Hydrogel Capacitors Based on MoS2 Nanosheets and Applications in Glucose Monitoring
Abstract
1. Introduction
2. Results
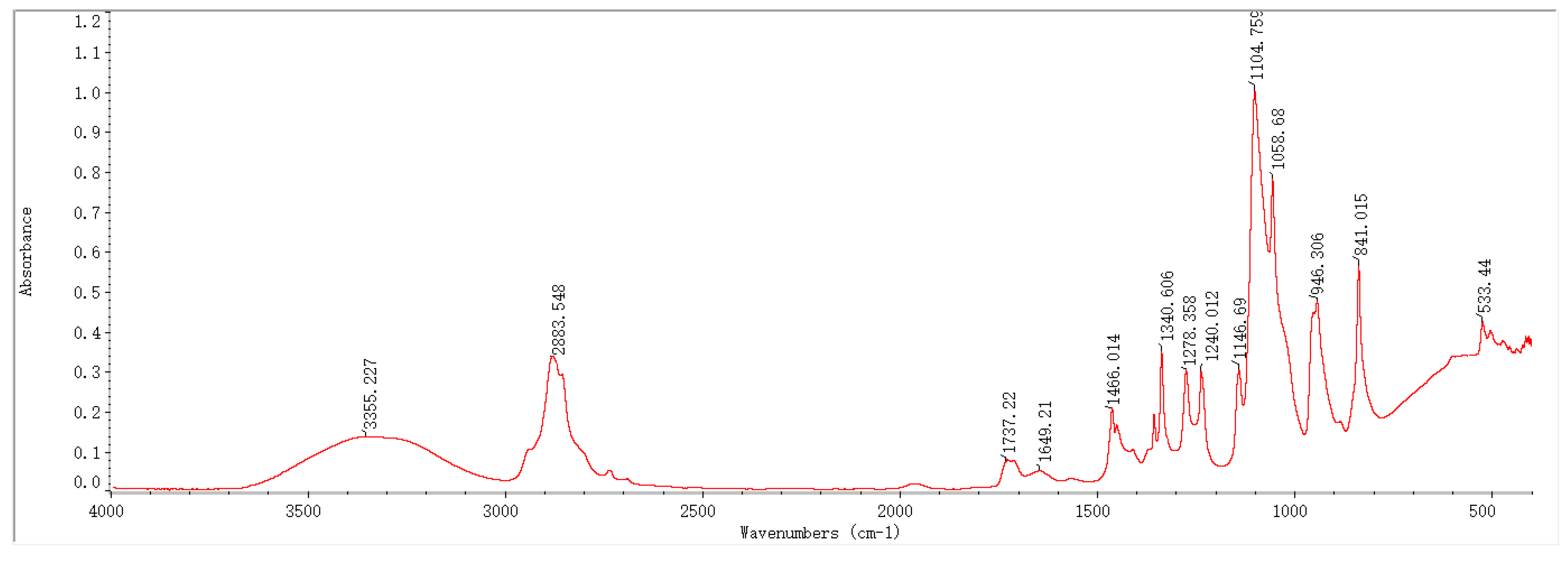
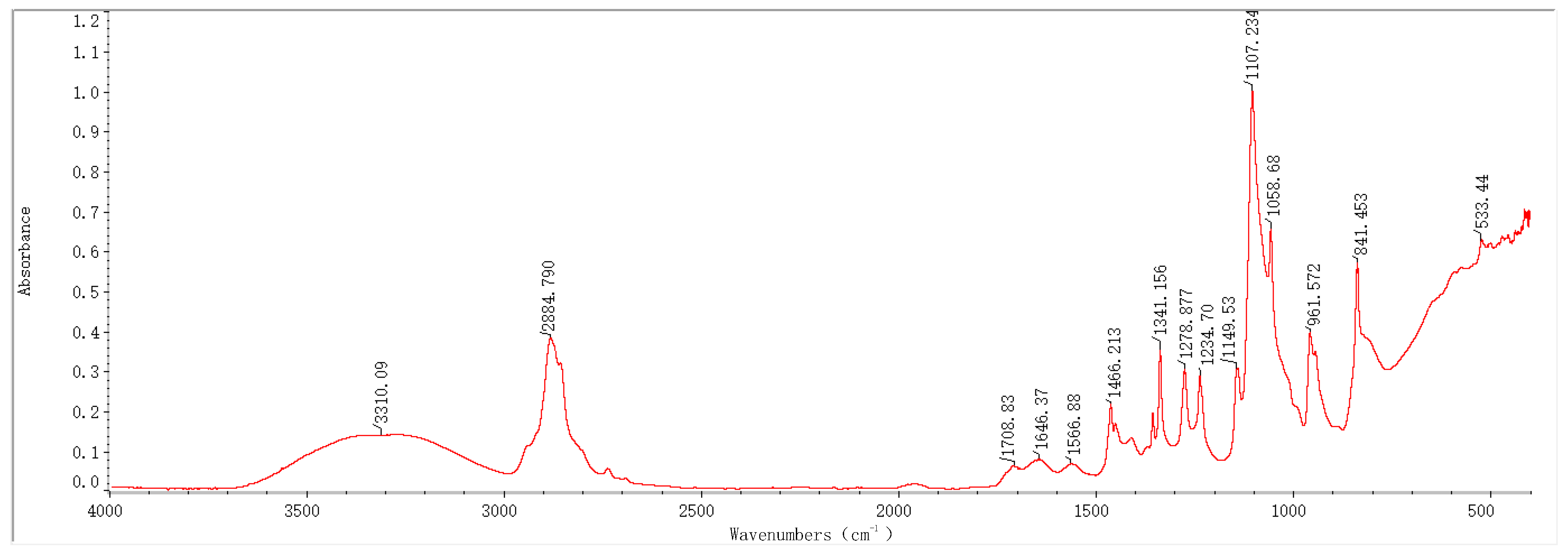
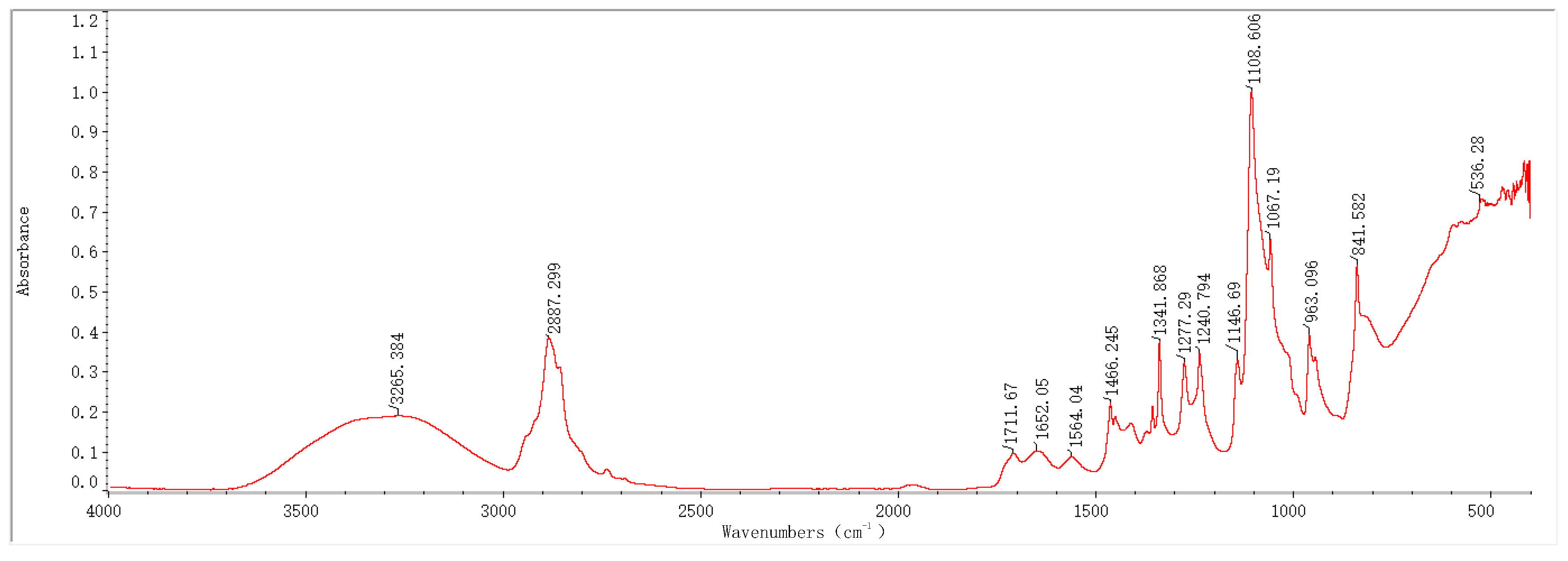
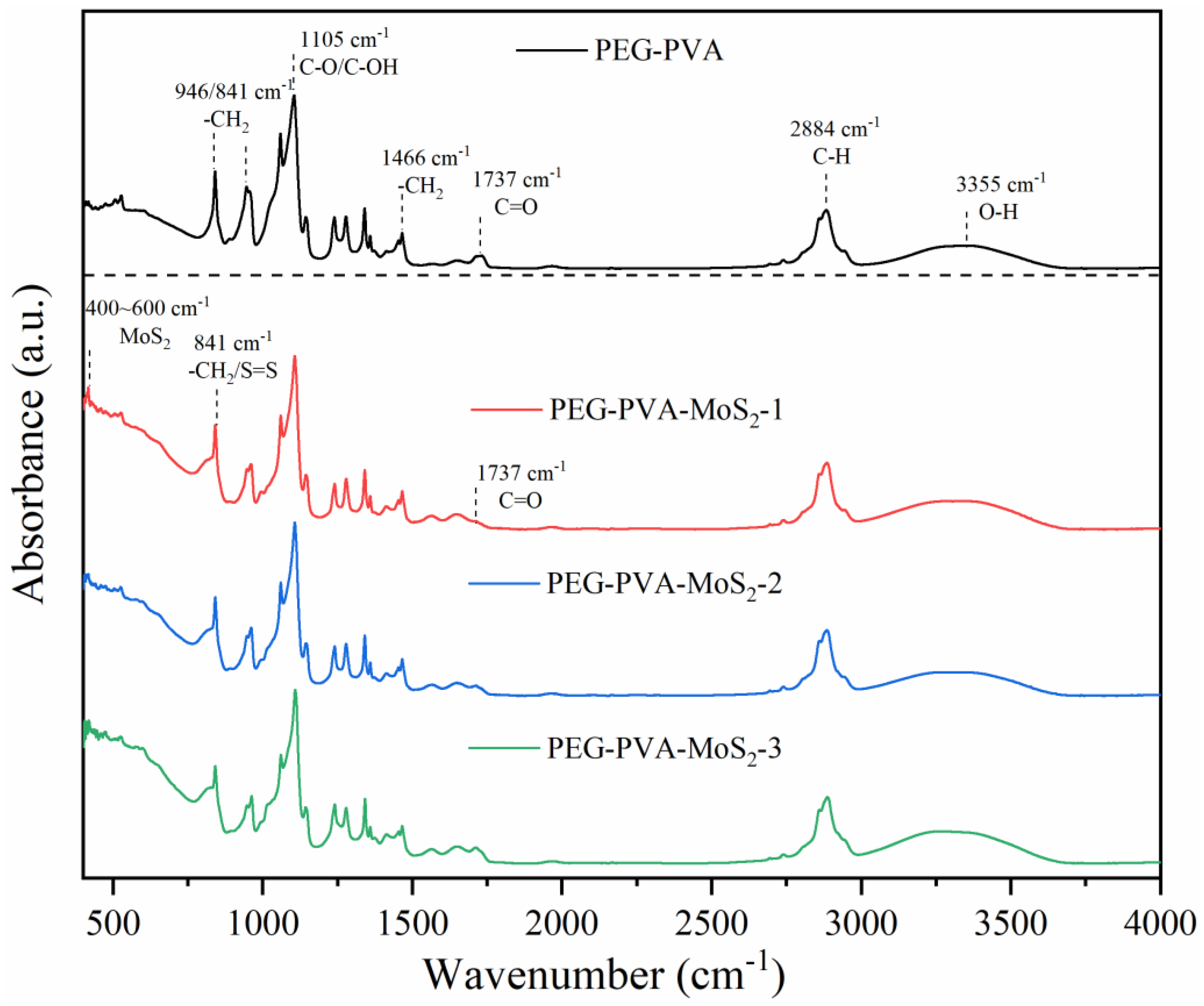
2.1. Infrared Spectroscopy Results and Analysis
2.2. SEM Analysis
2.3. Dielectric Constant Testing
2.4. Relationship between Real-Time Blood Glucose Concentration and the Output Period of the 555 Chip
2.4.1. Capacitors in Series
2.4.2. Capacitors in Parallel
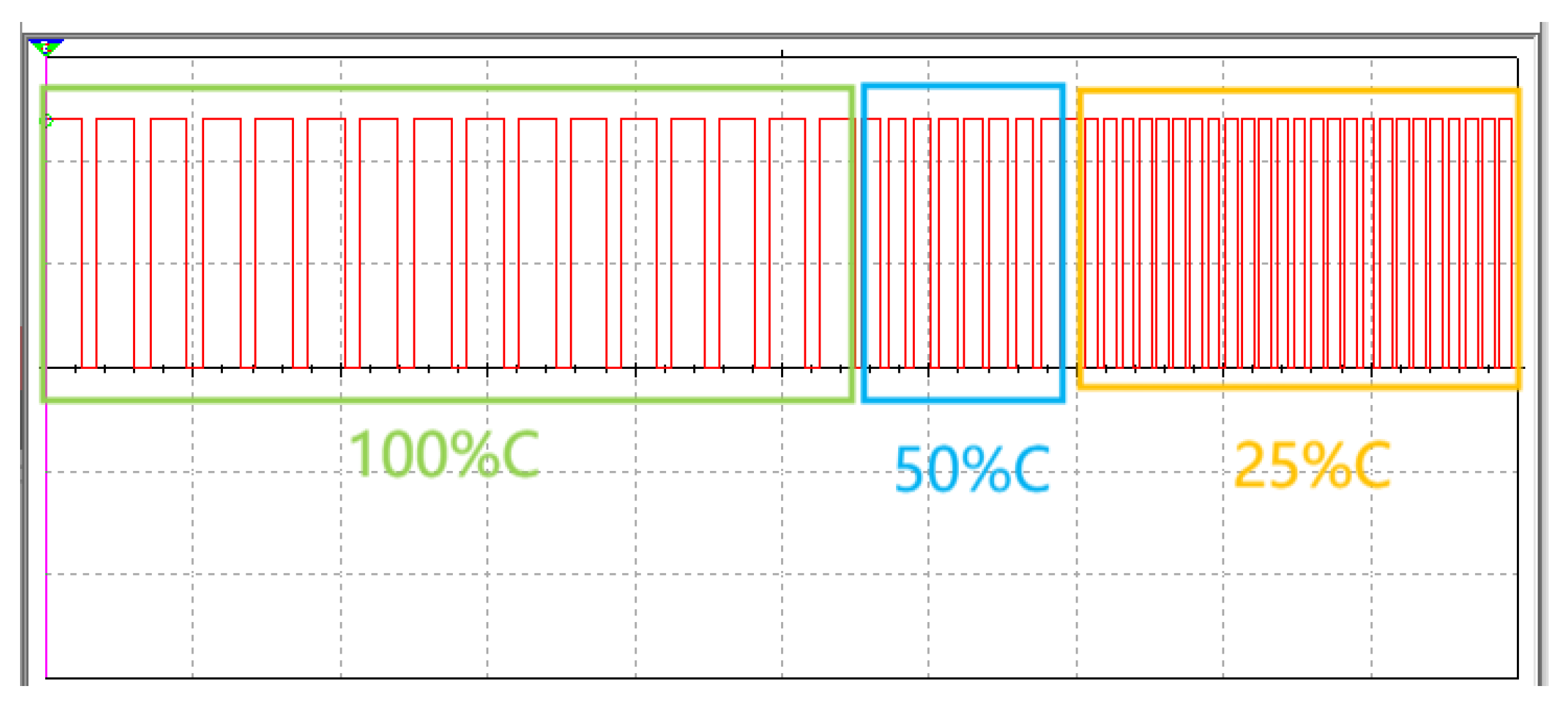
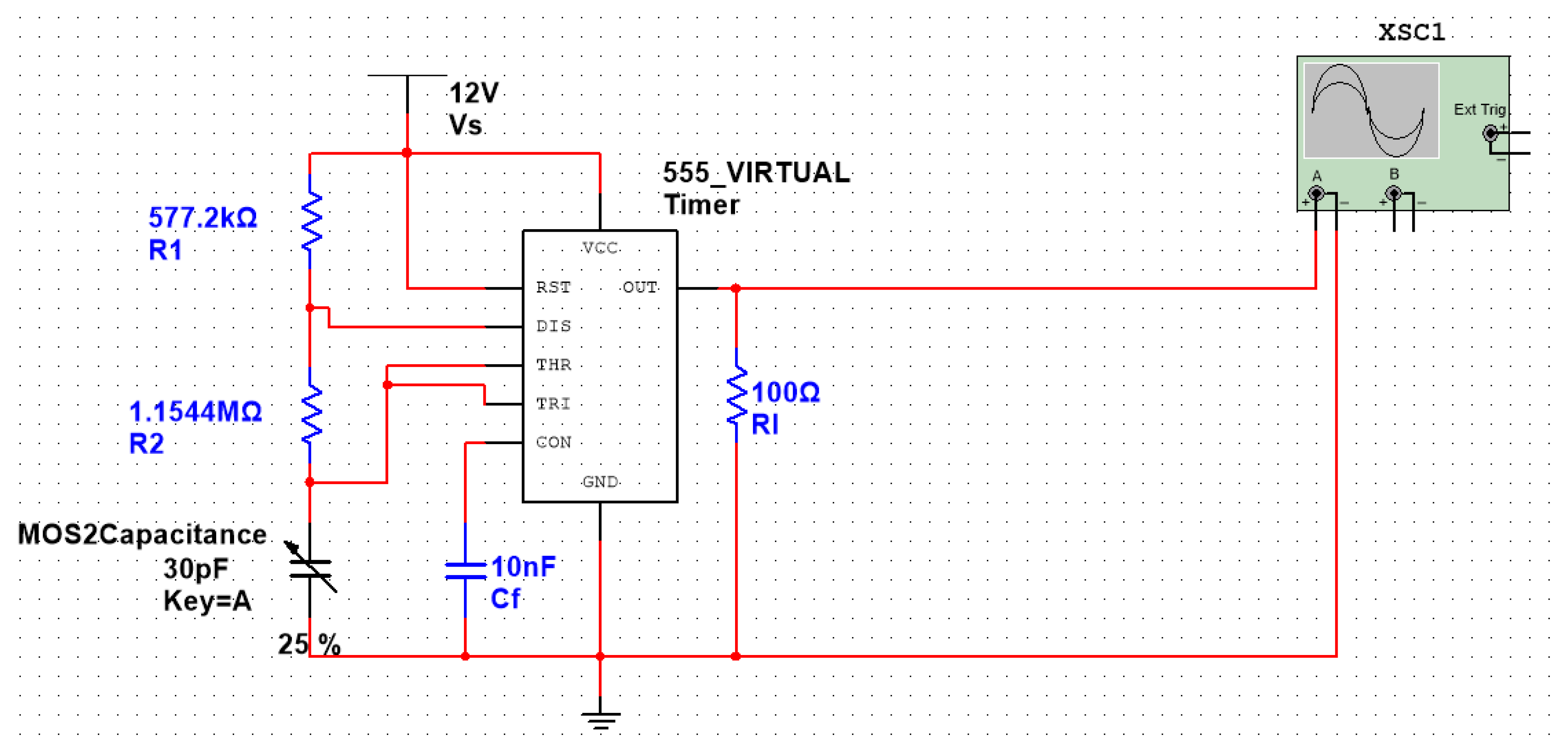
2.4.3. The Output Period of the 555 Chip
3. Discussion
3.1. Physical Structure Analysis of the PVA-PEG-MoS2 Hydrogel
3.2. Doping Concentrations of MoS2 and the Dielectric Constant
3.3. Parallel and Series Connections of Capacitors and Changes in the Output Periods of the 555 Chip
4. Materials and Methods
4.1. Chemicals
4.2. Instruments
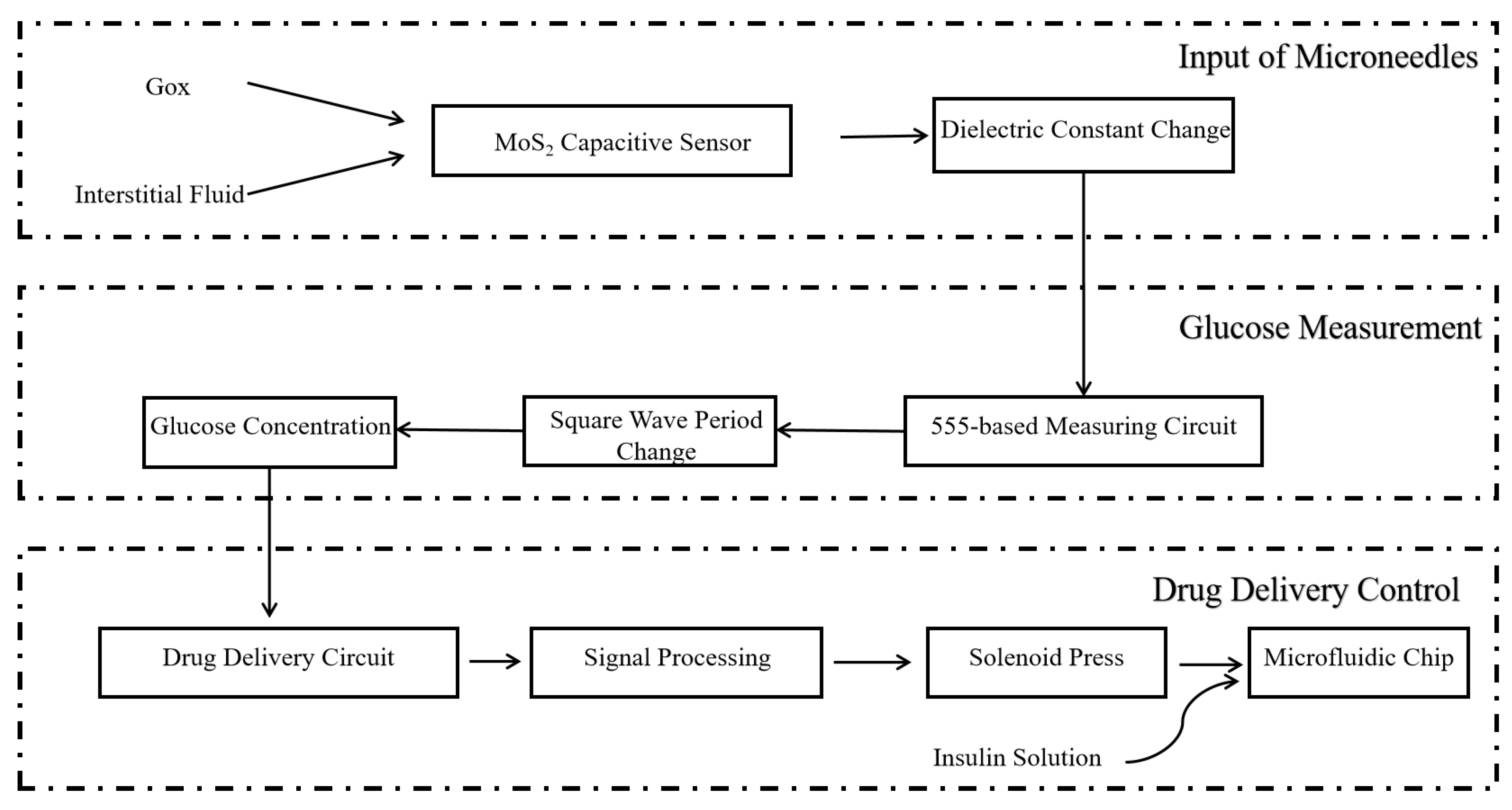
4.3. Overall Methodology
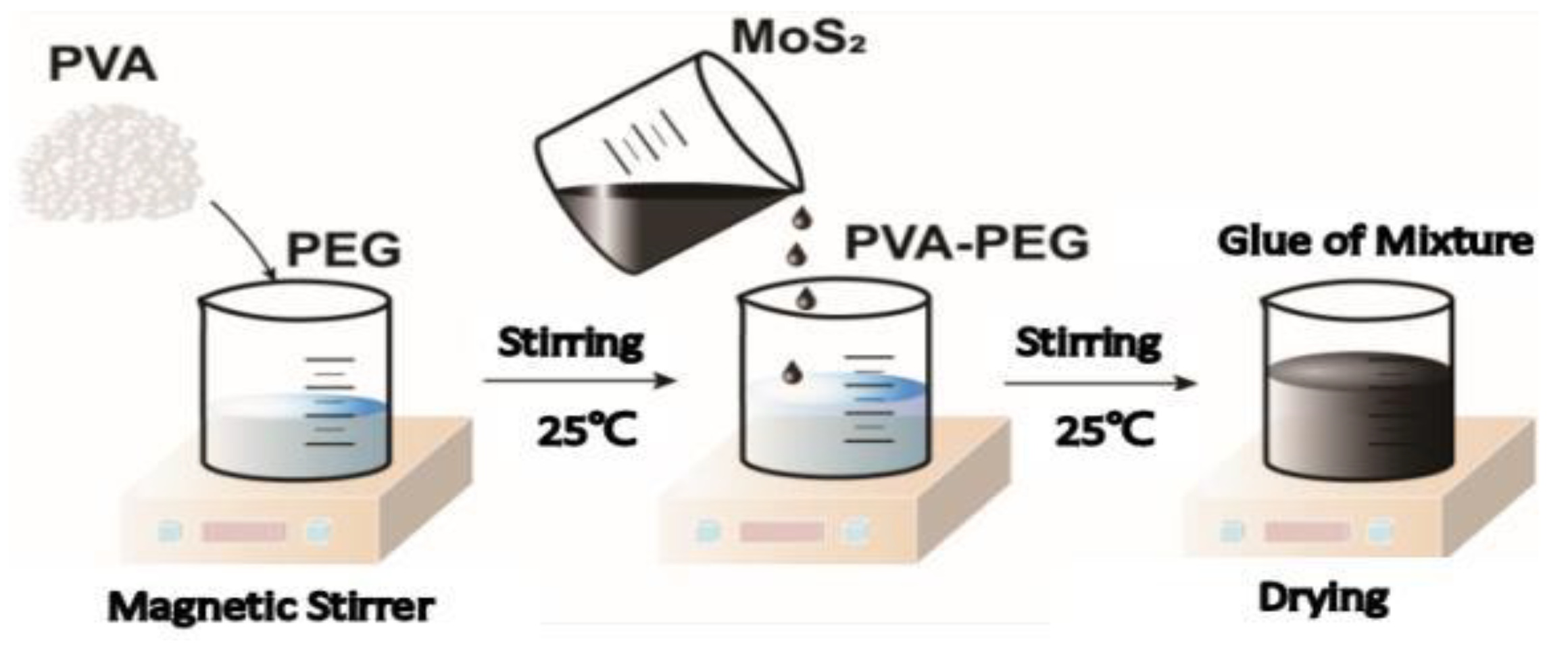
4.3.1. Preparation of the PVA-PEG-MoS2 Hydrogel
- (1)
- Preparation Results
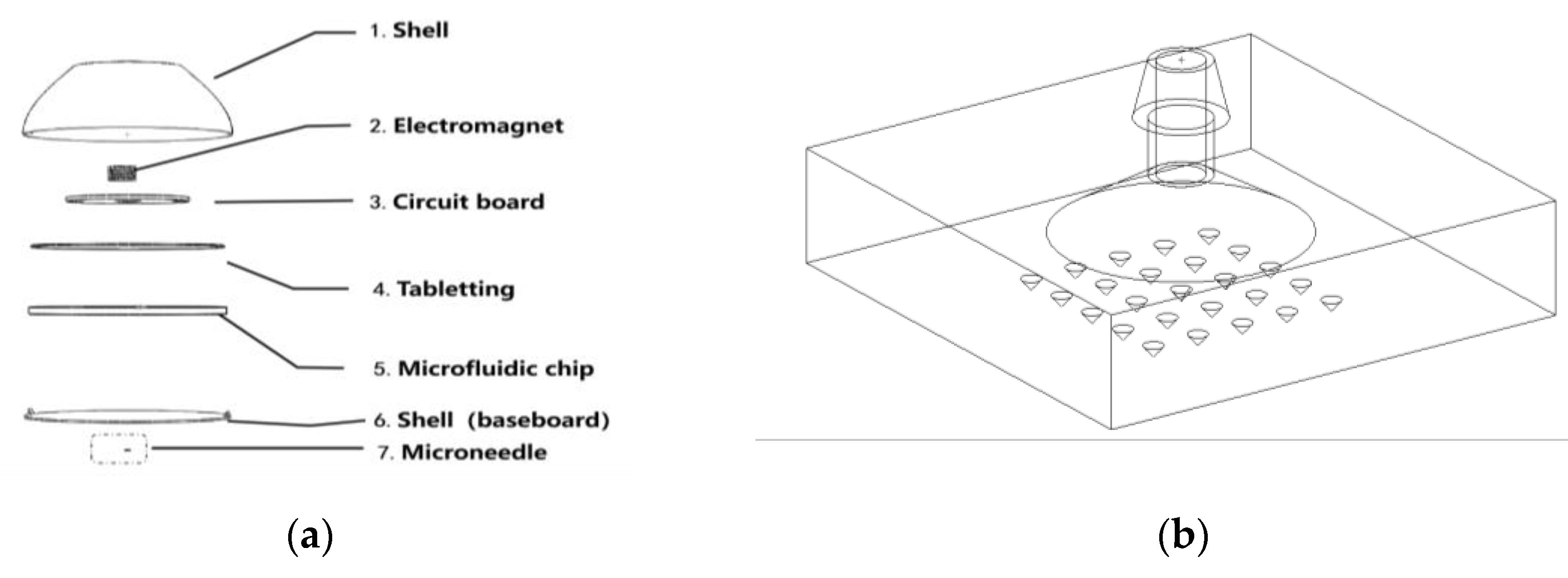
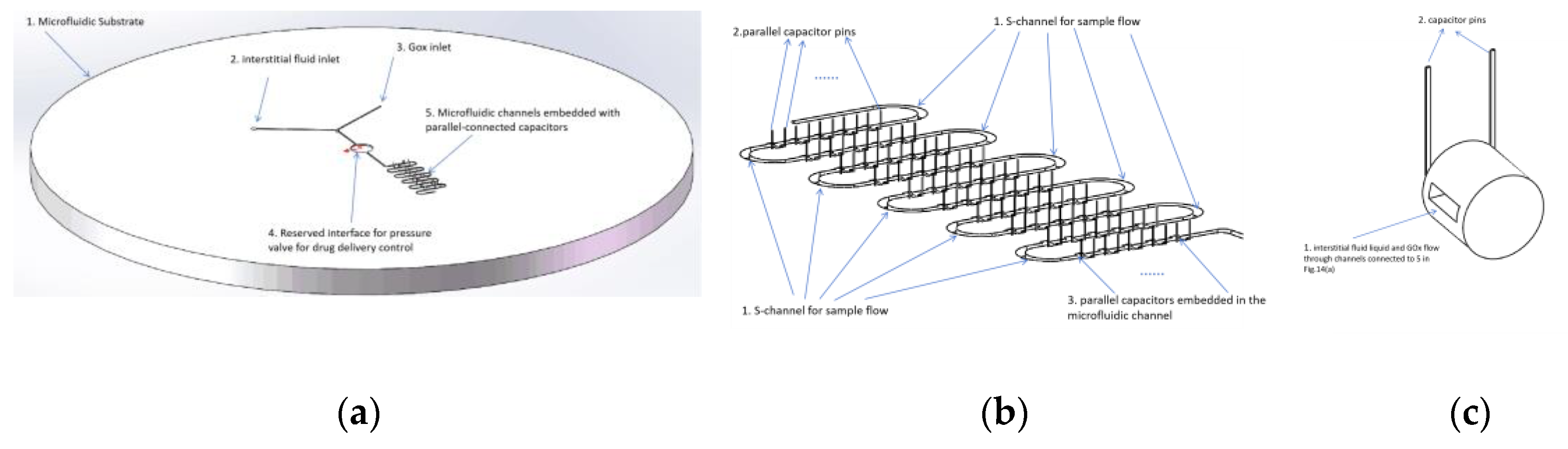
4.3.2. Improvement in Microfluidic Chip Design
4.3.3. Calibration of Microcapacitors to Glucose Concentration in Interstitial Fluid
- Capacitors connected in parallel.
- ▪
- Initial state: Defined as the state before the microfluidic chip is used. In this case, the total capacitance is specified in Equation (1):
- ▪
- Depleted state: Defined as the state in which all MoS2 nanosheets embedded in the microfluidic chip are consumed or changed in property, which results in an ignored capacitance change. In this case, the total capacitance is specified in Equation (2):where is the total capacitance of the capacitors connected in parallel in the depleted state.
- ▪
- Intermediate state: Defined as the state in which the capacitors embedded in the microfluidic chip start reacting with glucose in the interstitial fluid. In this case, the total capacitance is expressed in Equation (3):
- Capacitors connected in series
4.3.4. Circuit Design Based on the 555 Chip
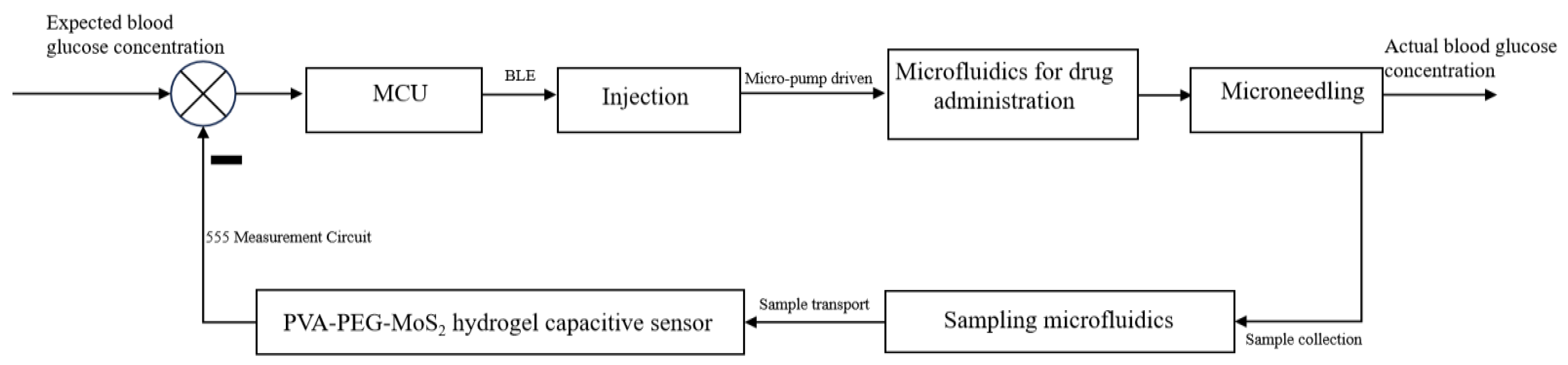
4.4. Drug Delivery Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Cao, C. Development and challenges of wearable flexible continuous glucose monitoring technology. Appl. Electron. Tech. 2023, 49, 75–84. [Google Scholar] [CrossRef]
- Wen, C.; Lin, Z.; Huang, L.; Qin, S.; Wang, Y.; Wen, H. Au@Cu2O composite nanorods as mimetic peroxidase for glucose colorimetric sensing. J. Photochem. Photobiol. A Chem. 2024, 455, 115786. [Google Scholar] [CrossRef]
- Fiedorova, K.; Augustynek, M.; Kubicek, J.; Kudrna, P.; Bibbo, D. Review of present method of glucose from human blood and body fluids assessment. Biosens. Bioelectron. 2022, 211, 114348. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, D.; Thomas, H.N.; Potter, M.; Dale, B.L.; Baum, J.V.; Toghill, K.E.; Hardy, J.G. Non-enzymatic Electrochemical Determination of Glucose Concentration. World J. Chem. Educ. 2020, 8, 107–113. [Google Scholar]
- Patra, S.; Purohit, S.S.; Swain, S.K. In vivo fluorescence non-enzymatic glucose sensing technique for diabetes management by CQDs incorporated dextran nanocomposites in human blood serums. Microchem. J. 2023, 190, 108646. [Google Scholar] [CrossRef]
- Peleg, Y.; Shefer, S.; Anavy, L.; Chudnovsky, A.; Israel, A.; Golberg, A.; Yakhini, Z. Sparse NIR Optimization method (SNIRO) to quantify analyte composition with visible (VIS)/near infrared (NIR) spectroscopy (350 nm–2500 nm). Anal. Chim. Acta 2018, 1051, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Lu, C.; Na, T.; Wang, T. Research of blood glucose control state detection based on percussion entropy. Anal. Chim. Acta 2023, 44, 657–663. [Google Scholar]
- Yang, M.; Liu, C. Clinical Application and Research Progress of Continuous Glucose Monitoring System. Chin. J. Pract. Intern. Med. 2023, 43, 688–693. [Google Scholar] [CrossRef]
- Chen, Z.; Lai, Y.; Xu, S.; Zhu, M.; Sun, Y.; Cheng, Y.; Zhao, G. A self-powered controllable microneedle drug delivery system for rapid blood pressure reduction. Nano Energy 2024, 123, 109344. [Google Scholar] [CrossRef]
- Meetoo, D.; Wong, L.; Ochieng, B. Smart tattoo: Technology for monitoring blood glucose in the future. Br. J. Nurs. 2019, 28, 110–115. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, H.; Huang, C.; Chen, Z.; Han, A. A gel-based separation-free point-of-care device for whole blood glucose detection. Anal. Chem. 2020, 92, 16122–16129. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Gong, X.; Yang, J.; Zheng, G.; Zheng, Y.; Li, Y.; Xu, Y.; Nie, G.; Xie, X.; Chen, M.; et al. A Touch-Actuated Glucose Sensor Fully Integrated with Microneedle Array and Reverse Iontophoresis for Diabetes Monitoring. Biosens. Bioelectron. 2022, 203, 114026. [Google Scholar] [CrossRef] [PubMed]
- Benavente, E.; Sánchez, V.; Ana, M.A.S.; González, G. Dialkylamines—Molybdenum Disulfide. Intercalates. Synthesis, Characterization, and Electrical Properties. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 2000, 354, 457–462. [Google Scholar] [CrossRef]
- Lemmon, P.J.; Lerner, M.M. Preparation and Characterization of Nanocomposites of Polyethers and Molybdenum Disulfide. Chem. Mater. 2002, 6, 207–210. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jiang, W.; Lee, D.; Moon, D.; Choi, H.; Shin, J.; Jeong, Y.; Kim, J.C.; Lee, J.; Huh, W.; et al. Boltzmann Switching MoS2 Metal–Semiconductor Field-Effect Transistors Enabled by Monolithic-Oxide-Gapped Metal Gates at the Schottky–Mott Limit. Adv. Mater. 2024, 36, 2314274. [Google Scholar] [CrossRef]
- Zhai, Y.J.; Chen, X.L.; Li, J.H.; Chu, X.Y.; Zhang, Y. Low-Temperature Photoluminescence Properties of the Monolayer MoS2 Nanomaterals. Integr. Ferroelectr. 2020, 212, 147–155. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Song, Y.; Yang, Q.; Shi, Z.; Xiong, C. Flexible and environment-friendly regenerated cellulose/MoS2 nanosheet nanogenerators with high piezoelectricity and output performance. Cellulose 2021, 28, 6513–6522. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, Z.; He, Q.; Wang, Z.; Wang, S.J.; Du, Y.; Yin, Z.; Sun, X.; Chen, W.; Zhang, H. Electrochemically reduced single-layer MoS2 nanosheets: Characterization, properties, and sensing applications. Small 2012, 8, 2264–2270. [Google Scholar] [CrossRef]
- Lin, T.; Zhong, L.; Chen, H.; Li, Z.; Song, Z.; Guo, L.; Fu, F. A sensitive colorimetric assay for cholesterol based on the peroxidase-like activity of MoS2 nanosheets. Microchim. Acta 2017, 184, 1233–1237. [Google Scholar] [CrossRef]
- Parlak, O.; İncel, A.; Uzun, L.; Turner, A.P.; Tiwari, A. Structuring Au nanoparticles on two-dimensional MoS2 nanosheets for electrochemical glucose biosensors. Biosens. Bioelectron. 2017, 89 Pt 1, 545–550. [Google Scholar] [CrossRef]
- Wang, T.; Dong, D.; Chen, T.; Zhu, J.; Wang, S.; Wen, W.; Zhang, X.; Tang, H.; Liang, J.; Wang, S.; et al. Acidity-responsive cascade nanoreactor based on metal-nanozyme and glucose oxidase combination for starving and photothermal-enhanced chemodynamic antibacterial therapy. Chem. Eng. J. 2022, 446 Pt 3, 137172. [Google Scholar] [CrossRef]
- Tiwari, P.; Nirala, N.R.; Prakash, R. Determination of the anti-HIV drug nevirapine using electroactive 2D material Pd@rGO decorated with MoS2 quantum dots. ChemistrySelect 2018, 3, 5341–5347. [Google Scholar] [CrossRef]
- Yang, T.; Chen, M.; Nan, F.; Chen, L.; Luo, X.; Jiao, K. Enhanced electropolymerization of poly(xanthurenic acid)–MoS2 film for specific electrocatalytic detection of guanine and adenine. J. Mater. Chem. B 2015, 3, 4884–4891. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, X. Molybdenum disulfide nanosheets supported Au-Pd bimetallic nanoparticles for non-enzymatic electrochemical sensing of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 239, 536–543. [Google Scholar] [CrossRef]
- Shi, R.; He, Q.; Cheng, S.; Chen, B.; Wang, Y. Determination of glucose by using MoS2 nanosheets as a peroxidase mimetic enzyme. New J. Chem. 2021, 45, 18048–18053. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Y.; Sun, W.; Wang, Y.; Chen, J.; Yang, W. Design of capacitance micro-displacement measurement system based on STM32 microcontroller. J. Tianjing Univ. Sci. Technol. 2022, 37, 38–44. [Google Scholar] [CrossRef]
- Lu, J.; Wang, S.; Wang, Y.; Hu, Q. Study on oil replacement time between plates of capacitive water-containing sensor. Hydraul. Pneum. Seals 2024, 44, 74–79. [Google Scholar]
- Cai, L.; Kong, L. Mathematical model of cylindrical capacitance grain moisture sensor and analysis of influential factors. Mathematical Model and Influencing Factor Analysis of Cylindrical Capacitive Grain Moisture Sensor. Anal. Instrum. 2009, 49–52. [Google Scholar]
- Zhao, L.; Shang, S.; Gao, L.; Hu, X.; Yin, Y.; Gong, L. Nondestructive measurement of moisture content of peanut kernels based on concentric cylindrical capacitance. Trans. Chin. Soc. Agric. Eng. 2016, 32, 212–218. [Google Scholar]
- Huang, H. Design and Application of 555-Based Chaotic Circuit. Master’s Thesis, Suzhou University, Suzhou, China, 2023. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Yan, H.; Song, Z.; Zhang, Y.; Dong, H.; Zhu, H.; Gu, S. Improved Fabrication Method and Application of Flexible Resistors Based on Molybdenum Disulfide Nanosheets. Chinese Materials Research Society. In Proceedings of the Chinese Materials Conference 2021, Xiamen, China, 9 June 2021; pp. 26–32. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Dong, H.; Ji, R.; Gu, S.; Chen, W.; Ge, S.; Zhang, Y. Research and Realization of Flexible Piezoelectric Sensors based on MoS2 Nanosheets-Films. In Proceedings of the EMIE 2022: The 2nd International Conference on Electronic Materials and Information Engineering, Hangzhou, China, 15–17 April 2022; pp. 1–5. [Google Scholar]


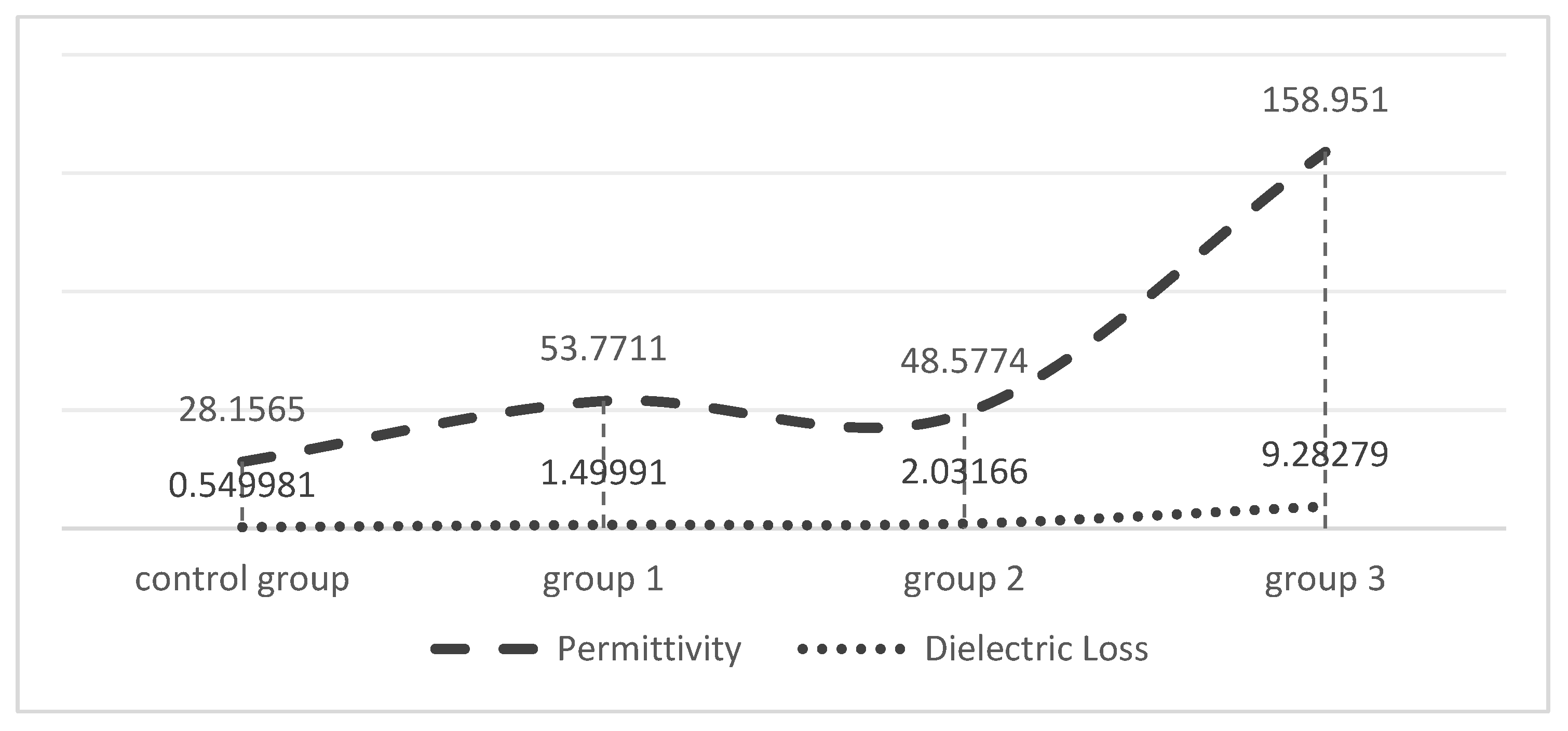


| Group | Measurement Frequency (Hz) | Permittivity | Dielectric Loss (S/CM) |
|---|---|---|---|
| Group 1 | 1000 | 53.7711 | 1.49991 |
| Group 2 | 1000 | 48.5774 | 2.03166 |
| Group 3 | 1000 | 158.951 | 9.28279 |
| Control Group | 1000 | 28.1465 | 0.549981 |
| Group | MoS2 Nanosheets * | PVA-PEG Embedded Media | Distilled Water |
|---|---|---|---|
| Group 1 (0.8%) | 40 mg | 5.000 g | 60 mL |
| Group 2 (1.2%) | 60 mg | 5.000 g | 40 mL |
| Group 3 (2%) | 100 mg | 5.000 g | 0 mL |
| Control Group (0%) | 0 mg | 5.000 g | 100 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, J.; Zhang, Y.; Wang, B.; Zhang, Y.; Lin, H. Hydrogel Capacitors Based on MoS2 Nanosheets and Applications in Glucose Monitoring. Molecules 2024, 29, 4401. https://doi.org/10.3390/molecules29184401
Wang Y, Zhang J, Zhang Y, Wang B, Zhang Y, Lin H. Hydrogel Capacitors Based on MoS2 Nanosheets and Applications in Glucose Monitoring. Molecules. 2024; 29(18):4401. https://doi.org/10.3390/molecules29184401
Chicago/Turabian StyleWang, Yizhi, Jinwen Zhang, Yusen Zhang, Bing Wang, Yang Zhang, and Hui Lin. 2024. "Hydrogel Capacitors Based on MoS2 Nanosheets and Applications in Glucose Monitoring" Molecules 29, no. 18: 4401. https://doi.org/10.3390/molecules29184401
APA StyleWang, Y., Zhang, J., Zhang, Y., Wang, B., Zhang, Y., & Lin, H. (2024). Hydrogel Capacitors Based on MoS2 Nanosheets and Applications in Glucose Monitoring. Molecules, 29(18), 4401. https://doi.org/10.3390/molecules29184401






