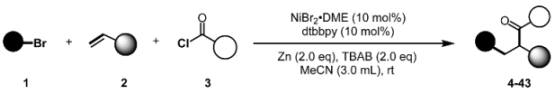
Nickel-Catalyzed Three-Component 1,2-Carboacylation of Alkenes
Abstract
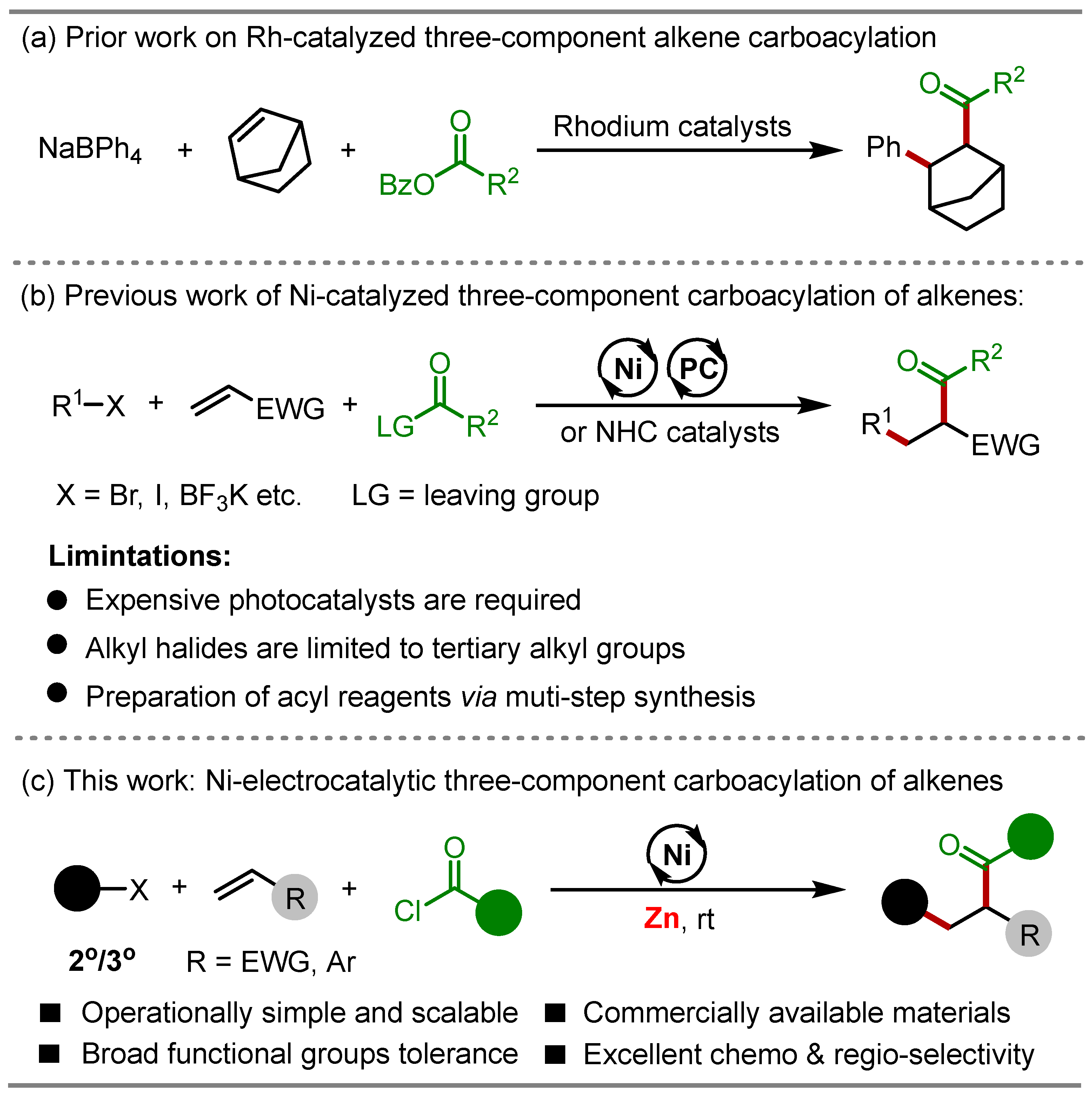
1. Introduction
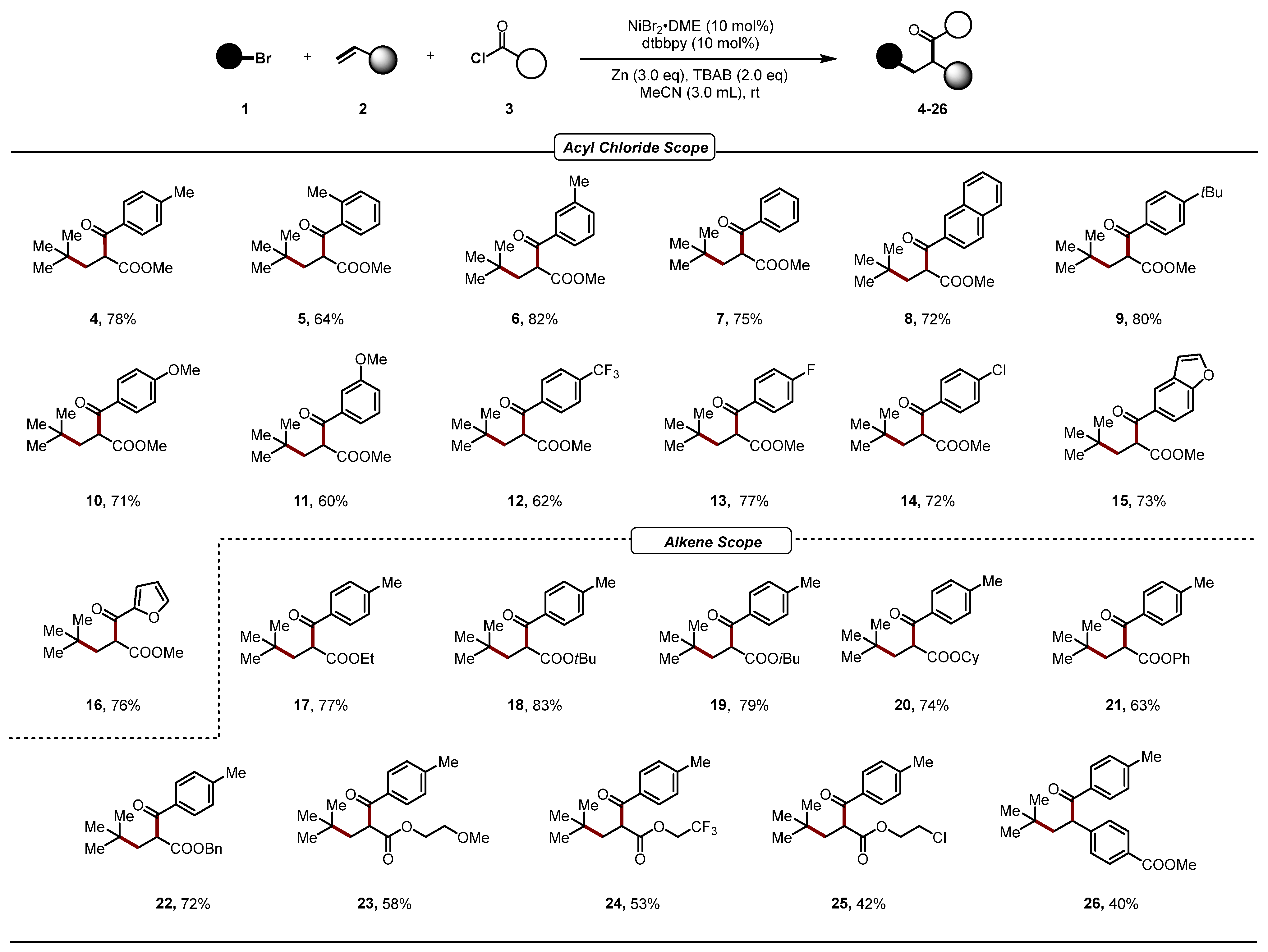
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
6. Characterization Data of Products
- Methyl-4,4-dimethyl-2-(4-methylbenzoyl)pentanoate (4). This compound was prepared according to the general procedures, 40.9 mg, 78% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.91 (d, J = 8.0 Hz, 2H), 7.28 (d, J = 8.6 Hz, 2H), 4.40 (t, J = 6.0 Hz, 1H), 3.67 (s, 3H), 2.42 (s, 3H), 2.03 (d, J = 6.0 Hz, 2H), 0.90 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 195.1, 171.1, 144.4, 133.5, 129.5, 128.9, 52.5, 50.5, 42.0, 30.9, 29.4, 21.7. HR-MS (ESI) C16H23O3 [M + H]+: 263.1642, found: 263.1644.
- Methyl-4,4-dimethyl-2-(2-methylbenzoyl)pentanoate (5). This compound was prepared according to the general procedures, 33.5 mg, 64% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.70 (d, J = 7.7 Hz, 1H), 7.39 (t, J = 7.4 Hz, 1H), 7.29–7.25 (m, 2H), 4.28 (dd, J = 7.0, 5.0 Hz, 1H), 3.68 (s, 3H), 2.47 (s, 3H), 2.04–1.91 (m, 2H), 0.89 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 198.9, 171.2, 139.1, 136.8, 132.1, 131.6, 128.3, 125.7, 53.4, 52.4, 41.7, 30.8, 29.2, 21.1. HR-MS (ESI) C16H23O3 [M + H]+: 263.1642, found: 263.1642.
- Methyl-4,4-dimethyl-2-(3-methylbenzoyl)pentanoate (6). This compound was prepared according to the general procedures, 43 mg, 82% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.80 (d, J = 7.2 Hz, 2H), 7.41–7.35 (m, 2H), 4.41 (t, J = 6.0 Hz, 1H), 3.68 (s, 3H), 2.42 (s, 3H), 2.03 (d, J = 6.0 Hz, 2H), 0.91 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 195.7, 171.1, 138.7, 136.0, 134.3, 129.2, 128.6, 125.9, 52.6, 50.7, 42.0, 30.9, 29.4, 21.4. HR-MS (ESI) C16H23O3 [M + H]+: 263.1642, found: 263.1644.
- Methyl-2-benzoyl-4,4-dimethylpentanoate (7). This compound was prepared according to the general procedures, 37.2 mg, 75% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.04–7.97 (m, 2H), 7.59 (t, J = 7.4 Hz, 1H), 7.52–7.45 (m, 2H), 4.42 (t, J = 6.0 Hz, 1H), 3.68 (s, 3H), 2.04 (d, J = 6.0 Hz, 2H), 0.91 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 195.5, 171.0, 136.0, 133.5, 128.8, 128.7, 52.6, 50.7, 42.0, 30.9, 29.4. HR-MS (ESI) C15H21O3 [M + H]+: 249.1485, found: 249.1486.
- Methyl-2-(2-naphthoyl)-4,4-dimethylpentanoate (8). This compound was prepared according to the general procedures, 43 mg, 72% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.56 (s, 1H), 8.06 (dd, J = 8.6, 1.7 Hz, 1H), 8.00 (d, J = 8.1 Hz, 1H), 7.90 (dd, J = 11.3, 8.4 Hz, 2H), 7.64–7.56 (m, 2H), 4.58 (t, J = 6.0 Hz, 1H), 3.69 (s, 3H), 2.10 (d, J = 5.9 Hz, 2H), 0.94 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 195.4, 171.1, 135.7, 133.4, 132.5, 130.6, 129.8, 128.8, 128.7, 127.8, 126.9, 124.3, 52.6, 50.8, 42.1, 31.0, 29.4. HR-MS (ESI) C19H23O3 [M + H]+: 299.1642, found: 299.1645.
- Methyl-2-(4-(tert-butyl)benzoyl)-4,4-dimethylpentanoate (9). This compound was prepared according to the general procedures, 48.7 mg, 80% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.96 (d, J = 8.5 Hz, 2H), 7.50 (d, J = 8.6 Hz, 2H), 4.42 (t, J = 6.0 Hz, 1H), 3.68 (s, 3H), 2.04 (d, J = 6.0 Hz, 2H), 1.35 (s, 9H), 0.91 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 195.0, 171.1, 157.3, 133.4, 128.7, 125.8, 52.5, 50.5, 42.0, 35.2, 31.1, 30.9, 29.4. HR-MS (ESI) C19H29O3 [M + H]+: 305.2111, found: 305.2112.
- Methyl-2-(4-methoxybenzoyl)-4,4-dimethylpentanoate (10). This compound was prepared according to the general procedures, 39.5 mg, 71% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.01 (d, J = 8.8 Hz, 2H), 6.96 (d, J = 8.8 Hz, 2H), 4.38 (t, J = 6.0 Hz, 1H), 3.88 (s, 3H), 3.67 (s, 3H), 2.06–1.99 (m, 2H), 0.91 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 193.9, 171.2, 163.8, 131.1, 129.0, 114.0, 55.5, 52.5, 50.4, 42.0, 30.9, 29.4. HR-MS (ESI) C16H23O4 [M + H]+: 279.1591, found: 279.1590.
- Methyl-2-(3-methoxybenzoyl)-4,4-dimethylpentanoate (11). This compound was prepared according to the general procedures, 33.4 mg, 60% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.60 (d, J = 7.7 Hz, 1H), 7.54–7.51 (m, 1H), 7.39 (t, J = 8.0 Hz, 1H), 7.13 (dd, J = 8.2, 2.1 Hz, 1H), 4.40 (t, J = 6.0 Hz, 1H), 3.86 (s, 3H), 3.68 (s, 3H), 2.03 (d, J = 6.0 Hz, 2H), 0.91 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 195.3, 171.0, 160.0, 137.4, 129.7, 121.2, 120.1, 113.0, 55.5, 52.6, 50.8, 42.0, 30.9, 29.4. HR-MS (ESI) C16H23O4 [M + H]+: 279.1591, found: 279.1591.
- Methyl-4,4-dimethyl-2-(4-(trifluoromethyl)benzoyl)pentanoate (12). This compound was prepared according to the general procedures, 39.2 mg, 62% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.11 (d, J = 8.1 Hz, 2H), 7.76 (d, J = 8.2 Hz, 2H), 4.39 (t, J = 6.0 Hz, 1H), 3.69 (s, 3H), 2.05 (d, J = 6.0 Hz, 2H), 0.91 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 194.6, 170.5, 138.8, 135.1, 129.1, 127.5, 125.9 (q, J = 4.0 HZ), 52.8, 51.0, 41.8, 30.9, 29.3. 19F NMR (376 MHz, Chloroform-d) δ -63.20. HR-MS (ESI) C16H20F3O3 [M + H]+: 317.1359, found: 317.1363.
- Methyl-2-(4-fluorobenzoyl)-4,4-dimethylpentanoate (13). This compound was prepared according to the general procedures, 41.0 mg, 77% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.07-8.03 (m, 2H), 7.20–7.11 (m, 2H), 4.38 (t, J = 6.0 Hz, 1H), 3.68 (s, 3H), 2.04 (d, J = 6.0 Hz, 2H), 0.91 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 193.8, 170.8, 165.9 (d, J = 255.7 Hz), 132.4 (d, J = 2.9 Hz), 131.4 (d, J = 9.4 Hz), 128.7 (d, J = 4.9 Hz), 115.9 (d, J = 21.9 Hz), 52.6, 50.7, 41.9, 30.8, 29.3. 19F NMR (376 MHz, Chloroform-d) δ -104.4. HR-MS (ESI) C15H20FO3 [M + H]+: 267.1391, found: 267.1392.
- Methyl-2-(4-chlorobenzoyl)-4,4-dimethylpentanoate (14). This compound was prepared according to the general procedures, 40.7 mg, 72% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.98–7.93 (m, 2H), 7.49–7.44 (m, 2H), 4.36 (t, J = 6.0 Hz, 1H), 3.68 (s, 3H), 2.03 (d, J = 6.0 Hz, 2H), 0.90 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 194.3, 170.7, 140.0, 134.3, 130.1, 129.1, 52.7, 50.7, 41.9, 30.8, 29.3. HR-MS (ESI) C15H20ClO3 [M + H]+: 283.1095, found: 283.1099.
- Methyl-2-(benzofuran-5-carbonyl)-4,4-dimethylpentanoate (15). This compound was prepared according to the general procedures, 42.1 mg, 73% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.33 (d, J = 1.5 Hz, 1H), 8.02 (dd, J = 8.7, 1.7 Hz, 1H), 7.71 (d, J = 2.1 Hz, 1H), 7.57 (d, J = 8.7 Hz, 1H), 6.90–6.88 (m, 1H), 4.51 (t, J = 6.0 Hz, 1H), 3.69 (s, 3H), 2.08 (dd, J = 6.0, 2.6 Hz, 2H), 0.92 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 194.9, 171.2, 157.7, 146.6, 131.6, 127.7, 125.5, 123.1, 111.8, 107.4, 52.6, 50.8, 42.2, 30.9, 29.4. HR-MS (ESI) C17H21O4 [M + H]+: 289.1434, found: 289.1440.
- Methyl-2-(furan-2-carbonyl)-4,4-dimethylpentanoate (16). This compound was prepared according to the general procedures, 36.2 mg, 76% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.64–7.62 (m, 1H), 7.31 (d, J = 3.6 Hz, 1H), 6.57 (dd, J = 3.6, 1.6 Hz, 1H), 4.22 (s, 1H), 3.69 (s, 3H), 2.02 (d, J = 6.0 Hz, 2H), 0.91 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 184.3, 170.7, 151.8, 147.0, 118.5, 112.6, 52.6, 50.9, 41.5, 30.8, 29.3. HR-MS (ESI) C12H17O4 [M + H]+: 225.1121, found: 225.1127.
- Ethyl-4,4-dimethyl-2-(4-methylbenzoyl)pentanoate (17). This compound was prepared according to the general procedures, 42.6 mg, 77% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.86–7.81 (m, 2H), 7.20 (d, J = 7.8 Hz, 2H), 4.29 (t, J = 6.0 Hz, 1H), 4.06 (q, J = 7.1 Hz, 2H), 2.34 (s, 3H), 1.94 (d, J = 5.9 Hz, 2H), 1.10 (t, J = 7.1 Hz, 3H), 0.84 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 194.1, 169.6, 143.2, 132.6, 128.4, 127.9, 60.3, 49.8, 40.9, 29.9, 28.4, 20.6, 12.9. HR-MS (ESI) C17H25O3 [M + H]+: 277.1798, found: 277.1800.
- Tert-butyl-4,4-dimethyl-2-(4-methylbenzoyl)pentanoate (18). This compound was prepared according to the general procedures, 50.5 mg, 83% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.90 (d, J = 8.2 Hz, 2H), 7.30–7.23 (m, 2H), 4.23 (t, J = 5.9 Hz, 1H), 2.42 (s, 3H), 1.98 (dd, J = 5.9, 1.2 Hz, 2H), 1.35 (s, 9H), 0.91 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 195.6, 169.8, 144.0, 133.9, 129.3, 128.9, 81.5, 52.0, 41.6, 30.8, 29.5, 27.7, 21.6. HR-MS (ESI) C19H29O3 [M + H]+: 305.2111, found: 305.2115.
- Isobutyl-4,4-dimethyl-2-(4-methylbenzoyl)pentanoate (19). This compound was prepared according to the general procedures, 48.1 mg, 79% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.92 (d, J = 8.1 Hz, 2H), 7.27 (d, J = 6.9 Hz, 2H), 4.39 (t, J = 6.0 Hz, 1H), 3.84 (d, J = 6.6 Hz, 2H), 2.42 (s, 3H), 2.10–1.99 (m, 2H), 1.85 (dt, J = 13.4, 6.7 Hz, 1H), 0.91 (s, 9H), 0.82 (d, J = 6.7 Hz, 6H). 13C NMR (101 MHz, Chloroform-d) δ 195.1, 170.7, 144.2, 133.7, 129.4, 128.9, 71.5, 50.7, 41.8, 30.8, 29.4, 27.6, 21.6, 18.9. HR-MS (ESI) C19H29O3 [M + H]+: 305.2111, found: 305.2114.
- Cyclohexyl-4,4-dimethyl-2-(4-methylbenzoyl)pentanoate (20). This compound was prepared according to the general procedures, 48.9 mg, 74% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.92 (d, J = 8.2 Hz, 2H), 7.27 (d, J = 4.9 Hz, 2H), 4.76 (td, J = 8.5, 3.9 Hz, 1H), 4.32 (t, J = 5.9 Hz, 1H), 2.42 (s, 3H), 2.01 (d, J = 6.0 Hz, 2H), 1.77–1.57 (m, 5H), 1.39–1.22 (m, 5H), 0.91 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 195.2, 170.1, 144.1, 133.7, 129.3, 128.9, 73.5, 51.2, 41.7, 31.2, 31.1, 30.9, 29.4, 25.3, 23.4, 23.4, 21.7. HR-MS (ESI) C21H31O3 [M + H]+: 331.2268, found: 331.2270.
- Phenyl-4,4-dimethyl-2-(4-methylbenzoyl)pentanoate (21). This compound was prepared according to the general procedures, 40.5 mg, 63% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.98 (d, J = 8.1 Hz, 2H), 7.34-7.29 (m, 4H), 7.18 (t, J = 7.4 Hz, 1H), 6.99 (d, J = 8.1 Hz, 2H), 4.59 (dd, J = 7.2, 4.7 Hz, 1H), 2.42 (s, 3H), 2.19 (dd, J = 14.4, 7.3 Hz, 1H), 2.05 (dd, J = 14.4, 4.6 Hz, 1H), 1.00 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 194.9, 169.4, 150.7, 144.6, 133.3, 129.6, 129.4, 129.0, 126.0, 121.3, 51.0, 41.9, 31.1, 29.5, 21.7. HR-MS (ESI) C21H25O3 [M + H]+: 325.1798, found: 325.1800.
- Benzyl-4,4-dimethyl-2-(4-methylbenzoyl)pentanoate (22). This compound was prepared according to the general procedures, 48.7 mg, 72% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.88 (d, J = 8.2 Hz, 2H), 7.29–7.19 (m, 7H), 5.14–5.07 (m, 2H), 4.41 (t, J = 6.0 Hz, 1H), 2.41 (s, 3H), 2.04 (d, J = 6.0 Hz, 2H), 0.89 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 195.0, 170.5, 144.3, 135.5, 133.5, 129.4, 128.9, 128.5, 128.2, 128.1, 67.1, 50.8, 41.9, 30.9, 29.4, 21.7. HR-MS (ESI) C22H27O3 [M + H]+: 339.1955, found: 339.1961.
- 2-Methoxyethyl-4,4-dimethyl-2-(4-methylbenzoyl)pentanoate (23). This compound was prepared according to the general procedures, 35.5 mg, 58% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.91 (d, J = 8.0 Hz, 2H), 7.29–7.26 (m, 2H), 4.43-4.40 (m, 1H), 4.30–4.19 (m, 2H), 3.55–3.47 (m, 2H), 3.28 (s, 3H), 2.42 (s, 3H), 2.07–1.96 (m, 2H), 0.92 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 195.0, 170.6, 144.3, 133.5, 129.4, 128.9, 70.2, 64.3, 58.8, 50.7, 41.9, 30.9, 29.4, 21.7. HR-MS (ESI) C18H27O4 [M + H]+: 307.1904, found: 307.1905.
- 2,2,2-Trifluoroethyl-4,4-dimethyl-2-(4-methylbenzoyl)pentanoate (24). This compound was prepared according to the general procedures, 35.0 mg, 53% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.93–7.88 (m, 2H), 7.28 (d, J = 7.7 Hz, 2H), 4.53 (dd, J = 7.0, 4.5 Hz, 1H), 2.51–2.44 (m, 2H), 2.42 (s, 3H), 2.20–2.15 (m, 1H), 1.84 (dd, J = 14.1, 4.5 Hz, 1H), 0.87 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 206.8, 196.1, 144.5, 134.0, 129.5, 128.9, 59.6, 41.8, 33.8, 31.1, 29.5, 21.6, 7.8. HR-MS (ESI) C17H22F3O3 [M + H]+: 331.1516, found: 331.1520.
- 2-Chloroethyl-4,4-dimethyl-2-(4-methylbenzoyl)pentanoate (25). This compound was prepared according to the general procedures, 26.1 mg, 42% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.91 (d, J = 8.2 Hz, 2H), 7.28 (d, J = 8.3 Hz, 2H), 4.43 (dd, J = 6.7, 5.3 Hz, 1H), 4.33 (4.37-4.28, m, 2H), 3.65–3.56 (m, 2H), 2.42 (s, 3H), 2.08–1.98 (m, 2H), 0.92 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 194.8, 170.3, 144.5, 133.3, 129.5, 128.9, 64.7, 50.5, 41.9, 41.2, 30.9, 29.4, 21.7. HR-MS (ESI) C17H24ClO3 [M + H]+: 311.1408, found: 311.1410.
- Methyl-4-(4,4-dimethyl-1-oxo-1-(p-tolyl)pentan-2-yl)benzoate (26). This compound was prepared according to the general procedures, 27.1 mg, 40% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.95–7.88 (m, 4H), 7.39 (d, J = 8.3 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 4.78-4.75 (m, 1H), 3.91-3.87 (m, 4H), 2.63-2.58 (m, 1H), 2.37 (s, 3H), 0.88 (s, 9H). 13C NMR (101 MHz, Chloroform-d) δ 199.0, 166.9, 146.5, 143.9, 134.2, 130.2, 129.4, 129.3, 128.7, 128.2, 52.1, 49.5, 47.4, 31.3, 29.8, 21.6. HR-MS (ESI) C22H27O3 [M + H]+: 339.1955, found: 339.1955.
- Methyl-6-(benzyloxy)-4,4-dimethyl-2-(4-methylbenzoyl)hexanoate (27). This compound was prepared according to the general procedures, 47.4 mg, 62% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.99–7.85 (m, 2H), 7.39–7.19 (m, 7H), 4.45 (d, J = 3.3 Hz, 2H), 3.73–3.48 (m, 5H), 2.41 (d, J = 2.9 Hz, 3H), 2.12–2.00 (m, 2H), 1.72 (s, 1H), 1.61-1.58 (m, 2H), 0.89 (d, J = 4.6 Hz, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.9, 171.0, 144.4, 138.5, 133.5, 129.5, 128.9, 128.3, 127.6, 127.5, 73.0, 67.1, 52.6, 50.0, 41.1, 40.5, 32.7, 27.4, 27.3, 21.7. HR-MS (ESI) C24H31O4 [M + H]+: 383.2217, found: 383.2222.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl benzoate (28). This compound was prepared according to the general procedures, 62.6 mg, 79% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.03–7.98 (m, 2H), 7.95–7.90 (m, 2H), 7.57–7.51 (m, 1H), 7.43-7.40 (m, 2H), 7.29–7.25 (m, 2H), 4.46 (t, J = 6.0 Hz, 1H), 4.41-4.38 (m, 2H), 3.66 (s, 3H), 2.41 (s, 3H), 2.21–2.09 (m, 2H), 1.73 (t, J = 7.0 Hz, 2H), 0.97 (s, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.7, 170.9, 166.6, 144.5, 133.4, 132.9, 130.3, 129.5, 128.9, 128.3, 62.0, 52.6, 49.9, 40.5, 40.2, 32.8, 27.1, 27.0, 21.7. HR-MS (ESI) C24H29O5 [M + H]+: 397.2010, found: 397.2011.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl 2-naphthoate (29). This compound was prepared according to the general procedures, 63.4 mg, 71% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.49 (s, 1H), 7.94 (dd, J = 8.6, 1.7 Hz, 1H), 7.85 (dd, J = 10.4, 8.5 Hz, 3H), 7.78 (dd, J = 8.3, 3.6 Hz, 2H), 7.52–7.44 (m, 2H), 7.17 (d, J = 7.8 Hz, 2H), 4.41–4.33 (m, 3H), 3.58 (s, 3H), 2.31 (s, 3H), 2.17–2.05 (m, 2H), 1.71 (t, J = 7.2 Hz, 2H), 0.92 (s, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.7, 171.0, 166.7, 144.5, 135.5, 133.5, 132.5, 131.0, 129.5, 129.4, 128.9, 128.2, 128.1, 127.7, 127.6, 126.6, 125.2, 62.1, 52.6, 50.0, 40.5, 40.3, 32.9, 27.1, 27.0, 21.7. HR-MS (ESI) C28H31O5 [M + H]+: 447.2166, found: 447.2175.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl 4-methylbenzoate (30). This compound was prepared according to the general procedures, 67.3 mg, 82% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.93-7.88 (m, 4H), 7.27 (d, J = 7.1 Hz, 2H), 7.21 (d, J = 7.9 Hz, 2H), 4.45 (t, J = 6.0 Hz, 1H), 4.38-4.32 (m, 2H), 3.66 (s, 3H), 2.40 (d, J = 5.7 Hz, 6H), 2.20–2.08 (m, 2H), 1.72 (t, J = 7.2 Hz, 2H), 0.97 (s, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.7, 170.9, 166.6, 144.5, 143.5, 133.5, 129.5, 129.5, 129.0, 128.9, 127.6, 61.7, 52.6, 50.0, 40.6, 40.2, 32.8, 27.1, 27.0, 21.7, 21.6. HR-MS (ESI) C25H31O5 [M + H]+: 411.2166, found: 411.2171.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl 3-methylbenzoate (31). This compound was prepared according to the general procedures, 63.2 mg, 77% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.95–7.90 (m, 2H), 7.84–7.77 (m, 2H), 7.37–7.26 (m, 4H), 4.47–4.32 (m, 3H), 3.66 (s, 3H), 2.40 (d, J = 7.6 Hz, 6H), 2.21–2.11 (m, 2H), 1.73 (t, J = 7.3 Hz, 2H), 0.97 (s, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.7, 170.9, 166.8, 144.5, 138.1, 133.6, 133.4, 130.2, 130.1, 129.5, 128.9, 128.2, 126.7, 61.9, 52.6, 50.0, 40.5, 40.2, 32.8, 27.1, 27.0, 21.7, 21.3. HR-MS (ESI) C25H31O5 [M + H]+: 411.2166, found: 411.2171.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl 2-methylbenzoate (32). This compound was prepared according to the general procedures, 62.4 mg, 76% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.84 (d, J = 8.3 Hz, 2H), 7.78 (d, J = 8.0 Hz, 1H), 7.32–7.28 (m, 1H), 7.21–7.18 (m, 2H), 7.15 (d, J = 7.5 Hz, 2H), 4.38 (t, J = 6.0 Hz, 1H), 4.31–4.21 (m, 2H), 3.58 (s, 3H), 2.50 (s, 3H), 2.34 (s, 3H), 2.12–2.01 (m, 2H), 1.67–1.62 (m, 2H), 0.89 (s, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.7, 170.9, 167.6, 144.5, 140.1, 133.5, 131.9, 131.7, 130.5, 129.5, 128.9, 125.7, 61.7, 52.6, 49.9, 40.5, 40.2, 32.8, 27.1, 27.0, 21.7, 21.7. HR-MS (ESI) C25H31O5 [M + H]+: 411.2166, found: 411.2171.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl 4-methoxybenzoate (33). This compound was prepared according to the general procedures, 75.0 mg, 64% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.99–7.90 (m, 4H), 7.27 (d, J = 6.8 Hz, 2H), 6.89 (d, J = 8.9 Hz, 2H), 4.45 (t, J = 6.0 Hz, 1H), 4.39-4.29 (m, 2H), 3.85 (s, 3H), 3.66 (s, 3H), 2.41 (s, 3H), 2.20–2.08 (m, 2H), 1.71 (t, J = 7.2 Hz, 2H), 0.96 (s, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.7, 170.9, 166.3, 163.3, 144.5, 133.5, 131.5, 129.5, 128.9, 122.8, 113.6, 61.6, 55.4, 52.6, 50.0, 40.6, 40.2, 32.8, 27.1, 27.0, 21.7. HR-MS (ESI) C25H31O6 [M + H]+: 427.2115, found: 427.2120.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl 4-(trifluoromethyl)benzoate (34). This compound was prepared according to the general procedures, 70.6 mg, 76% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.11 (d, J = 8.1 Hz, 2H), 7.92 (d, J = 8.0 Hz, 2H), 7.68 (d, J = 8.2 Hz, 2H), 7.29–7.26 (m, 2H), 4.47–4.37 (m, 3H), 3.67 (s, 3H), 2.41 (s, 3H), 2.22–2.10 (m, 2H), 1.74 (t, J = 7.2 Hz, 2H), 0.98 (d, J = 2.1 Hz, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.5, 170.9, 165.3, 144.6, 134.5, 134.2, 133.5, 133.4, 129.9, 129.5, 128.9, 128.1, 125.4, 125.4, 125.3, 62.5, 52.7, 50.0, 40.4, 40.2, 32.8, 27.1, 27.0, 21.6. 19F NMR (376 MHz, Chloroform-d) δ −63.11. HR-MS (ESI) C25H28F3O5 [M + H]+: 465.1883, found: 465.1889.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl 4-cyanobenzoate (35). This compound was prepared according to the general procedures, 53.1 mg, 63% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.02 (d, J = 8.6 Hz, 2H), 7.84 (d, J = 8.3 Hz, 2H), 7.64 (d, J = 8.6 Hz, 2H), 7.20 (d, J = 8.0 Hz, 2H), 4.39–4.30 (m, 3H), 3.59 (s, 3H), 2.34 (s, 3H), 2.15–2.00 (m, 2H), 1.69–1.64 (m, 2H), 0.90 (d, J = 4.3 Hz, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.5, 170.9, 164.9, 144.6, 134.1, 133.4, 132.2, 130.0, 129.5, 128.9, 118.0, 116.3, 62.8, 52.7, 49.9, 40.3, 40.2, 32.8, 27.1, 27.0, 21.7. HR-MS (ESI) C25H28NO5 [M + H]+: 422.1962, found: 422.1966.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl 4-fluorobenzoate (36). This compound was prepared according to the general procedures, 58.9 mg, 71% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 8.04–7.99 (m, 2H), 7.95–7.89 (m, 2H), 7.28 (d, J = 7.5 Hz, 2H), 7.09 (t, J = 8.6 Hz, 2H), 4.47–4.32 (m, 3H), 3.67 (s, 3H), 2.42 (s, 3H), 2.21-2.01 (m, 2H), 1.72 (t, J = 7.2 Hz, 2H), 0.97 (s, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.6, 170.9, 167.0, 165.6, 164.4, 144.5, 133.4, 132.1, 132.0, 129.5, 128.9, 126.6, 126.5, 115.6, 115.3, 62.1, 52.6, 50.0, 40.5, 40.2, 32.8, 27.1, 27.0, 21.7. HR-MS (ESI) C24H28FO5 [M + H]+: 415.1915, found:415.1918.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl 4-chlorobenzoate (37). This compound was prepared according to the general procedures, 56.0 mg, 65% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.94–7.91 (m, 4H), 7.38 (d, J = 8.6 Hz, 2H), 7.29–7.26 (m, 2H), 4.47–4.34 (m, 3H), 3.66 (s, 3H), 2.41 (s, 3H), 2.21–2.09 (m, 2H), 1.73 (d, J = 7.0 Hz, 2H), 0.97 (s, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.6, 170.9, 165.7, 144.5, 139.3, 133.4, 130.9, 129.5, 128.9, 128.8, 128.7, 62.2, 52.6, 50.0, 40.4, 40.2, 32.8, 27.1, 27.0, 21.7. HR-MS (ESI) C24H28ClO5 [M + H]+: 431.1620, found: 431.1627.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl thiophene-2-carboxylate (38). This compound was prepared according to the general procedures, 46.7 mg, 58% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.92 (d, J = 8.1 Hz, 2H), 7.76 (dd, J = 3.8, 1.2 Hz, 1H), 7.53 (dd, J = 5.0, 1.2 Hz, 1H), 7.28 (d, J = 7.6 Hz, 2H), 7.08 (dd, J = 5.0, 3.8 Hz, 1H), 4.44 (t, J = 6.0 Hz, 1H), 4.39-4.31 (m, 2H), 3.67 (s, 3H), 2.41 (s, 3H), 2.19–2.09 (m, 2H), 1.70 (t, J = 7.2 Hz, 2H), 0.96 (s, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.7, 170.9, 162.2, 144.5, 133.9, 133.5, 133.3, 132.3, 129.5, 128.9, 127.7, 62.1, 52.6, 49.9, 40.5, 40.1, 32.8, 27.1, 27.0, 21.7. HR-MS (ESI) C22H27SO5 [M + H]+: 403.1574, found: 403.1575.
- 6-methoxy-3,3-dimethyl-5-(4-methylbenzoyl)-6-oxohexyl furan-2-carboxylate (39). This compound was prepared according to the general procedures, 43.3 mg, 56% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.93–7.90 (m, 2H), 7.53-7.47(m, 1H), 7.29–7.26 (m, 2H), 7.21-7.17 (m, 1H), 7.15–7.06 (m, 1H), 4.48–4.33 (m, 3H), 3.66 (s, 3H), 2.41 (s, 3H), 2.22–2.07 (m, 2H), 1.73 (t, J = 7.1 Hz, 2H), 0.96 (d, J = 1.9 Hz, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.7, 170.9, 144.5, 134.4, 134.3, 133.4, 132.0, 129.5, 128.9, 123.9, 123.9, 117.1, 116.8, 62.3, 52.6, 49.9, 40.5, 40.0, 32.8, 27.1, 27.0, 21.7. HR-MS (ESI) C22H27O6 [M + H]+: 387.1802, found: 387.1810.
- Methyl-4-methyl-2-(4-methylbenzoyl)pentanoate (40). This compound was prepared according to the general procedures, 31.8 mg, 64% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.90 (d, J = 8.0 Hz, 2H), 7.28 (d, J = 7.7 Hz, 2H), 4.43–4.39 (m, 1H), 3.68 (s, 3H), 2.42 (s, 3H), 1.99–1.82 (m, 2H), 1.67-1.56 (m, 1H), 0.96–0.91 (m, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.9, 170.8, 144.5, 133.7, 129.5, 128.7, 52.4, 52.1, 37.9, 26.4, 22.6, 22.3, 21.7. HR-MS (ESI) C15H21O3 [M + H]+: 249.1485, found: 249.1485.
- Methyl 2-(cyclopentylmethyl)-3-oxo-3-(p-tolyl)propanoate (41). This compound was prepared according to the general procedures, 36.7 mg, 67% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.88 (d, J = 7.9 Hz, 2H), 7.26 (d, J = 7.6 Hz, 2H), 4.44–4.40 (m, 1H), 3.69 (s, 3H), 2.43 (s, 3H), 2.00–1.82 (m, 2H), 1.67-1.56 (m, 1H), 0.95-0.91 (m, 6H). 13C NMR (101 MHz, Chloroform-d) δ 195.0, 170.7, 144.5, 132.7, 129.5, 127.7, 52.3, 52.2, 37.8, 26.4, 22.5, 22.3, 21.6. HR-MS (ESI) C17H23O3 [M + H]+: 275.1642, found: 275.1645.
- Methyl 2-(cyclohexylmethyl)-3-oxo-3-(p-tolyl)propanoate (42). This compound was prepared according to the general procedures, 41.4 mg, 72% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.91 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 7.8 Hz, 2H), 4.44–4.39 (m, 1H), 3.67 (s, 3H), 2.43 (s, 3H), 2.00–1.82 (m, 2H), 1.68-1.57 (m, 1H), 0.97–0.92 (m, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.9, 170.8, 144.5, 134.1, 129.5, 128.7, 52.4, 52.1, 37.9, 26.4, 22.6, 22.3, 21.7. HR-MS (ESI) C18H25O3 [M + H]+: 289.1789, found: 289.1792.
- Methyl 2-(bicyclo[2.2.1]heptan-2-ylmethyl)-3-oxo-3-(p-tolyl)propanoate (43). This compound was prepared according to the general procedures, 36.6 mg, 61% yield as a colorless oil. 1H NMR (400 MHz, Chloroform-d) δ 7.90 (d, J = 87.9 Hz, 2H), 7.28 (d, J = 7.8 Hz, 2H), 4.43-4.39 (m, 1H), 3.69 (s, 3H), 2.42 (s, 3H), 1.97–1.82 (m, 2H), 1.67–1.57 (m, 1H), 0.96–0.92 (m, 6H). 13C NMR (101 MHz, Chloroform-d) δ 194.9, 170.7, 144.6, 133.8, 129.6, 128.8, 52.4, 52.1, 38.0, 26.4, 22.6, 22.4, 21.7. HR-MS (ESI) C19H25O3 [M + H]+: 301.1798, found: 301.1781.
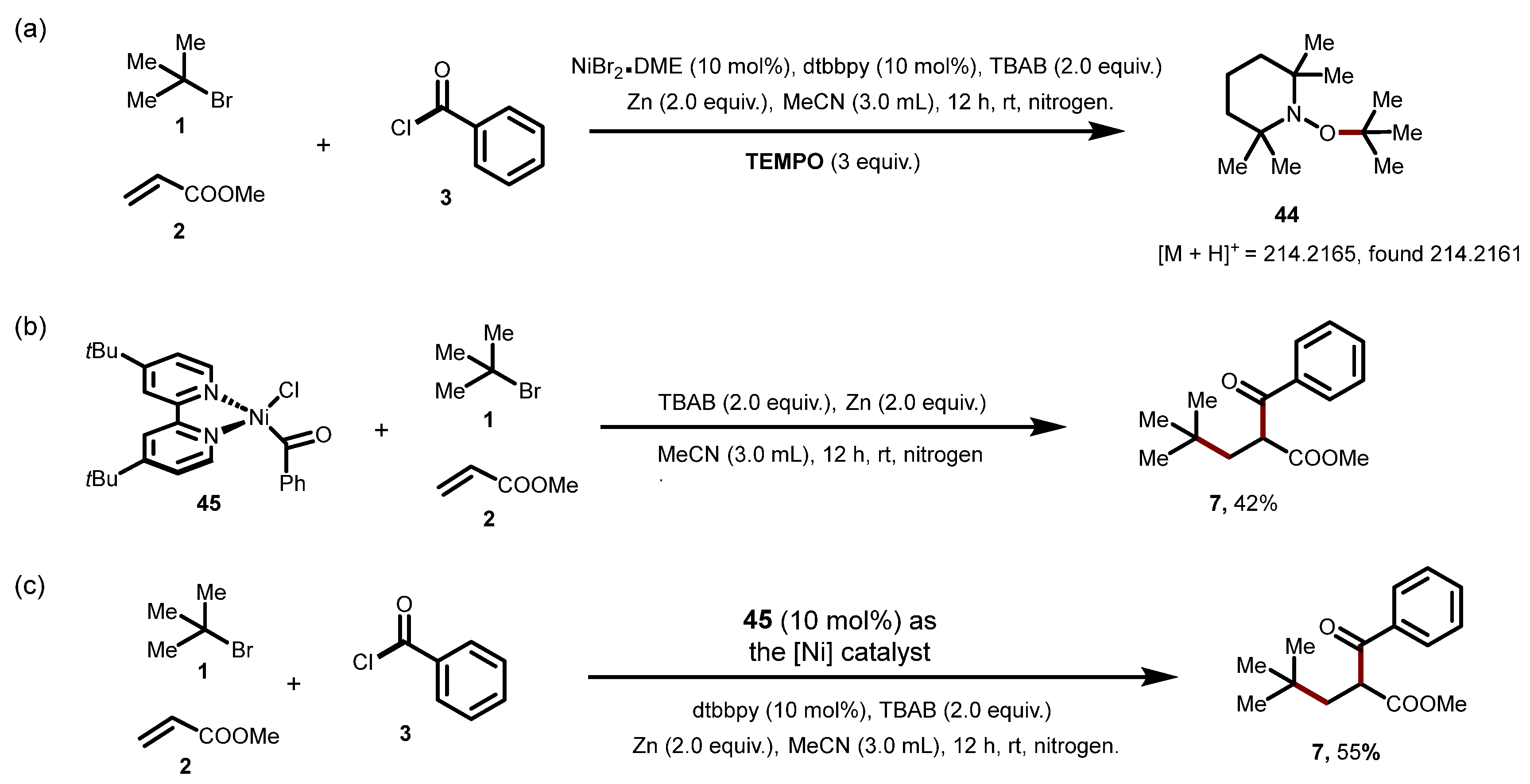
7. Mechanistic Investigations
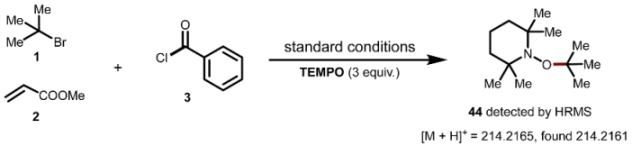
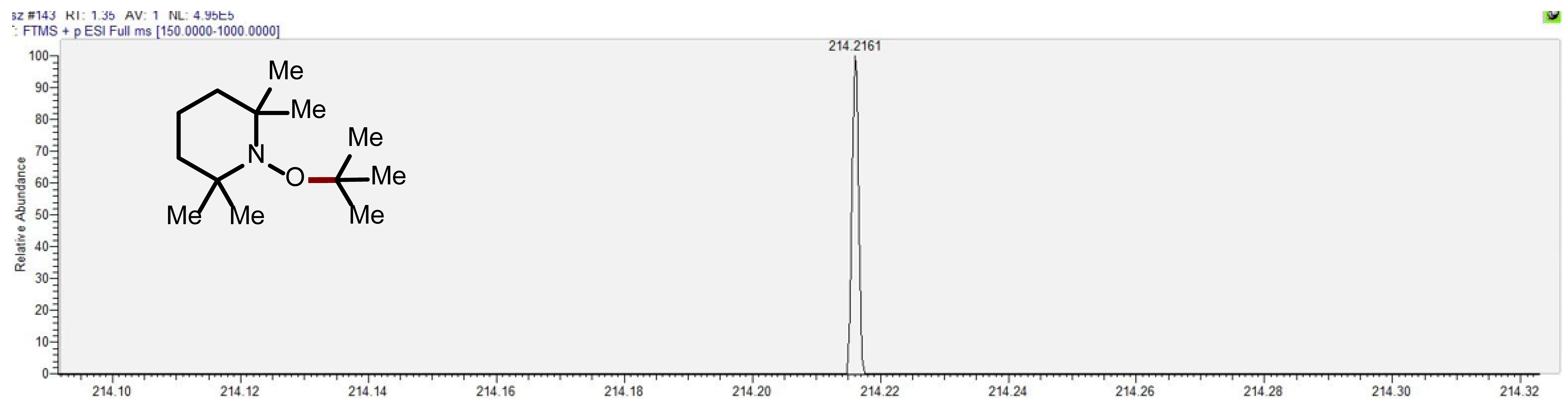
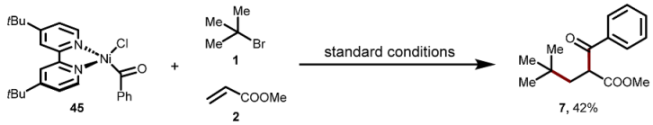

Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurth, M.C.; Adler, C.H.; St. Hilaire, M.; Singer, C.; Waters, C.; Le Witt, P.; Chernik, D.A.; Dorflinger, E.E.; Yoo, K. Tolcapone Improves Motor Function and Reduces Levodopa Requirement in Patients with Parkinson’s Disease Experiencing Motor Fluctuations: A Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. Neurology 1997, 48, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.W. Structure-based design of agrochemicals. Nat. Prod. Rep. 2002, 19, 278–291. [Google Scholar] [CrossRef]
- Koh, K.K.; Han, S.H.; Quon, M.J.; Yeal Ahn, J.; Shin, E.K. Beneficial Effects of Fenofibrate to Improve Endothelial Dysfunction and Raise Adiponectin Levels in Patients With Primary Hypertriglyceridemia. Diabetes Care 2005, 28, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.-Q.; Lim, H.N.; Dong, G. Intramolecular Acetyl Transfer to Olefins by Catalytic C-C Bond Activation of Unstrained Ketones. Angew. Chem. Int. Ed. 2018, 57, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.-H.; Song, R.-J.; Li, J.-H. Iron-Catalyzed Oxidative 1,2-Carboacylation of Activated Alkenes with Alcohols: A Tandem Route to 3-(2-Oxoethyl)-indolin-2-ones. Eur. J. Org. Chem. 2014, 2014, 3395–3401. [Google Scholar] [CrossRef]
- Walker, J.A., Jr.; Vickerman, K.L.; Humke, J.N.; Stanley, L.M. Ni-Catalyzed Alkene Carboacylation via Amide C-N Bond Activation. J. Am. Chem. Soc. 2017, 139, 10228–10231. [Google Scholar] [CrossRef]
- Deng, L.; Fu, Y.; Lee, S.Y.; Wang, C.; Liu, P.; Dong, G. Kinetic Resolution via Rh-Catalyzed C-C Activation of Cyclobutanones at Room Temperature. J. Am. Chem. Soc. 2019, 141, 16260–16265. [Google Scholar] [CrossRef]
- Oguma, K.; Miura, M.; Satoh, T.; Nomura, M. Rhodium Catalyzed Coupling of Sodium Tetraphenylborate with Acid Anhydrides in the Presence or Absence of Norbornene. J. Organomet. Chem. 2002, 648, 297–301. [Google Scholar] [CrossRef]
- Giri, R.; KC, S. Strategies toward Dicarbofunctionalization of Unactivated Olefins by Combined Heck Carbometalation and Cross-Coupling. J. Org. Chem. 2018, 83, 3013–3022. [Google Scholar] [CrossRef]
- Mizutani, K.; Shinokubo, H.; Oshima, K. Cobalt-Catalyzed Three-Component Coupling Reaction of Alkyl Halides, 1,3-Dienes, and Trimethylsilylmethylmagnesium Chloride. Org. Lett. 2003, 5, 3959–3961. [Google Scholar] [CrossRef]
- Terao, J.; Kato, Y.; Kambe, N. Titanocene-Catalyzed Regioselective Alkylation of Styrenes with Grignard Reagents Using β-Bromoethyl Ethers, Thioethers, or Amines. Chem.—Asian J. 2008, 3, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, F.; Wan, X.; Wang, D.; Chen, P.; Liu, G. Asymmetric Cu-Catalyzed Intermolecular Trifluoromethylarylation of Styrenes: Enantioselective Arylation of Benzylic Radicals. J. Am. Chem. Soc. 2017, 139, 2904–2907. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-S.; Li, T.-T.; Liu, J.-R.; Jiao, G.-Y.; Gu, Q.-S.; Cheng, J.-T.; Guo, Y.-L.; Hong, X.; Liu, X.-Y. Cu/Chiral Phosphoric Acid-Catalyzed Asymmetric Three-Component Radical-Initiated 1,2-Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2019, 141, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Klauck, F.J.R.; Yoon, H.; James, M.J.; Lautens, M.; Glorius, F. Visible-Light-MediatedDeaminative Three-Component Dicarbofunctionalization of Styrenes with Benzylic Radicals. ACS Catal. 2019, 9, 236–241. [Google Scholar] [CrossRef]
- Mega, R.S.; Duong, V.K.; Noble, A.; Aggarwal, V.K. Decarboxylative Conjunctive Cross-coupling of Vinyl Boronic Esters using Metallaphotoredox Catalysis. Angew. Chem. Int. Ed. 2020, 59, 4375. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, H.; Chen, B.; Xu, M.; Zhang, G. Copper-Catalyzed Enantioselective Arylalkynylation of Alkenes. Chem. Sci. 2020, 11, 1623–1628. [Google Scholar] [CrossRef]
- Sun, S.-Z.; Duan, Y.; Mega, R.S.; Somerville, R.J.; Martin, R. Site-Selective 1,2-Dicarbofunctionalization of Vinyl Boronates through Dual Catalysis. Angew. Chem. Int. Ed. 2020, 59, 4370–4374. [Google Scholar] [CrossRef]
- Yang, T.; Chen, X.; Rao, W.; Koh, M.J. Broadly Applicable Directed Catalytic Reductive Difunctionalization of Alkenyl Carbonyl Compounds. Chem 2020, 6, 738–751. [Google Scholar] [CrossRef]
- Zheng, Y.-L.; Newman, S.G. Nickel-Catalyzed Domino Heck-Type Reactions Using Methyl Esters as Cross-Coupling Electrophiles. Angew. Chem. Int. Ed. 2019, 58, 18159–18164. [Google Scholar] [CrossRef]
- Xu, S.; Wang, K.; Kong, W. Ni-Catalyzed Reductive Arylacylation of Alkenes toward Carbonyl-Containing Oxindoles. Org. Lett. 2019, 21, 7498–7503. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C. Nickel-Catalyzed Three-Component Reductive Alkylacylation of Electron-Deficient Activated Alkenes. Org. Lett. 2020, 22, 8829–8835. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.A.; Metz, T.L.; Qian, Y.; Stanley, L.M. Ni-Catalyzed Three-Component Alkene Carboacylation Initiated by Amide C-N Bond Activation. ACS Catal. 2019, 9, 5651–5656. [Google Scholar] [CrossRef]
- Ping, Y.; Kong, W. Ni-Catalyzed Reductive Difunctionalization of Alkenes. Synthesis 2020, 52, 979–992. [Google Scholar] [CrossRef]
- Luo, Y.-C.; Xu, C.; Zhang, X. Nickel-Catalyzed Dicarbofunctionalization of Alkenes. Chin. J. Chem. 2020, 38, 1371–1394. [Google Scholar] [CrossRef]
- Derosa, J.; Apolinar, O.; Kang, T.; Tran, V.T.; Engle, K.M. Recent Developments in NickelCatalyzed Intermolecular Dicarbofunctionalization of Alkenes. Chem. Sci. 2020, 11, 4287–4296. [Google Scholar] [CrossRef]
- Zhao, X.; Tu, H.-Y.; Guo, L.; Zhu, S.; Qing, F.-L.; Chu, L. Intermolecular selective carboacylation of alkenes via nickel-catalyzed reductive radical relay. Nat. Commun. 2018, 9, 3488. [Google Scholar] [CrossRef]
- Gu, J.-W.; Min, Q.-Q.; Yu, L.-C.; Zhang, X. Tandem Difluoroalkylation-Arylation of Enamides Catalyzed by Nickel. Angew. Chem. Int. Ed. 2016, 55, 12270–12274. [Google Scholar] [CrossRef]
- Shrestha, B.; Basnet, P.; Dhungana, R.K.; KC, S.; Thapa, S.; Sears, J.M.; Giri, R. Ni-Catalyzed Regioselective 1,2-Dicarbofunctionalization of Olefins by Intercepting Heck Intermediates as Imine-Stabilized Transient Metallacycles. J. Am. Chem. Soc. 2017, 139, 10653–10656. [Google Scholar] [CrossRef]
- Derosa, J.; Tran, V.T.; Boulous, M.N.; Chen, J.S.; Engle, K.M. Nickel-Catalyzed β, γ-Dicarbofunctionalization of Alkenyl Carbonyl Compounds via Conjunctive Cross-Coupling. J. Am. Chem. Soc. 2017, 139, 10657–10660. [Google Scholar] [CrossRef]
- Basnet, P.; Dhungana, R.K.; Thapa, S.; Shrestha, B.; KC, S.; Sears, J.M.; Giri, R. Ni-Catalyzed Regioselective β, δ-Diarylation of Unactivated Olefins in Ketimines via Ligand-Enabled Contraction of Transient Nickellacycles: Rapid Access to Remotely Diarylated Ketones. J. Am. Chem. Soc. 2018, 140, 7782–7786. [Google Scholar] [CrossRef]
- KC, S.; Dhungana, R.K.; Shrestha, B.; Thapa, S.; Khanal, N.; Basnet, P.; Lebrun, R.W.; Giri, R. Ni-Catalyzed Regioselective Alkylarylation of Vinylarenes via C(sp3)-C(sp3)/C(sp3)-C(sp2) Bond Formation and Mechanistic Studies. J. Am. Chem. Soc. 2018, 140, 9801–9805. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Chen, L.-A.; Brown, M.K. Nickel-Catalyzed Stereoselective Diarylation of Alkenylarenes. J. Am. Chem. Soc. 2018, 140, 10653–10657. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Zhou, S.; Wan, X.; Chen, P.; Liu, G. Enantioselective Trifluoromethylalkynylation of Alkenes via CopperCatalyzed Radical Relay. J. Am. Chem. Soc. 2018, 140, 10965–10969. [Google Scholar] [CrossRef] [PubMed]
- Luridiana, A.; Mazzarella, D.; Capaldo, L.; Rincón, J.A.; García-Losada, P.; Mateos, C.; Frederick, M.O.; Nuño, M.; Buma, W.J.; Noël, T. The Merger of Benzophenone HAT Photocatalysis and Silyl RadicalInduced XAT Enables Both Nickel-Catalyzed Cross-Electrophile Coupling and 1,2-Dicarbofunctionalization of Olefins. ACS Catal. 2022, 12, 11216–11225. [Google Scholar] [CrossRef]
- Derosa, J.; Kleinmans, R.; Tran, V.T.; Karunananda, M.K.; Wisniewski, S.R.; Eastgate, M.D.; Engle, K.M. Nickel-Catalyzed 1,2-Diarylation of Simple Alkenyl Amides. J. Am. Chem. Soc. 2018, 140, 17878–17883. [Google Scholar] [CrossRef]
- Li, W.; Boon, J.K.; Zhao, Y. Nickel-Catalyzed Difunctionalization of Allyl moieties using Organoboronic Acids and Halides with Divergent Regioselectivities. Chem. Sci. 2018, 9, 600–607. [Google Scholar] [CrossRef]
- GarcíaDomínguez, A.; Mondal, R.; Nevado, C. Dual Photoredox/NickelCatalyzed Three-Component Carbofunctionalization of Alkenes. Angew. Chem. Int. Ed. 2019, 58, 12286–12290. [Google Scholar] [CrossRef]
- Campbell, M.W.; Compton, J.-S.; Kelly, C.B.; Molander, G.A. Three-Component Olefin Dicarbofunctionalization Enabled by Nickel/Photoredox Dual Catalysis. J. Am. Chem. Soc. 2019, 141, 20069–20078. [Google Scholar] [CrossRef]
- Kc, S.; Dhungana, R.K.; Khanal, N.; Giri, R. Nickel Catalyzed α-Carbonylalkylarylation of Vinylarenes: Expedient Access to γ,γ-Diarylcarbonyl and Aryltetralone Derivatives. Angew. Chem. Int. Ed. 2020, 59, 8047–8051. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, R.; Luo, Y.-C.; Wang, M.-K.; Zhang, X. trans-Selective Aryldifluoroalkylation of Endocyclic Enecarbamates and Enamides via Nickel Catalysis. Angew. Chem. Int. Ed. 2020, 59, 18741–18747. [Google Scholar] [CrossRef]
- García-Domínguez, A.; Li, Z.; Nevado, C. Nickel-Catalyzed Reductive Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2017, 139, 6835–6838. [Google Scholar] [CrossRef]
- Shu, W.; García-Domínguez, A.; Quiros, M.T.; Mondal, R.; Cárdenas, D.J.; Nevado, C. Ni-Catalyzed Reductive Dicarbofunctionalization of Nonactivated Alkenes: Scope and Mechanistic Insights. J. Am. Chem. Soc. 2019, 141, 13812–13821. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tu, H.-Y.; Zhu, S.; Chu, L. Selective, Intermolecular Alkylarylation of Alkenes via Photoredox/Nickel Dual Catalysis. Org. Lett. 2019, 21, 4771–4776. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.-Y.; Wang, F.; Huo, L.-P.; Li, Y.; Zhu, S.; Zhao, X.; Li, H.; Qing, F.-L.; Chu, L. Enantioselective Three-Component Fluoroalkylarylation of Unactivated Olefins through Nickel-Catalyzed Cross-Electrophile Coupling. J. Am. Chem. Soc. 2020, 142, 9604–9611. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shu, W.; García-Domínguez, A.; Merino, E.; Nevado, C. Asymmetric Ni-Catalyzed Radical Relayed Reductive Coupling. J. Am. Chem. Soc. 2020, 142, 13515–13522. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Lu, X.; He, S.-J.; Fu, Y. Nickel-Catalyzed Three-Component Olefin Reductive Dicarbofunctionalization to Access Alkylborates. Chem. Sci. 2020, 11, 7950–7956. [Google Scholar] [CrossRef]
- Wang, Z.K.; Wang, Y.P.; Rao, Z.W.; Liu, C.-Y.; Pan, X.-H.; Guo, L. General Method for Selective Three-Component Carboacylation of Alkenes via Visible-Light Dual Photoredox/Nickel Catalysis. Org. Lett. 2023, 25, 1673–1677. [Google Scholar] [CrossRef]
- Fan, H.; Wang, B.; Wu, T.; Kang, Q.; Wang, H.; Sun, J.; Wei, X. Ball-milling-enabled nickel-catalyzed radical relayed reductive cross-coupling. Cell Rep. Phys. Sci. 2024, 5, 101831. [Google Scholar] [CrossRef]


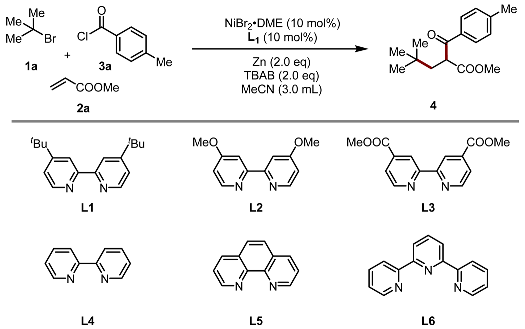 | ||
|---|---|---|
| Entry | Variation from “Standard Conditions” | 4 [%] [b] |
| 1 | none | 78 [c] |
| 2 | L2 as ligand | 47 |
| 3 | L3 as ligand | 12 |
| 4 | L4 as ligand | 55 |
| 5 | L5 as ligand | 10 |
| 6 | L6 as ligand | 0 |
| 7 | THF as solvent | trace |
| 8 | DMA as solvent | 24 |
| 9 | Mn powder as a reducing agent | 32 |
| 10 | w/o NiBr2•DME | 0 |
| 11 | w/o TBAB | 12 |
| 12 | w/o ligand | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Wang, L.; Jia, Y.; Ma, W.; Wang, D. Nickel-Catalyzed Three-Component 1,2-Carboacylation of Alkenes. Molecules 2024, 29, 4295. https://doi.org/10.3390/molecules29184295
Jin S, Wang L, Jia Y, Ma W, Wang D. Nickel-Catalyzed Three-Component 1,2-Carboacylation of Alkenes. Molecules. 2024; 29(18):4295. https://doi.org/10.3390/molecules29184295
Chicago/Turabian StyleJin, Shengzhou, Lanfen Wang, Yinggang Jia, Wenbo Ma, and Dingyi Wang. 2024. "Nickel-Catalyzed Three-Component 1,2-Carboacylation of Alkenes" Molecules 29, no. 18: 4295. https://doi.org/10.3390/molecules29184295
APA StyleJin, S., Wang, L., Jia, Y., Ma, W., & Wang, D. (2024). Nickel-Catalyzed Three-Component 1,2-Carboacylation of Alkenes. Molecules, 29(18), 4295. https://doi.org/10.3390/molecules29184295





