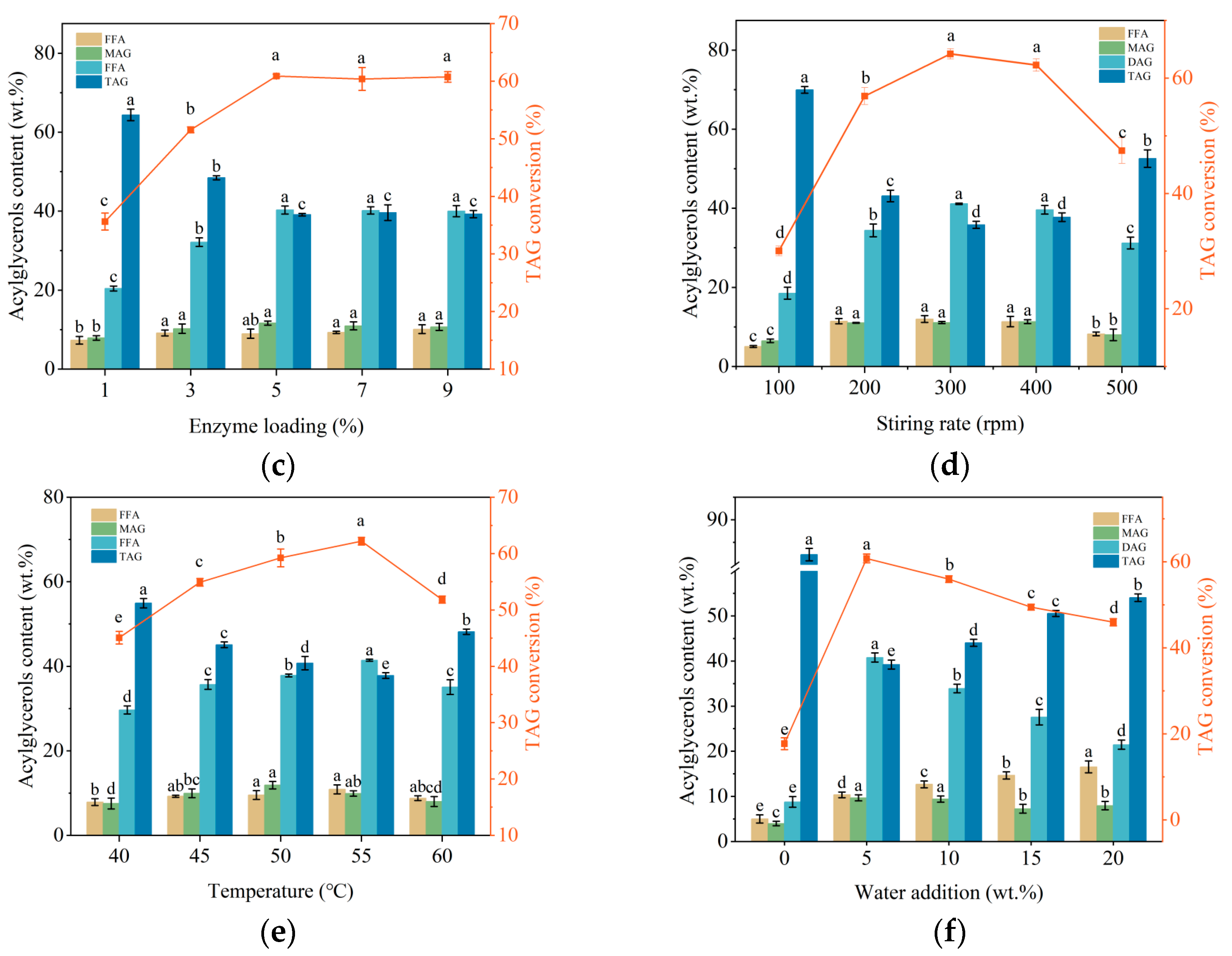
2.4. Immobilized Enzyme Catalyzed Glycerolysis for DAG Preparation
3. Materials and Methods
3.1. Materials
3.2. Pretreatment of Resin
3.3. Lipase Immobilization
3.4. Determination of Enzyme Activity
3.5. Protein Content of Lipase
3.6. Analysis of the Acylglycerol Compositions
3.7. Isomer Analysis
3.8. Characterization of the Immobilized Lipase
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo, S.-K.; Tan, C.-P.; Long, K.; Yusoff, M.S.A.; Lai, O.-M. Diacylglycerol Oil—Properties, Processes and Products: A Review. Food Bioprocess Technol. 2008, 1, 223–233. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Tang, T.-K.; Phuah, E.-T.; Tan, C.-P.; Wang, Y.; Li, Y.; Cheong, L.-Z.; Lai, O.-M. Production, safety, health effects and applications of diacylglycerol functional oil in food systems: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 2509–2525. [Google Scholar] [CrossRef] [PubMed]
- Sivakanthan, S.; Madhujith, T. Current trends in applications of enzymatic interesterification of fats and oils: A review. LWT 2020, 132, 109880. [Google Scholar] [CrossRef]
- Nicholson, R.A.; Marangoni, A.G. Enzymatic glycerolysis converts vegetable oils into structural fats with the potential to replace palm oil in food products. Nat. Food 2020, 1, 684–692. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, F.; Zhong, N. Production of diacylglycerols through glycerolysis with SBA-15 supported Thermomyces lanugi-nosus lipase as catalyst. J. Sci. Food Agric. 2020, 100, 1426–1435. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.-H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Finco, G.F.; Fiametti, K.G.; Lobo, V.d.S.; da Silva, E.A.; Palú, F.; Wancura, J.H.C.; Rodrigues, M.L.F.; Valério, A.; de Oliveira, J.V. Kinetic study of liquid lipase-catalyzed glycerolysis of olive oil using Lipozyme TL 100L. J. Am. Oil Chem. Soc. 2022, 99, 559–568. [Google Scholar] [CrossRef]
- Zeng, C.; Zhong, N. Encapsulation of lipases on coordination polymers and their catalytic performance in glycerolysis and esterification. Grain Oil Sci. Technol. 2023, 6, 113–119. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, J.; Xu, H.; Sun, W.; Pan, X.; Cui, S.; Lv, S.; Zhang, Y. Enhanced Enzymatic Performance of Immobilized Pseudomonas fluorescens Lipase on ZIF-8@ZIF-67 and Its Application to the Synthesis of Neryl Acetate with Transesterification Reaction. Molecules 2024, 29, 2922. [Google Scholar] [CrossRef] [PubMed]
- Quayson, E.; Amoah, J.; Hama, S.; Kondo, A.; Ogino, C. Immobilized lipases for biodiesel production: Current and future greening opportunities. Renew. Sustain. Energy Rev. 2020, 134, 110355. [Google Scholar] [CrossRef]
- Abdulmalek, S.A.; Yan, Y. Recent developments of lipase immobilization technology and application of immobilized lipase mixtures for biodiesel production. Biofuels, Bioprod. Biorefining 2022, 16, 1062–1094. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Han, J.; Zhang, T.; Zhou, Z.; Zhang, H. Development of a novel ultrasound- and biocrosslinking-enhanced immobilization strategy with application to food enzymes. Food Chem. 2023, 417, 135810. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef] [PubMed]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Taheri-Kafrani, A.; Kharazmi, S.; Nasrollahzadeh, M.; Soozanipour, A.; Ejeian, F.; Etedali, P.; Mansouri-Tehrani, H.-A.; Razmjou, A.; Yek, S.M.-G.; Varma, R.S. Recent developments in enzyme immobilization technology for high-throughput pro-cessing in food industries. Crit. Rev. Food Sci. Nutr. 2021, 61, 3160–3196. [Google Scholar] [CrossRef] [PubMed]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of enzymes on nanoinorganic support materials: An update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef]
- Anand, A.; Gnanasekaran, P.; Allgeier, A.M.; Weatherley, L.R. Study and deployment of methacrylate-based polymer resins for immobilized lipase catalyzed triglyceride hydrolysis. Food Bioprod. Process. 2020, 123, 164–176. [Google Scholar] [CrossRef]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T. Immobilization of Enzymes by Polymeric Materials. Catalysts 2021, 11, 1211. [Google Scholar] [CrossRef]
- He, L.; Zeng, C.; Wei, L.; Xu, L.; Song, F.; Huang, J.; Zhong, N. Fabrication of immobilized lipases for efficient preparation of 1,3-dioleoyl-2-palmitoylglycerol. Food Chem. 2023, 408, 135236. [Google Scholar] [CrossRef]
- Feng, K.; Huang, Z.; Peng, B.; Dai, W.; Li, Y.; Zhu, X.; Chen, Y.; Tong, X.; Lan, Y.; Cao, Y. Immobilization of Aspergillus niger lipase onto a novel macroporous acrylic resin: Stable and recyclable biocatalysis for deacidification of high-acid soy sauce residue oil. Bioresour. Technol. 2020, 298, 122553. [Google Scholar] [CrossRef]
- Jones, P.O.; Vasudevan, P.T. Cellulose hydrolysis by immobilized Trichoderma reesei cellulase. Biotechnol. Lett. 2009, 32, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, Y.; Jiang, B.; Zhang, T.; Chen, J. Dual-enzyme co-immobilization for the one-pot production of glucose 6-phosphate from maltodextrin. Biochem. Eng. J. 2020, 161, 107654. [Google Scholar] [CrossRef]
- Wan, D.; Tian, L.; Li, X.; Li, B.; Zhang, Q. A versatile strategy for enzyme immobilization: Fabricating lipase/inorganic hybrid nanostructures on macroporous resins with enhanced catalytic properties. Biochem. Eng. J. 2018, 139, 101–108. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Luo, G. Immobilization of Penicillin G Acylase on Mesostructured Cellular Foams through a Cross-Linking Network Method. Ind. Eng. Chem. Res. 2014, 53, 1947–1953. [Google Scholar] [CrossRef]
- Yang, J.; Ma, X.; Zhang, Z.; Chen, B.; Li, S.; Wang, G. Lipase immobilized by modification-coupled and adsorption–cross-linking methods: A comparative study. Biotechnol. Adv. 2010, 28, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lee, Y.Y.; Wang, Y.; Qiu, C. Interfacial behavior, gelation and foaming properties of diacyglycerols with different acyl chain lengths and isomer ratios. Food Chem. 2023, 136696. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, S.; Lu, C.; Xu, J.; Pei, J.; Zhao, L. Immobilization of Thermostable β-Glucosidase and α-l-Rhamnosidase from Dictyoglomus thermophilum DSM3960 and Their Cooperated Biotransformation of Total Flavonoids Extract from Epimedium into Icaritin. Catal. Lett. 2021, 151, 2950–2963. [Google Scholar] [CrossRef]
- Miao, Q.; Zhang, C.; Zhou, S.; Meng, L.; Huang, L.; Ni, Y.; Chen, L. Immobilization and Characterization of Pectinase onto the Cationic Polystyrene Resin. ACS Omega 2021, 6, 31683–31688. [Google Scholar] [CrossRef]
- Sun, X.; Zou, S.; Xie, X.; Wang, Y.; Zhang, Z. Immobilization of Lecitase® Ultra for production of flaxseed oil-based diacyl-glycerols by glycerolysis and its operational stability. LWT 2023, 183, 114964. [Google Scholar] [CrossRef]
- Guo, H.; Lei, B.; Yu, J.; Chen, Y.; Qian, J. Immobilization of lipase by dialdehyde cellulose crosslinked magnetic nanoparticles. Int. J. Biol. Macromol. 2021, 185, 287–296. [Google Scholar] [CrossRef]
- Chen, N.; Chang, B.; Shi, N.; Yan, W.; Lu, F.; Liu, F. Cross-linked enzyme aggregates immobilization: Preparation, characterization, and applications. Crit. Rev. Biotechnol. 2022, 43, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Secundo, F.; Sun, J.; Mao, X. Advances in enzyme biocatalysis for the preparation of functional lipids. Biotechnol. Adv. 2022, 61, 108036. [Google Scholar] [CrossRef]
- Hueso, D.; Fontecha, J.; Gómez-Cortés, P. Comparative study of the most commonly used methods for total protein determi-nation in milk of different species and their ultrafiltration products. Front. Nutr. 2022, 9, 925565. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, J.; Ma, X.; Zhang, Z.; Liu, N.; Wang, Y. Enzymatic preparation and facile purification of medium-chain, and medium- and long-chain fatty acid diacylglycerols. LWT 2018, 92, 227–233. [Google Scholar] [CrossRef]
| CN-TL | TL-PEGDGE-LX | |||||
|---|---|---|---|---|---|---|
| sn-1,2 DAG | sn-1,3 DAG | sn-1,3 DAG/sn-1,2 DAG | sn-1,2 DAG | sn-1,3 DAG | sn-1,3 DAG/sn-1,2 DAG | |
| Olive Oil | 1.75 ± 0.12 | 8.25 ± 0.12 | 4.72 ± 0.25 a | 1.96 ± 0.12 | 8.04 ± 0.12 | 4.20 ± 0.23 b |
| Canola Oil | 2.72 ± 0.13 | 7.28 ± 0.13 | 2.69 ± 0.26 a | 2.77 ± 0.18 | 7.23 ± 0.18 | 2.62 ± 0.36 a |
| Peanut Oil | 2.34 ± 0.09 | 7.66 ± 0.09 | 3.34 ± 0.19 a | 2.33 ± 0.14 | 7.67 ± 0.14 | 3.30 ± 0.26 a |
| Soybean oil | 2.21 ± 0.15 | 7.79 ± 0.15 | 3.57 ± 0.28 a | 2.34 ± 0.23 | 7.66 ± 0.23 | 3.36 ± 0.44 a |
| Corn Oil | 2.09 ± 0.08 | 7.91 ± 0.08 | 3.78 ± 0.18 a | 2.18 ± 0.14 | 7.82 ± 0.14 | 3.60 ± 0.29 a |
| Palm Oil | 2.43 ± 0.15 | 7.57 ± 0.15 | 3.11 ± 0.21 a | 2.51 ± 0.11 | 7.49 ± 0.11 | 2.98 ± 0.16 a |
| Almond Oil | 2.67 ± 0.09 | 7.33 ± 0.09 | 2.74 ± 0.28 a | 2.69 ± 0.23 | 7.31 ± 0.23 | 2.71 ± 0.44 a |
| Sample | Olive Oil | Canola Oil | Peanut Oil | Corn Oil | Soybean Oil | Palm Oil | Almond Oil |
|---|---|---|---|---|---|---|---|
| Initial DAG | 1.98 ± 0.11 | 2.01 ± 0.05 | 1.40 ± 0.01 | 2.38 ± 0.06 | 1.57 ± 0.09 | 2.95 ± 0.05 | 1.78 ± 0.11 |
| DAG | 38.98 ± 0.35 | 32.24 ± 0.88 | 39.76 ± 0.70 | 41.65 ± 0.13 | 34.57 ± 0.89 | 36.18 ± 1.07 | 37.31 ± 0.35 |
| TAG Conversion | 56.45 ± 0.86 | 47.97 ± 0.65 | 60.87 ± 1.12 | 62.09 ± 0.97 | 52.43 ± 0.54 | 55.86 ± 0.77 | 56.01 ± 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, R.; Lee, Y.-Y.; Xie, P.; Tan, C.-P.; Wang, Y.; Zhang, Z. Immobilization of Lipase from Thermomyces Lanuginosus and Its Glycerolysis Ability in Diacylglycerol Preparation. Molecules 2024, 29, 4141. https://doi.org/10.3390/molecules29174141
Xie R, Lee Y-Y, Xie P, Tan C-P, Wang Y, Zhang Z. Immobilization of Lipase from Thermomyces Lanuginosus and Its Glycerolysis Ability in Diacylglycerol Preparation. Molecules. 2024; 29(17):4141. https://doi.org/10.3390/molecules29174141
Chicago/Turabian StyleXie, Rui, Yee-Ying Lee, Pengkai Xie, Chin-Ping Tan, Yong Wang, and Zhen Zhang. 2024. "Immobilization of Lipase from Thermomyces Lanuginosus and Its Glycerolysis Ability in Diacylglycerol Preparation" Molecules 29, no. 17: 4141. https://doi.org/10.3390/molecules29174141






