Use of Molecular Logic Gates for the Tuning of Chemosensor Dynamic Range
Abstract
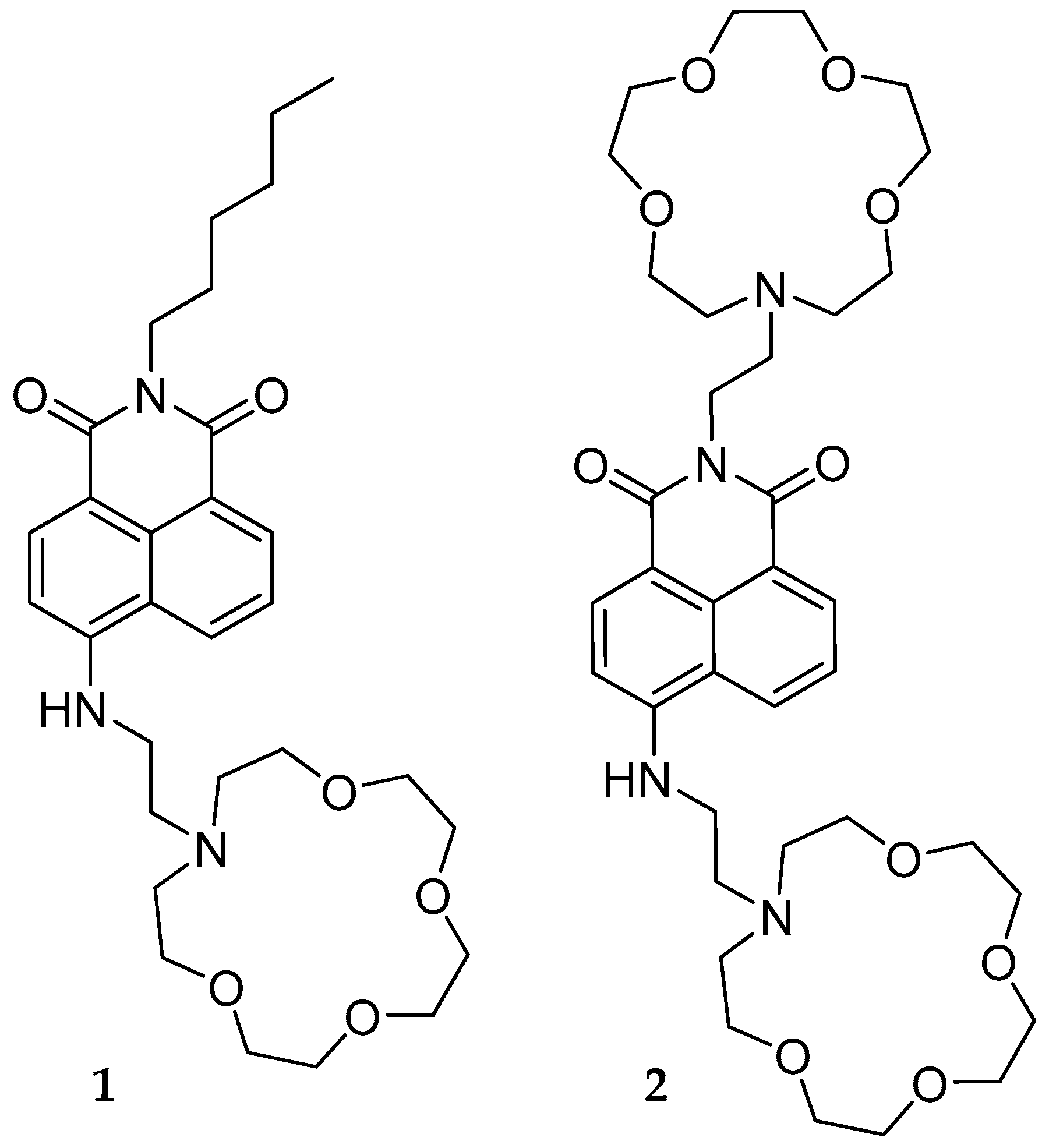
1. Introduction
2. Results
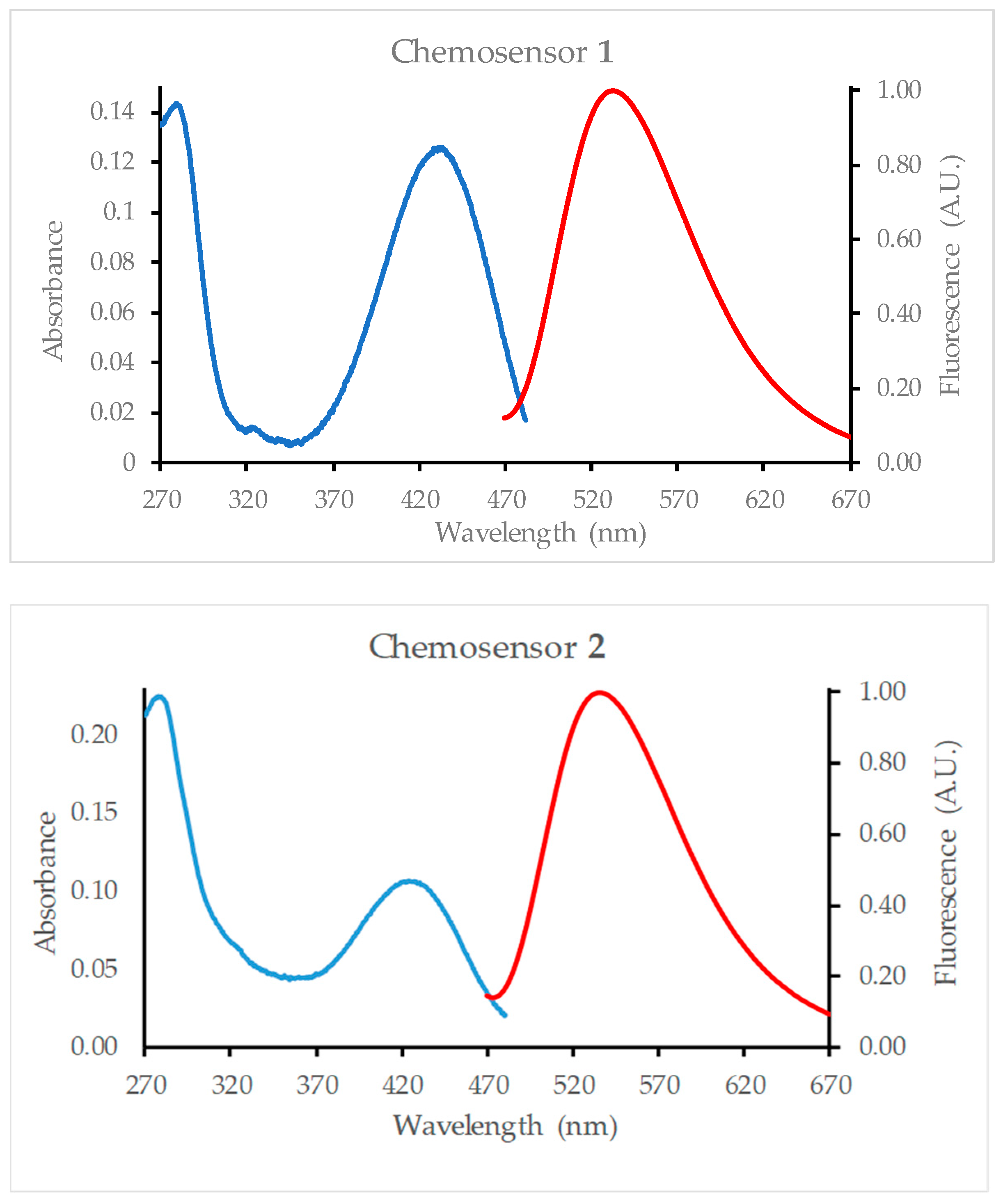
2.1. Absorption Spectroscopy
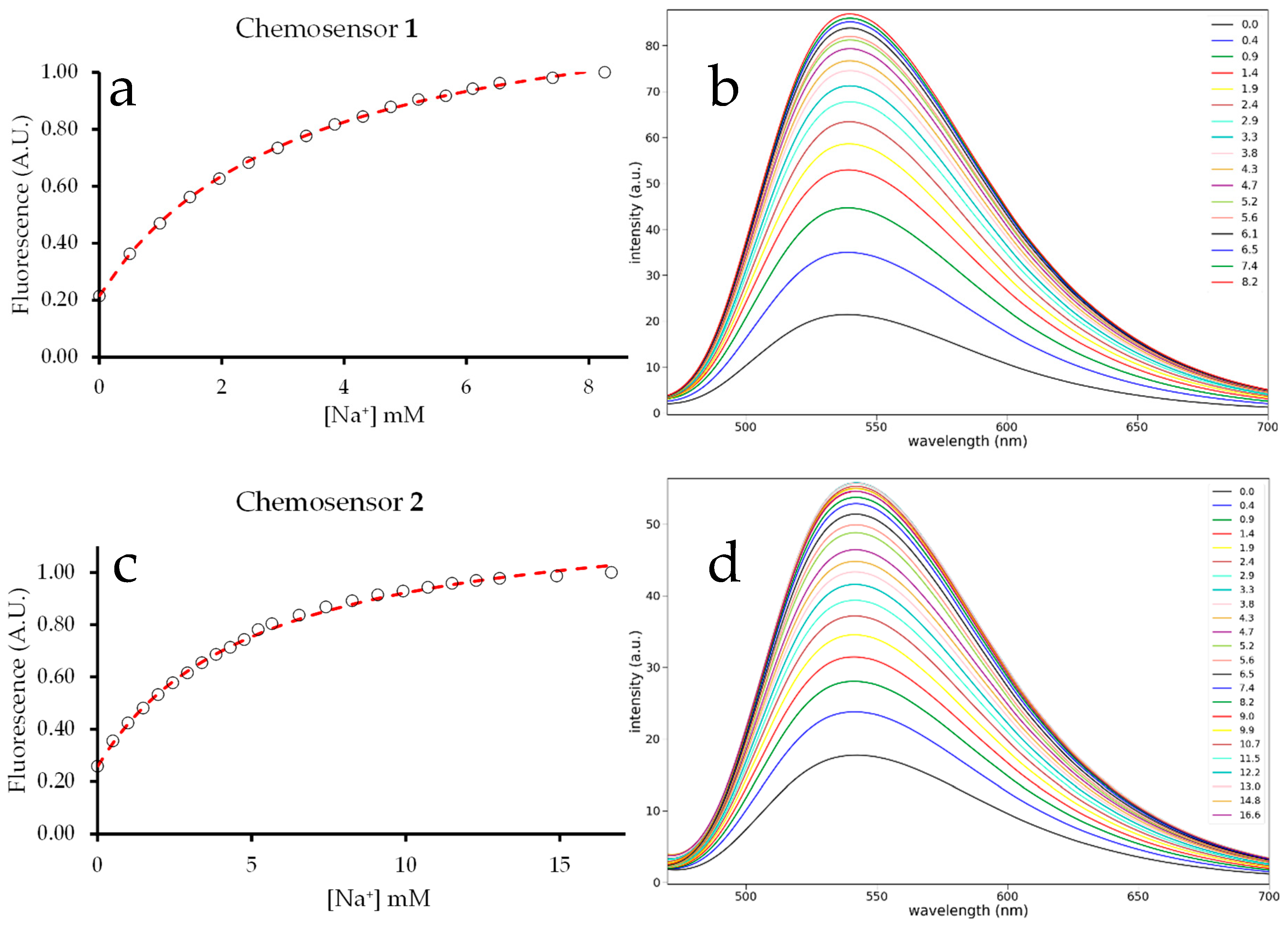
2.2. Fluorescence Spectroscopy
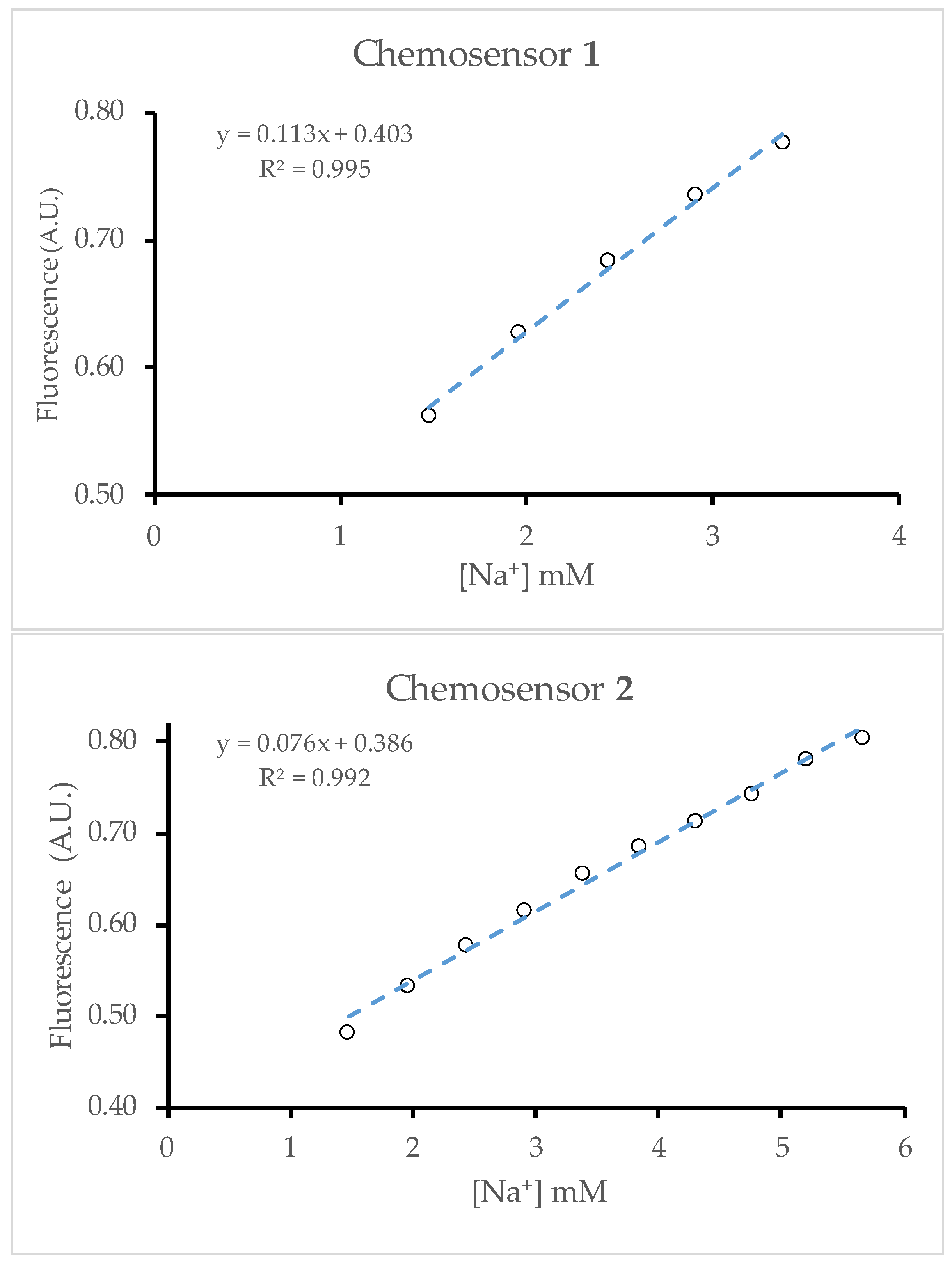
2.3. Binding Constant
2.4. Dynamic Range
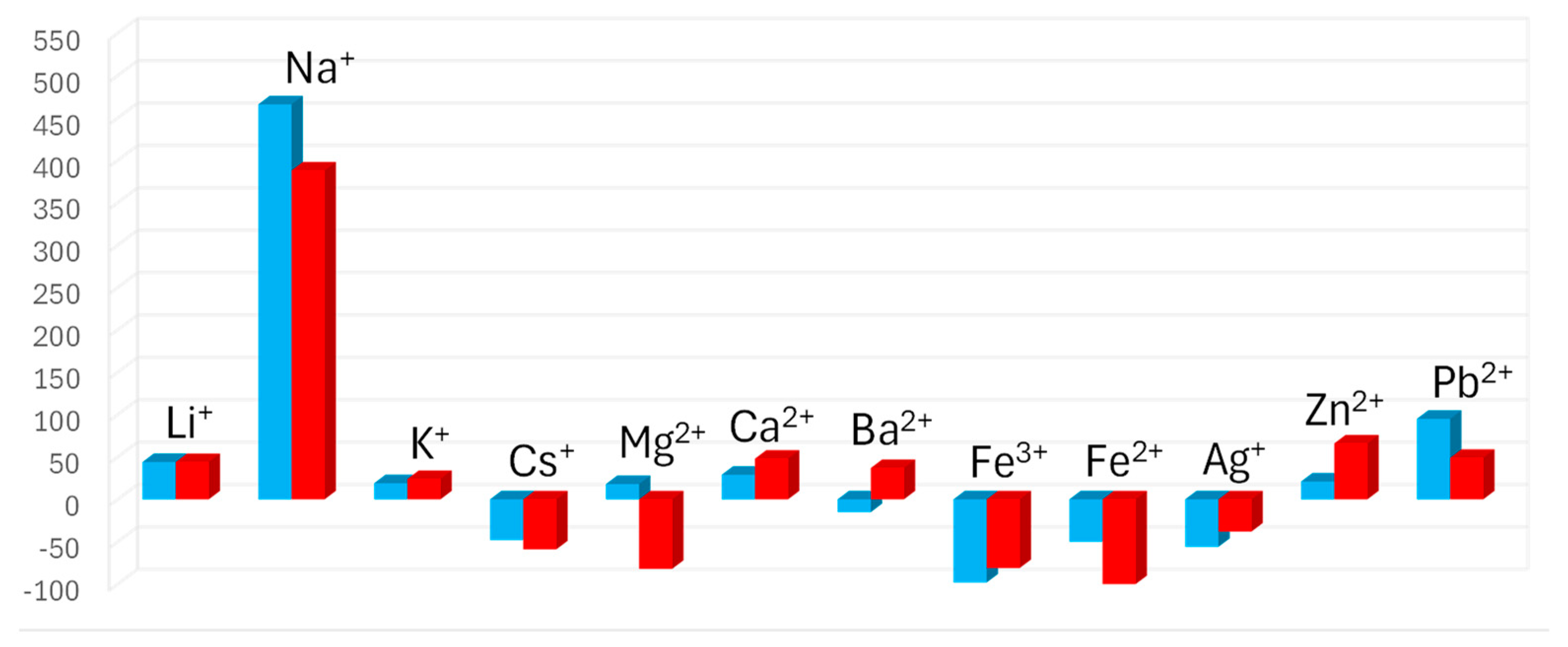
2.5. Chemoselectivity
3. Discussion
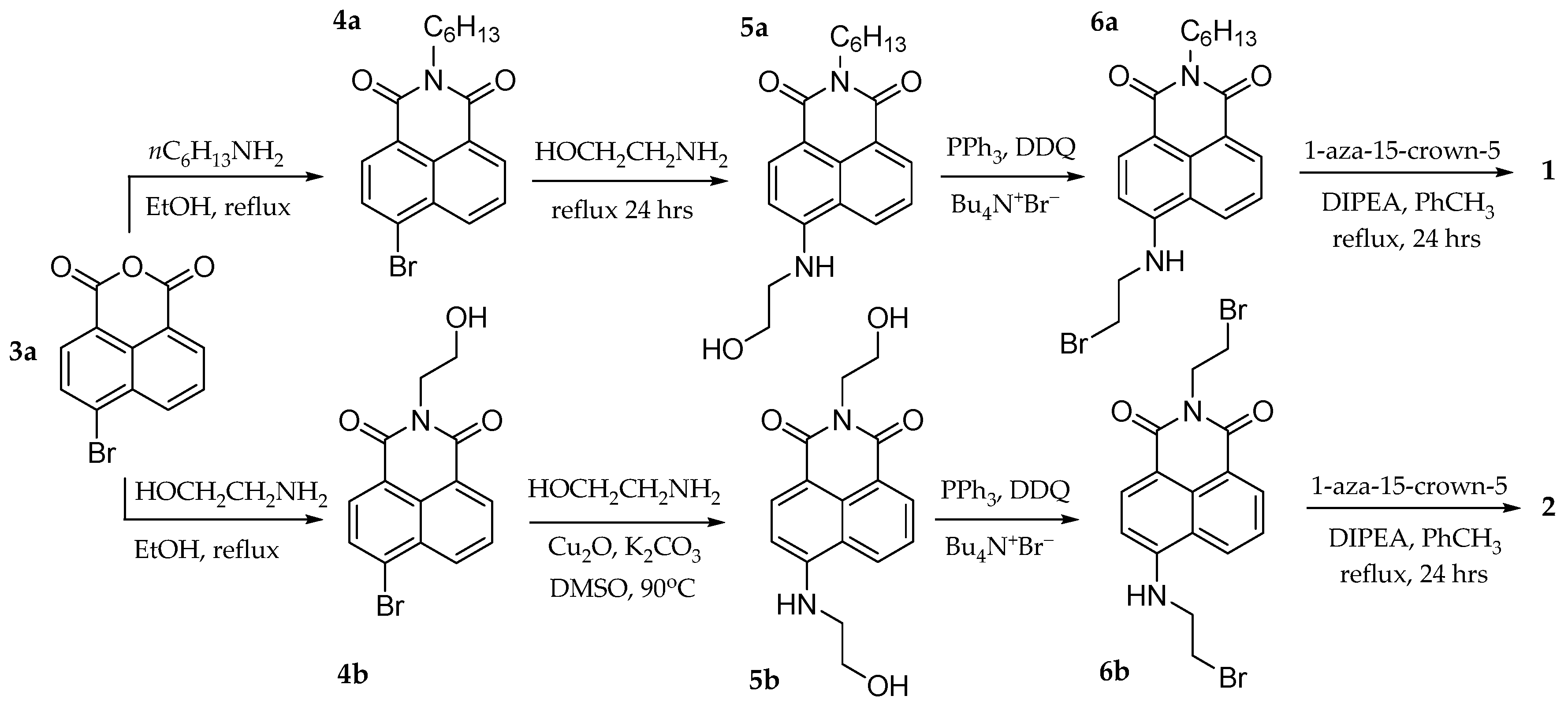
4. Materials and Methods
4.1. Experimental
4.2. Spectroscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Desvergne, J.P.; Czarnik, A.W. Fluorescent Chemosensors for Ion and Molecule Recognition; American Chemical Society: Washington, DC, USA, 1993; Volume 538, pp. i–vi. ISBN 978-0-8412-2728-6. [Google Scholar]
- Wang, X.; Ding, Q.; Groleau, R.R.; Wu, L.; Mao, Y.; Che, F.; Kotova, O.; Scanlan, E.M.; Lewis, S.E.; Li, P.; et al. Fluorescent probes for disease diagnosis. Chem. Rev. 2024, 124, 7106–7164. [Google Scholar] [CrossRef]
- Dongare, P.R.; Gore, A.H. Recent advances in colorimetric and fluorescent chemosensors for Ionic species: Design, principle and optical signalling mechanism. ChemistrySelect 2021, 6, 5657–5669. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design Strategies for Water-Soluble Small Molecular Chromogenic and Fluorogenic Probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef]
- Gao, G.; Cao, Y.; Liu, W.; Li, D.; Zhou, W.; Liu, J. Fluorescent Sensors for Sodium Ions. Anal. Methods 2017, 9, 5570–5579. [Google Scholar] [CrossRef]
- Chen, W.; Ma, X.; Chen, H.; Hua Liu, S.; Yin, J. Fluorescent Probes for pH and Alkali Metal Ions. Coord. Chem. Rev. 2021, 427, 213584. [Google Scholar] [CrossRef]
- Minta, A.; Tsien, R.Y. Fluorescent Indicators for Cytosolic Sodium. J. Biol. Chem. 1989, 264, 19449–19457. [Google Scholar] [CrossRef]
- de Silva, P.A.; Gunaratne, N.H.Q.; McCoy, C.P. A Molecular Photoionic AND Gate Based on Fluorescent Signalling. Nature 1993, 364, 42–44. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Kolemen, S.; Sedgwick, A.C.; Gunnlaugsson, T.; James, T.D.; Yoon, J.; Akkaya, E.U. Molecular Logic Gates: The Past, Present and Future. Chem. Soc. Rev. 2018, 47, 2228–2248. [Google Scholar] [CrossRef]
- Bozdemir, O.A.; Guliyev, R.; Buyukcakir, O.; Selcuk, S.; Kolemen, S.; Gulseren, G.; Nalbantoglu, T.; Boyaci, H.; Akkaya, E.U. Selective Manipulation of ICT and PET Processes in Styryl-Bodipy Derivatives: Applications in Molecular Logic and Fluorescence Sensing of Metal Ions. J. Am. Chem. Soc. 2010, 132, 8029–8036. [Google Scholar] [CrossRef]
- Spiteri, J.C.; Johnson, A.D.; Denisov, S.A.; Jonusauskas, G.; McClenaghan, N.D.; Magri, D.C. A Fluorescent AND Logic Gate Based on a Ferrocene-Naphthalimide-Piperazine Format Responsive to Acidity and Oxidizability. Dye. Pigment. 2018, 157, 278–283. [Google Scholar] [CrossRef]
- Acikgoz, O. Use of Molecular Logic Gates for the Tuning of Chemosensor Dynamic Range. Bachelor’s Thesis, College of William and Mary, Williamsburg, VA, USA, 2024. Available online: https://scholarworks.wm.edu/honorstheses/2127/ (accessed on 12 July 2024).
- Green, A.M.; Abelt, C.J. Dual-Sensor Fluorescent Probes of Surfactant-Induced Unfolding of Human Serum Albumin. J. Phys. Chem. B 2015, 119, 3912–3919. [Google Scholar] [CrossRef]
- Gui, S.; Huang, Y.; Hu, F.; Jin, Y.; Zhang, G.; Yan, L.; Zhang, D.; Zhao, R. Fluorescence Turn-On Chemosensor for Highly Selective and Sensitive Detection and Bioimaging of Al3+ in Living Cells Based on Ion-Induced Aggregation. Anal. Chem. 2015, 87, 1470–1474. [Google Scholar] [CrossRef]
- Qin, J.; Fan, L.; Wang, B.; Yang, Z.; Li, T. The Design of a Simple Fluorescent Chemosensor for Al3+/Zn2+ via Two Different Approaches. Anal. Methods 2015, 7, 716–722. [Google Scholar] [CrossRef]
- Hiraoka, M. (Ed.) Crown Ethers and Analogous Compounds; Elsevier: Amsterdam, The Netherlands, 1992; ISBN 0-444-88191-3. [Google Scholar]
- Yu, H.-R.; Hu, J.-Q.; Lu, X.-H.; Ju, X.-J.; Liu, Z.; Xie, R.; Wang, W.; Chu, L.-Y. Insights into the effects of 2:1 “sandwich-type” crown-ether/metal-ion complexes in responsive host–guest systems. J. Phys. Chem. B 2015, 119, 1696–1705. [Google Scholar] [CrossRef]
- Credi, A.; Balzani, V.; Langford, S.J.; Stoddart, J.F. Logic Operations at the Molecular Level. An XOR Gate Based on a Molecular Machine. J. Am. Chem. Soc. 1997, 119, 2679–2681. [Google Scholar] [CrossRef]
- de Silva, A.P.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E. Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef]
- Kim, S.Y.; Hong, J.-I. Naphthalimide-Based Fluorescent Zn2+ Chemosensors Showing PET Effect According to Their Linker Length in Water. Tetrahedron Lett. 2009, 50, 2822–2824. [Google Scholar] [CrossRef]
- Dong, H.-Q.; Wei, T.-B.; Ma, X.-Q.; Yang, Q.-Y.; Zhang, Y.-F.; Sun, Y.-J.; Shi, B.-B.; Yao, H.; Zhang, Y.-M.; Lin, Q. 1,8-Naphthalimide-Based Fluorescent Chemosensors: Recent Advances and Perspectives. J. Mater. Chem. C 2020, 8, 13501–13529. [Google Scholar] [CrossRef]
- He, H.; Mortellaro, M.A.; Leiner, M.J.P.; Young, S.T.; Fraatz, R.J.; Tusa, J.K. A Fluorescent Chemosensor for Sodium Based on Photoinduced Electron Transfer. Anal. Chem. 2003, 75, 549–555. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Dimitrova, M.D.; Todorova, Y.D.; Bojinov, V.B. Synthesis, chemosensing properties and logic behaviour of a novel ratiometric 1,8-naphthalimide probe based on ICT and pet. Dye. Pigm 2016, 131, 9–17. [Google Scholar] [CrossRef]
- Gokel, G.W. Crown Ethers and Cryptands; The Royal Society of Chemistry: London, UK, 1991; ISBN 978-0-85186-996-4. [Google Scholar]
- Poronik, Y.M.; Clermont, G.; Blanchard-Desce, M.; Gryko, D.T. Nonlinear Optical Chemosensor for Sodium Ion Based on Rhodol Chromophore. J. Org. Chem. 2013, 78, 11721–11732. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Hu, Y.; Bi, S.; Wu, S.; Wang, L. Micellization of 4-Hydroxynaphthalimides: The Solvent-Induced Aggregation and the Detection of Low-Level Water in THF. Chem. Lett. 2016, 45, 1162–1164. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Tong, L.; Tan, S.; Zhou, W.; Peng, T.; Han, K.; Ding, J.; Xie, H.; Xu, Y. Naphthalimides Exhibit in Vitro Antiproliferative and Antiangiogenic Activities by Inhibiting Both Topoisomerase II (Topo II) and Receptor Tyrosine Kinases (RTKs). Eur. J. Med. Chem. 2013, 65, 477–486. [Google Scholar] [CrossRef]
- Ravi, A.; Krishnarao, P.S.; Shumilova, T.A.; Khrustalev, V.N.; Rüffer, T.; Lang, H.; Kataev, E.A. Cation Molecular Exchanger Based on a Conformational Hinge. Org. Lett. 2018, 20, 6211–6214. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, H.; Jin, B.; Liu, X.; Fu, H.; Shangguan, D. A Guanidine Derivative of Naphthalimide with Excited-State Deprotonation Coupled Intramolecular Charge Transfer Properties and Its Application. J. Mater. Chem. C 2013, 1, 4427–4436. [Google Scholar] [CrossRef]
- Fang, C.; Zhou, J.; Liu, X.; Cao, Z.; Shangguan, D. Mercury(II)-Mediated Formation of Imide-Hg-Imide Complexes. Dalton Trans. 2011, 40, 899–903. [Google Scholar] [CrossRef]
- Chand, T.; Khamari, L.; Chopra, D.; Mukherjee, S.; Kapur, M. Rh(III)-Catalyzed One-Step Synthesis of Ortho-Alkynylated Perylene Imide Dyes: Optical and Electrochemical Properties of New Derivatives. Chem.–A Eur. J. 2022, 28, e202200723. [Google Scholar] [CrossRef]
- Tripathi, N.P.; Jain, S.; Singh, R.K.; Sengupta, S. Tripodal Triazine and 1,8-Naphthalimide-Based Small Molecules as Efficient Photocatalysts for Visible-Light Oxidative Condensation. Chem.–A Eur. J. 2024, 30, e202303244. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Aldred, M.P.; Gong, W.-L.; Li, C.; Zhu, M.-Q. Utilising Tetraphenylethene as a Dual Activator for Intramolecular Charge Transfer and Aggregation Induced Emission. Chem. Commun. 2012, 48, 7711–7713. [Google Scholar] [CrossRef]
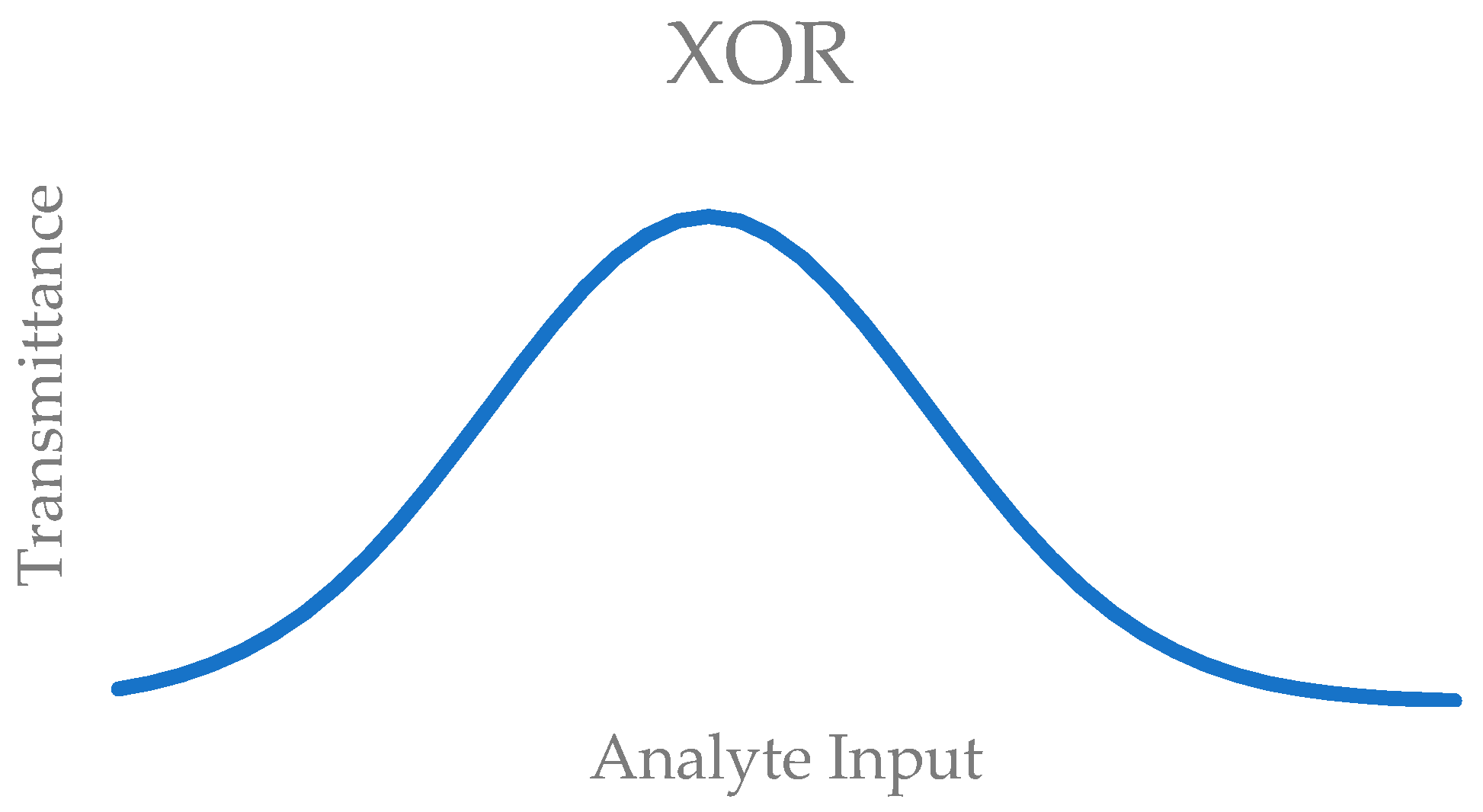
| Input I | Input II | Output |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 0 | 0 |
| 0 | 1 | 0 |
| 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acikgoz, O.; Abelt, C. Use of Molecular Logic Gates for the Tuning of Chemosensor Dynamic Range. Molecules 2024, 29, 4330. https://doi.org/10.3390/molecules29184330
Acikgoz O, Abelt C. Use of Molecular Logic Gates for the Tuning of Chemosensor Dynamic Range. Molecules. 2024; 29(18):4330. https://doi.org/10.3390/molecules29184330
Chicago/Turabian StyleAcikgoz, Orhan, and Christopher Abelt. 2024. "Use of Molecular Logic Gates for the Tuning of Chemosensor Dynamic Range" Molecules 29, no. 18: 4330. https://doi.org/10.3390/molecules29184330
APA StyleAcikgoz, O., & Abelt, C. (2024). Use of Molecular Logic Gates for the Tuning of Chemosensor Dynamic Range. Molecules, 29(18), 4330. https://doi.org/10.3390/molecules29184330









