Theoretical Investigation of the Effects of Aldehyde Substitution with Pyran Groups in D-π-A Dye on Performance of DSSCs
Abstract
1. Introduction
2. Chemical Design
3. Results
4. Computational Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, H.; Zhu, X. Development of small-molecule materials for high-performance organic solar cells. Sci. China Chem. 2015, 58, 922–936. [Google Scholar] [CrossRef]
- Gao, W.; Ma, R.; Peña, T.A.D.; Yan, C.; Li, H.; Li, M.; Wu, J.; Cheng, P.; Zhong, C.; Wei, Z.; et al. Efficient all-small-molecule organic solar cells processed with non-halogen solvent. Nat. Commun. 2024, 15, 1946. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, D.; Yang, Q.; Gao, J.; Fu, J.; Yang, K.; He, H.; Chen, S.; Kan, Z.; Duan, T.; et al. All-Small-Molecule Organic Solar Cells with an Ordered Liquid Crystalline Donor. Joule 2019, 3, 3034–3047. [Google Scholar] [CrossRef]
- Lee, C.P.; Li, C.T.; Ho, K.C. Use of organic materials in dye-sensitized solar cells. Mater. Today Proc. 2017, 20, 267–283. [Google Scholar] [CrossRef]
- Obotowo, I.; Obot, I.; Ekpe, U.J. Organic sensitizers for dye-sensitized solar cell (DSSC): Properties from computation, progress and future perspectives. J. Mol. Struct. 2016, 1122, 80–87. [Google Scholar] [CrossRef]
- Sen, A.; Putra, M.H.; Biswas, A.K.; Behera, A.K.; Groβ, A. A Brief History of and Insight in the Choice of Sensitizers/Dyes for Dye Sensitized Solar Cells. Available online: https://chemrxiv.org/engage/chemrxiv/article-details/62a21e04bb751904a5487692 (accessed on 15 July 2024).
- Yahya, M.; Bouziani, A.; Ocak, C.; Seferoğlu, Z.; Sillanpää, M. Organic/metal-organic photosensitizers for dye-sensitized solar cells (DSSC): Recent developments, new trends, and future perceptions. Dye. Pigment. 2021, 192, 109227. [Google Scholar] [CrossRef]
- Tan, C.J.; Yang, C.S.; Sheng, Y.C.; Amini, H.; Tsai, H.H. Spacer Effects of Donor-π Spacer-Acceptor Sensitizers on Photophysical Properties in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 21272–21284. [Google Scholar] [CrossRef]
- Chawla, P.; Tripathi, M. Novel improvements in the sensitizers of dye-sensitized solar cells for enhancement in efficiency—A review. Int. J. Energy Res. 2015, 39, 1579–1596. [Google Scholar] [CrossRef]
- Yen, Y.S.; Indumathi, V. Effect of π-Conjugated Spacer in N-Alkylphenoxazine-Based Sensitizers Containing Double Anchors for Dye-Sensitized Solar Cells. Polymers 2021, 13, 1304. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Z.H.; Bai, F.Q.; Zhang, H.X. Performance Regulation of Thieno [3, 2-b] benzothiophene π-Spacer-Based D-π-A Organic Dyes for Dye-Sensitized Solar Cell Applications: Insights from Computational Study. Front Chem. 2019, 6, 676. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Song, P.; Ma, F.; Kungwan, N.; Sun, M. Physical Insight on Mechanism of Photoinduced Charge Transfer in Multipolar Photoactive Molecules. Sci. Rep. 2018, 8, 10089. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Testoff, T.T.; Wang, L.; Zhou, X. Cause, Regulation and Utilization of Dye Aggregation in Dye-Sensitized Solar Cells. Molecules 2020, 25, 4478. [Google Scholar] [CrossRef]
- Farokhi, A.; Shahroosvand, H.; Zisti, F.; Pilkington, M.; Nazeeruddin, M.K. Influence of triphenylamine derivatives in efficient dye-sensitized/organic solar cells. J. Mater. Chem. A 2023, 11, 25136–25215. [Google Scholar] [CrossRef]
- Kalipriyadharshini, M.; Raman, A.; Ajantha, J.; Gowthaman, J.B.; Easwaramoorthi, S. Triarylamines—A Versatile Molecular Building Block for Optoelectronic Materials. In Handbook of Materials Science, Volume 1: Optical Materials; Ningthoujam, R.S., Tyagi, A.K., Eds.; Springer Nature: Singapore, 2024; pp. 183–214. [Google Scholar] [CrossRef]
- Bibi, S.; Imtiaz, A.; Muhammad, S.; Urrehman, S.; Chaudhry, A.R.; Al-Sehemi, A.G.; Alqurashy, B.A.; Gilani, M.A. Tailoring the donor moieties in TPA -based organic dyes for efficient photovoltaic, optical and nonlinear optical response properties. Int. J. Quantum Chem. 2024, 124, e27362. [Google Scholar] [CrossRef]
- Lee, S.W.; Hussain, W.; Shome, S.; Ha, S.R.; Oh, J.T.; Whang, D.R.; Kim, Y.; Kim, D.-Y.; Choi, H.; Chang, D.W. Effect of electron-withdrawing fluorine and cyano substituents on photovoltaic properties of two-dimensional quinoxaline-based polymers. Sci. Rep. 2021, 11, 24381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhao, J.; Chow, P.C.Y.; Jiang, K.; Zhang, J.; Zhu, Z.; Zhang, J.; Huang, F.; Yan, H. Nonfullerene Acceptor Molecules for Bulk Heterojunction Organic Solar Cells. Chem. Rev. 2018, 118, 3447–3507. [Google Scholar] [CrossRef]
- Arunkumar, A.; Shanavas, S.; Acevedo, R.; Anbarasan, P.M. Acceptor tuning effect on TPA-based organic efficient sensitizers for optoelectronic applications—Quantum chemical investigation. Struct. Chem. 2020, 31, 1029–1042. [Google Scholar] [CrossRef]
- Thavasi, V.; Renugopalakrishnan, V.; Jose, R.; Ramakrishna, S. Controlled electron injection and transport at materials interfaces in dye sensitized solar cells. Mater. Sci. Eng. R Rep. 2009, 63, 81–99. [Google Scholar] [CrossRef]
- Segal-Peretz, T.; Jahnke, J.P.; Berenson, A.; Neeman, L.; Oron, D.; Rossini, A.J.; Chmelka, B.F.; Frey, G.L. Understanding and Promoting Molecular Interactions and Charge Transfer in Dye-Mediated Hybrid Photovoltaic Materials. J. Phys. Chem. C 2014, 118, 25374–25391. [Google Scholar] [CrossRef]
- Roy, A.; Mallick, T.K.; Tahir, A.A.; Gondal, M.A.; Sundaram, S. Chapter Seven—Advanced fabrication strategies to enhance the performance of dye-sensitized solar cells. In Photovoltaics Beyond Silicon; Sundaram, S., Subramaniam, V., Raffaelle, R.P., Nazeeruddin, M.K., Morales-Acevedo, A., Navarro, M.B., Hepp, A.F., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 223–254. [Google Scholar] [CrossRef]
- Arbouch, I.; Cornil, D.; Karzazi, Y.; Hammouti, B.; Lazzaroni, R.; Cornil, J. Influence of the Nature of the Anchoring Group on Electron Injection Processes at Dye-Titania Interfaces. Phys. Chem. Chem. Phys. 2017, 19, 29389–29401. [Google Scholar] [CrossRef]
- Sharma, S.J.; Prasad, J.; Soni, S.S.; Sekar, N. The impact of anchoring groups on the efficiency of dye-sensitized solar cells: 2-Cyanoacrylic acid vs. ethyl 2-cyanoacrylate. J. Photochem. Photobiol. A Chem. 2023, 444, 114915. [Google Scholar] [CrossRef]
- Liu, S.; Jiao, Y.; Ding, Y.; Fan, X.; Song, J.; Mi, B.; Gao, Z. Position engineering of cyanoacrylic-acid anchoring group in a dye for DSSC applications. Dye. Pigment. 2020, 180, 108470. [Google Scholar] [CrossRef]
- Rahman, S.; Haleem, A.; Siddiq, M.; Hussain, M.K.; Qamar, S.; Hameed, S.; Waris, M. Research on dye sensitized solar cells: Recent advancement toward the various constituents of dye sensitized solar cells for efficiency enhancement and future prospects. RSC Adv. 2023, 13, 19508–19529. [Google Scholar] [CrossRef]
- Nilsing, M.; Persson, P.; Lunell, S.; Ojamäe, L. Dye-Sensitization of the TiO2 Rutile (110) Surface by Perylene Dyes: Quantum- Chemical Periodic B3LYP Computations. J. Phys. Chem. C 2007, 111, 12116–12123. [Google Scholar] [CrossRef]
- Chiu, C.C.; Sheng, Y.C.; Lin, W.J.; Juwita, R.; Tan, C.J.; Tsai, H.H.G. Effects of Internal Electron-Withdrawing Moieties in D–A−π–A Organic Sensitizers on Photophysical Properties for DSSCs: A Computational Study. ACS Omega. 2018, 3, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.; Kumbhani, J.; DBhatt, K. Pyran Heterocyclic Compound as the Prosperous Scaffolds for Biological Sensor (A-Review). Orient. J. Chem. 2021, 37, 1280–1286. [Google Scholar] [CrossRef]
- Wolinski, K.; Brzyska, A. Theoretical studies of the pyranose ring under mechanical stress. Carbohydr. Res. 2018, 470, 64–72. [Google Scholar] [CrossRef]
- Almalki, F.A. An overview of structure-based activity outcomes of pyran derivatives against Alzheimer’s disease. Saudi Pharm. J. 2023, 31, 998–1018. [Google Scholar] [CrossRef]
- Fairlamb, I.; Marrison, L.; Dickinson, J.; Lu, F.J.; Schmidt, J. 2-Pyrones possessing antimicrobial and cytotoxic activities. Bioorganic Med. Chem. 2004, 12, 4285–4299. [Google Scholar] [CrossRef]
- Grover, P.; Bhardwaj, M.; Mehta, L.; Kapoor, G.; Chawla, P. Current Developments in the Pyran-Based Analogues as Anticancer Agents. Anti-Cancer Agents Med. Chem. 2021, 22, 3239–3268. [Google Scholar] [CrossRef]
- Navarro-Tovar, G.; Vega-Rodríguez, S.; Leyva, E.; Loredo-Carrillo, S.; De Loera, D.; López-López, L.I. The Relevance and Insights on 1,4-Naphthoquinones as Antimicrobial and Antitumoral Molecules: A Systematic Review. Pharmaceuticals. 2023, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Darling, S.B.; You, Y. Density functional theory as a guide for the design of pyran dyes for dye-sensitized solar cells. Monatsh. Chem. 2011, 142, 45–52. [Google Scholar] [CrossRef]
- Maglione, C.; Carella, A.; Carbonara, C.; Centore, R.; Fusco, S.; Velardo, A.; Peluso, A.; Colonna, D.; Lanuti, A.; Di Carlo, A. Novel pyran based dyes for application in dye sensitized solar cells. Dye. Pigments. 2016, 133, 395–405. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, W.; Tian, H. Dicyanomethylene-4H-pyran chromophores for OLED emitters, logic gates and optical chemosensors. Chem. Commun. 2012, 48, 6073–6084. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Elroby, S.A.; Asiri, A.M.; Hilal, R.H. Pyran-Squaraine as Photosensitizers for Dye-Sensitized Solar Cells: DFT/TDDFT Study of the Electronic Structures and Absorption Properties. Int. J. Photoenergy 2014, 2014, 136893. [Google Scholar] [CrossRef]
- Tejada, R.P.; Pellejà, L.; Palomares, E.; Franco, S.; Orduna, J.; Garín, J.; Andreu, R. Novel 4H-pyranylidene organic dyes for dye-sensitized solar cells: Effect of different heteroaromatic rings on the photovoltaic properties. Org. Electron. 2014, 15, 3237–3250. [Google Scholar] [CrossRef]
- Raftani, M.; Abram, T.; Azaid, A.; Kacimi, R.; Bennani, M.N.; Bouachrine, M. New Organic Dyes with Low Bandgap Based on Heterocyclic Compounds for Dye-sensitized Solar Cells Applications. Biointerface Res. Appl. Chem. 2022, 13, 54. [Google Scholar] [CrossRef]
- Napiórkowska, E.; Milcarz, K.; Szeleszczuk, Ł. Review of Applications of Density Functional Theory (DFT) Quantum Mechanical Calculations to Study the High-Pressure Polymorphs of Organic Crystalline Materials. Int. J. Mol. Sci. 2023, 24, 14155. [Google Scholar] [CrossRef]
- Bursch, M.; Mewes, J.M.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202205735. [Google Scholar] [CrossRef]
- Abu Alrub, S.; Ali, A.I.; Hussein, R.K.; Alghamdi, S.K.; Eladly, S.A. DFT and TD-DFT Investigations for the Limitations of Lengthening the Polyene Bridge between N, N-dimethylanilino Donor and Dicyanovinyl Acceptor Molecules as a D-π-A Dye-Sensitized Solar Cell. Int. J. Mol. Sci. 2024, 25, 5586. [Google Scholar] [CrossRef]
- Tommalieh, M.J.; Aljameel, A.I.; Hussein, R.K.; Al-heuseen, K.; Alghamdi, S.K.; Alrub, S.A. The Effect of Conjugated Nitrile Structures as Acceptor Moieties on the Photovoltaic Properties of Dye-Sensitized Solar Cells: DFT and TD-DFT Investigation. Int. J. Mol. Sci. 2024, 25, 7138. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, A.; Ajibade, P. Towards the Development of Functionalized PolypyridineLigands for Ru (II) Complexes as Photosensitizers inDye-Sensitized Solar Cells (DSSCs). Molecules 2014, 19, 12421–12460. [Google Scholar] [CrossRef] [PubMed]
- Diez-Cabanes, V.; Fantacci, S.; Pastore, M. First-principles modeling of dye-sensitized solar cells: From the optical properties of standalone dyes to the charge separation at dye/TiO2 interfaces. In Theoretical and Computational Photochemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 215–245. [Google Scholar] [CrossRef]
- Gudim, N.S.; Knyazeva, E.A.; Rakitin, O.A. 5-(9-(p-Tolyl)-2,3,4,4a,9,9a-hexahydro-1H-1,4-methanocarbazol-6-yl) thiophene-2-carbaldehyde. Molbank 2024, 2024, M1792. [Google Scholar] [CrossRef]
- Martinez, A. Astaxanthin interacting with metal clusters: Free radical scavenger and photovoltaic materials. Theor. Chem. Acc. 2016, 135, 1–15. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res Lett. 2018, 13, 381. [Google Scholar] [CrossRef]
- Oliva, R.; Luque, F.J.; Orozco, M. Reliability of MEP and MEP-derived properties computed from DFT methods for molecules containing P, S and CL. Theor. Chem. Acc. 1997, 98, 42–49. [Google Scholar] [CrossRef]
- Ebenso, E.E.; Isabirye, D.E.; Eddy, N.O. Adsorption and Quantum Chemical Studies on the Inhibition Potentials of Some Thiosemicarbazides for the Corrosion of Mild Steel in Acidic Medium. Int. J. Mol. Sci. 2010, 11, 2473–2498. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mi, L.; Wang, H.; Li, Y.; Liang, J. Design, Electron Transfer Process, and Opto-Electronic Property of Solar Cell Using Triphenylamine-Based D-π-A Architectures. Materials 2019, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Q.; Liu, D.; Su, R.; Liu, J.; Li, Y. Computational Prediction of Electronic and Photovoltaic Properties of Anthracene-Based Organic Dyes for Dye-Sensitized Solar Cells. Int. J. Photoenergy 2018, 2018, 4764830. [Google Scholar] [CrossRef]
- Biswas, A.K.; Das, A.; Ganguly, B. Can fused-pyrrole rings act as better π-spacer units than fused-thiophene in dye-sensitized solar cells? A computational study. New J. Chem. 2016, 40, 9304–9312. [Google Scholar] [CrossRef]
- Katoh, R.; Furube, A. Electron injection efficiency in dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 1–16. [Google Scholar] [CrossRef]
- Ren, S.; Habibi, A.; Ni, P.; Zhang, Y.; Yassar, A. Tuning the Photophysical Properties of Acceptor–Donor–Acceptor Di-2-(2-oxindolin-3-ylidene) Malononitrile Materials via Extended π–Conjugation: A Joint Experimental and Theoretical Study. Materials 2023, 16, 6410. [Google Scholar] [CrossRef]
- Koops, S.; O’Regan, B.; Barnes, P.; Durrant, J. Parameters Influencing the Efficiency of Electron Injection in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2009, 131, 4808–4818. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhang, W.; Wang, Z.; Yassar, A.; Chen, J.; Zeng, M.; Yi, Z. Preparation of Dye Semiconductors via Coupling Polymerization Catalyzed by Two Catalysts and Application to Transistor. Molecules 2023, 29, 71. [Google Scholar] [CrossRef] [PubMed]
- Daeneke, T.; Mozer, A.J.; Uemura, Y.; Makuta, S.; Fekete, M.; Tachibana, Y.; Koumura, N.; Bach, U.; Spiccia, L. Dye Regeneration Kinetics in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2012, 134, 16925–16928. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, Y.; Zhou, J.; Li, N.; Ren, S.; Zeng, M. Preparation of Novel Organic Polymer Semiconductor and Its Properties in Transistors through Collaborative Theoretical and Experimental Approaches. Polymers 2023, 15, 4421. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, version A.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- O’boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Dassault Systemes Biovia Corp. BIOVIA Materials Studio 2017; Dassault Systemes Biovia Corp.: San Diego, CA, USA, 2017. [Google Scholar]


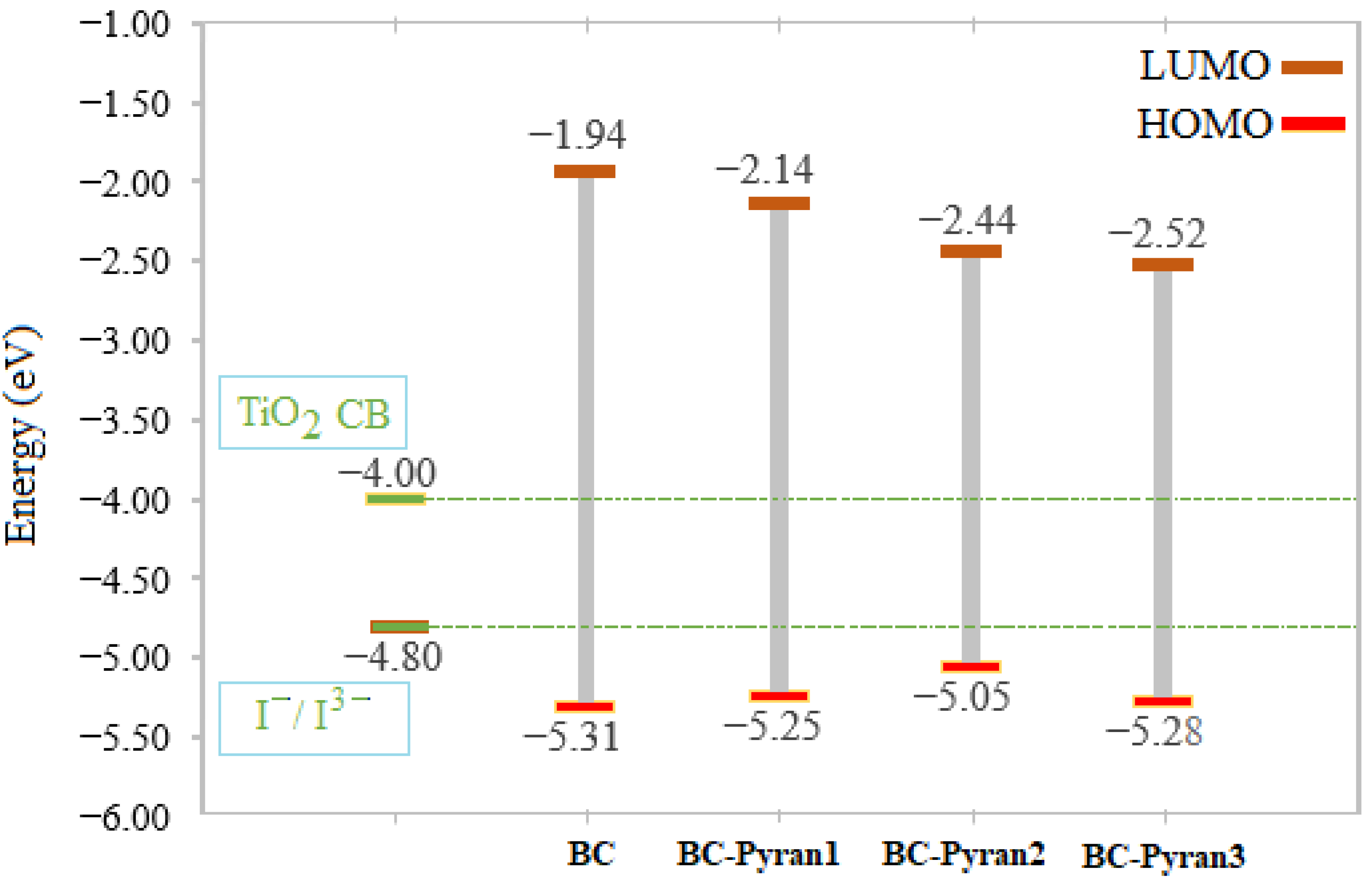
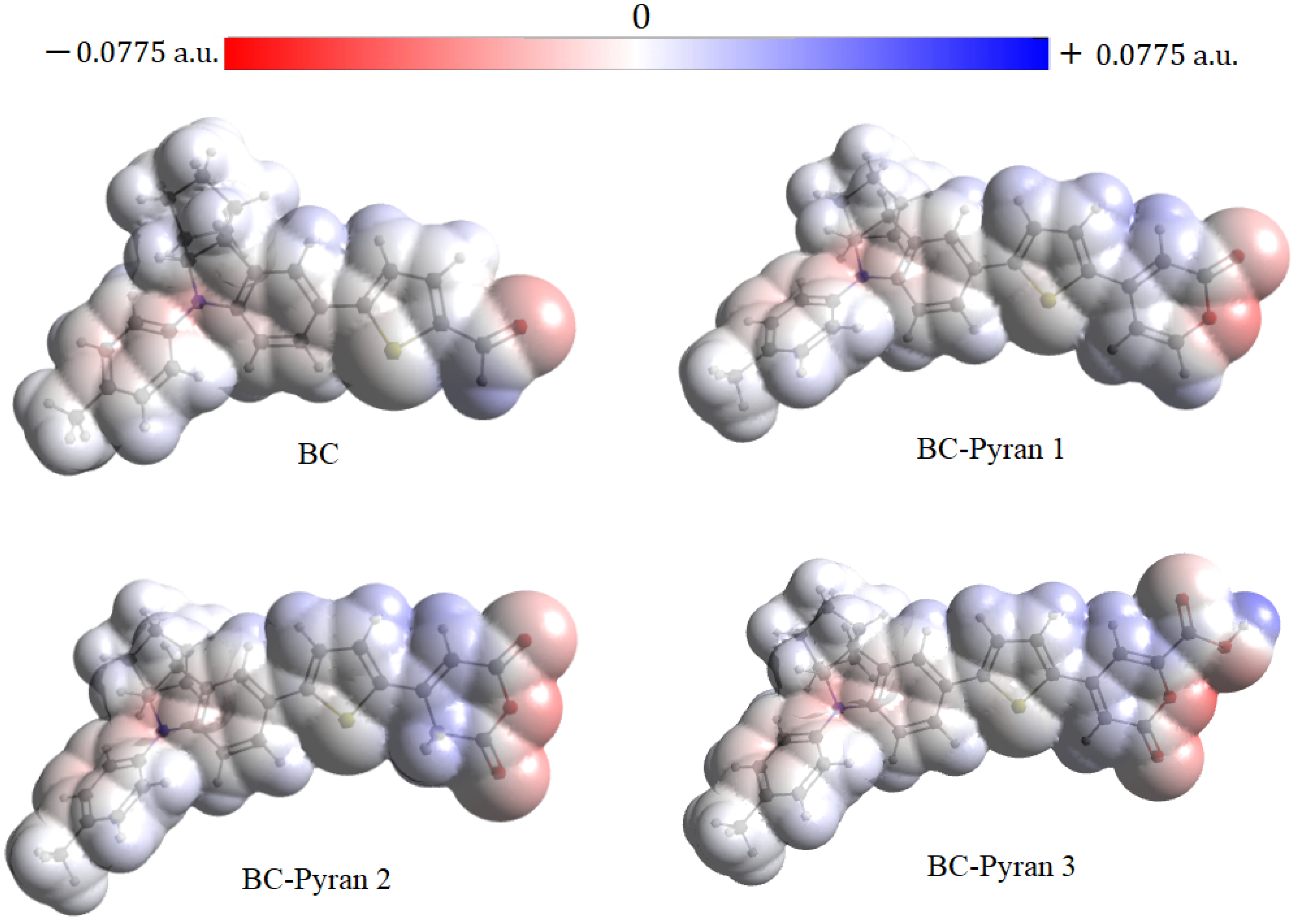
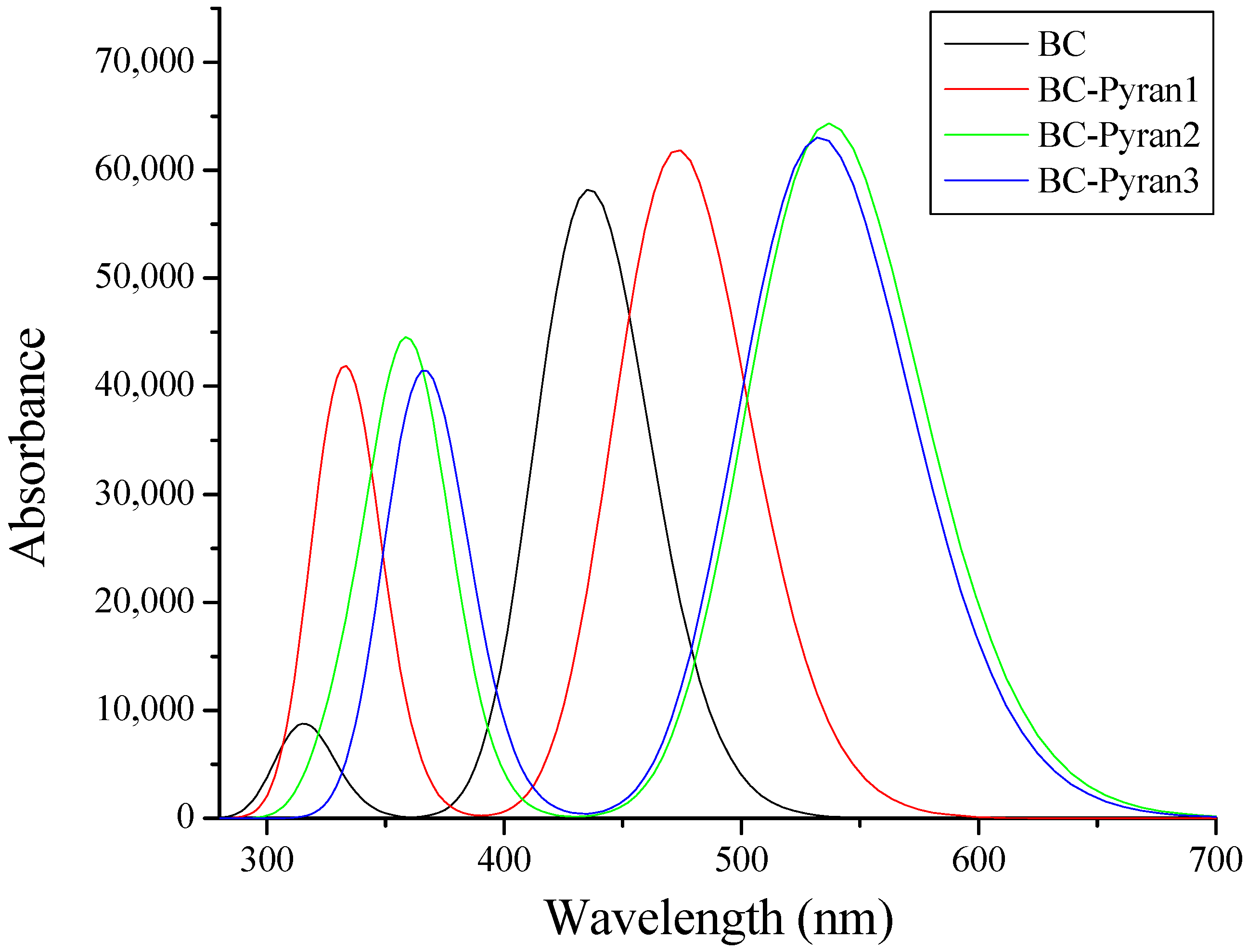

| Compound | EHOMO (eV) | ELUMO (eV) | (eV) | IP (eV) | EA (eV) |
|---|---|---|---|---|---|
| BC | −5.31 | −1.94 | 3.37 | 5.31 | 1.94 |
| BC-Pyran1 | −5.25 | −2.14 | 3.11 | 5.25 | 2.14 |
| BC-Pyran2 | −5.05 | −2.44 | 2.61 | 5.05 | 2.44 |
| BC-Pyran3 | −5.28 | −2.52 | 2.76 | 5.28 | 2.52 |
| BC + cyanoacrylic group | −5.34 | −2.61 | 2.73 | 5.34 | 2.61 |
| Compound | (eV) | Wavelength (nm) | Oscillator Strength (ƒ) | Transition | Major Contribution |
|---|---|---|---|---|---|
| BC | 2.85 | 435.49 | 0.81 | HOMO→LUMO | 99% |
| BC-Pyran1 | 2.63 | 472.82 | 0.85 | HOMO→LUMO | 99% |
| BC-Pyran2 | 2.32 | 536.66 | 0.89 | HOMO→LUMO | 99% |
| BC-Pyran3 | 2.33 | 532.696 | 0.87 | HOMO→LUMO | 99% |
| Compound | (eV) | (eV) | (eV) | (eV) | (eV) | LHE | (eV) | τ (ns) |
|---|---|---|---|---|---|---|---|---|
| BC | 2.85 | 5.31 | 2.46 | −1.54 | 0.51 | 0.85 | 2.06 | 0.23 |
| BC-Pyran1 | 2.63 | 5.25 | 2.63 | −1.38 | 0.45 | 0.86 | 1.86 | 0.25 |
| BC-Pyran2 | 2.32 | 5.05 | 2.74 | −1.27 | 0.25 | 0.87 | 1.56 | 0.31 |
| BC-Pyran3 | 2.33 | 5.28 | 2.95 | −1.05 | 0.48 | 0.87 | 1.48 | 0.32 |
| Best candidate | BC-Pyran2 | - | - | BC | BC | BC-Pyran2 and BC-Pyran3 | BC | BC-Pyran2 |
| Total Energy (Kcal/mol) | Adsorption Energy (Kcal/mol) | Rigid Adsorption Energy (Kcal/mol) | Deformation Energy (Kcal/mol) | Dye: | DCM: | |
|---|---|---|---|---|---|---|
| BC | −2063.38 | −4173.05 | −2135.33 | −2037.72 | −2100.65 | −8.63 |
| BC-Pyran1 | −2068.47 | −4363.10 | −2138.70 | −2224. 40 | −2289.77 | −10.18 |
| BC-Pyran2 | −2063.26 | −6399.78 | −2132.81 | −4266.97 | −4325.64 | −8.92 |
| BC-Pyran3 | −2076.47 | −6365.01 | −2161.74 | −4203.27 | −4258.79 | −9.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, S.K.; Aljameel, A.I.; Hussein, R.K.; Al-heuseen, K.; Aljaafreh, M.J.; Ezzat, D. Theoretical Investigation of the Effects of Aldehyde Substitution with Pyran Groups in D-π-A Dye on Performance of DSSCs. Molecules 2024, 29, 4175. https://doi.org/10.3390/molecules29174175
Alghamdi SK, Aljameel AI, Hussein RK, Al-heuseen K, Aljaafreh MJ, Ezzat D. Theoretical Investigation of the Effects of Aldehyde Substitution with Pyran Groups in D-π-A Dye on Performance of DSSCs. Molecules. 2024; 29(17):4175. https://doi.org/10.3390/molecules29174175
Chicago/Turabian StyleAlghamdi, Suzan K., Abdulaziz I. Aljameel, Rageh K. Hussein, Khalled Al-heuseen, Mamduh J. Aljaafreh, and Dina Ezzat. 2024. "Theoretical Investigation of the Effects of Aldehyde Substitution with Pyran Groups in D-π-A Dye on Performance of DSSCs" Molecules 29, no. 17: 4175. https://doi.org/10.3390/molecules29174175
APA StyleAlghamdi, S. K., Aljameel, A. I., Hussein, R. K., Al-heuseen, K., Aljaafreh, M. J., & Ezzat, D. (2024). Theoretical Investigation of the Effects of Aldehyde Substitution with Pyran Groups in D-π-A Dye on Performance of DSSCs. Molecules, 29(17), 4175. https://doi.org/10.3390/molecules29174175






