The Importance of Biotinylation for the Suitability of Cationic and Neutral Fourth-Generation Polyamidoamine Dendrimers as Targeted Drug Carriers in the Therapy of Glioma and Liver Cancer
Abstract
:1. Introduction
2. Results and Discussion
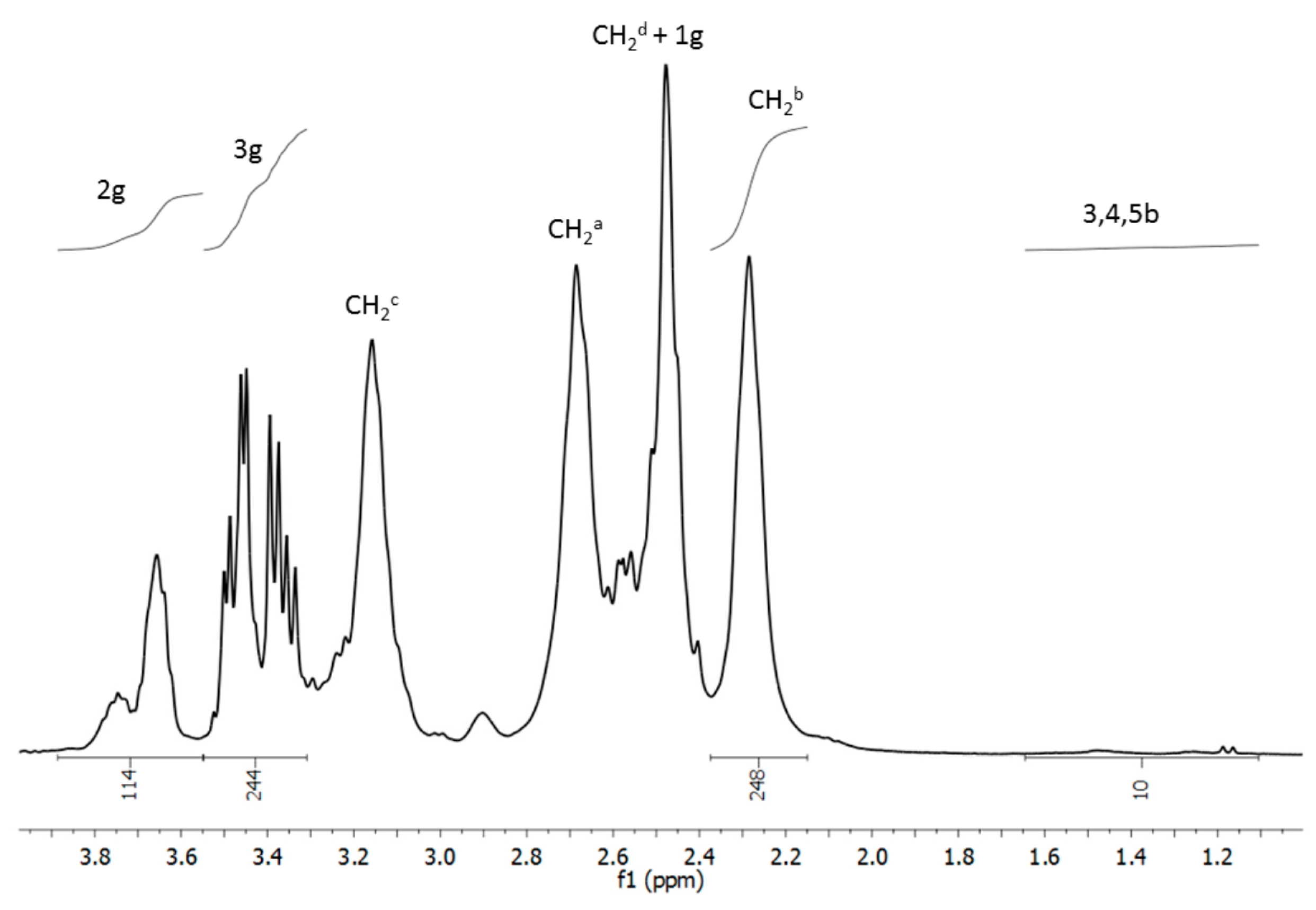
2.1. Chemistry
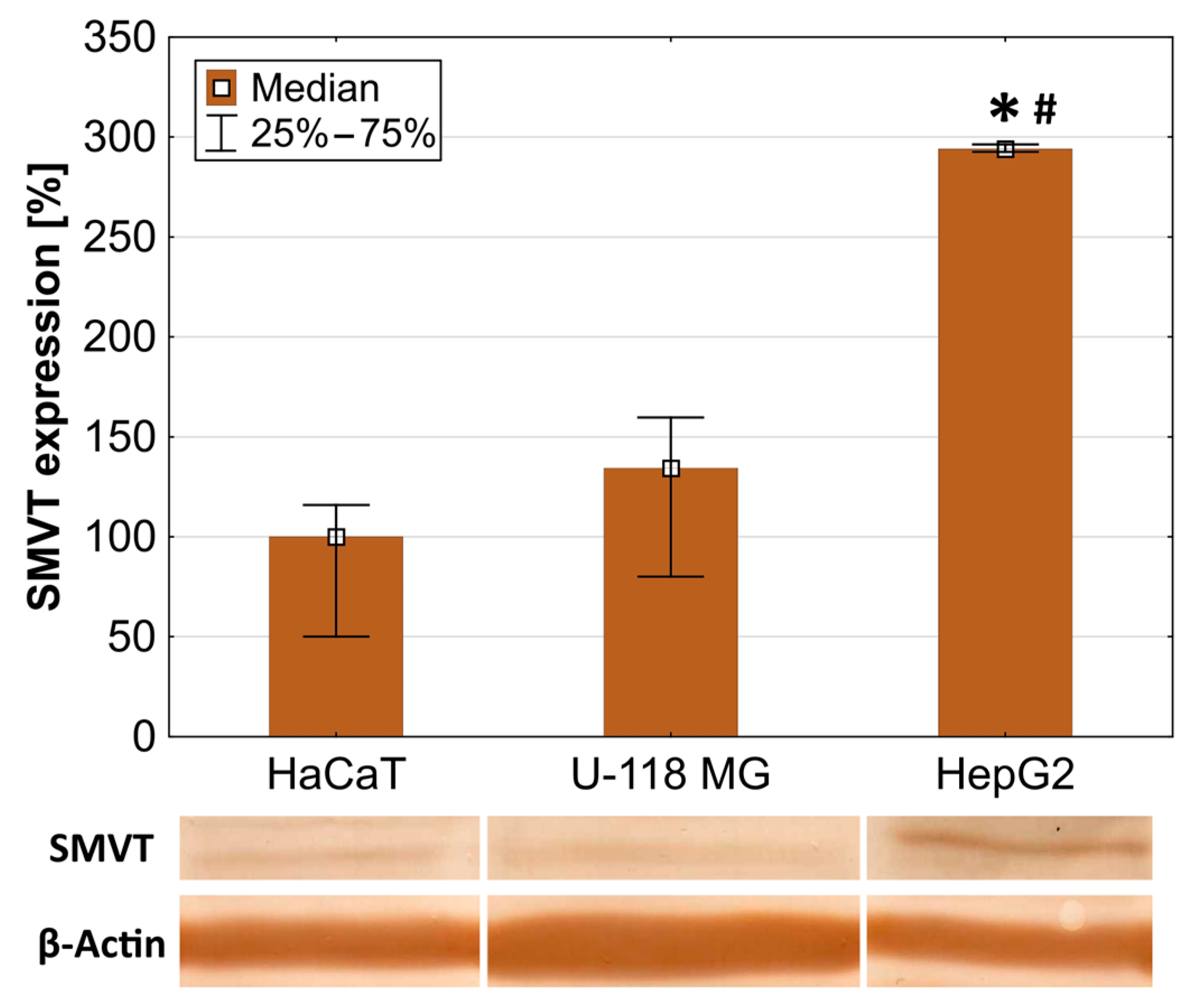
2.2. SMVT Expression
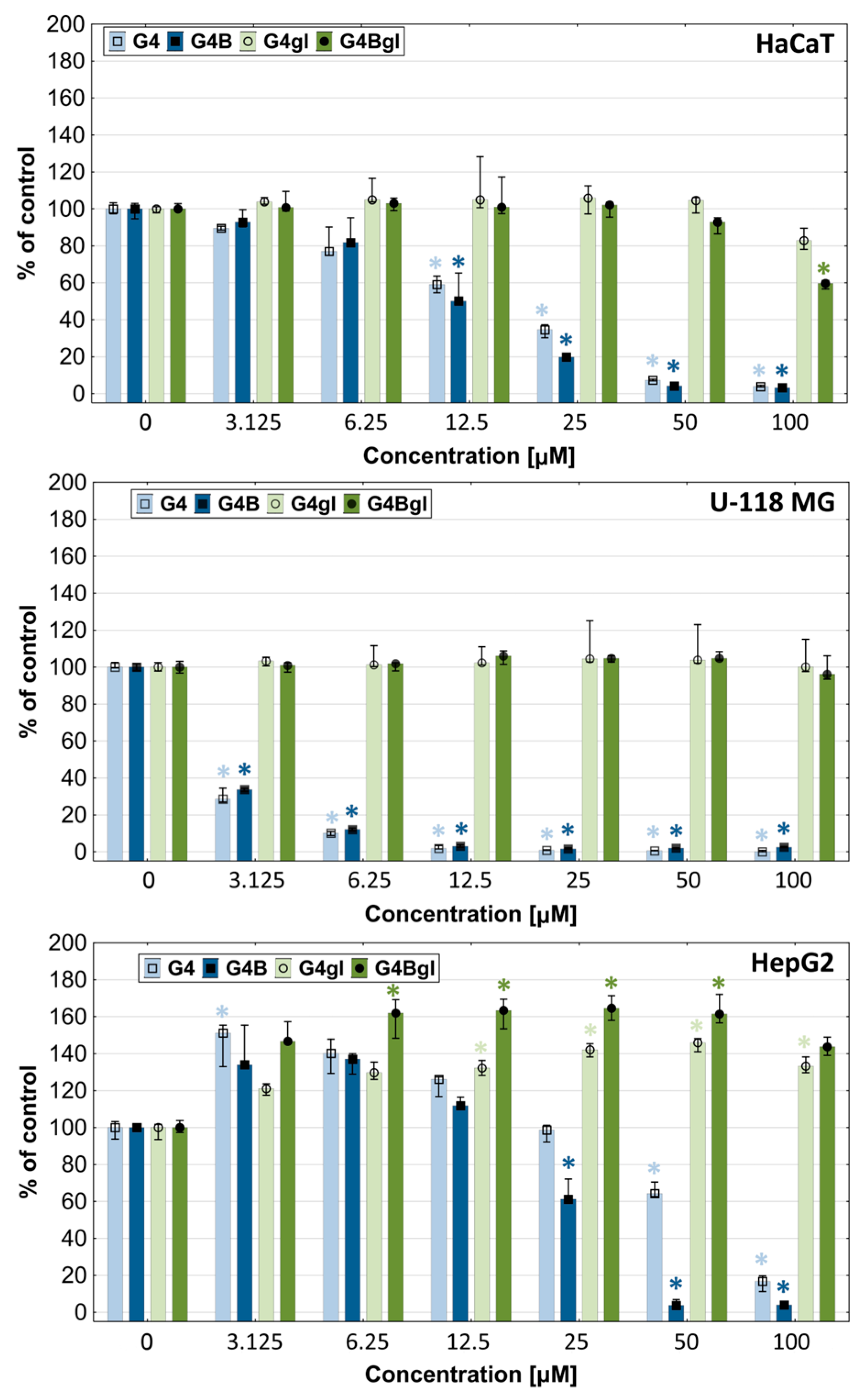
2.3. Dendrimers Cytotoxicity
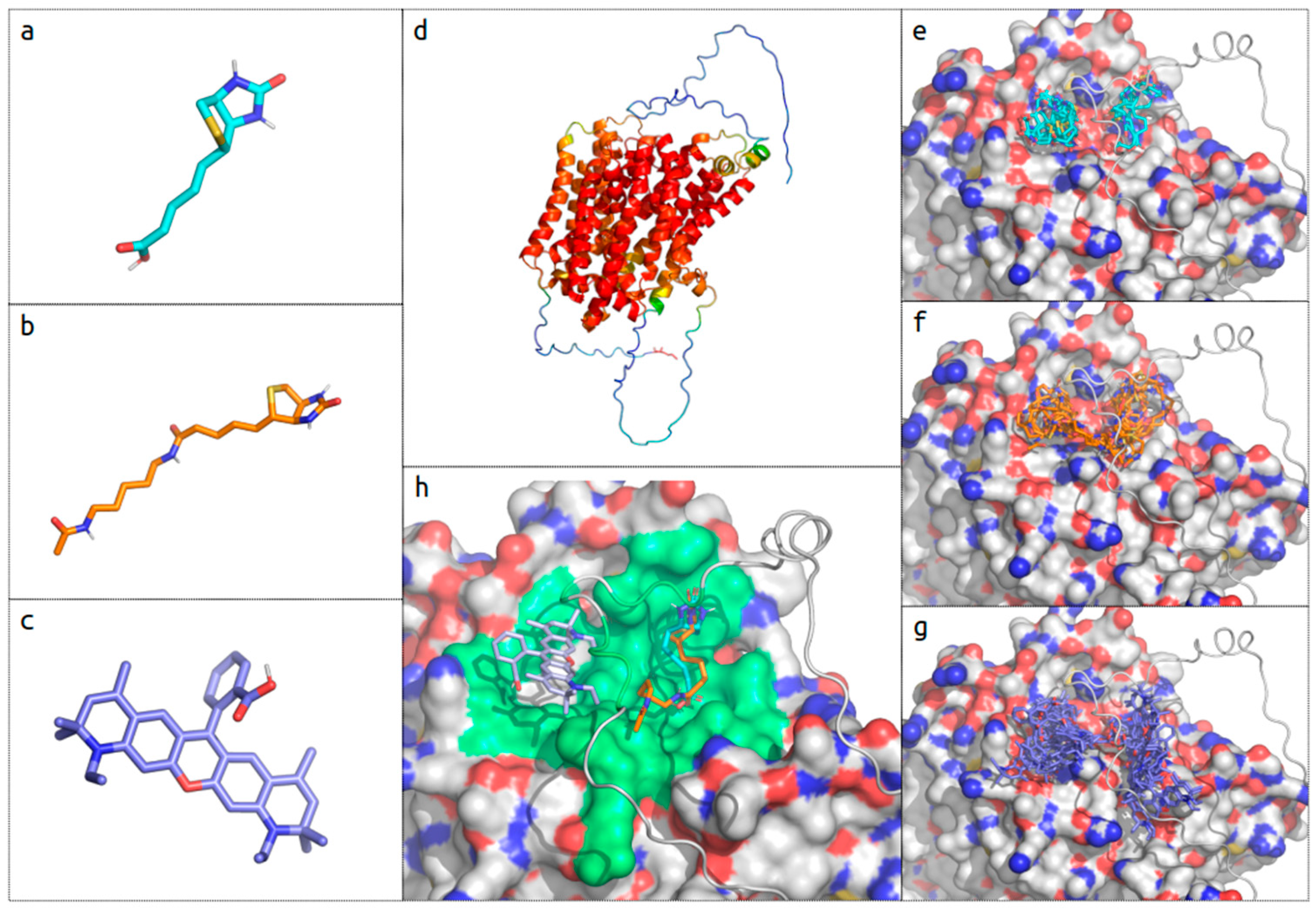
2.4. In Silico Biotin Binding Site Mapping
2.5. Biotin Uptake
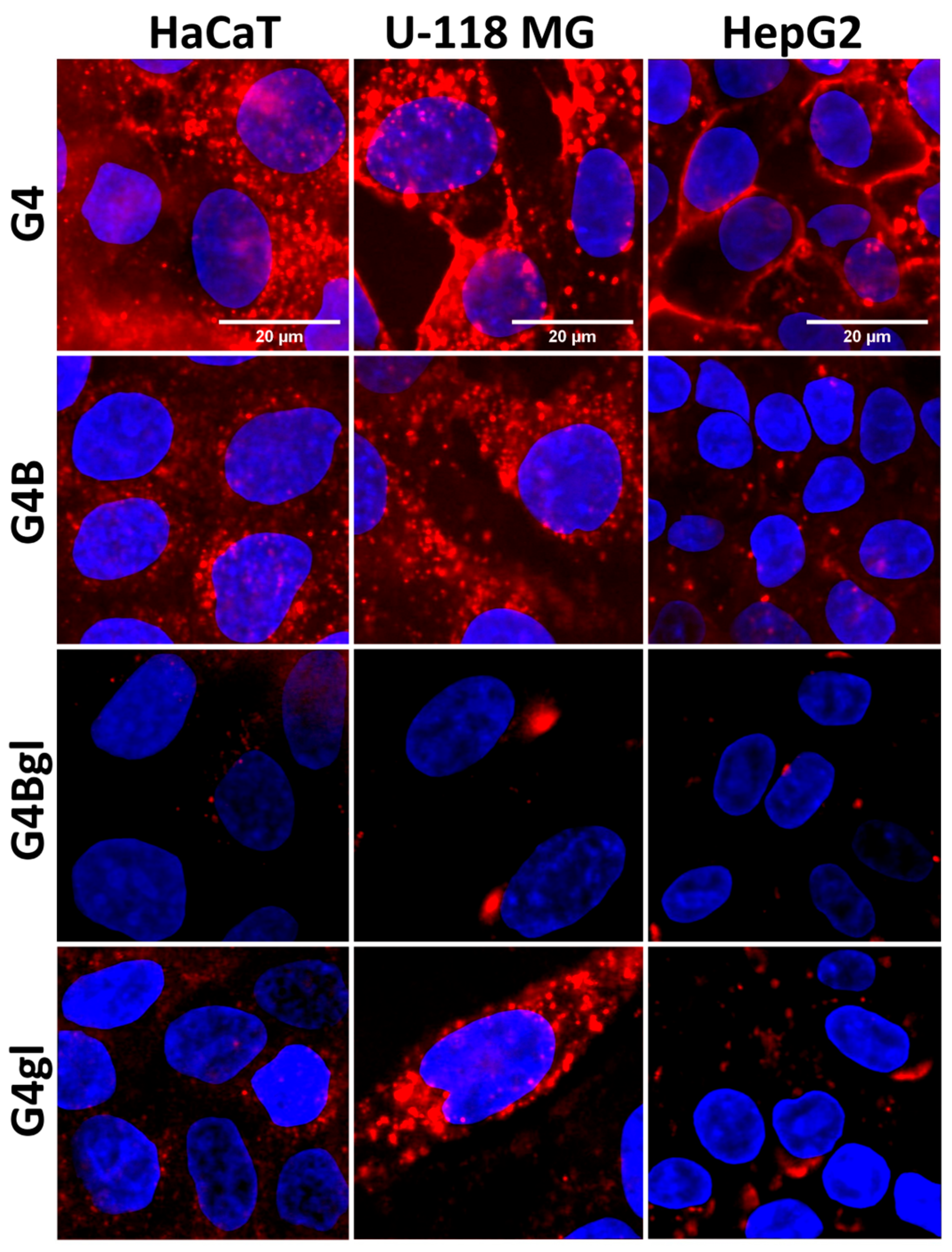
2.6. Time-Dependent Dendrimer Uptake
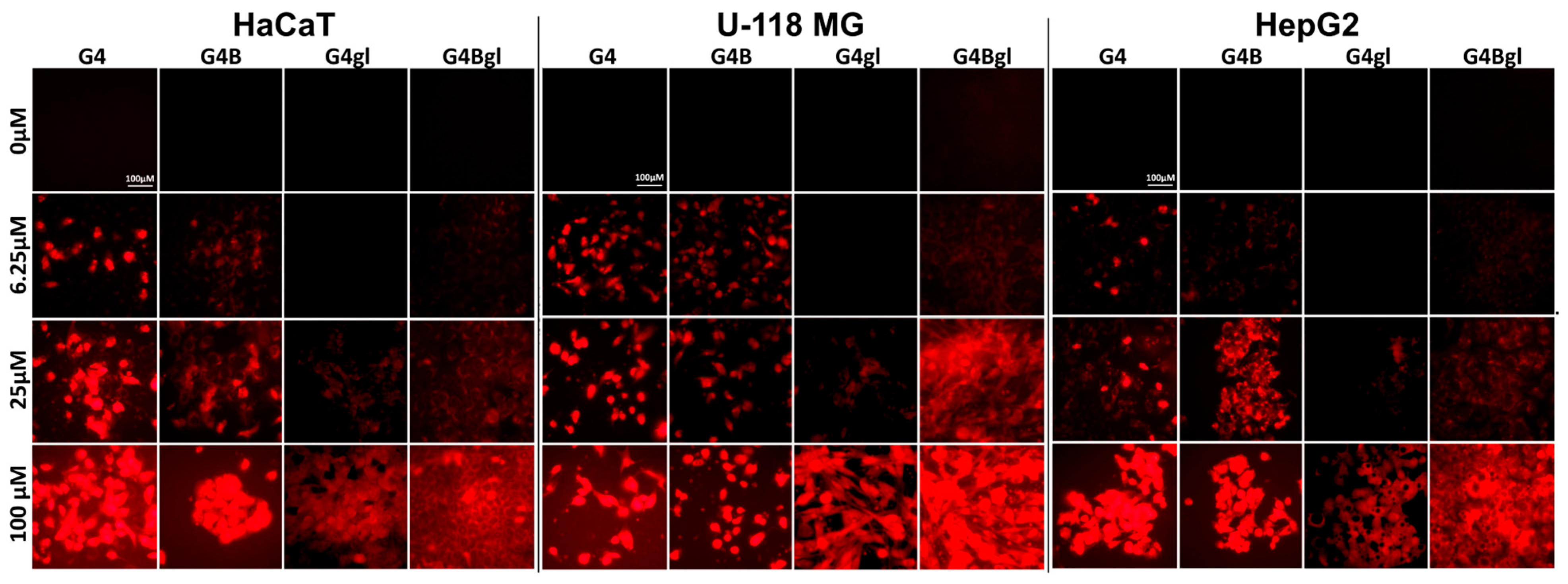
2.7. Dendrimer Accumulation
2.8. Toxicity against C. elegans
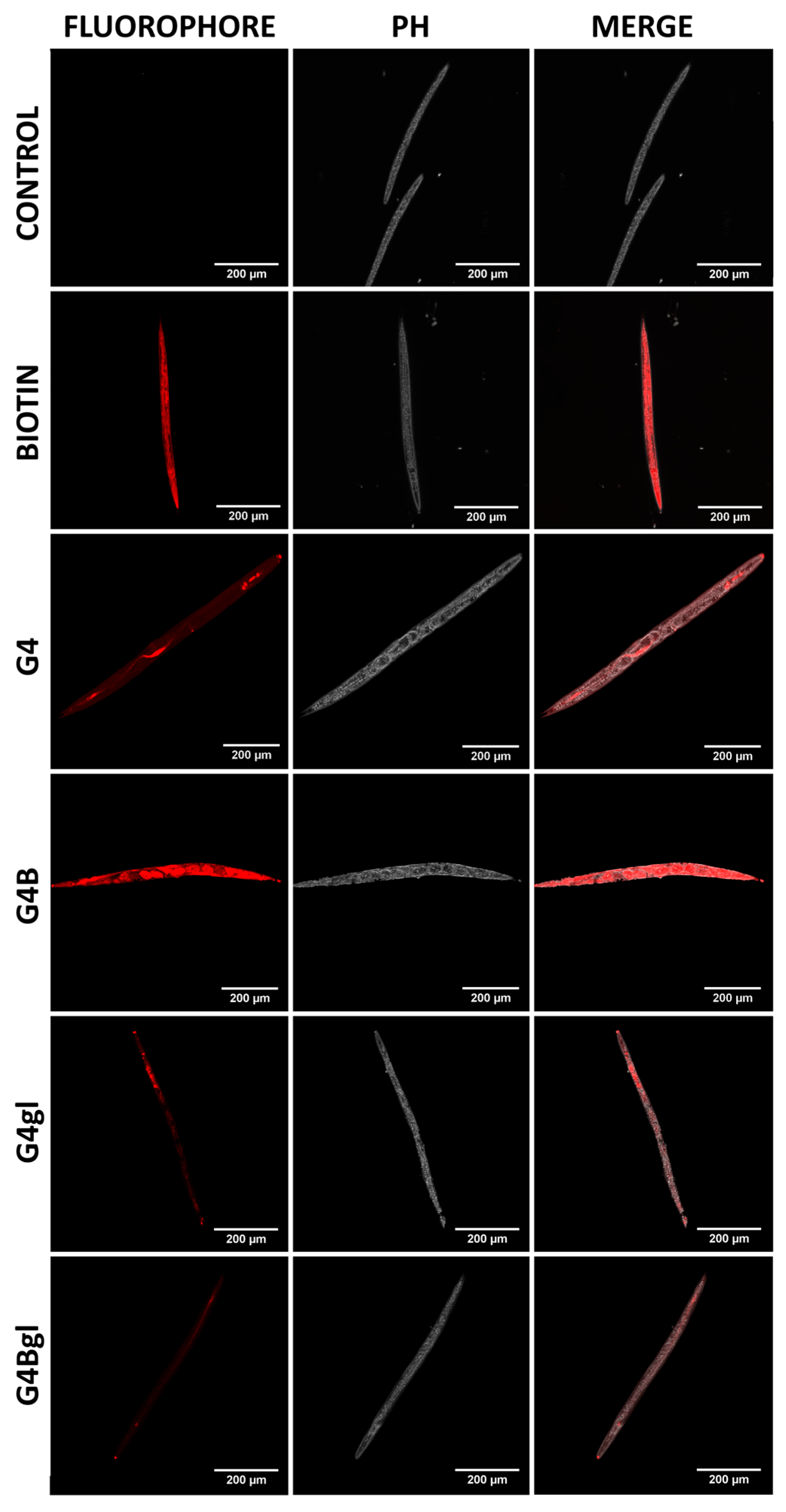
2.9. Penetration into the Rat’s Brain
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Syntheses
3.4. Cell Cultures
3.5. SMVT Expression
3.6. Cytotoxicity
3.7. In Silico Biotin-Binding Site Mapping
3.8. Time-Dependent Biotin Uptake
3.9. Time-Dependent Dendrimer Uptake
3.10. Proliferation and Dendrimer Accumulation
3.11. Toxicity against Caenorhabditis elegans
3.12. Penetration into the Rat Central Nervous System
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heley, K.; Vanderpool, R.C.; Vedham, V. Global Cancer Stigma Research: A U.S. National Cancer Institute Workshop Report. JNCI Monogr. 2024, 2024, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.S.; Trapani, D.; Cao, B.; Bray, F.; Murthy, S.; Kingham, T.P.; Are, C.; Ilbawi, A.M. Premature Mortality Trends in 183 Countries by Cancer Type, Sex, WHO Region, and World Bank Income Level in 2000–2019: A Retrospective, Cross-Sectional, Population-Based Study. Lancet Oncol. 2024, 25, 969–978. [Google Scholar] [CrossRef]
- Obrador, E.; Moreno-Murciano, P.; Oriol-Caballo, M.; López-Blanch, R.; Pineda, B.; Gutiérrez-Arroyo, J.L.; Loras, A.; Gonzalez-Bonet, L.G.; Martinez-Cadenas, C.; Estrela, J.M.; et al. Glioblastoma Therapy: Past, Present and Future. Int. J. Mol. Sci. 2024, 25, 2529. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in Adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) Consensus Review on Current Management and Future Directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Ahsan, H.; Asghar, M.; Malik, S.I. Potential Diagnostic and Drug Target Markers in Glioblastoma. Sci. Rep. 2024, 14, 7292. [Google Scholar] [CrossRef] [PubMed]
- Ganz, J.C. Low Grade Gliomas. Prog. Brain Res. 2022, 268, 271–277. [Google Scholar] [CrossRef]
- Salvato, I.; Marchini, A. Immunotherapeutic Strategies for the Treatment of Glioblastoma: Current Challenges and Future Perspectives. Cancers 2024, 16, 1276. [Google Scholar] [CrossRef]
- Mao, M.; Wu, Y.; He, Q. Recent Advances in Targeted Drug Delivery for the Treatment of Glioblastoma. Nanoscale 2024, 16, 8689–8707. [Google Scholar] [CrossRef]
- Su, X.; Li, Y.; Ren, Y.; Cao, M.; Yang, G.; Luo, J.; Hu, Z.; Deng, H.; Deng, M.; Liu, B.; et al. A New Strategy for Overcoming Drug Resistance in Liver Cancer: Epigenetic Regulation. Biomed. Pharmacother. 2024, 176, 116902. [Google Scholar] [CrossRef]
- Bakrania, A.; Joshi, N.; Zhao, X.; Zheng, G.; Bhat, M. Artificial Intelligence in Liver Cancers: Decoding the Impact of Machine Learning Models in Clinical Diagnosis of Primary Liver Cancers and Liver Cancer Metastases. Pharmacol. Res. 2023, 189, 106706. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhou, X.; Chu, Q. Mechanisms and Therapeutic Prospect of the JAK-STAT Signaling Pathway in Liver Cancer. Mol. Cell. Biochem. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Martinez, E.; Landazuri-Navas, S.; Vilchez, E.; Cantu-Hernandez, R.; Mosquera-Moscoso, J.; Encalada, S.; Iami, Z.A.; Zevallos-Delgado, C.; Cinicola, J. Prognostic Scores and Survival Rates by Etiology of Hepatocellular Carcinoma: A Review. J. Clin. Med. Res. 2023, 15, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, M.; Zhang, D.; Zhang, C.; Liu, P. Advances in Targeted Drug Resistance Associated with Dysregulation of Lipid Metabolism in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2024, 11, 113–129. [Google Scholar] [CrossRef]
- Ladd, A.D.; Duarte, S.; Sahin, I.; Zarrinpar, A. Mechanisms of Drug Resistance in HCC. Hepatology 2024, 79, 926–940. [Google Scholar] [CrossRef]
- Le Rhun, E.; Preusser, M.; Roth, P.; Reardon, D.A.; van den Bent, M.; Wen, P.; Reifenberger, G.; Weller, M. Molecular Targeted Therapy of Glioblastoma. Cancer Treat. Rev. 2019, 80, 101896. [Google Scholar] [CrossRef]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef]
- Marchal, S.; Hor, A.E.; Millard, M.; Gillon, V.; Bezdetnaya, L. Anticancer Drug Delivery: An Update on Clinically Applied Nanotherapeutics. Drugs 2015, 75, 1601–1611. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.-H.; Chen, Z.-S. Overcoming ABC Transporter-Mediated Multidrug Resistance: Molecular Mechanisms and Novel Therapeutic Drug Strategies. Drug Resist. Updates 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of Conventional Anti-Cancer Drugs as New Tools against Multidrug Resistant Tumors. Drug Resist. Updates 2020, 50, 100682. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Iyer, A.K. Recent Advances in Dendrimer-Based Nanovectors for Tumor-Targeted Drug and Gene Delivery. Drug Discov. Today 2015, 20, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Sarode, R.J.; Mahajan, H.S. Dendrimers for Drug Delivery: An Overview of Its Classes, Synthesis, and Applications. J. Drug Deliv. Sci. Technol. 2024, 98, 105896. [Google Scholar] [CrossRef]
- Chaudhari, H.S.; Popat, R.R.; Adhao, V.S.; Shrikhande, V.N. Dendrimers: Novel Carriers for Drug Delivery. J. Appl. Pharm. Res. 2016, 4, 01–19. [Google Scholar]
- Araújo, R.V.d.; Santos, S.d.S.; Igne Ferreira, E.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef]
- Ban, J.; Li, S.; Zhan, Q.; Li, X.; Xing, H.; Chen, N.; Long, L.; Hou, X.; Zhao, J.; Yuan, X. PMPC Modified PAMAM Dendrimer Enhances Brain Tumor-Targeted Drug Delivery. Macromol. Biosci. 2021, 21, 2000392. [Google Scholar] [CrossRef]
- Sadekar, S.; Ghandehari, H. Transepithelial Transport and Toxicity of PAMAM Dendrimers: Implications for Oral Drug Delivery. Adv. Drug Deliv. Rev. 2012, 64, 571–588. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Byrne, H.J. Polyamidoamine Dendrimer Nanoparticle Cytotoxicity, Oxidative Stress, Caspase Activation and Inflammatory Response: Experimental Observation and Numerical Simulation. Nanomedicine 2013, 9, 202–211. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Tamayo-Belda, M.; González-Pleiter, M.; Pulido-Reyes, G.; Martin-Betancor, K.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Mechanism of the Toxic Action of Cationic G5 and G7 PAMAM Dendrimers in the Cyanobacterium Anabaena sp. PCC7120. Environ. Sci. Nano 2019, 6, 863–878. [Google Scholar] [CrossRef]
- Naha, P.C.; Byrne, H.J. Generation of Intracellular Reactive Oxygen Species and Genotoxicity Effect to Exposure of Nanosized Polyamidoamine (PAMAM) Dendrimers in PLHC-1 Cells in Vitro. Aquat. Toxicol. 2013, 132–133, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Bodewein, L.; Schmelter, F.; Di Fiore, S.; Hollert, H.; Fischer, R.; Fenske, M. Differences in Toxicity of Anionic and Cationic PAMAM and PPI Dendrimers in Zebrafish Embryos and Cancer Cell Lines. Toxicol. Appl. Pharmacol. 2016, 305, 83–92. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM Dendrimers: Enhancing Efficacy and Mitigating Toxicity for Effective Anticancer Drug and Gene Delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Diaz, C.; Benitez, C.; Vidal, F.; Barraza, L.F.; Jiménez, V.A.; Guzman, L.; Fuentealba, J.; Yevenes, G.E.; Alderete, J.B. Cytotoxicity and in Vivo Plasma Kinetic Behavior of Surface-Functionalized PAMAM Dendrimers. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2227–2234. [Google Scholar] [CrossRef]
- Qi, X.; Fan, Y.; He, H.; Wu, Z. Hyaluronic Acid-Grafted Polyamidoamine Dendrimers Enable Long Circulation and Active Tumor Targeting Simultaneously. Carbohydr. Polym. 2015, 126, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Rai, D.B.; Solanki, R.; Patel, S.; Pooja, D.; Kulhari, H. Designing of Fucosylated Dendrimers as a Biocompatible Carrier for the Targeted Delivery of Chrysin to Human Lung Cancer Cells. Next Mater. 2024, 5, 100257. [Google Scholar] [CrossRef]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-Based Postnatal Therapy for Neuroinflammation and Cerebral Palsy in a Rabbit Model. Sci. Transl. Med. 2012, 4, 130ra46. [Google Scholar] [CrossRef]
- Sharma, A.; Liaw, K.; Sharma, R.; Spriggs, T.; Appiani La Rosa, S.; Kannan, S.; Kannan, R.M. Dendrimer-Mediated Targeted Delivery of Rapamycin to Tumor-Associated Macrophages Improves Systemic Treatment of Glioblastoma. Biomacromolecules 2020, 21, 5148–5161. [Google Scholar] [CrossRef]
- Tao, A.J.; Jiang, J.; Gadbois, G.E.; Goyal, P.; Boyle, B.T.; Mumby, E.J.; Myers, S.A.; English, J.G.; Ferguson, F.M. A Biotin Targeting Chimera (BioTAC) System to Map Small Molecule Interactomes in Situ. Nat. Commun. 2023, 14, 8016. [Google Scholar] [CrossRef]
- Pretzer, I.; Bushiri, D.; Weberskirch, R. Biotin-Functionalized Block Catiomers as an Active Targeting Approach in Gene Delivery. Macromol. Mater. Eng. 2023, 308, 2200627. [Google Scholar] [CrossRef]
- Raza, A.; Singh, A.; Amin, S.; Spallholz, J.E.; Sharma, A.K. Identification and Biotin Receptor-Mediated Activity of a Novel Seleno-Biotin Compound That Inhibits Viability of and Induces Apoptosis in Ovarian Cancer Cells. Chem. Biol. Interact. 2022, 365, 110071. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Javaid, F.; Chudasama, V. Advances in Targeting the Folate Receptor in the Treatment/Imaging of Cancers. Chem. Sci. 2018, 9, 790–810. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, V.; Lenaers, G.; Urbanski, G. Diagnostic and Therapeutic Perspectives Associated to Cobalamin-Dependent Metabolism and Transcobalamins’ Synthesis in Solid Cancers. Nutrients 2022, 14, 2058. [Google Scholar] [CrossRef] [PubMed]
- Bartmann, L.; Schumacher, D.; von Stillfried, S.; Sternkopf, M.; Alampour-Rajabi, S.; van Zandvoort, M.A.M.J.; Kiessling, F.; Wu, Z. Evaluation of Riboflavin Transporters as Targets for Drug Delivery and Theranostics. Front. Pharmacol. 2019, 10, 79. [Google Scholar] [CrossRef]
- Tripodo, G.; Mandracchia, D.; Collina, S.; Rui, M.; Rossi, D. New Perspectives in Cancer Therapy: The Biotin-Antitumor Molecule Conjugates. Med. Chem. 2014, 8, 1–4. [Google Scholar] [CrossRef]
- Peterson, C.T.; Rodionov, D.A.; Osterman, A.L.; Peterson, S.N. B Vitamins and Their Role in Immune Regulation and Cancer. Nutrients 2020, 12, 3380. [Google Scholar] [CrossRef]
- Karachaliou, C.-E.; Livaniou, E. Biotin Homeostasis and Human Disorders: Recent Findings and Perspectives. Int. J. Mol. Sci. 2024, 25, 6578. [Google Scholar] [CrossRef]
- Polyak, S.W. Mechanisms of Biotin Transport. Biochem. Anal. Biochem. 2015, 4, 1000210. [Google Scholar] [CrossRef]
- Hemmer, R.; Hall, A.; Spaulding, R.; Rossow, B.; Hester, M.; Caroway, M.; Haskamp, A.; Wall, S.; Bullen, H.A.; Morris, C.; et al. Analysis of Biotinylated Generation 4 Poly(Amidoamine) (PAMAM) Dendrimer Distribution in the Rat Brain and Toxicity in a Cellular Model of the Blood-Brain Barrier. Molecules 2013, 18, 11537–11552. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, Y.; Xu, T.; Wang, X.; Wen, L.-P. Targeting Cancer Cells with Biotin-Dendrimer Conjugates. Eur. J. Med. Chem. 2009, 44, 862–868. [Google Scholar] [CrossRef]
- Maiti, S.; Park, N.; Han, J.H.; Jeon, H.M.; Lee, J.H.; Bhuniya, S.; Kang, C.; Kim, J.S. Gemcitabine–Coumarin–Biotin Conjugates: A Target Specific Theranostic Anticancer Prodrug. J. Am. Chem. Soc. 2013, 135, 4567–4572. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Chen, J.; Chen, J.; Kuznetsova, L.; Wong, S.S.; Ojima, I. Mechanism-Based Tumor-Targeting Drug Delivery System. Validation of Efficient Vitamin Receptor-Mediated Endocytosis and Drug Release. Bioconjugate Chem. 2010, 21, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Bullen, H.A.; Hemmer, R.; Haskamp, A.; Cason, C.; Wall, S.; Spaulding, R.; Rossow, B.; Hester, M.; Caroway, M.; Haik, K.L. Evaluation of Biotinylated PAMAM Dendrimer Toxicity in Models of the Blood Brain Barrier: A Biophysical and Cellular Approach. J. Biomater. Nanobiotechnol. 2011, 2, 485–493. [Google Scholar] [CrossRef]
- Vadlapudi, A.D.; Vadlapatla, R.K.; Mitra, A.K. Sodium Dependent Multivitamin Transporter (SMVT): A Potential Target for Drug Delivery. Curr. Drug Targets 2012, 13, 994–1003. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Wróbel, K.; Wołowiec, S. Synthesis and Characterization of Fulvestrant and Paclitaxel Conjugates with Polyamidoamine Dendrimer Fourth Generation. Eur. J. Clin. Exp. Med. 2023, 21, 442–449. [Google Scholar] [CrossRef]
- Uram, Ł.; Wróbel, K.; Walczak, M.; Szymaszek, Ż.; Twardowska, M.; Wołowiec, S. Exploring the Potential of Lapatinib, Fulvestrant, and Paclitaxel Conjugated with Glycidylated PAMAM G4 Dendrimers for Cancer and Parasite Treatment. Molecules 2023, 28, 6334. [Google Scholar] [CrossRef]
- Albertazzi, L.; Gherardini, L.; Brondi, M.; Sulis Sato, S.; Bifone, A.; Pizzorusso, T.; Ratto, G.M.; Bardi, G. In Vivo Distribution and Toxicity of PAMAM Dendrimers in the Central Nervous System Depend on Their Surface Chemistry. Mol. Pharm. 2013, 10, 249–260. [Google Scholar] [CrossRef]
- Malinga-Drozd, M.; Uram, Ł.; Wróbel, K.; Wołowiec, S. Chiral Recognition of Homochiral Poly (Amidoamine) Dendrimers Substituted with R- and S-Glycidol by Keratinocyte (HaCaT) and Squamous Carcinoma (SCC-15) Cells In Vitro. Polymers 2021, 13, 1049. [Google Scholar] [CrossRef]
- Pacheco-Alvarez, D.; Solórzano-Vargas, R.S.; González-Noriega, A.; Michalak, C.; Zempleni, J.; León-Del-Río, A. Biotin Availability Regulates Expression of the Sodium-Dependent Multivitamin Transporter and the Rate of Biotin Uptake in HepG2 Cells. Mol. Genet. Metab. 2005, 85, 301–307. [Google Scholar] [CrossRef]
- Grafe, F.; Brandsch, M.; Wohlrab, W.; Neubert, R.H. Transport of Biotin in Human Keratinocytes. J. Investig. Dermatol. 2003, 120, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Takechi, R.; Taniguchi, A.; Ebara, S.; Fukui, T.; Watanabe, T. Biotin Deficiency Affects the Proliferation of Human Embryonic Palatal Mesenchymal Cells in Culture12. J. Nutr. 2008, 138, 680–684. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000138074-SLC5A6/subcellular#rna (accessed on 26 July 2024).
- Mosquera, J.; García, I.; Liz-Marzán, L.M. Cellular Uptake of Nanoparticles versus Small Molecules: A Matter of Size. Acc. Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Hassan, Y.; Wijeratne, S. Biotin and Biotinidase Deficiency. Expert Rev. Endocrinol. Metab. 2008, 3, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Madaan, K.; Lather, V.; Pandita, D. Evaluation of Polyamidoamine Dendrimers as Potential Carriers for Quercetin, a Versatile Flavonoid. Drug Deliv. 2016, 23, 254–262. [Google Scholar] [CrossRef]
- Teow, H.M.; Zhou, Z.; Najlah, M.; Yusof, S.R.; Abbott, N.J.; D’Emanuele, A. Delivery of Paclitaxel across Cellular Barriers Using a Dendrimer-Based Nanocarrier. Int. J. Pharm. 2013, 441, 701–711. [Google Scholar] [CrossRef]
- Myc, A.; Douce, T.B.; Ahuja, N.; Kotlyar, A.; Kukowska-Latallo, J.; Thomas, T.P.; Baker, J.R. Preclinical Antitumor Efficacy Evaluation of Dendrimer-Based Methotrexate Conjugates. Anticancer Drugs 2008, 19, 143–149. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Wang, M.; Xu, H.; Wang, Z.; Li, Y.; Ding, B.; Gao, J. Effects of the Surface Charge of Polyamidoamine Dendrimers on Cellular Exocytosis and the Exocytosis Mechanism in Multidrug-Resistant Breast Cancer Cells. J. Nanobiotechnol. 2021, 19, 135. [Google Scholar] [CrossRef]
- Patil, Y.B.; Toti, U.S.; Khdair, A.; Ma, L.; Panyam, J. Single-Step Surface Functionalization of Polymeric Nanoparticles for Targeted Drug Delivery. Biomaterials 2009, 30, 859–866. [Google Scholar] [CrossRef]
- Tripathi, R.; Guglani, A.; Ghorpade, R.; Wang, B. Biotin Conjugates in Targeted Drug Delivery: Is It Mediated by a Biotin Transporter, a yet to Be Identified Receptor, or (an)Other Unknown Mechanism(s)? J. Enzym. Inhib. Med. Chem. 2023, 38, 2276663. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM Dendrimer—Cell Membrane Interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liaw, K.; Gök, O.; DeRidder, L.B.; Kannan, S.; Kannan, R.M. Quantitative Assessment of Surface Functionality Effects on Microglial Uptake and Retention of PAMAM Dendrimers. J. Nanopart. Res. 2018, 20, 111. [Google Scholar] [CrossRef]
- Perumal, O.P.; Inapagolla, R.; Kannan, S.; Kannan, R.M. The Effect of Surface Functionality on Cellular Trafficking of Dendrimers. Biomaterials 2008, 29, 3469–3476. [Google Scholar] [CrossRef]
- Lee, H.M.; Joh, J.W.; Seo, S.-R.; Kim, W.-T.; Kim, M.K.; Choi, H.S.; Kim, S.Y.; Jang, Y.-J.; Sinn, D.H.; Choi, G.S.; et al. Cell-Surface Major Vault Protein Promotes Cancer Progression through Harboring Mesenchymal and Intermediate Circulating Tumor Cells in Hepatocellular Carcinomas. Sci. Rep. 2017, 7, 13201. [Google Scholar] [CrossRef]
- Said, H.M.; Redha, R.; Nylander, W. A Carrier-Mediated, Na+ Gradient-Dependent Transport for Biotin in Human Intestinal Brush-Border Membrane Vesicles. Am. J. Physiol. 1987, 253, G631–G636. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; Luo, S.; Balasubrahmanyam, B.; Pal, D.; Mitra, A.K. Biotin Uptake and Cellular Translocation in Human Derived Retinoblastoma Cell Line (Y-79): A Role of hSMVT System. Int. J. Pharm. 2006, 312, 43–52. [Google Scholar] [CrossRef]
- Chen, H.; Xie, L.Q.; Qin, J.; Jia, Y.; Cai, X.; Nan, W.; Yang, W.; Lv, F.; Zhang, Q.Q. Surface Modification of PLGA Nanoparticles with Biotinylated Chitosan for the Sustained in Vitro Release and the Enhanced Cytotoxicity of Epirubicin. Colloids Surf. B Biointerfaces 2016, 138, 1–9. [Google Scholar] [CrossRef]
- Patel, M.; Vadlapatla, R.K.; Shah, S.; Mitra, A.K. Molecular Expression and Functional Activity of Sodium Dependent Multivitamin Transporter in Human Prostate Cancer Cells. Int. J. Pharm. 2012, 436, 324–331. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans Model in Toxicity Testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef]
- Wittkowski, P.; Marx-Stoelting, P.; Violet, N.; Fetz, V.; Schwarz, F.; Oelgeschläger, M.; Schönfelder, G.; Vogl, S. Caenorhabditis elegans as a Promising Alternative Model for Environmental Chemical Mixture Effect Assessment—A Comparative Study. Environ. Sci. Technol. 2019, 53, 12725–12733. [Google Scholar] [CrossRef]
- Konopka, M.; Janaszewska, A.; Klajnert-Maculewicz, B. Intrinsic Fluorescence of PAMAM Dendrimers—Quenching Studies. Polymers 2018, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghpour, E.; Moztarzadeh, F.; Rabiee, M.; Tahriri, M.; Ashuri, M.; Sameie, H.; Salimi, R.; Moghadas, S. Micro-Emulsion Synthesis, Surface Modification, and Photophysical Properties of Zn(1-x) Mn(x)S Nanocrystals for Biomolecular Recognition. IEEE Trans. NanoBiosci. 2012, 11, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Liaw, K.; Zhang, F.; Mangraviti, A.; Kannan, S.; Tyler, B.; Kannan, R.M. Dendrimer Size Effects on the Selective Brain Tumor Targeting in Orthotopic Tumor Models upon Systemic Administration. Bioeng. Transl. Med. 2020, 5, e10160. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.A.; Fleming, J.T. Basic Culture Methods. Methods Cell Biol. 1995, 48, 3–29. [Google Scholar]
- Scanlan, L.D.; Lund, S.P.; Coskun, S.H.; Hanna, S.K.; Johnson, M.E.; Sims, C.M.; Brignoni, K.; Lapasset, P.; Petersen, E.J.; Elliott, J.T.; et al. Counting Caenorhabditis elegans: Protocol Optimization and Applications for Population Growth and Toxicity Studies in Liquid Medium. Sci. Rep. 2018, 8, 904. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uram, Ł.; Twardowska, M.; Szymaszek, Ż.; Misiorek, M.; Łyskowski, A.; Setkowicz, Z.; Rauk, Z.; Wołowiec, S. The Importance of Biotinylation for the Suitability of Cationic and Neutral Fourth-Generation Polyamidoamine Dendrimers as Targeted Drug Carriers in the Therapy of Glioma and Liver Cancer. Molecules 2024, 29, 4293. https://doi.org/10.3390/molecules29184293
Uram Ł, Twardowska M, Szymaszek Ż, Misiorek M, Łyskowski A, Setkowicz Z, Rauk Z, Wołowiec S. The Importance of Biotinylation for the Suitability of Cationic and Neutral Fourth-Generation Polyamidoamine Dendrimers as Targeted Drug Carriers in the Therapy of Glioma and Liver Cancer. Molecules. 2024; 29(18):4293. https://doi.org/10.3390/molecules29184293
Chicago/Turabian StyleUram, Łukasz, Magdalena Twardowska, Żaneta Szymaszek, Maria Misiorek, Andrzej Łyskowski, Zuzanna Setkowicz, Zuzanna Rauk, and Stanisław Wołowiec. 2024. "The Importance of Biotinylation for the Suitability of Cationic and Neutral Fourth-Generation Polyamidoamine Dendrimers as Targeted Drug Carriers in the Therapy of Glioma and Liver Cancer" Molecules 29, no. 18: 4293. https://doi.org/10.3390/molecules29184293
APA StyleUram, Ł., Twardowska, M., Szymaszek, Ż., Misiorek, M., Łyskowski, A., Setkowicz, Z., Rauk, Z., & Wołowiec, S. (2024). The Importance of Biotinylation for the Suitability of Cationic and Neutral Fourth-Generation Polyamidoamine Dendrimers as Targeted Drug Carriers in the Therapy of Glioma and Liver Cancer. Molecules, 29(18), 4293. https://doi.org/10.3390/molecules29184293






