The Effects of Different Thiol-Containing Compounds on the Degradation of Sulforaphene
Abstract
1. Introduction
2. Results and Discussion
2.1. SFE Analysis by High-Performance Liquid Chromatography (HPLC)
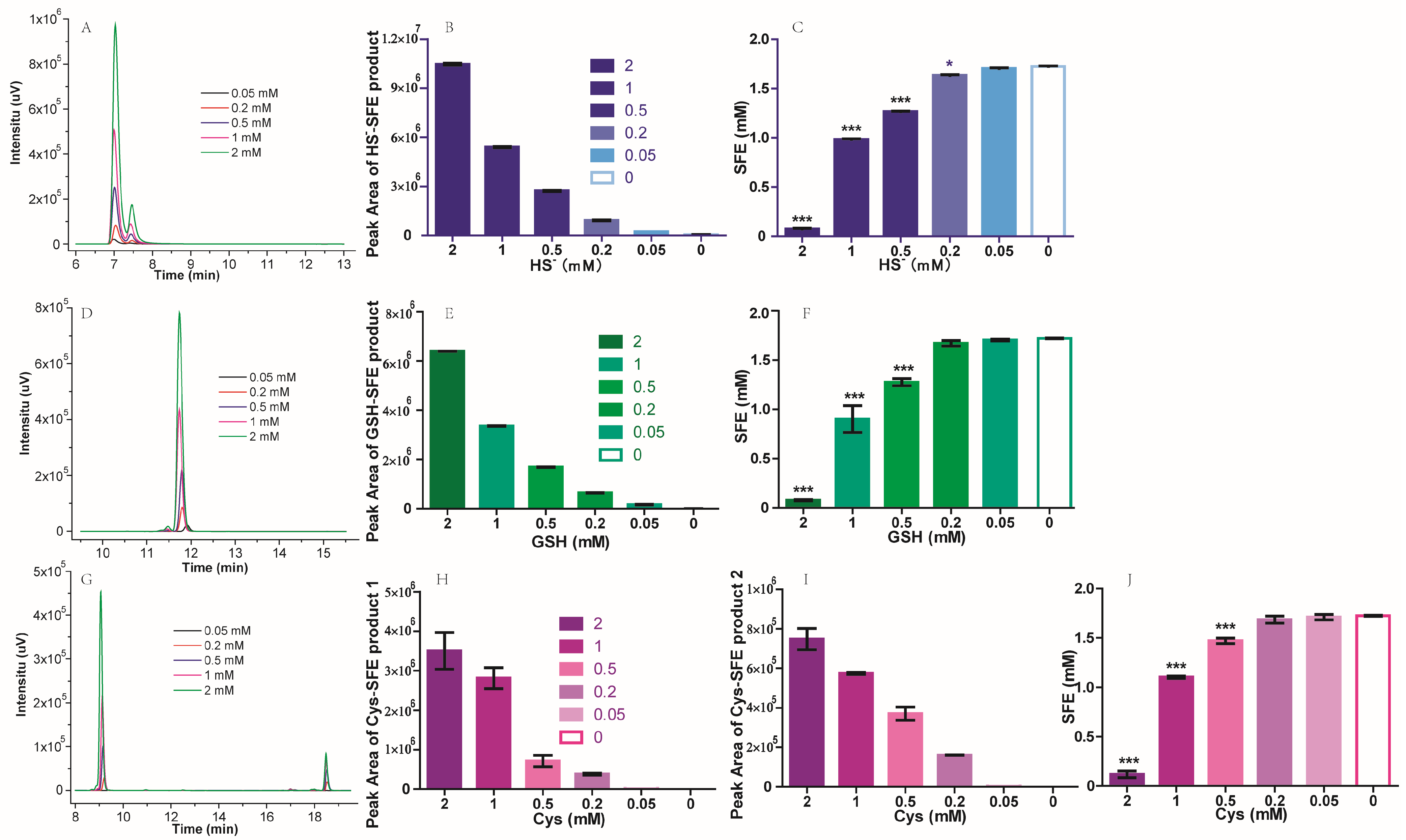
2.2. The Influence of Thiol-Containing Compounds on SFE
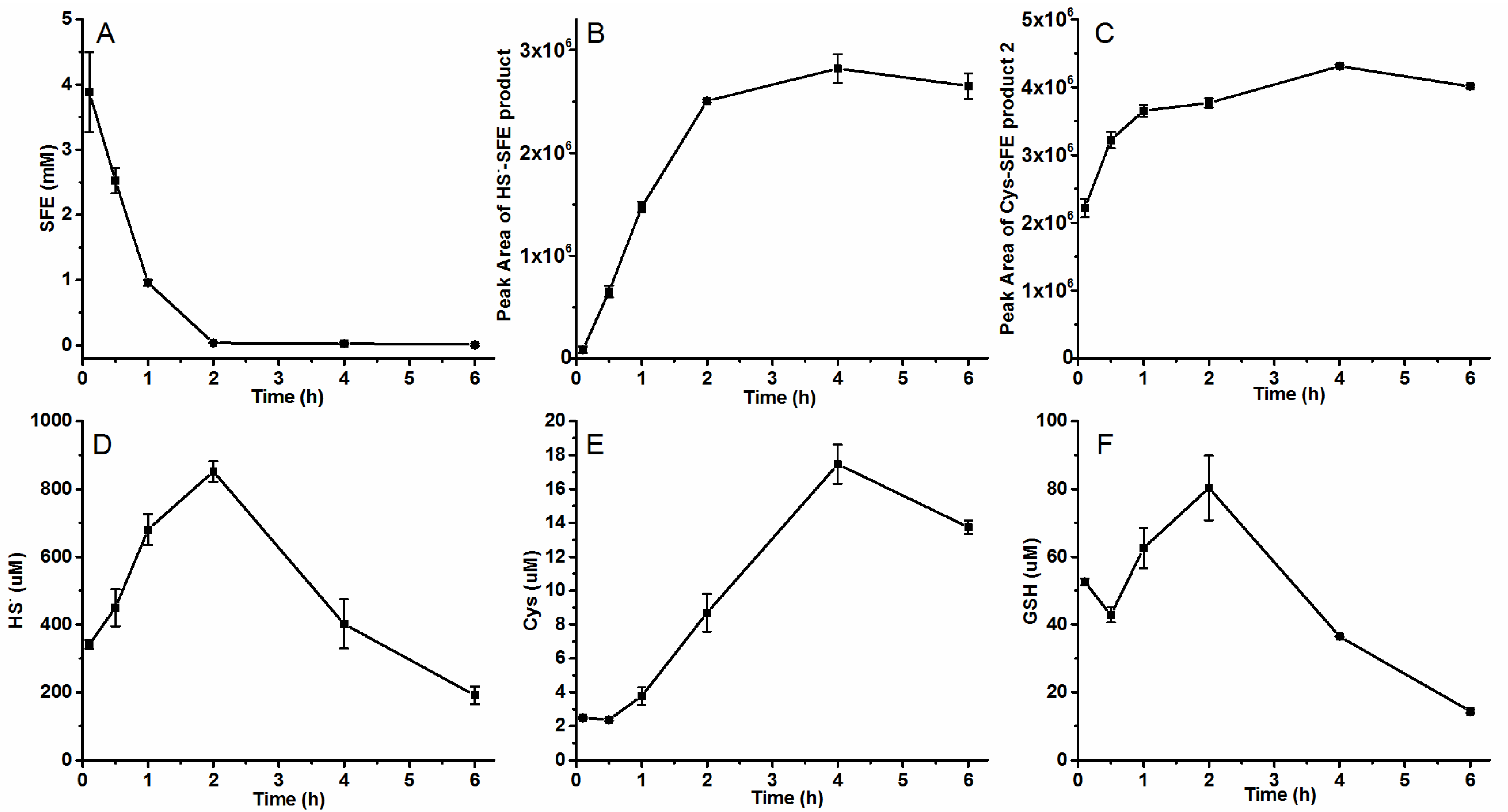
2.3. The Generation of SFE in Radish Seeds’ Hydrolytic Process
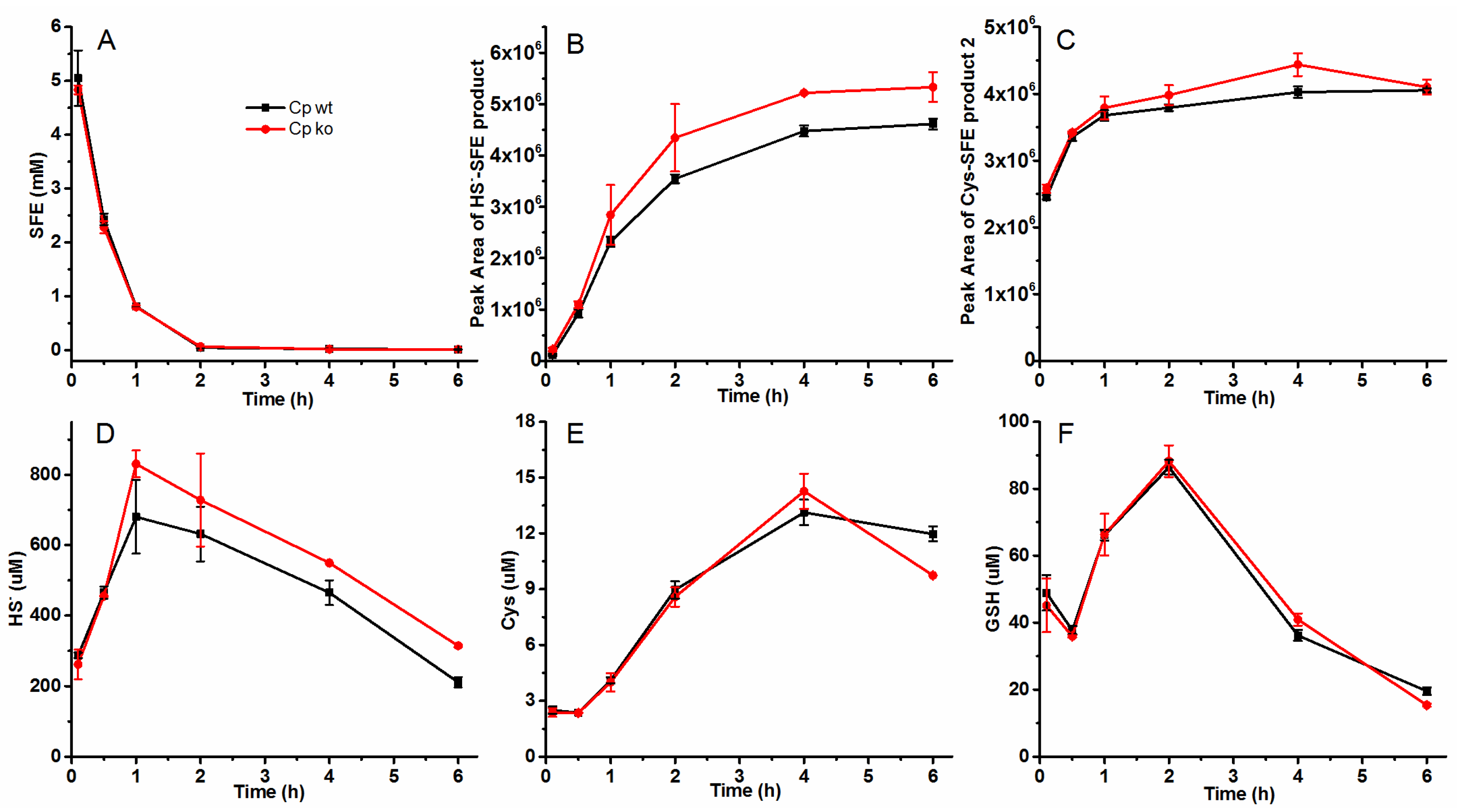
2.4. Effect of Sulfide-Oxidizing Bacteria Cupriavidus Pinatubonensis JMP134 on the SFE Degradation in Radish Seed Hydrolytic Process
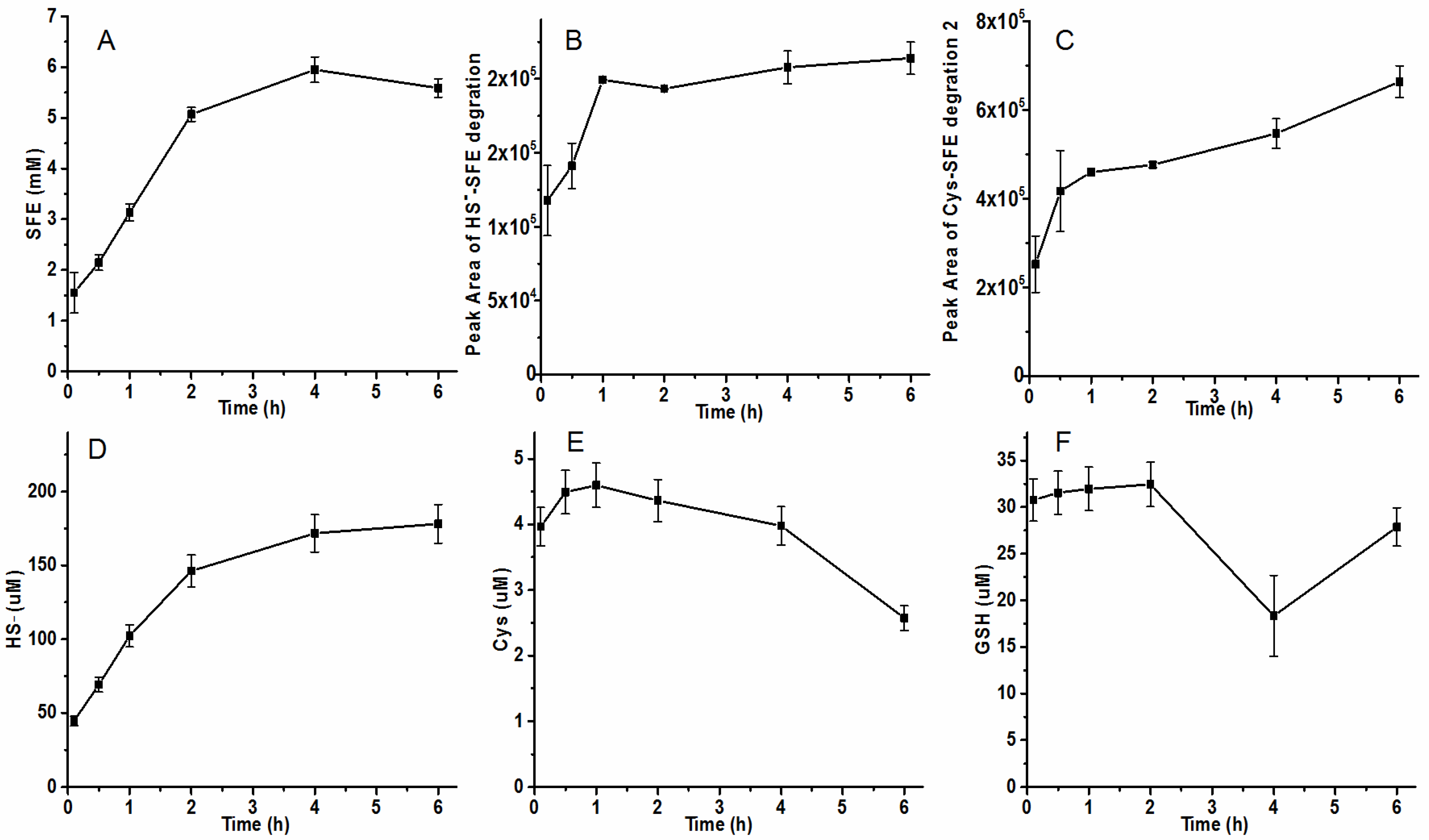
2.5. Effect of Heat-Treatment on the SFE Degradation in Radish Seed Hydrolytic Process
3. Materials and Methods
3.1. Materials
3.2. HPLC Analysis
3.3. The Influence of Thiol-Containing Compounds on the Stability of SFE
3.4. The Radish Seed Hydrolysis Process
3.5. Preparation of Cell Suspension and Detection of Sulfide HS−
3.6. Data Processing and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dholwani, K.K.; Saluja, A.K.; Gupta, A.R.; Shah, D.R. A review on plant-derived natural products and their analogs with anti-tumor activity. Indian J. Pharmacol. 2008, 40, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Motyka, S.; Jafernik, K.; Ekiert, H.; Sharifi-Rad, J.; Calina, D.; Al-Omari, B.; Szopa, A.; Cho, W.C. Podophyllotoxin and its derivatives: Potential anticancer agents of natural origin in cancer chemotherapy. Biomed. Pharmacother. 2023, 158, 114145. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; Noor, N.M. Cruciferous vegetables: Dietary phytochemicals for cancer prevention. Asian Pac. J. Cancer Prev. 2013, 14, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.G.; Luu, H.N.; Liu, Y.; Wang, D.W.; Guo, X. High intake of cruciferous vegetables reduces the risk of gastrointestinal cancers: Results from observational studies. Crit. Rev. Food Sci. Nutr. 2023, 5, 1–7. [Google Scholar] [CrossRef]
- Cardozo, L.F.M.F.; Alvarenga, L.A.; Ribeiro, M.; Dai, L.; Shiels, P.G.; Stenvinkel, P.; Lindholm, B.; Mafra, D. Cruciferous vegetables: Rationale for exploring potential salutary effects of sulforaphane-rich foods in patients with chronic kidney disease. Nutr. Rev. 2021, 79, 1204–1224. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Şahin, T.Ö.; Yılmaz, B.; Ekenci, K.D.; Duyar Özer, Ş.; Capasso, R. Cruciferous vegetables and their bioactive metabolites: From prevention to novel therapies of colorectal cancer. Evid. Based Complement. Altern. Med 2022, 2022, 1534083. [Google Scholar] [CrossRef]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous vegetables, isothiocyanates, and bladder cancer prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef]
- Hudlikar, R.; Wang, L.; Wu, R.; Li, S.; Peter, R.; Shannar, A.; Chou, P.J.; Liu, X.; Liu, Z.; Kuo, H.-C.D.; et al. Epigenetics/epigenomics and prevention of early stages of cancer by isothiocyanates. Cancer Prev. Res. 2020, 14, 151–164. [Google Scholar] [CrossRef]
- Na, G.; He, C.; Zhang, S.; Tian, S.; Bao, Y.; Shan, Y. Dietary isothiocyanates: Novel insights into the potential for cancer prevention and therapy. Int. J. Mol. Sci. 2023, 24, 1962. [Google Scholar] [CrossRef]
- Bhat, R.; Vyas, D. Myrosinase: Insights on structural, catalytic, regulatory, and environmental interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Q.; Yao, K.; Jin, G.; Zhao, S.; Zhang, J.; Zheng, W.; Xu, e.; Zu, Y.; Yuan, J.; et al. Sulforaphene suppresses oesophageal cancer growth through mitogen- and stress-activated kinase 2 in a PDX mouse model. Am. J. Cancer Res. 2023, 13, 4708–4720. [Google Scholar] [PubMed]
- Yu, H.Y.; Yang, L.; Liu, Y.C.; Yu, A.J. Sulforaphene suppressed cell proliferation and promoted apoptosis of COV362 cells in endometrioid ovarian cancer. PeerJ 2023, 11, e16308. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, H.; Zhou, M.; Liu, W.; Kuang, P.; Liang, H.; Yuan, Q. The natural compound sulforaphene, as a novel anticancer reagent, targeting PI3K-AKT signaling pathway in lung cancer. Oncotarget 2016, 7, 76656–76666. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Shin, S.H.; Park, J.; Lim, S.; Lee, E.; Lee, C.; Sung, D.; Farrand, L.; Lee, S.R.; Kim, K.H.; et al. Sulforaphene suppresses growth of colon cancer-derived tumors via induction of glutathione depletion and microtubule depolymerization. Mol. Nutr. Food Res. 2016, 60, 1068–1078. [Google Scholar] [CrossRef]
- Bao, C.; Kim, M.C.; Chen, J.; Song, J.; Ko, H.W.; Lee, H.J. Sulforaphene interferes with Human breast cancer cell migration and invasion through inhibition of hedgehog signaling. J. Agric. Food Chem. 2016, 64, 5515–5524. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Xu, Q.-Q.; Xian, Y.-F.; Lin, Z.-X. Sulforaphene ameliorates neuroinflammation and hyperphosphorylated tau protein via regulating the PI3K/Akt/GSK-3 β pathway in experimental models of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2020, 2020, 4754195. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, J.-E.; Lee, K.W. Sulforaphene attenuates Cutibacterium acnes-induced inflammation. J. Microbiol. Biotechnol. 2022, 32, 1390–1395. [Google Scholar] [CrossRef]
- Song, D.; Liang, H.; Kuang, P.; Tang, P.; Hu, G.; Yuan, Q. Instability and structural change of 4-methylsulfinyl-3-butenyl isothiocyanate in the hydrolytic process. J. Agric. Food Chem. 2013, 61, 5097–5102. [Google Scholar] [CrossRef]
- Tian, G.; Tang, P.; Xie, R.; Cheng, L.; Yuan, Q.; Hu, J. The stability and degradation mechanism of sulforaphene in solvents. Food Chem. 2016, 199, 301–306. [Google Scholar] [CrossRef]
- Tian, G.; Li, Y.; Cheng, L.; Yuan, Q.; Tang, P.; Kuang, P.; Hu, J. The mechanism of sulforaphene degradation to different water contents. Food Chem. 2016, 194, 1022–1027. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, M.; Rosen, R.T.; Ho, C.T. Thermal degradation of sulforaphane in aqueous solution. J. Agric. Food Chem. 1999, 47, 3121–3123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P. Anticarcinogenic activities of organic isothiocyanates: Chemistry and mechanisms. Cancer Res. 1994, 54, 1976s–1981s. [Google Scholar] [PubMed]
- Bonnot, T.; Bachelet, F.; Boudet, J.; Le Signor, C.; Bancel, E.; Vernoud, V.; Ravel, C.; Gallardo, K. Sulfur in determining seed protein composition: Present understanding of its interaction with abiotic stresses and future directions. J. Exp. Bot. 2023, 74, 3276–3285. [Google Scholar] [CrossRef] [PubMed]
- Kuang, P.; Song, D.; Yuan, Q.; Yi, R.; Lv, X.; Liang, H. Separation and purification of sulforaphene from radish seeds using macroporous resin and preparative high-performance liquid chromatography. Food Chem. 2013, 136, 342–347. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, H.; Liang, X.; Zhou, W.; Qiu, Y.; Xue, C.; Sun, J.; Mao, X. Preparation of sulforaphene from Radish seed extracts with recombinant food-grade Yarrowia lipolytica harboring high myrosinase activity. J. Agric. Food Chem. 2021, 69, 5363–5371. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Li, X.; Xiao, J.; Guo, L. Enhancement of ultrasound-assisted extraction of sulforaphane from broccoli seeds via the application of microwave pretreatment. Ultrason. Sonochem. 2022, 87, 106061. [Google Scholar] [CrossRef]
- Fuentes-Lara, L.O.; Medrano-Macias, J.; Perez-Labrada, F.; Rivas-Martinez, E.N.; Garcia-Enciso, E.L.; Gonzalez-Morales, S.; Juarez-Maldonado, A.; Rincon-Sanchez, F.; Benavides-Mendoza, A. From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef]
- Ito, T.; Ohkama-Ohtsu, N. Degradation of glutathione and glutathione conjugates in plants. J. Exp. Bot. 2023, 74, 3313–3327. [Google Scholar] [CrossRef]
- Ciacka, K.; Tyminski, M.; Gniazdowska, A.; Krasuska, U. Cold stratification-induced dormancy removal in apple (Malus domestica Borkh.) seeds is accompanied by an increased glutathione pool in embryonic axes. J. Plant Physiol. 2022, 274, 153736. [Google Scholar] [CrossRef]
- Xia, Y.; Lu, C.; Hou, N.; Xin, Y.; Liu, J.; Liu, H.; Xun, L. Sulfide production and oxidation by heterotrophic bacteria under aerobic conditions. ISME J. 2017, 11, 2754–2766. [Google Scholar] [CrossRef]
- Newton, G.L.; Dorian, R.; Fahey, R.C. Analysis of biological thiols: Derivatization with monobromobimane and separation by reverse-phase high-performance liquid chromatography. Anal. Biochem. 1981, 114, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Liu, H.; Cui, F.; Liu, H.; Xun, L. Recombinant Escherichia coli with sulfide:quinone oxidoreductase and persulfide dioxygenase rapidly oxidises sulfide to sulfite and thiosulfate via a new pathway. Environ. Microbiol. 2016, 18, 5123–5136. [Google Scholar] [CrossRef] [PubMed]
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Ocean. 1969, 14, 454–458. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Liu, P.; Bi, J.; Jiang, Y.; Zhao, T.; Yuan, X.; Zhang, C.; Wang, Y. The Effects of Different Thiol-Containing Compounds on the Degradation of Sulforaphene. Molecules 2024, 29, 4328. https://doi.org/10.3390/molecules29184328
Gao R, Liu P, Bi J, Jiang Y, Zhao T, Yuan X, Zhang C, Wang Y. The Effects of Different Thiol-Containing Compounds on the Degradation of Sulforaphene. Molecules. 2024; 29(18):4328. https://doi.org/10.3390/molecules29184328
Chicago/Turabian StyleGao, Rui, Pingxiang Liu, Jingxiu Bi, Yuying Jiang, Tong Zhao, Xuexia Yuan, Chao Zhang, and Yutao Wang. 2024. "The Effects of Different Thiol-Containing Compounds on the Degradation of Sulforaphene" Molecules 29, no. 18: 4328. https://doi.org/10.3390/molecules29184328
APA StyleGao, R., Liu, P., Bi, J., Jiang, Y., Zhao, T., Yuan, X., Zhang, C., & Wang, Y. (2024). The Effects of Different Thiol-Containing Compounds on the Degradation of Sulforaphene. Molecules, 29(18), 4328. https://doi.org/10.3390/molecules29184328






