Research on the Cell Wall Breaking and Subcritical Extraction of Astaxanthin from Phaffia rhodozyma
Abstract
:1. Introduction
2. Results
2.1. Construction of the Astaxanthin Standard Curve
2.2. Astaxanthin Content Obtained by Different Wall Disruption Methods
2.3. Subcritical Extraction
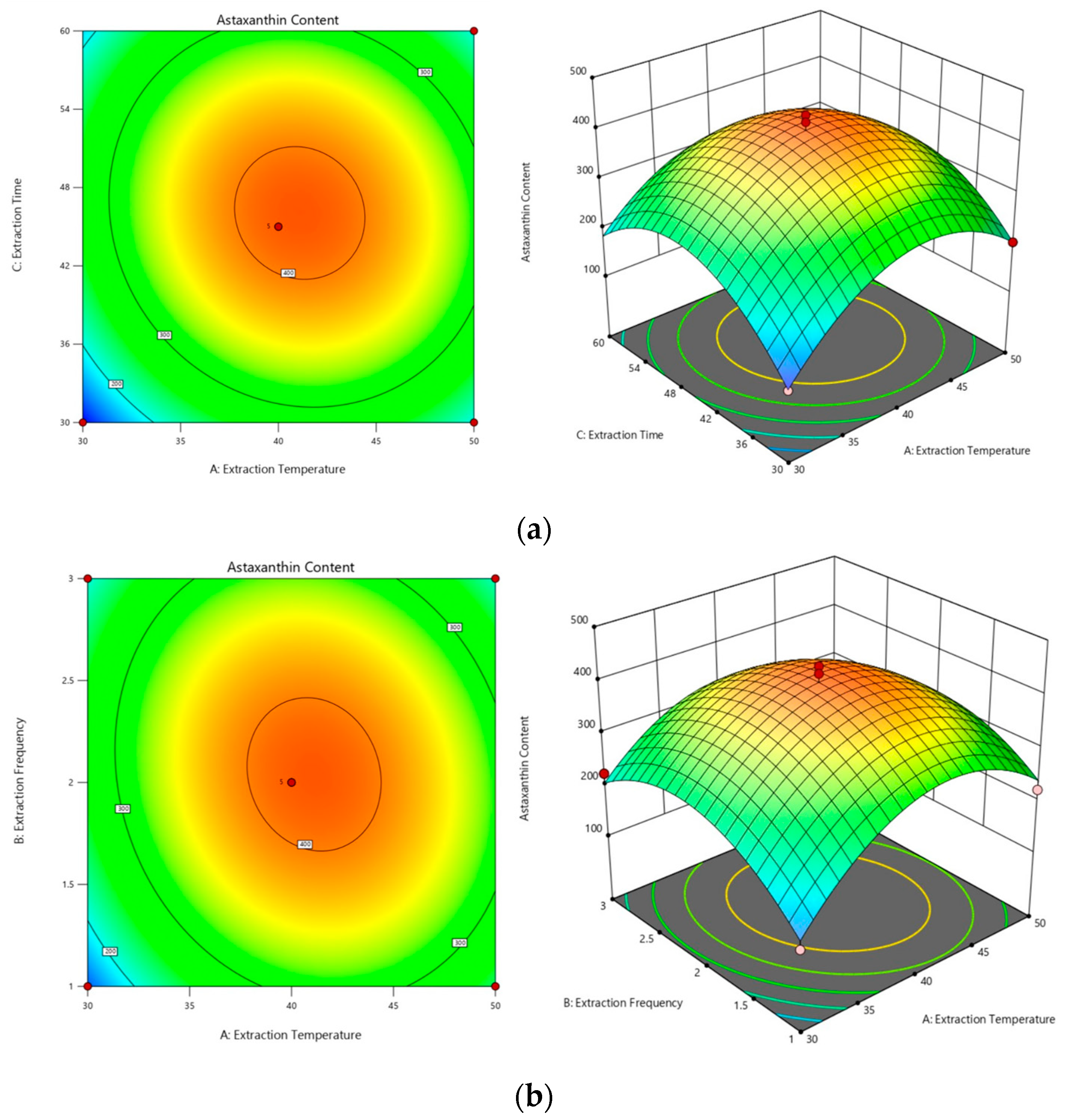
2.3.1. Optimization of the Subcritical Extraction Method
2.3.2. Optimal Process Conditions and Validation Experiments
2.4. Astaxanthin Content Detection Results
UPLC-Q-TOF MS Detection of Astaxanthin
2.5. Detection of Astaxanthin Samples by Liquid Chromatography
3. Materials and Methods
3.1. Main Instruments and Reagents
3.2. Strains and Cultivation Conditions
3.3. P. rhodozyma Cell Wall Disruption
3.3.1. Lactic Acid Method
3.3.2. DMSO Method
3.3.3. NaOH Method
3.3.4. Enzymatic Methods
3.4. Subcritical Fluid Extraction
3.5. Detection of Astaxanthin Content
3.5.1. Preparation of the Astaxanthin Standard Mother Liquor
3.5.2. Liquid Chromatography Conditions
3.5.3. Construction of the Astaxanthin Standard Curve
3.5.4. Astaxanthin Content Determination
3.5.5. UPLC-Q-TOF MS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.-Y.; Lee, P.-C.; Wu, Y.-L.; Liu, L.-Y. In Vivo Effects of Free Form Astaxanthin Powder on Anti-Oxidation and Lipid Metabolism with High-Cholesterol Diet. PLoS ONE 2015, 10, e0134733. [Google Scholar] [CrossRef] [PubMed]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Einafshar, S.; Ramaswamy, H.S. Optimization of ultrasonic-assisted extraction of astaxanthin from green tiger (Penaeus semisulcatus) shrimp shell. Ultrason. Sonochem. 2021, 76, 105666. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Cho, S.-H.; Lee, H.; Choi, H.-K.; Kim, D.-M.; Lee, C.-G.; Cho, S.; Cho, B.-K. Current Status and Future Strategies to Increase Secondary Metabolite Production from Cyanobacteria. Microorganisms 2020, 8, 1849. [Google Scholar] [CrossRef]
- Zhao, T.; Yan, X.; Sun, L.; Yang, T.; Hu, X.; He, Z.; Liu, F.; Liu, X. Research progress on extraction, biological activities and delivery systems of natural astaxanthin. Trends Food Sci. Technol. 2019, 91, 354–361. [Google Scholar] [CrossRef]
- Ahmed, I. Microalgae as a source of high-value bioactive compounds. Front. Biosci. 2018, 10, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Eggersdorfer, M.; Rimbach, G.; Esatbeyoglu, T. Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin. Int. J. Mol. Sci. 2016, 17, 103. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant Activities of Astaxanthin and Related Carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Nishida, Y.; Berg, P.C.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N.; et al. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. [Google Scholar] [CrossRef]
- Lim, S.-R.; Kim, D.-W.; Sung, J.; Kim, T.H.; Choi, C.-H.; Lee, S.-J. Astaxanthin Inhibits Autophagic Cell Death Induced by Bisphenol A in Human Dermal Fibroblasts. Antioxidants 2021, 10, 1273. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, e05993. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, C.; Wu, J. Astaxanthin in Liver Health and Disease: A Potential Therapeutic Agent. Drug Des. Dev. Ther. 2020, 14, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; Rathod, N.; Čagalj, M.; Hamed, I.; Mekinić, I. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A. Phaffia rhodozyma: Colorful odyssey. Int. Microbiol. 2003, 6, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A.; Lewis, M.J. Astaxanthin Formation by the Yeast Phaffia rhodozyma. J. Gen. Microbiol. 1979, 115, 173–183. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.-D.; Li, K.; Yin, W.-T.; Liu, H.-M.; Zhu, X.-L.; Hu, P. Characterisation of flavourous sesame oil obtained from microwaved sesame seed by subcritical propane extraction. Food Chem. X 2024, 21, 101087. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical Water Extraction of Phenolic Compounds from Onion Skin Wastes (Allium cepa cv. Horcal): Effect of Temperature and Solvent Properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef]
- Atanasova, A.; Petrova, A.; Teneva, D.; Ognyanov, M.; Georgiev, Y.; Nenov, N.; Denev, P. Subcritical Water Extraction of Rosmarinic Acid from Lemon Balm (Melissa officinalis L.) and Its Effect on Plant Cell Wall Constituents. Antioxidants 2023, 12, 888. [Google Scholar] [CrossRef]
- Ni, H. Optimization of Astaxanthin Fermentation Conditions and Extraction and Analysis Research of Phaffia Yeast. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2005. [Google Scholar]
- Sedmak, J.J.; Weerasinghe, D.K.; Jolly, S.O. Extraction and quantitation of astaxanthin from Phaffia rhodozyma. Biotechnol. Tech. 1990, 4, 107–112. [Google Scholar] [CrossRef]
- Song, G.J.; Jian, H.L. Advances in the Extraction and Separation Technology of Astaxanthin from Phaffia Rhodozyma. Pop. Sci. 2005, 6, 60–61+56. [Google Scholar] [CrossRef]
- Harith, Z.T.; de Andrade Lima, M.; Charalampopoulos, D.; Chatzifragkou, A. Optimised Production and Extraction of Astaxanthin from the Yeast Xanthophyllomyces dendrorhous. Microorganisms 2020, 8, 430. [Google Scholar] [CrossRef]
- Amponsah, S.K.; Boadu, J.A.; Dwamena, D.K.; Opuni, K.F. Bioanalysis of Aminoglycosides Using High-Performance Liquid Chromatography. ADMET DMPK 2022, 10, 27–62. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Ni, H. Establishing aqueous two-phase flotation coupled with preparative high performance liquid chromatography and its application for the purification of astragalin from Flaveria bidentis. J. Chromatogr. B 2024, 1240, 124141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Chen, F.; Yu, J.; Liu, X.; Liu, Y.; Ouyang, J.; Liang, M.; Zhu, Y.; Zou, L. Insight into the mechanisms of therapeutic hypothermia for asphyxia cardiac arrest using a comprehensive approach of GC-MS/MS and UPLC-Q-TOF-MS/MS based on serum metabolomics. Heliyon 2023, 9, e16247. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-D.; Song, C.-G.; Yang, J.; Zhou, T.; Zhao, Y.-Y.; Qin, J.-C.; Guo, L.-P.; Ding, G. Accurate Identification of Degraded Products of Aflatoxin B1 Under UV Irradiation Based on UPLC-Q-TOF-MS/MS and NMR Analysis. Front. Chem. 2021, 9, 789249. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Yang, J.; Li, Q.; Li, Y.-Y.; Tan, X.-M.; Yao, S.-Y.; Niu, S.-B.; Deng, H.; Guo, L.-P.; Ding, G. UPLC-Q-TOF-MS/MS Analysis of Seco-Sativene Sesquiterpenoids to Detect New and Bioactive Analogues From Plant Pathogen Bipolaris sorokiniana. Front. Microbiol. 2022, 13, 807014. [Google Scholar] [CrossRef]
- Shen, Z.J.; Fu, Y.B.; Hou, J.L.; Lin, L.N.; Wang, X.Y.; Li, C.Y.; Yang, Y.X. Integrating network pharmacology, UPLC-Q–TOF–MS and molecular docking to investigate the effect and mechanism of Chuanxiong Renshen decoction against Alzheimer’s disease. Chin. Med. 2022, 17, 143. [Google Scholar] [CrossRef]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast Cell Disruption Strategies for Recovery of Intracellular Bio-Active Compounds—A Review. Innov. Food Sci. Amp Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Ni, H.; Chen, Q.H.; He, G.Q.; Wu, G.B.; Yang, Y.F. Optimization of acidic extraction of astaxanthin from Phaffia rhodozyma. J. Zhejiang Univ. Science B 2008, 9, 51–59. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, K.; Dong, C.C.; Ni, H.; Yang, Y.F.; Huang, G.L.; Cai, H.N.; Du, X.P. Effect of dimethyl sulfoxide method on the extraction of astaxanthin from Phaffia rhodozyma. Food Ind. Sci. Technol. 2017, 38, 212–217. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Cao, X.H.; Lin, Q. Optimization of the extraction process of astaxanthin from Phaffia rhodozyma. Food Sci. Technol. 2011, 36, 196–199. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhou, P.P.; Zhu, F.Y.; Yu, L.J. Effect of wall-breaking conditions on the extraction rate of carotenoids from Phaffia rhodozyma. China Feed 2006, 4, 20–22+25. [Google Scholar] [CrossRef]
- Zeng, Z.D.; Yao, S.Y. Study on the conditions for enzymatic extraction of red Fife yeast. Food Ind. Sci. Technol. 2007, 8, 91–93. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, E.; Ramos, C.; Alonso-Riaño, P.; Beltrán, S.; Sanz, M.T. Subcritical Water Treatment for Valorization of the Red Algae Residue after Agar Extraction: Scale-Up from Laboratory to Pilot Plant. Ind. Eng. Chem. Res. 2023, 62, 3503–3514. [Google Scholar] [CrossRef]
- Choi, S.K.; Kim, J.H.; Park, Y.S.; Kim, Y.J.; Chang, H.I. An efficient method for the extraction of astaxanthin from the Phaffia rhodozyma Xanthophyllomyces dendrorhous. Microbiol. Biotechnol. 2007, 17, 847–852. [Google Scholar]
- Ni, H.; He, G.Q.; Wu, G.B.; Yang, Y.F.; Chen, S.R.; Cai, H.N. Optimization of Astaxanthin Extraction Process from Phaffia rhodozyma. Trans. Chin. Soc. Agric. Eng. 2004, 2, 204–208. [Google Scholar] [CrossRef]
- Yu, X.; Shang, X.M.; Liu, Y.; Wu, Z.Q.; Xu, H.L.; Jiang, G.L. Papain enzyme digestion of Antarctic krill shells for astaxanthin extraction. Food Ind. Sci. Technol. 2013, 34, 212–217. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Hou, H.Z.; Kong, L.W. Study on enzymatic hydrolysis process of combined astaxanthin. Food Res. Dev. 2017, 38, 35–39. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Shu, A.; Zhang, S.M.; Jia, H.H.; Xie, S.Y.; Zhang, H.R.; Li, R.F. Identification of all-trans, 9-cis, and 13-cis geometrical isomers of astaxanthin by high-performance liquid chromatography. Chin. Mar. Drugs 2022, 41, 73–81. [Google Scholar]
- Zhang, J.X.; Zhou, Y.T.; Li, J.X.; Liu, J.P. Determination of astaxanthin in astaxanthin softgels by HPLC combined with enzymatic digestion. Mod. Food 2022, 28, 185–188+193. [Google Scholar] [CrossRef]
- Xiao, D.G.; Li, X.Y.; Hao, Y. Determination of intracellular astaxanthin in Phaffia rhodozyma by high performance liquid chromatography. J. Tianjin Univ. Sci. Technol. 2005, 1, 9–12. [Google Scholar] [CrossRef]
- Kai, S.; Kosuge, N.; Waki, M.; Miyazawa, M.; Kanazawa, H. Analysis of of Fusarium Toxins in Processed Grain Products Using High-Performance Liquid Chromatography/Tandem Mass Spectrometry. Chromatography 2016, 37, 79–85. [Google Scholar] [CrossRef]
- Sharmili, K.; Jinap, S.; Sukor, R. Development, optimization and validation of QuEChERS based liquid chromatography tandem mass spectrometry method for determination of multimycotoxin in vegetable oil. Food Control 2016, 70, 152–160. [Google Scholar] [CrossRef]
- Ul Haq, F.; Ali, A.; Akhtar, N.; Aziz, N.; Khan, M.N.; Ahmad, M.; Musharraf, S.G. A high-throughput method for dereplication and assessment of metabolite distribution in Salvia species using LC-MS/MS. J. Adv. Res. 2020, 24, 79–90. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.S.; Zhang, T.F.; Wang, X.D. Extraction and Characterization of Flaxseed Oil Obtained with Subcritical n-Butane. J. Oleo Sci. 2020, 69, 1011–1020. [Google Scholar] [CrossRef]
- Feng, F.F.; Liu, S.S.; Liu, R.K.; Yi, Y.J.; Qi, K.; Yang, Y. Optimization of process conditions for subcritical extraction of gardenia jasminoides (Gardenia jasminoides) fruit oil. Hunan For. Sci. Technol. 2024, 51, 67–72. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Połaska, M.; Marszałek, K.; Skąpska, S. Photosensitizing Furocoumarins: Content in Plant Matrices and Kinetics of Supercritical Carbon Dioxide Extraction. Molecules 2020, 25, 3805. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. Supercritical Carbon Dioxide (scCO2) Extraction of Phenolic Compounds from Lavender (Lavandula angustifolia) Flowers: A Box-Behnken Experimental Optimization. Molecules 2019, 24, 3354. [Google Scholar] [CrossRef]
- Miękus, N.; Iqbal, A.; Marszałek, K.; Puchalski, C.; Świergiel, A. Green Chemistry Extractions of Carotenoids from Daucus carota L.—Supercritical Carbon Dioxide and Enzyme-Assisted Methods. Molecules 2019, 24, 4339. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, B. Bio-based chemicals from biorefining: Lipid and wax conversion and utilization. Adv. Biorefin. 2014, 693–720. [Google Scholar]
- Wu, Y.C.; Yang, H.R.; Fang, Y.; Ren, X.L. Progress of natural astaxanthin extraction and purification. Food Res. Dev. 2014, 25, 117–120. [Google Scholar]
- Najafi, M.; Abousadi, Z.A.; Honarvar, B.; Sajadian, S.A. Separation of hydrocarbons from activated carbon as a porous substance in a glycol regeneration process using supercritical carbon dioxide. Sci. Rep. 2022, 12, 19910. [Google Scholar] [CrossRef]
- Zhang, H.M.; Yang, J.Q.; Ma, X.X.; Shi, L.; Teng, J.; Feng, Q.; Zhang, K. A rapid method for the determination of astaxanthin in Haematococcus pluvialis. Food Res. Dev. 2016, 37, 4. [Google Scholar] [CrossRef]
- Li, R.; Xia, Z.; Li, B.; Tian, Y.; Zhang, G.; Li, M.; Dong, J. Advances in Supercritical Carbon Dioxide Extraction of Bioactive Substances from Different Parts of Ginkgo biloba L. Molecules 2021, 26, 4011. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsani, S.; Patel, A.S.; Kar, A.; Dash, S. Process optimization for the supercritical carbondioxide extraction of lycopene from ripe grapefruit (Citrus paradisi) endocarp. Sci. Rep. 2021, 11, 10273. [Google Scholar] [CrossRef]
- Coelho, J.P.; Cristino, A.F.; Matos, P.G.; Rauter, A.P.; Nobre, B.P.; Mendes, R.L.; Barroso, J.G.; Mainar, A.; Urieta, J.S.; Fareleira, J.M.N.A.; et al. Extraction of Volatile Oil from Aromatic Plants with Supercritical Carbon Dioxide: Experiments and Modeling. Molecules 2012, 17, 10550–10573. [Google Scholar] [CrossRef]
- Melgosa, R.; Trigueros, E.; Sanz, M.T.; Cardeira, M.; Rodrigues, L.; Fernández, N.; Matias, A.A.; Bronze, M.R.; Marques, M.; Paiva, A.; et al. Supercritical CO2 and subcritical water technologies for the production of bioactive extracts from sardine (Sardina pilchardus) waste. J. Supercrit. Fluids 2020, 164, 104943. [Google Scholar] [CrossRef]
- Temerdashev, Z.A.; Milevskaya, V.V.; Ryabokon’, L.P.; Latin, N.N.; Kiseleva, N.V.; Nagalevskii, M.V. Identification and Determination of the Components of Garden Sage (Salvia officinalis L.) Essential Oil, Isolated by Different Extraction Methods. J. Anal. Chem. 2020, 75, 1451–1460. [Google Scholar] [CrossRef]






| Sample | Astaxanthin Content (mg/L) |
|---|---|
| Lactic acid method | 474.32 |
| DMSO method | 102.65 |
| NaOH method (30 °C, 3 h) | 97.35 |
| NaOH method (40 °C, 2 h) | 105.34 |
| Enzymatic method: β-mannanase | 82.08 |
| Enzymatic method: β-glucosidase | 78.80 |
| Enzymatic method: composite enzyme | 78.09 |
| Serial Number | A | B | C | Y |
|---|---|---|---|---|
| Extraction Temperature (°C) | Extraction Frequency (Times) | Extraction Time (min) | Astaxanthin Content (mg/L) | |
| 1 | 30.00 | 2.00 | 30.00 | 111.21 |
| 2 | 50.00 | 2.00 | 30.00 | 202.34 |
| 3 | 30.00 | 3.00 | 45.00 | 223.62 |
| 4 | 40.00 | 3.00 | 30.00 | 184.42 |
| 5 | 40.00 | 2.00 | 45.00 | 426.78 |
| 6 | 40.00 | 2.00 | 45.00 | 380.23 |
| 7 | 40.00 | 1.00 | 60.00 | 234.84 |
| 8 | 40.00 | 2.00 | 45.00 | 409.37 |
| 9 | 40.00 | 2.00 | 45.00 | 398.44 |
| 10 | 30.00 | 1.00 | 45.00 | 123.04 |
| 11 | 50.00 | 3.00 | 45.00 | 231.19 |
| 12 | 40.00 | 1.00 | 30.00 | 188.57 |
| 13 | 40.00 | 3.00 | 60.00 | 207.65 |
| 14 | 50.00 | 1.00 | 45.00 | 221.99 |
| 15 | 40.00 | 2.00 | 45.00 | 440.65 |
| 16 | 50.00 | 2.00 | 60.00 | 205.56 |
| Serial Number | A | B | C | Y |
|---|---|---|---|---|
| Extraction Temperature (°C) | Extraction Frequency (Times) | Extraction Time (min) | Astaxanthin Content (mg/L) | |
| 1 | 30.00 | 2.00 | 30.00 | 111.21 |
| 2 | 50.00 | 2.00 | 30.00 | 202.34 |
| 3 | 30.00 | 3.00 | 45.00 | 223.62 |
| 4 | 40.00 | 3.00 | 30.00 | 184.42 |
| 5 | 40.00 | 2.00 | 45.00 | 426.78 |
| 6 | 40.00 | 2.00 | 45.00 | 380.23 |
| 7 | 40.00 | 1.00 | 60.00 | 234.84 |
| 8 | 40.00 | 2.00 | 45.00 | 409.37 |
| 9 | 40.00 | 2.00 | 45.00 | 398.44 |
| 10 | 30.00 | 1.00 | 45.00 | 123.04 |
| 11 | 50.00 | 3.00 | 45.00 | 231.19 |
| 12 | 40.00 | 1.00 | 30.00 | 188.57 |
| 13 | 40.00 | 3.00 | 60.00 | 207.65 |
| 14 | 50.00 | 1.00 | 45.00 | 221.99 |
| 15 | 40.00 | 2.00 | 45.00 | 440.65 |
| 16 | 50.00 | 2.00 | 60.00 | 205.56 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 182,945.80 | 9.00 | 20,327.31 | 30.26 | <0.0001 | significant |
| A-extraction temperature | 6272.00 | 1.00 | 6272.00 | 9.34 | 0.02 | |
| B-extraction frequency | 780.13 | 1.00 | 780.13 | 1.16 | 0.32 | |
| C-extraction time | 2415.13 | 1.00 | 2415.13 | 3.60 | 0.10 | |
| AB | 2025.00 | 1.00 | 2025.00 | 3.01 | 0.13 | |
| AC | 1024.00 | 1.00 | 1024.00 | 1.52 | 0.26 | |
| BC | 132.25 | 1.00 | 132.25 | 0.20 | 0.67 | |
| A2 | 60,808.55 | 1.00 | 60,808.55 | 90.52 | <0.0001 | |
| B2 | 34,809.92 | 1.00 | 34,809.92 | 51.82 | 0.00 | |
| C2 | 57,072.76 | 1.00 | 57,072.76 | 84.96 | <0.0001 | |
| Residual | 4702.45 | 7.00 | 671.78 | |||
| Lack-of-fit | 2503.25 | 3.00 | 834.42 | 1.52 | 0.34 | not significant |
| Pure error | 2199.20 | 4.00 | 549.80 | |||
| Cor total | 187,648.20 | 16.00 |
| Level | Factor | ||
|---|---|---|---|
| A | B | C | |
| Extraction Temperature (°C) | Extraction Frequency (Times) | Extraction Time (min) | |
| −1 | 30 | 1 | 30 |
| 0 | 40 | 2 | 45 |
| 1 | 50 | 3 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Deng, X.; Qin, L.; Jiang, D.; Lu, M.; Chen, K.; Yang, M.; Zhang, L.; Jiang, J.; Lu, L. Research on the Cell Wall Breaking and Subcritical Extraction of Astaxanthin from Phaffia rhodozyma. Molecules 2024, 29, 4201. https://doi.org/10.3390/molecules29174201
Jiang W, Deng X, Qin L, Jiang D, Lu M, Chen K, Yang M, Zhang L, Jiang J, Lu L. Research on the Cell Wall Breaking and Subcritical Extraction of Astaxanthin from Phaffia rhodozyma. Molecules. 2024; 29(17):4201. https://doi.org/10.3390/molecules29174201
Chicago/Turabian StyleJiang, Wenxuan, Xiangrong Deng, Lanxian Qin, Dahai Jiang, Mengqi Lu, Kai Chen, Manqi Yang, Liangliang Zhang, Jianchun Jiang, and Liming Lu. 2024. "Research on the Cell Wall Breaking and Subcritical Extraction of Astaxanthin from Phaffia rhodozyma" Molecules 29, no. 17: 4201. https://doi.org/10.3390/molecules29174201






