Application of the Wittig Rearrangement of N-Butyl-2-benzyloxybenzamides to Synthesis of Phthalide Natural Products and 3-Aryl-3-benzyloxyisoindolinone Anticancer Agents
Abstract
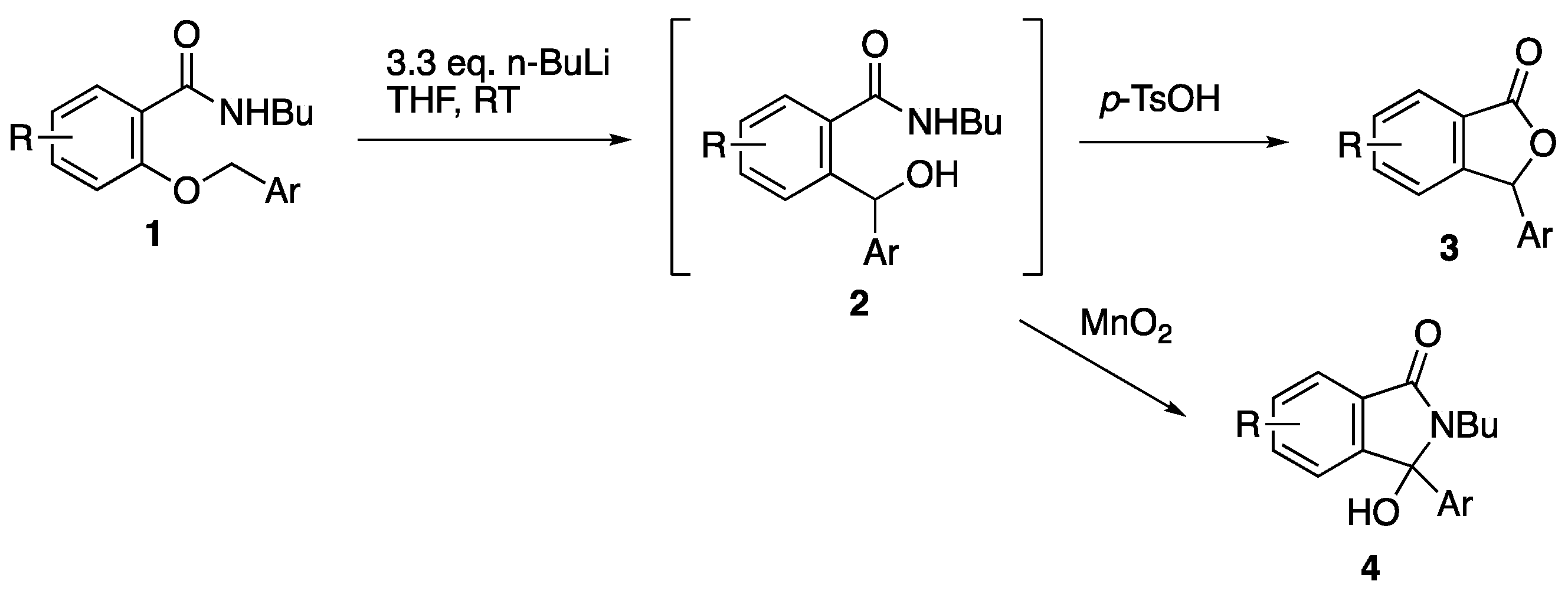
1. Introduction
2. Results and Discussion
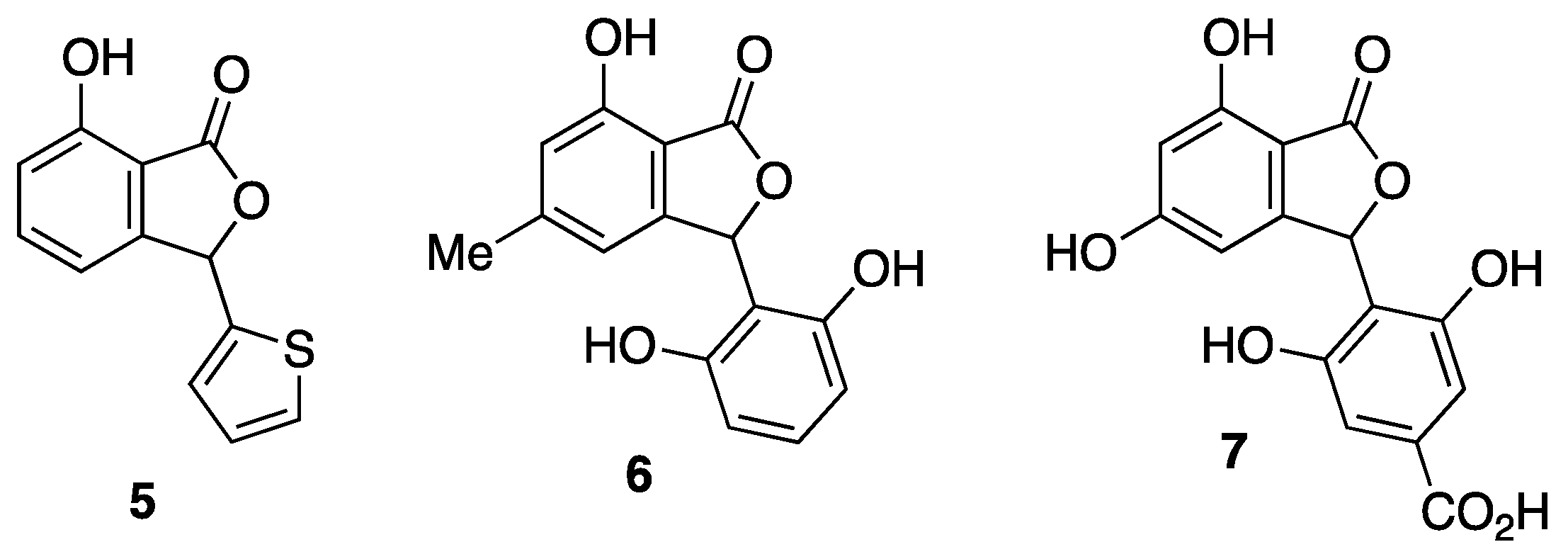
2.1. Synthesis of 3-Arylphthalide Natural Products
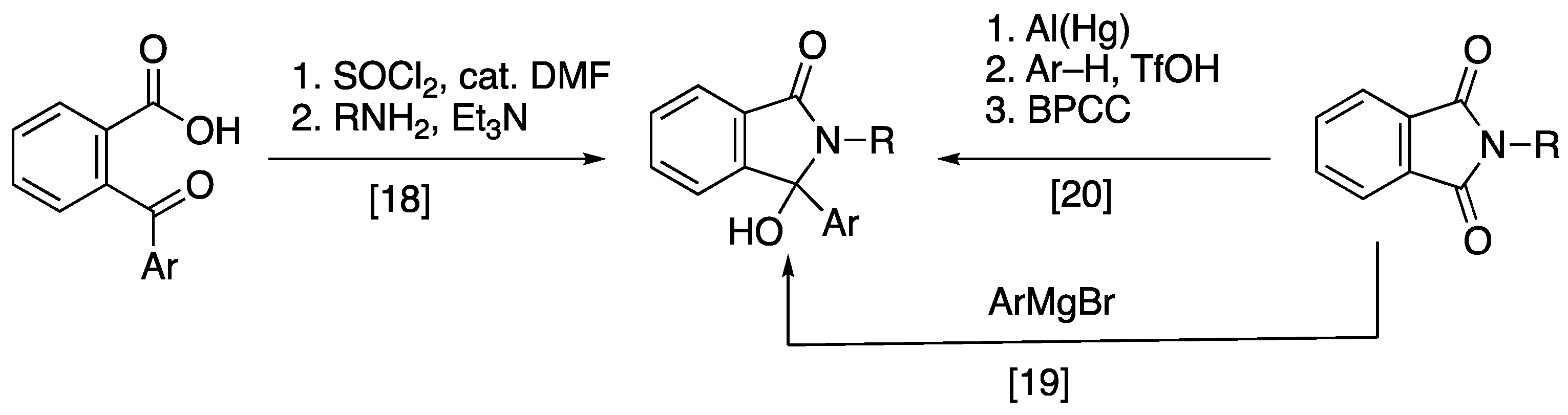
2.2. Synthesis of 3-Aryl-3-hydroxyisoindolin-2-ones and Their Benzyl Derivatives
3. Experimental
3.1. General Experimental Details
3.2. Trial Deprotection of a Methoxyphthalide
Preparation of 6-Hydroxy-3-phenylisobenzofuran-1(3H)-one 9
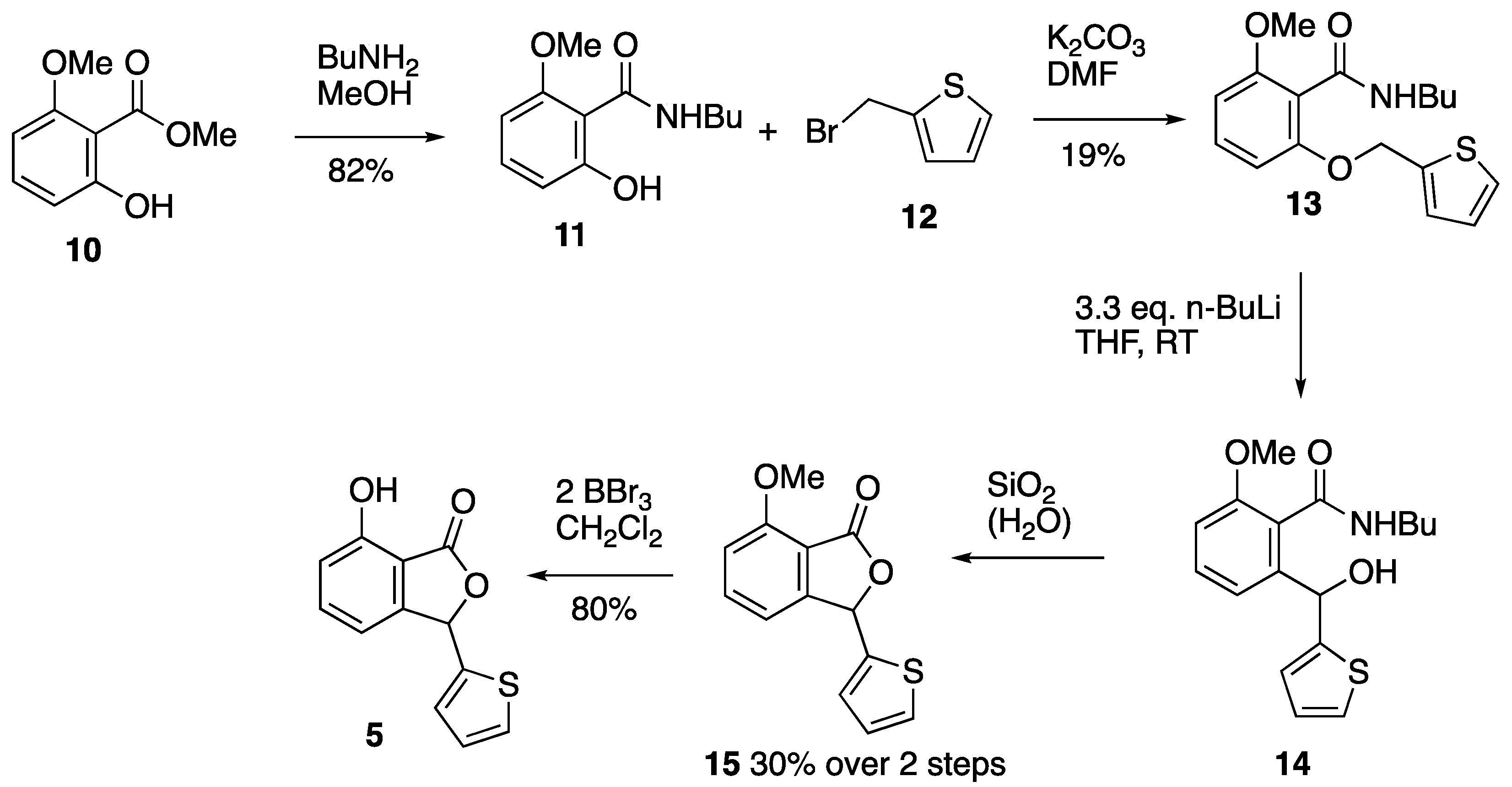
3.3. Synthesis of Chrycolide 5
3.3.1. Methyl 2-Hydroxy-6-methoxybenzoate 10
3.3.2. N-Butyl-2-hydroxy-6-methoxybenzamide 11
3.3.3. N-Butyl-2-methoxy-6-(2-thienylmethoxy)benzamide 13
3.3.4. N-Butyl-2-methoxy-6-(2-thienyl(hydroxy)methyl)benzamide 14 and Cyclisation to 7-Methoxy-3-(2-thienyl)isobenzofuran-1(3H)-one 15
3.3.5. Chrycolide 5
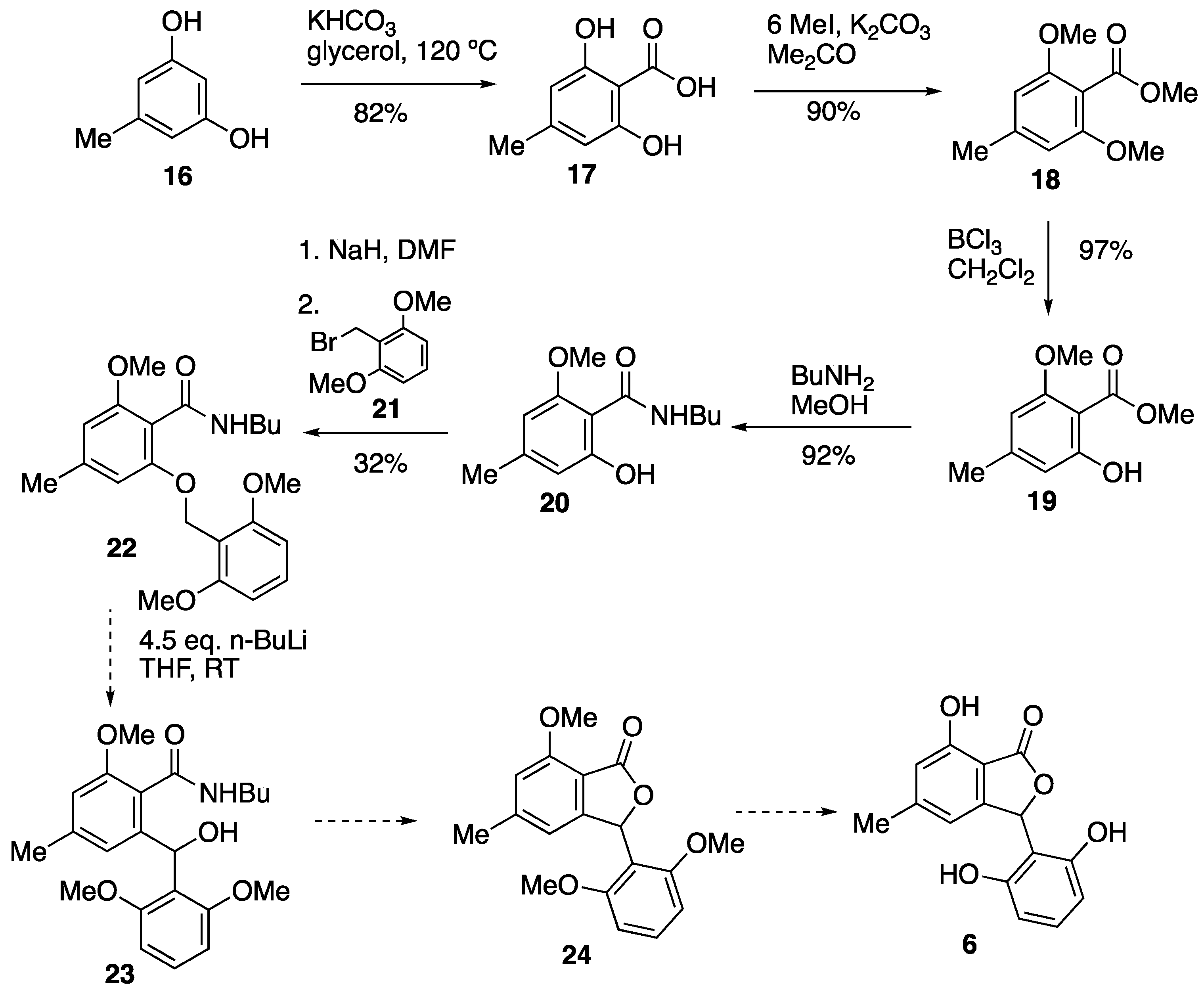
3.4. Attempted Synthesis of Isopestacin 6
3.4.1. 2,6-Dihydroxy-4-methylbenzoic Acid 17
3.4.2. Methyl 2,6-Dimethoxy-4-methylbenzoate 18
3.4.3. Methyl 2-Hydroxy-6-methoxy-4-methylbenzoate 19
3.4.4. N-Butyl-2-hydroxy-6-methoxy-4-methylbenzamide 20
3.4.5. N-Butyl-2-(2,6-dimethoxybenzyloxy)-6-methoxy-4-methylbenzamide 22
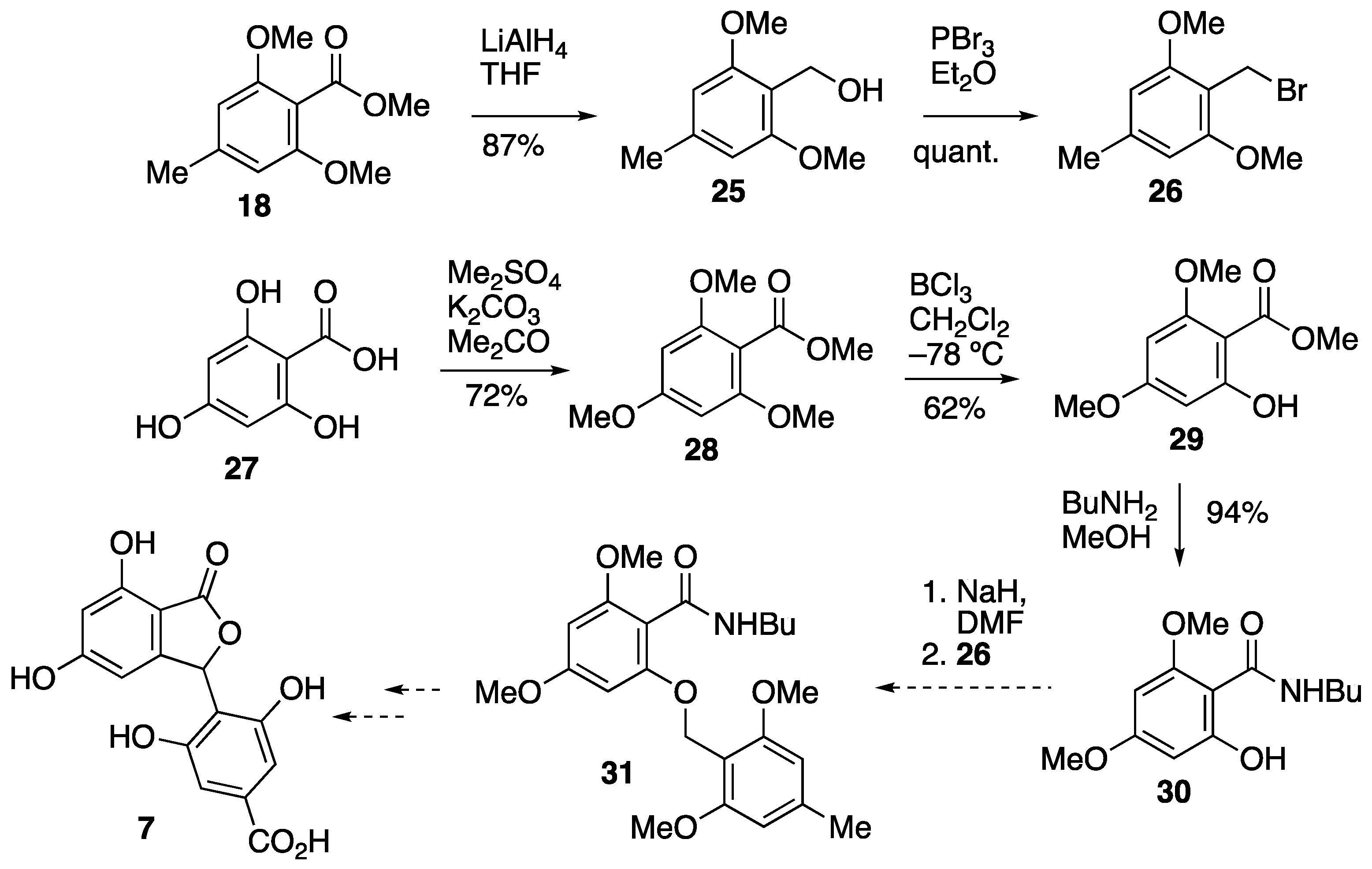
3.5. Attempted Synthesis of Cryphonectric Acid 7
3.5.1. 2,6-Dimethoxy-4-methylbenzyl Alcohol 25
3.5.2. 2,6-Dimethoxy-4-methylbenzyl Bromide 26
3.5.3. Methyl 2,4,6-Trimethoxybenzoate 28
3.5.4. Methyl 2-Hydroxy-4,6-dimethoxybenzoate 29
3.5.5. N-Butyl-2-hydroxy-4,6-dimethoxybenzamide 30
3.5.6. N-Butyl-2,4-dimethoxy-6-(2,6-dimethoxy-4-methylbenzyloxy)benzamide 31
3.6. Synthesis 3-Aryl-3-hydroxyisoindolin-1-ones and Their Benzyl Derivatives
3.6.1. Preparation of 2-Butyl-3-hydroxy-3-phenylisoindolin-1(3H)-one 34
3.6.2. 2-Hydroxy-N-propylbenzamide 38
3.6.3. 2-Benzyloxy-N-propylbenzamide 39
3.6.4. 2-(4-Chlorobenzyloxy)-N-propylbenzamide 40
3.6.5. 2-(Hydroxy(phenyl)methyl)-N-propylbenzamide 41
3.6.6. 2-(Hydroxy(4-chlorophenyl)methyl)-N-propylbenzamide 42
3.6.7. 3-Hydroxy-3-phenyl-2-propylisoindolin-1(3H)-one 43
3.6.8. 3-(4-Chlorophenyl)-3-hydroxy-2-propylisoindolin-1(3H)-one 44
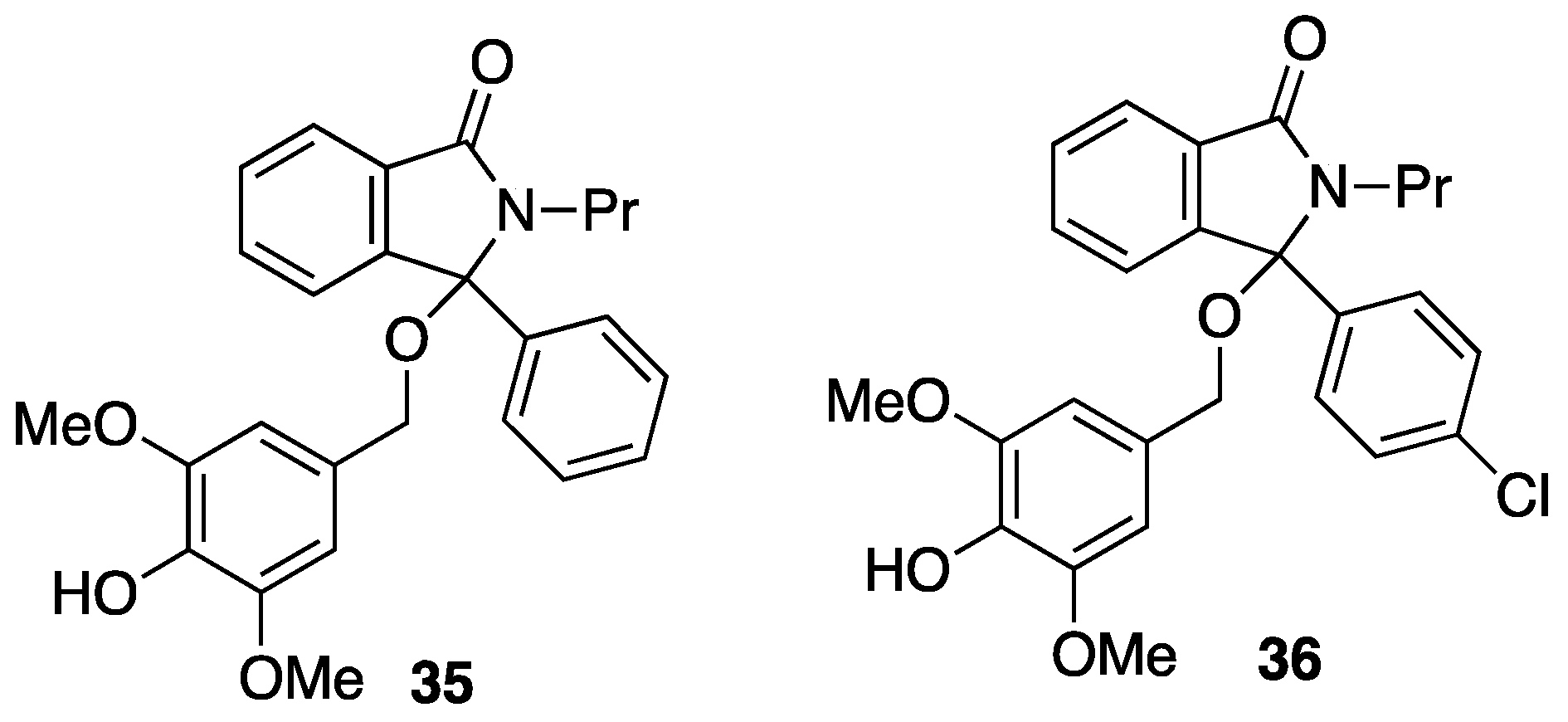
3.6.9. 3-(4-Hydroxy-3,5-dimethoxybenzyloxy)-3-phenyl-2-propylisoindolin-1(3H)-one 35
3.6.10. 3-(4-Chlorophenyl)-3-(4-hydroxy-3,5-dimethoxybenzyloxy)-2-propylisoindolin-1(3H)-one 36
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aitken, R.A.; Harper, A.D.; Inwood, R.A.; Slawin, A.M.Z. Access to diarylmethanols by Wittig rearrangement of ortho-, meta- and para-benzyloxy-N-butylbenzamides. J. Org. Chem. 2022, 87, 4692–4701. [Google Scholar] [CrossRef]
- Aitken, R.A.; Inwood, R.A. Synthesis and Wittig rearrangement of 3- and 4-benzyloxyphenylphosphonamidates. Organics 2023, 4, 59–69. [Google Scholar] [CrossRef]
- Aitken, R.A.; Ait Moulay, K.; Cordes, D.B.; Inwood, R.A.; Jamieson, F.G.; Nelson, A.J.B.; McKay, A.P. Synthesis and butyllithium-induced cyclisation of 2-benzyloxyphenylphosphonamidates giving 2,3-dihydrobenzo[d][1,3]oxaphospholes. Organics 2024, 5, 12–31. [Google Scholar] [CrossRef]
- Awasthi, A.; Singh, M.; Rathee, G.; Chandra, R. Recent advancements in synthetic methodologies of 3-substituted phthalides and their application in the total synthesis of biologically active natural products. RSC Adv. 2020, 10, 12626–12652. [Google Scholar] [CrossRef]
- Topolovcan, N.; Gredicak, M. Synthesis and stereoselective catalytic transformations of 3-hydroxyisoindolinones. Org. Biomol. Chem. 2021, 19, 4637–4651. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, I.R.; Ahmed, S.U.; Atkins, H.; Farnie, G.; Golding, B.T.; Griffin, R.J.; Guyenne, S.; Hutton, C.; Källblad, P.; Kemp, S.J.; et al. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction based on an isoindoline scaffold. J. Med. Chem. 2006, 49, 6209–6221. [Google Scholar] [CrossRef]
- Hardcastle, I.R.; Liu, J.; Valeur, E.; Watson, A.; Ahmed, S.U.; Blackburn, T.J.; Bennaceur, K.; Clegg, W.; Drummond, C.; Endicott, J.A.; et al. Isoindoline inhibitors of the murine double minute 2 (MDM2)-p53 protein-protein interaction: Structure-activity studies leading to improved potency. J. Med. Chem. 2011, 54, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Chiba, K. Novel plant growth inhibitors and an insect antifeedant from Chrysanthemum coronarium (Japanese name: Shungiku). Agric. Biol. Chem. 1984, 48, 1367–1369. [Google Scholar] [CrossRef]
- Strobel, G.; Ford, E.; Worapong, J.; Harper, J.K.; Arif, A.M.; Grant, D.M.; Fung, P.C.W.; Chau, R.M.W. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora possessing antifungal and antioxidant activities. Phytochemistry 2002, 60, 179–183. [Google Scholar] [CrossRef]
- Arnone, A.; Assante, G.; Nasini, G.; Strada, S.; Vercesi, A. Cryphonectric acid and other minor metabolites from a hypervirulent strain of Cryphonectria parasitica. J. Nat. Prod. 2002, 65, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, F.M.; Farimani, M.M. A simple and efficient total synthesis of (±)-danshexinkun A, a bioactive diterpenoid from Salvia miltiorrhiza. Tetrahedron Lett. 2010, 51, 540–542. [Google Scholar] [CrossRef]
- Biagetti, M.; Bellina, F.; Carpita, A.; Stabile, P.; Rossi, R. New procedures for the selective synthesis of 2(2H)-pyranone derivatives and 3-aryl-4-iodoisocoumarins. Tetrahedron 2002, 58, 5023–5038. [Google Scholar] [CrossRef]
- Kuethe, J.T.; Maloney, K.M. A concise synthesis of 3,4-fused spiro[isobenzofuran-3-ones], spiro[furo [3,4-b]pyridin-5(7H)-ones], 3-aryl-, and alkylphthalides. Tetrahedron 2013, 69, 5248–5258. [Google Scholar] [CrossRef]
- Crombie, L.; Games, D.E.; James, A.W.G. Reactions of fused and unfused α-pyrones with magnesium alkoxide, sodium alkoxide and water as the nucleophile: Effect of chelation. J. Chem. Soc. Perkin Trans. 1 1996, 2715–2724. [Google Scholar] [CrossRef]
- Kikuchi, H.; Hoshikawa, T.; Kurata, S.; Katou, Y.; Oshima, Y. Design and synthesis of structure-simplified derivatives of gonytolide for the promotion of innate immune responses. J. Nat. Prod. 2016, 79, 1259–1266. [Google Scholar] [CrossRef]
- Aitken, R.A.; Saab, E.A.; Slawin, A.M.Z. 2,6-Dimethoxybenzyl bromide. Molbank 2021, 2021, M1277. [Google Scholar] [CrossRef]
- Mal, D.; Pahari, P.; De, S.R. Regiospecific synthesis of 3-(2,6-dihydroxyphenyl)phthalides: Application to the synthesis of isopestacin and cryphonectric acid. Tetrahedron 2007, 63, 11781–11792. [Google Scholar] [CrossRef]
- Eddebbarh, E.; Moutardier, L.; Thibonnet, J.; Camiade, E.; Petrignet, J. Direct synthesis of 3-arylphthalides promoted by Eaton’s reagent. Eur. J. Org. Chem. 2024, 27, e202301303. [Google Scholar] [CrossRef]
- Kitching, M.S.; Clegg, W.; Elsegood, M.R.J.; Griffin, R.J.; Golding, B.T. Synthesis of 3-alkoxy- and 3-alkylamino-2-alkyl-3-arylisoindolinones. Synlett 1999, 1, 997–999. [Google Scholar] [CrossRef]
- Sekiya, M.; Terao, Y. Formic acid reduction XIV. Formation of 3-arylphthalimidines from 3-aryl-3-hydroxyphthalimidines. Chem. Pharm. Bull. 1972, 20, 2128–2133. [Google Scholar] [CrossRef][Green Version]
- Dempster, R.K.; Luzzio, F.A. A direct arylation-oxidation route to 3-arylisoindolinone inhibitors of MDM2-p53 interaction. Tetrahedron Lett. 2011, 52, 4992–4995. [Google Scholar] [CrossRef]
- Herzig, J.; Wenzel, F. Studien über Kernalkylierung bei Phenolen. Monatsh. Chem. 1906, 27, 781–802. [Google Scholar] [CrossRef]
- Bloomer, J.L.; Brosz, C.S. Synthesis of an 11-deoxypretetramide derivative. Tetrahedron 1985, 41, 3241–3252. [Google Scholar] [CrossRef]
- Lam, J.K.K.; Sargent, M.V. Synthesis of methyl tri-o-methylptilometrate (methyl 1,6,8-trimethoxy-3-propylanthraquinone-2-carboxylate). J. Chem. Soc. Perkin Trans. 1 1974, 1417–1421. [Google Scholar] [CrossRef]
- Góngora-Benítez, M.; Mendive-Tapia, L.; Ramos-Tomillero, I.; Breman, A.C.; Tulla-Puche, J.; Alberico, F. Acid-labile Cys-protecting groups for the Fmoc/t-Bu strategy: Filling the gap. Org. Lett. 2012, 14, 5472–5475. [Google Scholar] [CrossRef]
- Wehlan, H.; Jezek, E.; Lebrasseur, N.; Pavé, G.; Roullard, E.; White, A.J.P.; Burrows, J.N.; Barrett, A.G.M. Studies on the total synthesis of lactonomycin: Synthesis of the CDEF ring system. J. Org. Chem. 2006, 71, 8151–8158. [Google Scholar] [CrossRef] [PubMed]
- Herzig, J.; Wenzel, F. Über Carbonsäureester der Phloroglucine. Monatsh. Chem. 1902, 23, 81–118. [Google Scholar] [CrossRef]
- Li, P.; Zhao, J.; Lang, R.; Xia, C.; Li, F. Copper-catalyzed methyl esterification of aromatic aldehydes and benzoic alcohols by TBHP as both oxidant and methyl source. Tetrahedron Lett. 2014, 55, 390–393. [Google Scholar] [CrossRef]
- Rossi, R.; Carpita, A.; Bellina, F.; Stabile, P.; Mannina, L. Synthesis of 3-arylisocoumarins, including thunberginols A and B, unsymmetrical 3,4-disubstituted isocoumarins, and 3-ylidenephthalides via iodolactonization of methyl 2-ynylbenzoates or the corresponding carboxylic acids. Tetrahedron 2003, 59, 2067–2081. [Google Scholar] [CrossRef]
- Shan, G.; Han, X.; Lin, Y.; Yu, S.; Rao, Y. Broadening the catalyst and reaction scope of regioselective and chemoselective C–H oxygenation: A convenient and scalable approach to 2-acylphenols by intriguing Rh(II) and Ru(II) catalysis. Org. Biomol. Chem. 2013, 11, 2318–2322. [Google Scholar] [CrossRef] [PubMed]



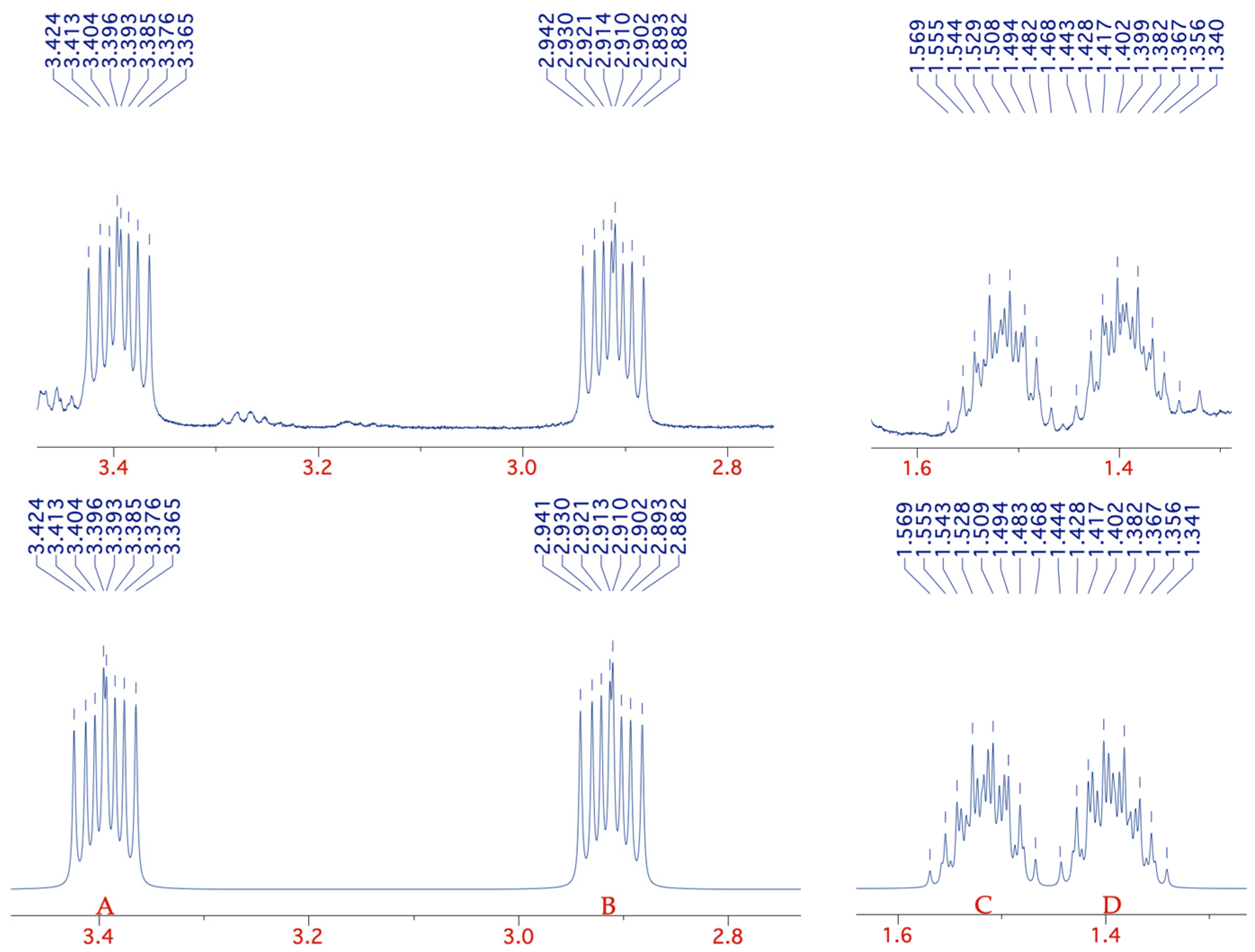
| Proton (*) | δ | J*–H1b | J*–H2a | J*–H2b | J*–H3 |
|---|---|---|---|---|---|
| H-1a | 3.394 | 14 | 10 | 5.5 | — |
| H-1b | 2.912 | 5.5 | 10 | — | |
| H-2a | 1.517 | 13 | 7.5 | ||
| H-2b | 1.393 | 7.5 | |||
| 3 × H-3 | 0.785 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Cooper, F.K.; Harper, A.D.; Inwood, R.A.; Saab, E.A.; Soutar, E.J. Application of the Wittig Rearrangement of N-Butyl-2-benzyloxybenzamides to Synthesis of Phthalide Natural Products and 3-Aryl-3-benzyloxyisoindolinone Anticancer Agents. Molecules 2024, 29, 4722. https://doi.org/10.3390/molecules29194722
Aitken RA, Cooper FK, Harper AD, Inwood RA, Saab EA, Soutar EJ. Application of the Wittig Rearrangement of N-Butyl-2-benzyloxybenzamides to Synthesis of Phthalide Natural Products and 3-Aryl-3-benzyloxyisoindolinone Anticancer Agents. Molecules. 2024; 29(19):4722. https://doi.org/10.3390/molecules29194722
Chicago/Turabian StyleAitken, R. Alan, Francesca K. Cooper, Andrew D. Harper, Ryan A. Inwood, Elizabeth A. Saab, and Ewan J. Soutar. 2024. "Application of the Wittig Rearrangement of N-Butyl-2-benzyloxybenzamides to Synthesis of Phthalide Natural Products and 3-Aryl-3-benzyloxyisoindolinone Anticancer Agents" Molecules 29, no. 19: 4722. https://doi.org/10.3390/molecules29194722
APA StyleAitken, R. A., Cooper, F. K., Harper, A. D., Inwood, R. A., Saab, E. A., & Soutar, E. J. (2024). Application of the Wittig Rearrangement of N-Butyl-2-benzyloxybenzamides to Synthesis of Phthalide Natural Products and 3-Aryl-3-benzyloxyisoindolinone Anticancer Agents. Molecules, 29(19), 4722. https://doi.org/10.3390/molecules29194722








