Antibacterial and Anticorrosive Hydrogel Coating Based on Complementary Functions of Sodium Alginate and g-C3N4
Abstract
:1. Introduction
2. Results
2.1. Form and Structure
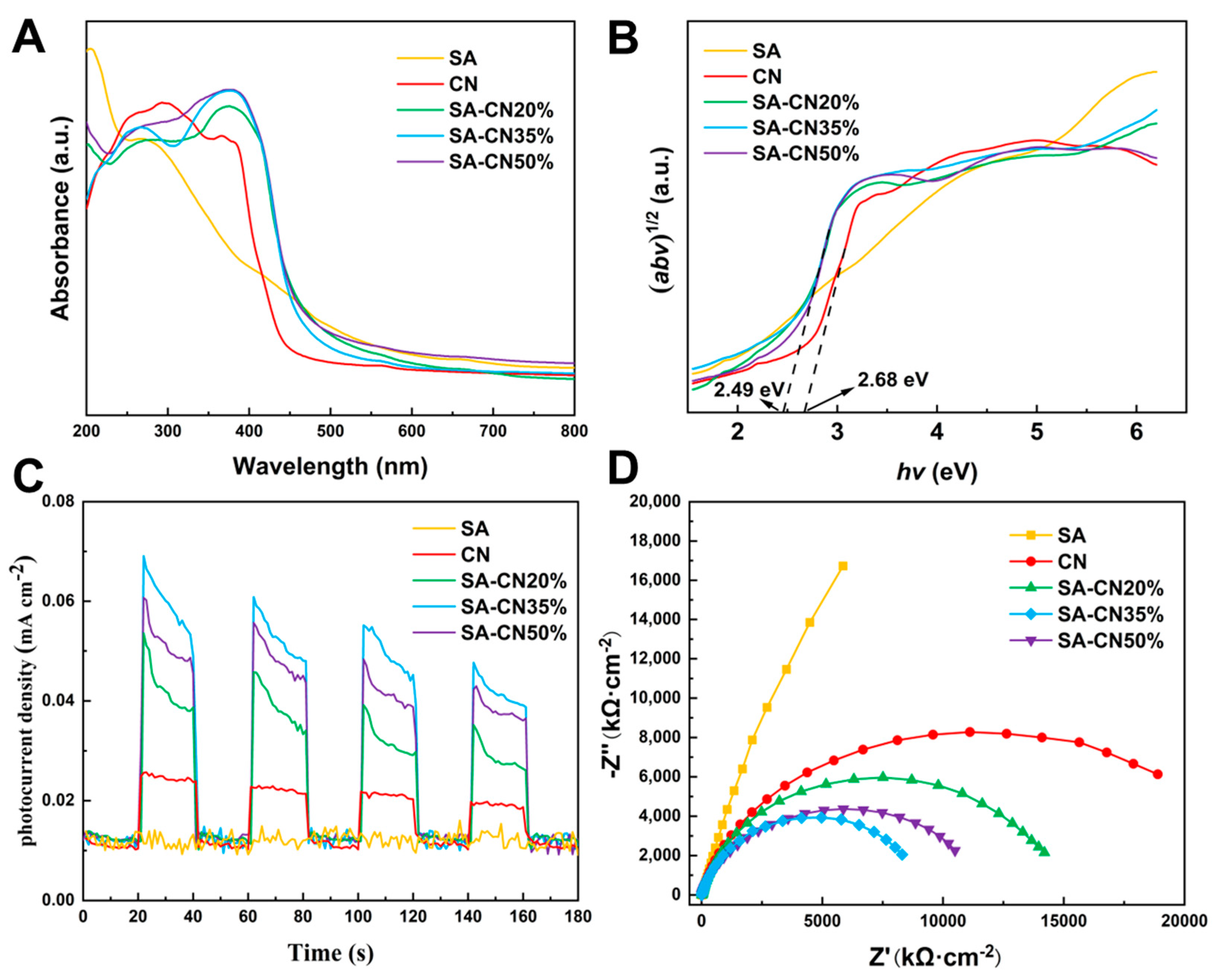
2.2. Light Absorption and Optoelectronic Properties
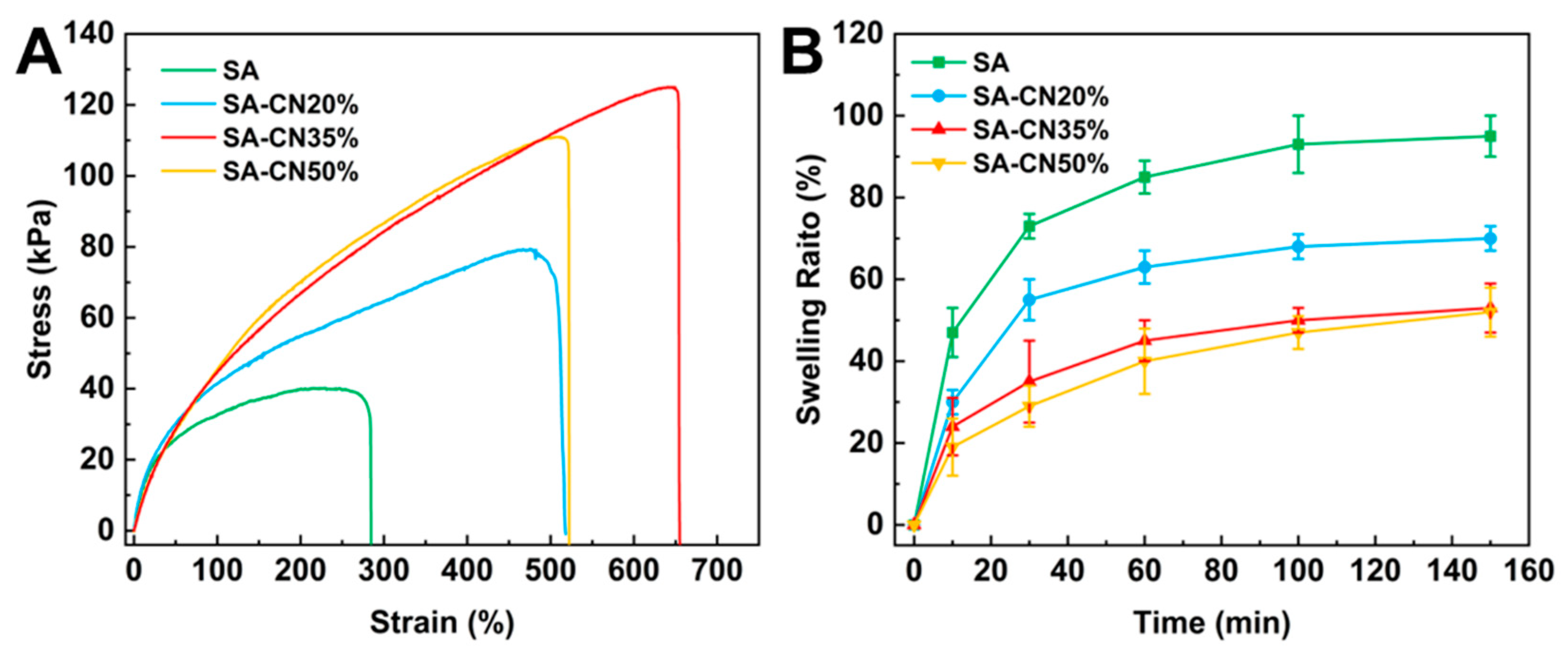
2.3. Mechanical and Swelling Properties
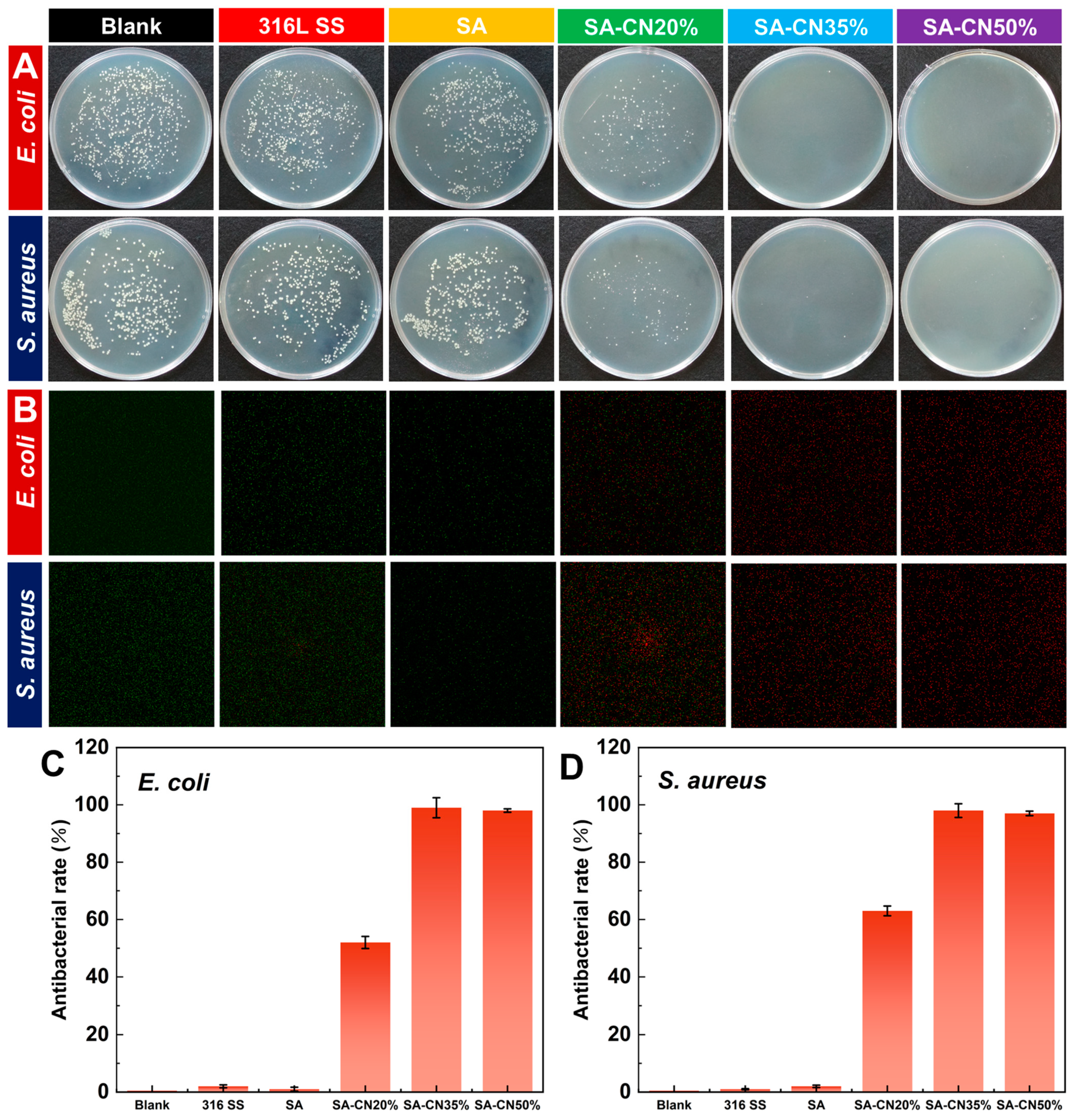
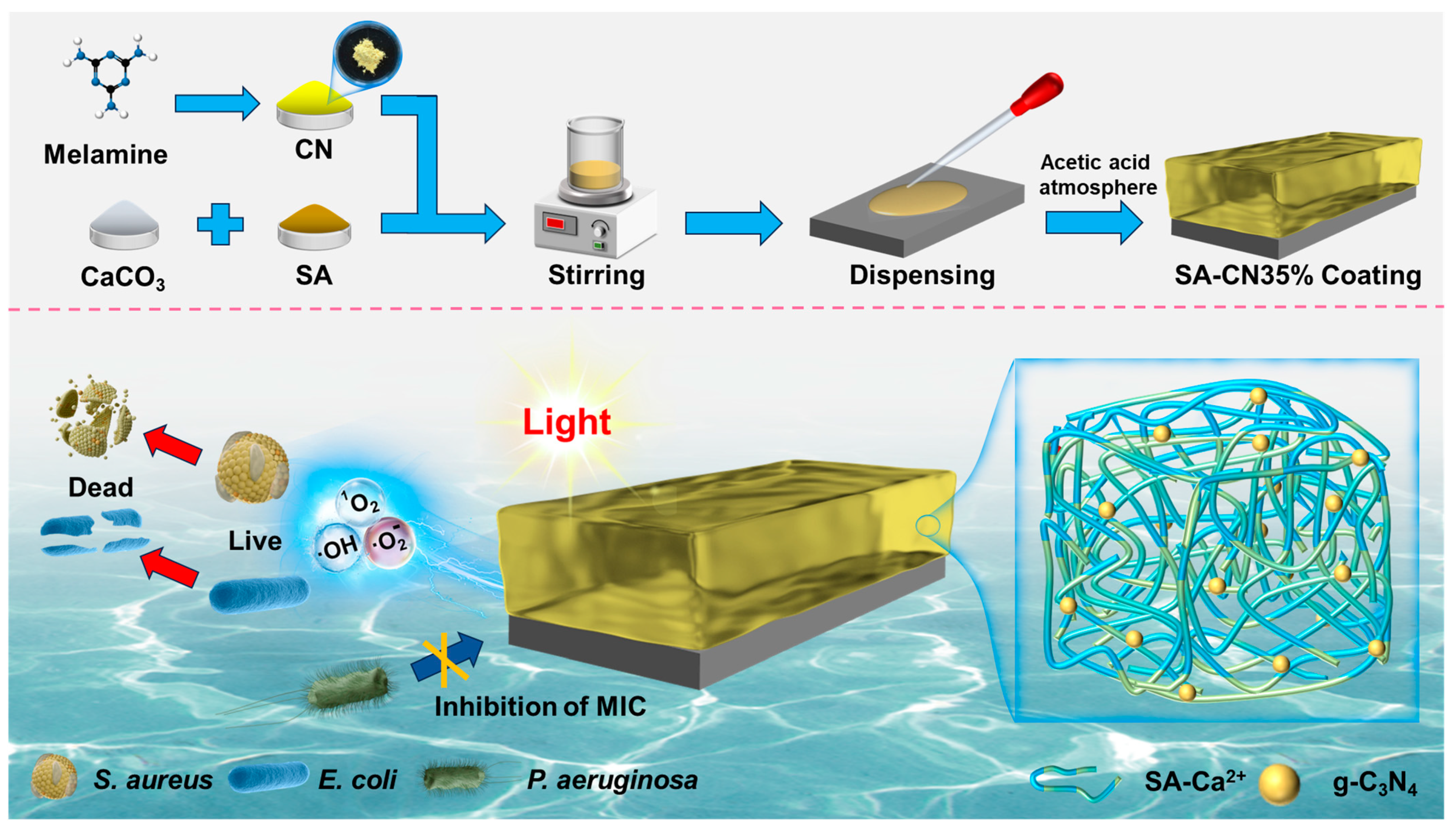
2.4. Photocatalytic Antibacterial Performance
2.5. Photocatalytic Antibacterial Mechanism
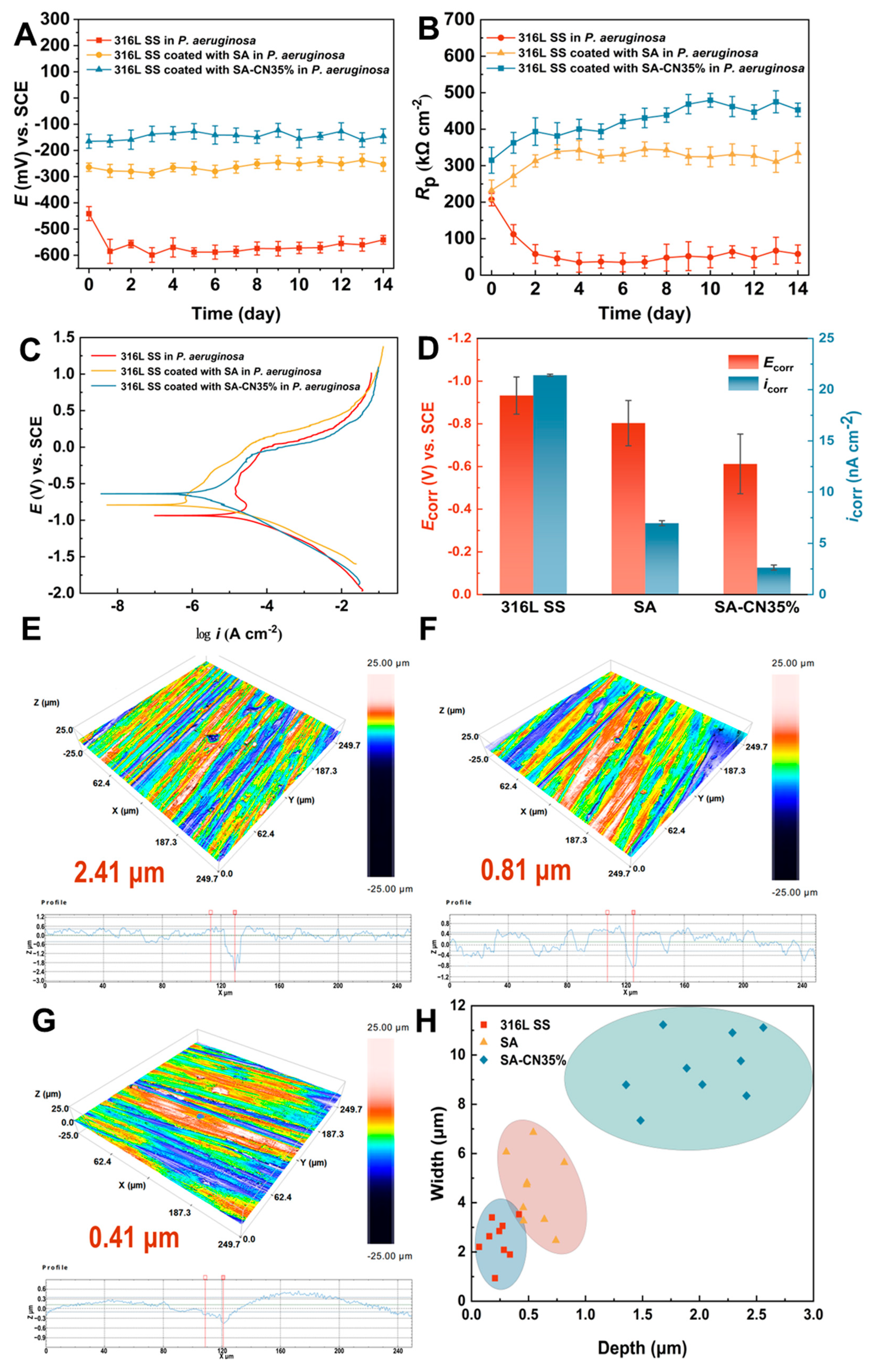
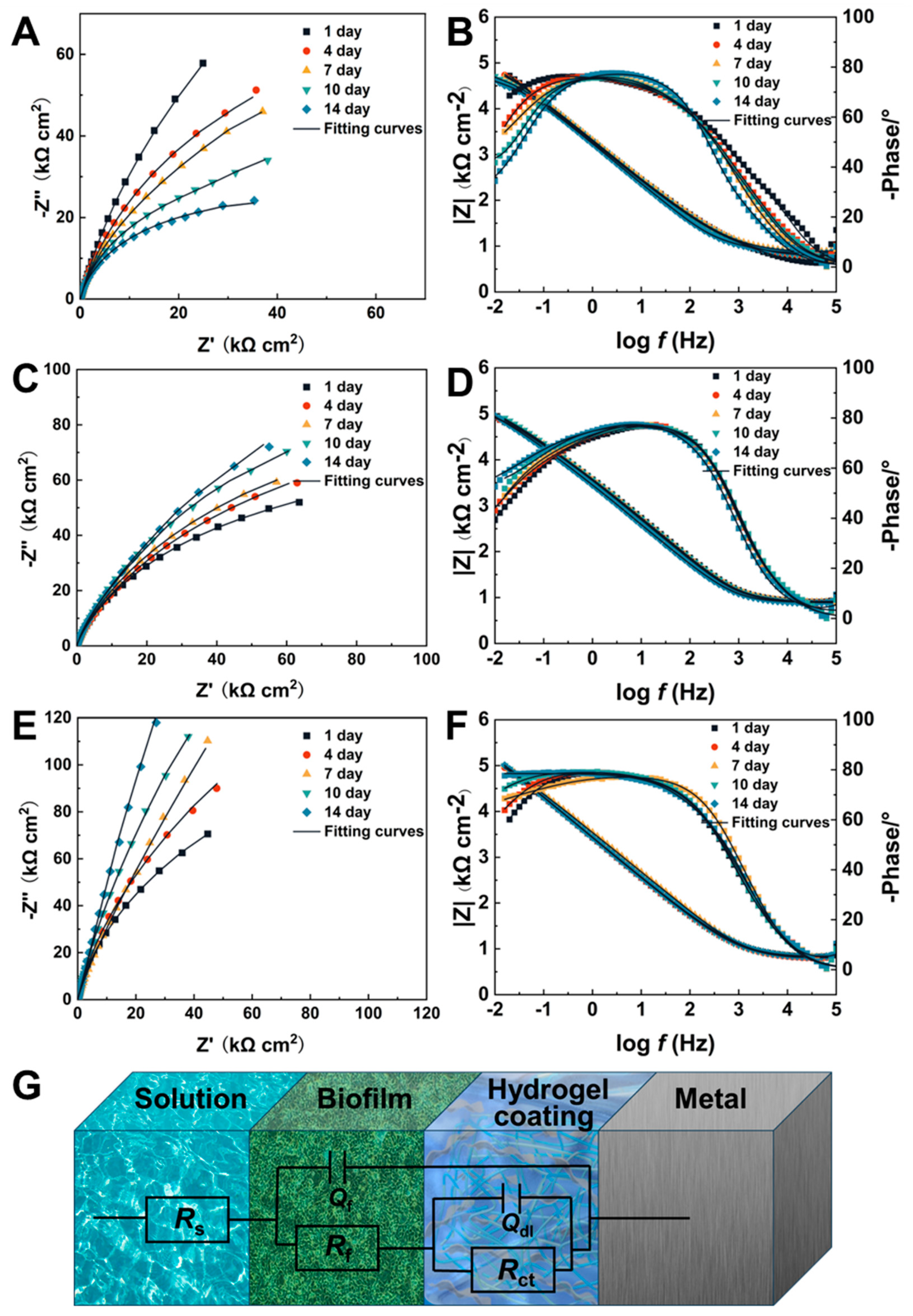
2.6. Corrosion Resistance
3. Materials and Methods
3.1. Materials and Microorganisms
3.2. Preparation of SA-CN Coating
3.2.1. Preparation of CN
3.2.2. Preparation of SA-CN Composite Coating
3.3. Characterization
3.4. Mechanical Performance Testing
3.5. Antibacterial Performance Testing
3.6. Photocatalytic Performance Testing
3.7. Optoelectronic Performance Testing
3.8. Corrosion Resistance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jing, J.; Guo, J.; Li, B.; Jia, S.; Ren, Y. Relationship between Microstructure and Corrosion Behavior of High-Grade Pipeline Steel in a Low-Temperature Environment. J. Iron Steel Res. Int. 2021, 28, 1037–1046. [Google Scholar] [CrossRef]
- Wang, J.; Du, M.; Shan, X. Effect of Marine Streptomyces on Corrosion Behavior of X65 Steel in Simulated Offshore Oilfield Produced Water System. J Mater Res Technol 2023, 24, 7925–7937. [Google Scholar] [CrossRef]
- Bhandari, J.; Khan, F.; Abbassi, R.; Garaniya, V.; Ojeda, R. Modelling of Pitting Corrosion in Marine and Offshore Steel Structures—A Technical Review. J. Loss Prev. Process. Ind. 2015, 37, 39–62. [Google Scholar] [CrossRef]
- Feng, J.; Feng, Q.; Xin, J.; Liang, Q.; Li, X.; Chen, K.; Teng, J.; Wang, S.; Feng, L.; Liu, J. Fabrication of Durable Self-Cleaning Photocatalytic Coating with Long-Term Effective Natural Light Photocatalytic Degradation Performance. Chemosphere 2023, 336, 139316. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Peng, H.; Yang, M.; Hu, K.; Lin, Y.; Zhu, Y.; Chen, H.; Zhao, J.; Han, S.; Wang, Y.; et al. Dual-Functional Acridine-Based Coatings with Anti-Bacterial Adhesion and Durable Photocatalytic Antibacterial. Prog. Org. Coat. 2024, 194, 108593. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Chaharmahali, R.; Alizad, S.; Babaei, K.; Stojadinović, S. A Review on the Revealed Improved Photocatalytic Activity of PEO Coatings Applied on Al Alloys. Nano-Struct. Nano-Objects 2024, 39, 101233. [Google Scholar] [CrossRef]
- Cao, Q.; Kumru, B.; Antonietti, M.; Schmidt, B.V.K.J. Graphitic Carbon Nitride and Polymers: A Mutual Combination for Advanced Properties. Mater. Horiz. 2020, 7, 762–786. [Google Scholar] [CrossRef]
- Yuan, S.; Dai, L.; Xie, M.; Liu, J.; Peng, H. Modification Optimization and Application of Graphitic Carbon Nitride in Photocatalysis: Current Progress and Future Prospects. Chem. Eng. Sci. 2024, 296, 120245. [Google Scholar] [CrossRef]
- Hao, J.; Yan, S.; Yuan, H.; Du, C.; Tan, Y. High-Strength Alginate Fibers Wet-Spun from Pre-Crosslinked Sodium Alginate Solutions. Carbohydr. Polym. 2024, 342, 122386. [Google Scholar] [CrossRef]
- Xie, F. Alginate-Based Nanocomposites for Food Preservation: Recent Progress Showcasing Heightened Material Properties and Functionalities. Adv. Nanocompos. 2024, 1, 248–274. [Google Scholar] [CrossRef]
- Mandal, S.; Sarkar, A.; Mukherjee, P.; Das, S.; Banerjee, D.; Ganguly, S.; Kargupta, K. Organic Alginate Encapsulated rGO-CdS Millispheres for Remarkable Photocatalytic Solar Hydrogen Production. Int. J. Hydrogen Energy 2024, 51, 1167–1185. [Google Scholar] [CrossRef]
- Khan, S.B.; Ahmad, S.; Kamal, T.; Asiri, A.M.; Bakhsh, E.M. Metal Nanoparticles Decorated Sodium Alginate-carbon Nitride Composite Beads as Effective Catalyst for the Reduction of Organic Pollutants. Int. J. Biol. Macromol. 2020, 164, 1087–1098. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, Z.; Lin, J.; Xu, J.; Wang, X.; Hong, B.; Xia, Y.; Zeng, Y. Surface Atom Rearrangement Enabling Graphitic Carbon Nitride/Sodium Alginate Gel Monolith for Ultrafast Completely Photodegrading Ciprofloxacin under Visible Light. Chem. Eng. J. 2024, 489, 151218. [Google Scholar] [CrossRef]
- Kong, D.; Yang, B.; Yuan, H.; Du, C.; Tan, Y. 3D Printing of Glycerol-Mediated Alginate Hydrogels with High Strength and Stiffness. J. Mater. Sci. Technol. 2024, 213, 268–275. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Tan, Y.; Tong, Q.; Liu, D.; Yi, Z.; Li, X. Soft yet Mechanically Robust Injectable Alginate Hydrogels with Processing Versatility Based on Alginate/Hydroxyapatite Hybridization. Int. J. Biol. Macromol. 2024, 270, 132458. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Zhu, J.; Tian, M.; Zheng, S.; Wang, F.; Wang, X.; Wang, L. Ion Sieving by a Two-Dimensional Ti3C2Tx Alginate Lamellar Membrane with Stable Interlayer Spacing. Nat. Commun. 2020, 11, 3540. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.D.; Aminabhavi, D.T.M. Pervaporation Separation Using Sodium Alginate and Its Modified Membranes—A Review. Sep. Purif. Rev. 2007, 36, 203–229. [Google Scholar] [CrossRef]
- Ye, C.; Gong, Q.-M.; Lu, F.-P.; Liang, J. Preparation of Carbon Nanotubes/Phenolic-Resin-Derived Activated Carbon Spheres for the Removal of Middle Molecular Weight Toxins. Sep. Purif. Technol. 2008, 61, 9–14. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Lin, Z.; Zhao, B.; Wang, J.; Xie, J.; Zhang, A. Preparation of a Novel Bio-Adsorbent of Sodium Alginate Grafted Polyacrylamide/Graphene Oxide Hydrogel for the Adsorption of Heavy Metal Ion. Sci. Total Environ. 2020, 744, 140653. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, T.; Wang, X.-Y.; Xue, B. Photo-Crosslinked Composite Hydrogels with Silver-Deposited Polymeric Carbon Nitride for Boosting Antibacterial Activity. Colloid. Surface A 2024, 680, 132668. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, G.; Yang, C.; Li, S. Recent Progress on S-Scheme Heterojunction Strategy Enabling Polymer Carbon Nitrides CN and CN Enhanced Photocatalysis in Energy Conversion and Environmental Remediation3435. Curr. Opin. Chem. Eng. 2024, 45, 101040. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, J.; Cao, S.; Cui, C.; Cheng, B. Efficient Photocatalytic Reduction of CO2 by Amine-Functionalized g-C3N4. Appl. Surf. Sci. 2015, 358, 350–355. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.; Ouyang, S.; Lu, D.; Wang, X.; Wang, D.; Ye, J. In Situ Surface Alkalinized G-C3N4 toward Enhancement of Photocatalytic H2 Evolution under Visible-Light Irradiation. J. Mater. Chem. A 2016, 4, 2943–2950. [Google Scholar] [CrossRef]
- You, Z.; Wang, C.; Hu, P.; Zhang, W.; Li, Q.; Zheng, Y. Construction of Dual Driving Force in Carbon Nitride for Highly Efficient Hydrogen Evolution: Simultaneously Manipulating Carriers Transport in Intra- and Interlayer. J. Colloid. Interface Sci. 2024, 676, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yang, J.; Lin, L.; Peng, K.; Chen, Y.; Jin, S.; Yao, W. Construction of Physically Crosslinked Chitosan/Sodium Alginate/Calcium Ion Double-Network Hydrogel and Its Application to Heavy Metal Ions Removal. Chem. Eng. J. 2020, 393, 124728. [Google Scholar] [CrossRef]
- Hao, D.; Huang, Q.; Wei, W.; Bai, X.; Ni, B.-J. A Reusable, Separation-Free and Biodegradable Calcium Alginate/g-CN Microsphere for Sustainable Photocatalytic Wastewater Treatment34. J. Clean. Prod. 2021, 314, 128033. [Google Scholar] [CrossRef]
- Fan, J.; Shi, Z.; Lian, M.; Li, H.; Yin, J. Mechanically Strong Graphene Oxide/Sodium Alginate/Polyacrylamide Nanocomposite Hydrogel with Improved Dye Adsorption Capacity. J. Mater. Chem. A 2013, 1, 7433–7443. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Ye, X.; Guo, F.; Wang, X. Helical Graphitic Carbon Nitrides with Photocatalytic and Optical Activities. Angew. Chem. Int. Ed. Engl. 2014, 53, 11926–11930. [Google Scholar] [CrossRef]
- Dalponte, I.; de Sousa, B.C.; Mathias, A.L.; Jorge, R.M.M. Formulation and Optimization of a Novel TiO2/Calcium Alginate Floating Photocatalyst. Int. J. Biol. Macromol. 2019, 137, 992–1001. [Google Scholar] [CrossRef]
- Nawaz, M.; Khan, A.A.; Hussain, A.; Jang, J.; Jung, H.-Y.; Lee, D.S. Reduced Graphene oxide-TiO2/Sodium Alginate 3-Dimensional Structure Aerogel for Enhanced Photocatalytic Degradation of Ibuprofen and Sulfamethoxazole. Chemosphere 2020, 261, 127702. [Google Scholar] [CrossRef]
- Nawaz, M.; Moztahida, M.; Kim, J.; Shahzad, A.; Jang, J.; Miran, W.; Lee, D.S. Photodegradation of Microcystin-LR Using Graphene-TiO2/Sodium Alginate Aerogels. Carbohyd Polym. 2018, 199, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Feng, C.; Li, T.; Wang, Y.; Zhu, S.; Ren, H.; Huang, H. G-C3N4/Polyvinyl Alcohol-Sodium Alginate Aerogel for Removal of Typical Heterocyclic Drugs from Water. Environ. Pollut. 2023, 319, 121057. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M.; Polagani, R.K. 3D Kaolinite/g-C3N4-Alginate Beads as an Affordable and Sustainable Photocatalyst for Wastewater Remediation. Carbohydr. Polym. 2024, 323, 121420. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.D.; Arias, E.R.; Gutierrez, L.B. Enhancing Copper and Lead Adsorption in Water by In-Situ Generation of Calcium Carbonate on Alginate/Chitosan Biocomposite Surfaces. Int. J. Biol. Macromol. 2024, 266, 131110. [Google Scholar] [CrossRef]
- Divakar, S.; Sampatkumar, H.G.; Naik, S.S.; Malladi, S.; Padaki, M.; Patil, S.A.; Balakrishna, R. Graphitic Carbon Nitride Enriched Phytochemicals-Based Integrated Membranes for Perilous Chromium (VI) Ion Removal. Sep. Purif. Technol. 2023, 334, 125953. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, G.; Feng, F.; Gao, C.; Jin, X.; Zhang, X.; Xing, W. Synergetic Effects of In-Plane and Interlayer Dual Regulation on Sandworm-like Graphitic Carbon Nitride for High-Efficiency Photocatalytic Performance. Opt. Mater. 2024, 147, 114742. [Google Scholar] [CrossRef]
- Valiyathur, M.F.; Kottur, A.B.; Sakvai, M.S. Alginate Reinforced Composite of CuO-gCN: Synthesis, Characterization and Photocatalytic Activity34. Mater Lett 2024, 371, 136901. [Google Scholar] [CrossRef]
- Falletta, E.; Longhi, M.; Di Michele, A.; Boffito, D.C.; Bianchi, C.L. Floatable Graphitic Carbon Nitride/Alginate Beads for the Photodegradation of Organic Pollutants under Solar Light Irradiation. J. Clean. Prod. 2022, 371, 133641. [Google Scholar] [CrossRef]
- Li, P.; Xie, S.; Wang, G.; Qiu, L.; Liu, Y.; Liu, J. Surface Electric Dipole and Coordinate Microenvironment Mediated Charge Separation and Transfer for Patterned Carbon Nitride Photocatalysis. Appl. Surf. Sci. 2024, 665, 160304. [Google Scholar] [CrossRef]
- Sun, S.; Li, C.; Sun, Z.; Wang, J.; Wang, X.; Ding, H. In-Situ Design of Efficient Hydroxylated SiO2/g-C3N4 Composite Photocatalyst: Synergistic Effect of Compounding and Surface Hydroxylation. Chem. Eng. J. 2021, 416, 129107. [Google Scholar] [CrossRef]
- Xia, L.; Huang, H.; Fan, Z.; Hu, D.; Zhang, D.; Khan, A.S.; Usman, M.; Pan, L. Hierarchical Macro-/Meso-/Microporous Oxygen-Doped Carbon Derived from Sodium Alginate: A Cost-Effective Biomass Material for Binder-Free Supercapacitors. Mater. Design 2019, 182, 108048. [Google Scholar] [CrossRef]
- Zhao, R.; Ke, Y.; Maschmeyer, T.; Li, X. O2-Promoted Photodoping for Enhanced Photocurrent on Polymeric Carbon Nitride. Mater. Today Sustain. 2022, 21, 100268. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Lv, Y.; Zhao, W.; Dong, Y.; Wang, L. Sodium Alginate Intercalated 2D G-CN Membrane: Efficient Dye Removal and Photocatalytic Self-Cleaning34. Sep. Purif. Technol. 2023, 320, 124177. [Google Scholar] [CrossRef]
- Saeidi Tabar, F.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Rahdar, A.; Fathi-karkan, S.; Romanholo Ferreira, L.F. Ultrasensitive Aptamer-Based Electrochemical Nanobiosensor in Diagnosis of Prostate Cancer Using 2D:2D Reduced Graphene Oxide/Graphitic Carbon Nitride Decorated with Au Nanoparticles. Eur. J. Med. Chem. Rep. 2024, 12, 100192. [Google Scholar] [CrossRef]
- Peng, M.; Zhang, Q.; Zhang, M.; Yang, Z.; Wan, Y.; Deng, X.; Luo, H. Dealloying and Polydopamine/Silver Coating on NiTi Alloy for Improved Antibacterial Activity. Mater. Chem. Phys. 2023, 305, 127939. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Zheng, H.; Meng, F.; Wang, F. Thermoresponsive PNIPAm on Anti-Corrosion Antibacterial Coating for Controlled Ag Ions Release. Compos. Commun. 2022, 35, 101327. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Hashem, A.H.; Khattab, T.A.; Kamel, S. New Antibacterial Hydrogels Based on Sodium Alginate. Int. J. Biol. Macromol. 2023, 248, 125872. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Liu, C.; Lu, Z.; Li, M.; Hurren, C.; Wang, D. Photopolymerized Multifunctional Sodium Alginate-Based Hydrogel for Antibacterial and Coagulation Dressings. Int. J. Biol. Macromol. 2024, 260, 129428. [Google Scholar] [CrossRef]
- Feyissa, Z.; Edossa, G.D.; Gupta, N.K.; Negera, D. Development of Double Crosslinked Sodium Alginate/Chitosan Based Hydrogels for Controlled Release of Metronidazole and Its Antibacterial Activity. Heliyon 2023, 9, e20144. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Preethi, J.; Meenakshi, S. Removal of Chlorpyrifos, an Insecticide Using Metal Free Heterogeneous Graphitic Carbon Nitride (g-CN) Incorporated Chitosan as Catalyst: Photocatalytic and Adsorption Studies34. Int. J. Biol. Macromol. 2019, 132, 289–299. [Google Scholar] [CrossRef]
- Shen, H.; Durkin, D.P.; Aiello, A.; Diba, T.; Lafleur, J.; Zara, J.M.; Shen, Y.; Shuai, D. Photocatalytic Graphitic Carbon Nitride-Chitosan Composites for Pathogenic Biofilm Control under Visible Light Irradiation. J. Hazard. 2021, 408, 124890. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.J.; Ali, S.; Jamil, S.; Bibi, S.; Jafar, T.; Rasheed, A.; Noreen, S.; Bashir, A.; Rauf Khan, S. Comparative Catalytic Reduction and Degradation with Biodegradable Sodium Alginate Based Nanocomposite: Zinc Oxide/N-Doped Carbon Nitride/Sodium Alginate. Int. J. Biol. Macromol. 2024, 254, 127954. [Google Scholar] [CrossRef] [PubMed]
- Chitra, V.P.; Vasantharani, P.; Sivakumar, G.; Arif Dar, M.; Majid, S.R.; Rezaul Karim, M.; Arularasan, P.; Guganathan, L. Structural, Morphological and Optical Properties of CuAl2O4 Nanoparticles and Their Applications for Photocatalytic and Antibacterial Activities. Inorg. Chem. Commun. 2024, 166, 112658. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Zeng, Z.; Lv, M.; Wang, K.; Wang, H.; Tang, X. Towards Molecular Understanding of Surface and Interface Catalytic Engineering in TiO2/TiOF2 Nanosheets Photocatalytic Antibacterial under Visible Light Irradiation. J. Hazard. 2024, 465, 133429. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, S.; Gao, X.; Jiang, X.; Zhang, E.; Fan, H.; Zhu, S. Highly Flexible Carbon Nitride-Polyethylene Glycol-Cellulose Acetate Film with Photocatalytic Antibacterial Activity for Fruit Preservation. Int. J. Biol. Macromol. 2024, 266, 131161. [Google Scholar] [CrossRef]
- Wang, B.-B.; Quan, Y.-H.; Xu, Z.-M.; Zhao, Q. Preparation of Highly Effective Antibacterial Coating with Polydopamine/Chitosan/Silver Nanoparticles via Simple Immersion. Prog. Org. Coat. 2020, 149, 105967. [Google Scholar] [CrossRef]
- Liu, J.; Jia, R.; Zhou, E.; Zhao, Y.; Dou, W.; Xu, D.; Yang, K.; Gu, T. Antimicrobial Cu-Bearing 2205 Duplex Stainless Steel against MIC by Nitrate Reducing Pseudomonas aeruginosa Biofilm. Int. Biodeterior. 2018, 132, 132–138. [Google Scholar] [CrossRef]
- Liu, D.; Yang, H.; Li, J.; Li, J.; Dong, Y.; Yang, C.; Jin, Y.; Yassir, L.; Li, Z.; Hernandez, D.; et al. Electron Transfer Mediator PCN Secreted by Aerobic Marine Pseudomonas aeruginosa Accelerates Microbiologically Influenced Corrosion of TC4 Titanium Alloy. J. Mater. Sci. Technol. 2021, 79, 101–108. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Z.; Wu, T.; Li, C.; Li, Z. Effects of Non-Viable Microbial Film on Corrosion of Pipeline Steel in Soil Environment. Corr. Comm. 2021, 3, 23–33. [Google Scholar] [CrossRef]
- Liu, D.; Hu, Z.; Li, M.; Han, B.; Liang, Y.; Dilawer Hayat, M.; Sun, Y.; Jin, D.; Li, J.; Wang, B.; et al. Synergistic Effect on Corrosion Behavior of X80 Steel Influenced by Pseudomonas aeruginosa and Acetobacter aceti. Sep. Purif. Technol. 2024, 351, 128135. [Google Scholar] [CrossRef]
- Li, M.; Hu, Z.; Liu, D.; Liang, Y.; Liu, S.; Wang, B.; Niu, C.; Xu, D.; Li, J.; Han, B. Efficient Antibacterial and Microbial Corrosion Resistant Photocatalytic Coating: Enhancing Performance with S-Type Heterojunction and Cu Synergy. Chem. Eng. J. 2024, 495, 153519. [Google Scholar] [CrossRef]
- Li, M.; Zhou, J.; Li, Y.; Zhu, G.; Hu, Z.; Liu, S.; Han, B.; Zhao, H.; Liang, Y.; Liu, D.; et al. Enhanced Antibacterial and Corrosion Resistance of Copper-Containing 2205 Duplex Stainless Steel against the Corrosive Bacterium Shewanella algae. Bioelectrochemistry 2024, 160, 108768. [Google Scholar] [CrossRef]
- Liu, D.; Liang, Y.; Wei, H.; Liu, P.; Jin, D.; Yassir, L.; Han, B.; Li, J.; Xu, D. Enhanced Corrosion of 2205 Duplex Stainless Steel by Acetobacter aceti through Synergistic Electron Transfer and Organic Acids Acceleration. Bioelectrochemistry 2024, 157, 108665. [Google Scholar] [CrossRef] [PubMed]
- Jubu, P.R.; Danladi, E.; Ndeze, U.I.; Adedokun, O.; Landi, S.; Haider, A.J.; Adepoju, A.T.; Yusof, Y.; Obaseki, O.S.; Yam, F.K. Comment about the Use of Unconventional Tauc Plots for Bandgap Energy Determination of Semiconductors Using UV–Vis Spectroscopy. Results Opt. 2024, 14, 100606. [Google Scholar] [CrossRef]
- Wan, J.; Wang, R.; Liu, Z.; Zhang, S.; Hao, J.; Mao, J.; Li, H.; Chao, D.; Zhang, L.; Zhang, C. Hydrated Eutectic Electrolyte Induced Bilayer Interphase for High-Performance Aqueous Zn-Ion Batteries with 100 °C Wide-Temperature Range. Adv. Mater. 2024, 36, 2310623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-X.; Liu, J.-N.; Li, B.-Q.; Ren, D.; Chen, X.; Yu, J.; Zhang, Q. Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc–Air Batteries. Adv. Funct. 2020, 30, 2003619. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.; Zhou, X.; Tan, Z.; Zhang, M.; Luo, J.; Wang, Y.; Wu, T. Effects of Carbon Source Starvation and Riboflavin Addition on Selective Corrosion of Welded Joint by Desulfovibrio vulgaris. Corros. Sci. 2024, 230, 111931. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Han, B.; Li, J.; Liu, D.; Qi, J. Antibacterial and Anticorrosive Hydrogel Coating Based on Complementary Functions of Sodium Alginate and g-C3N4. Molecules 2024, 29, 4192. https://doi.org/10.3390/molecules29174192
Hu Z, Han B, Li J, Liu D, Qi J. Antibacterial and Anticorrosive Hydrogel Coating Based on Complementary Functions of Sodium Alginate and g-C3N4. Molecules. 2024; 29(17):4192. https://doi.org/10.3390/molecules29174192
Chicago/Turabian StyleHu, Zishuai, Baochen Han, Jianhui Li, Dan Liu, and Jian Qi. 2024. "Antibacterial and Anticorrosive Hydrogel Coating Based on Complementary Functions of Sodium Alginate and g-C3N4" Molecules 29, no. 17: 4192. https://doi.org/10.3390/molecules29174192





