Synthesis, Physicochemical Characterization, and Antimicrobial Evaluation of Halogen-Substituted Non-Metal Pyridine Schiff Bases
Abstract
1. Introduction
2. Results and Discussion
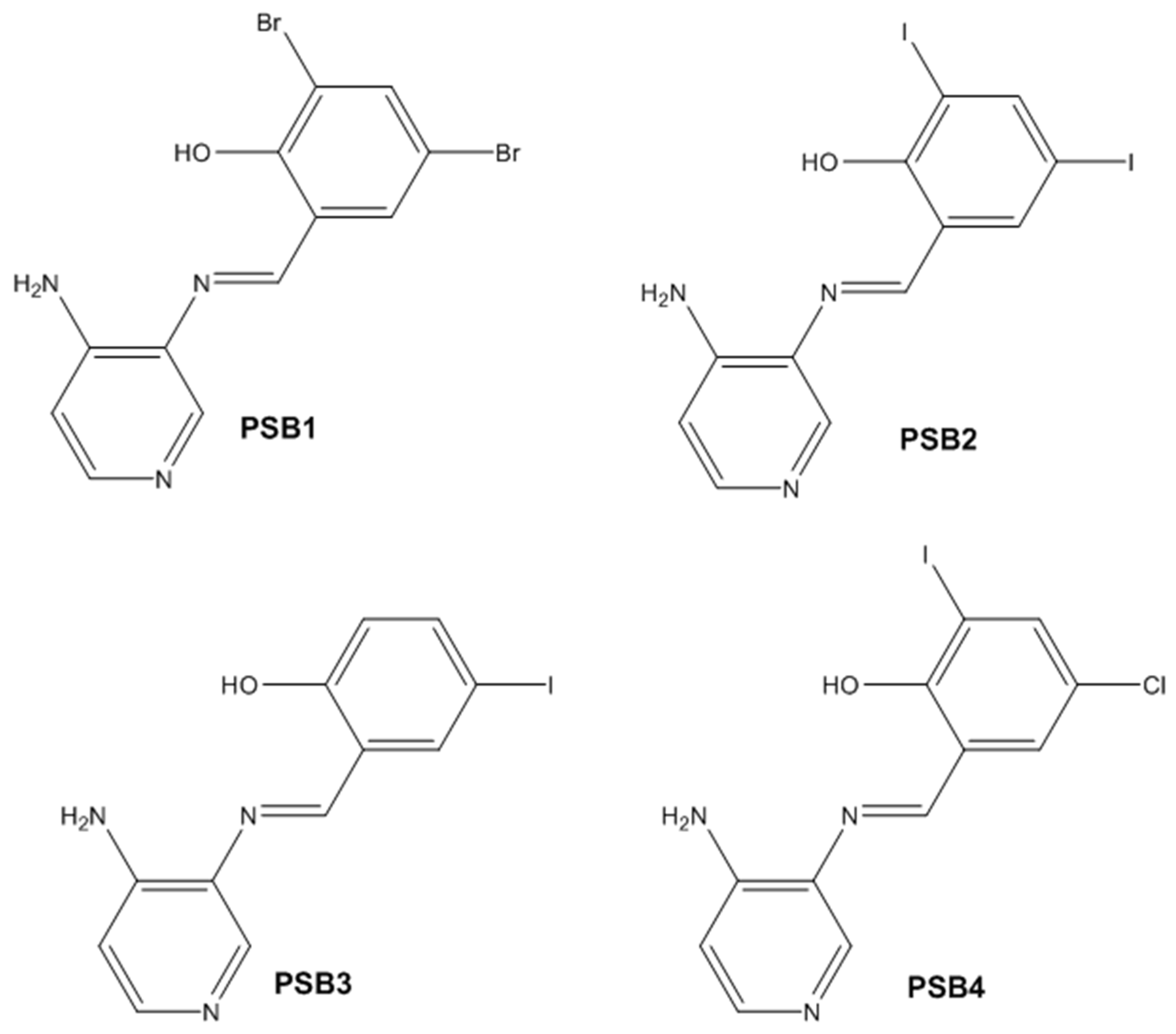
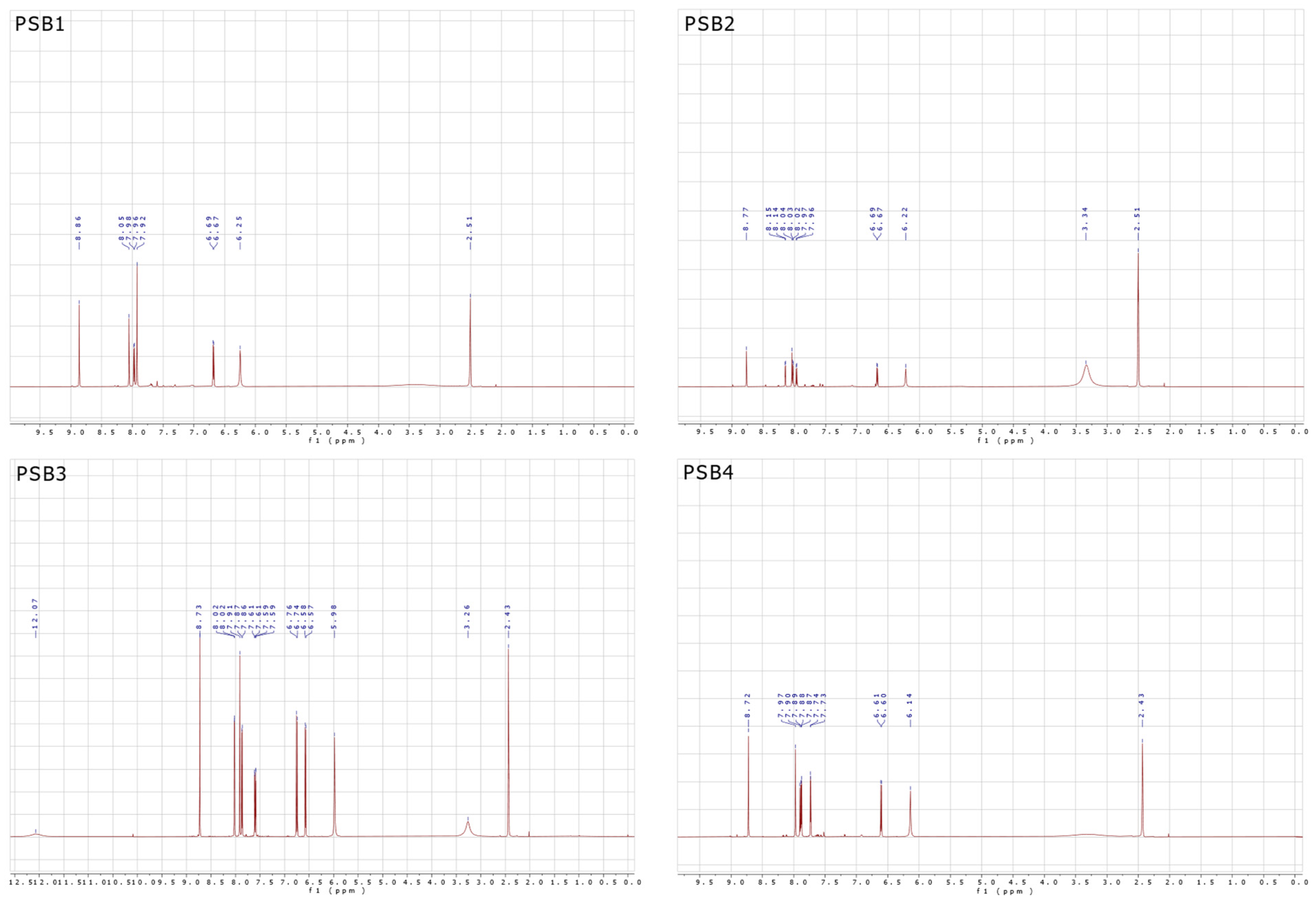
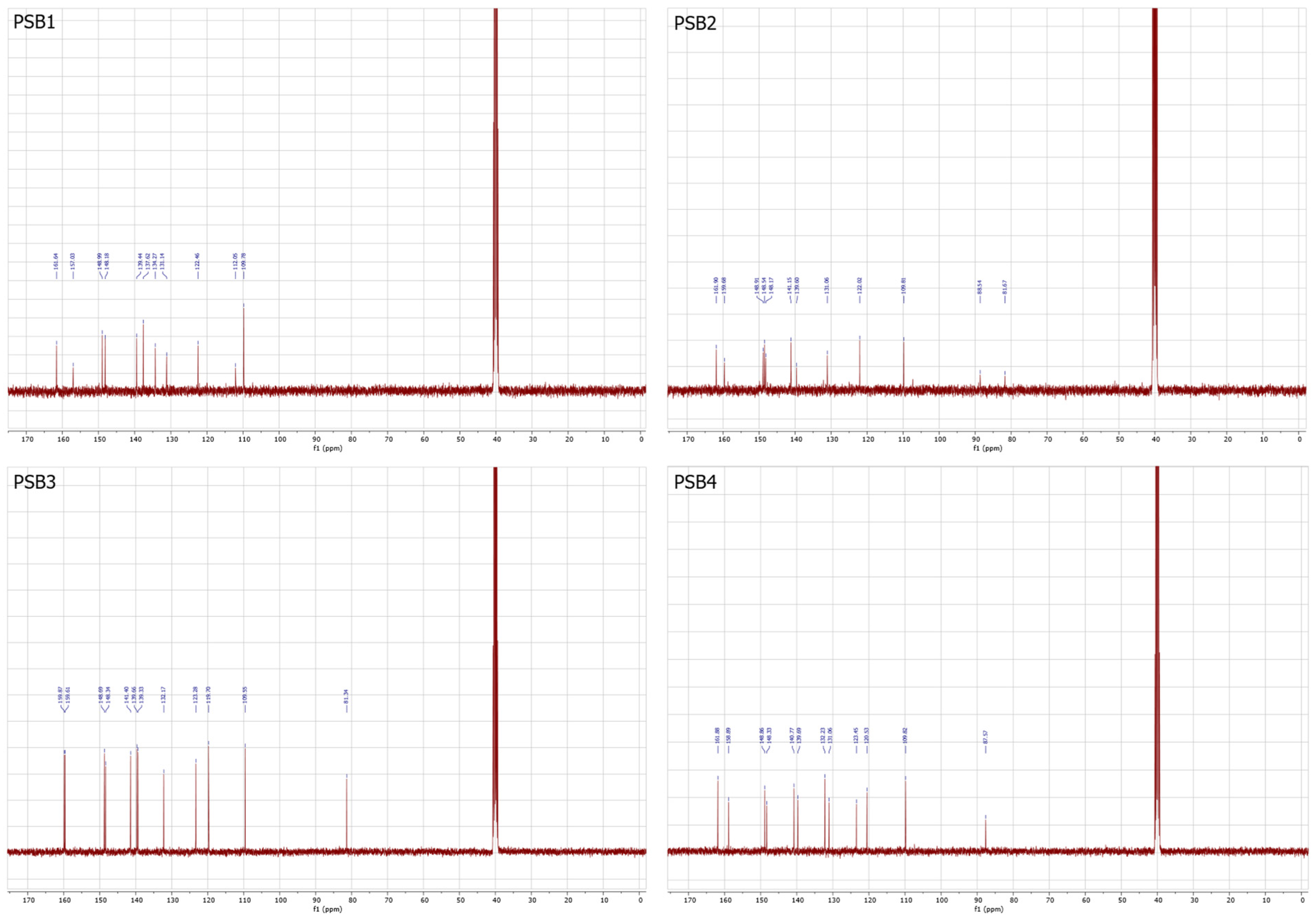
2.1. Synthesis and Characterizations
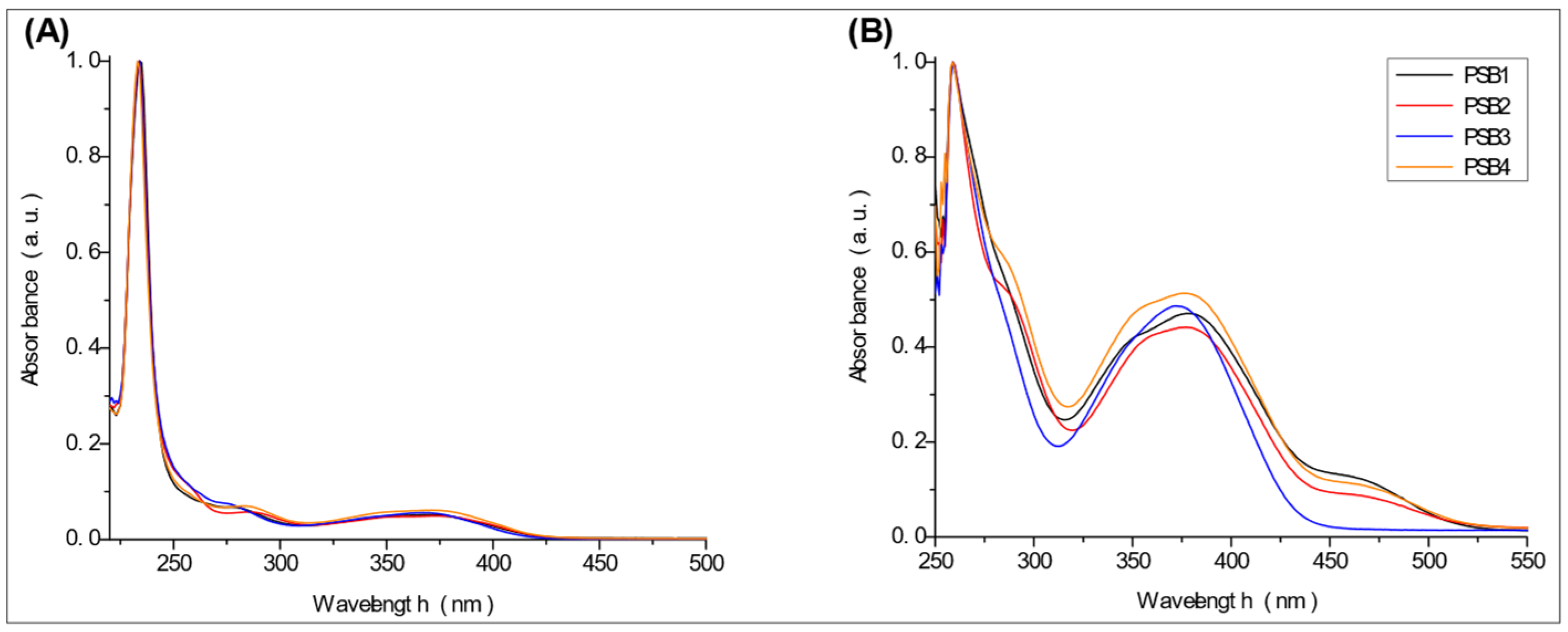
2.2. UV-Vis Studies
2.3. Theoretical Analysis
2.3.1. NBO Analysis
2.3.2. Optical Properties
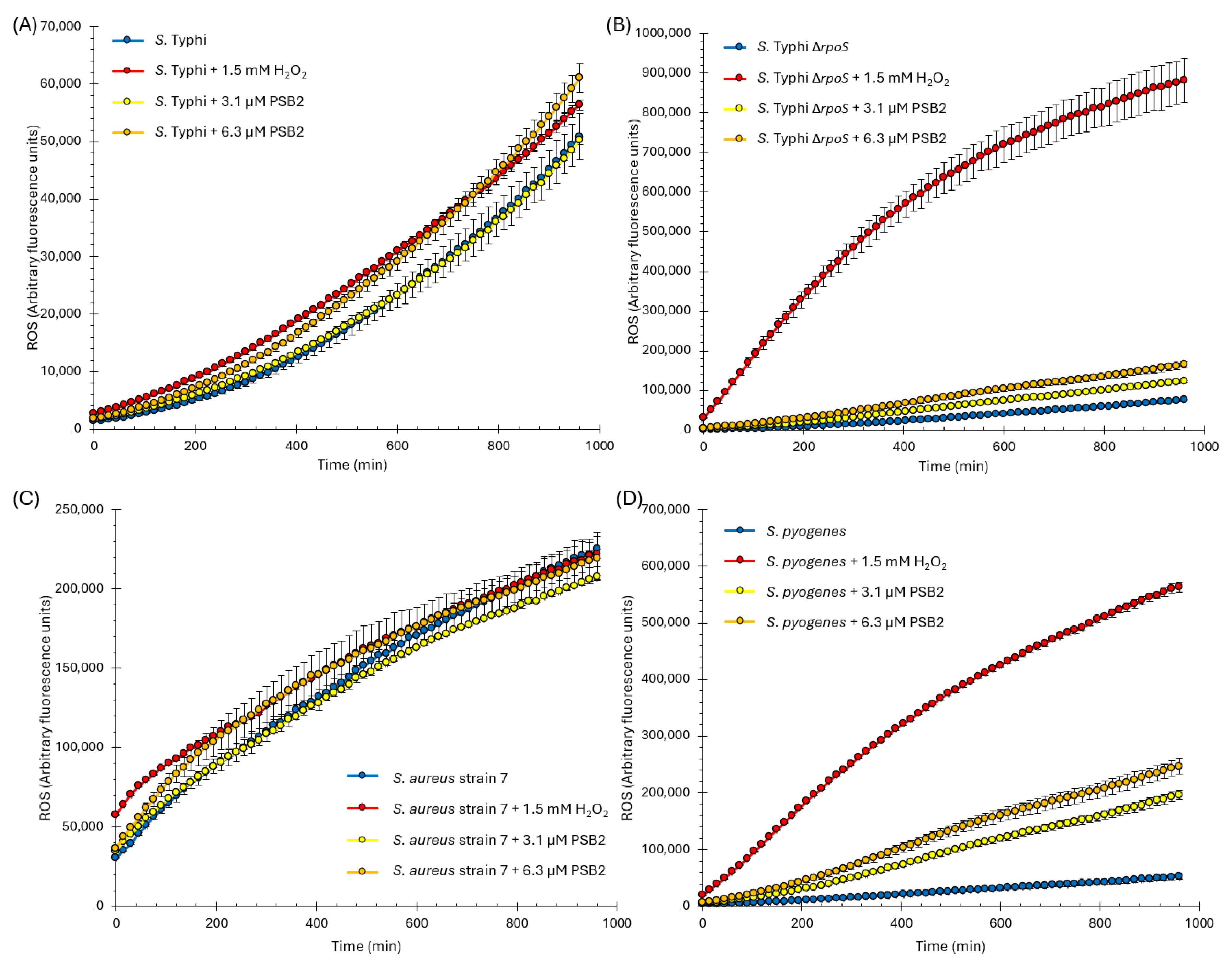
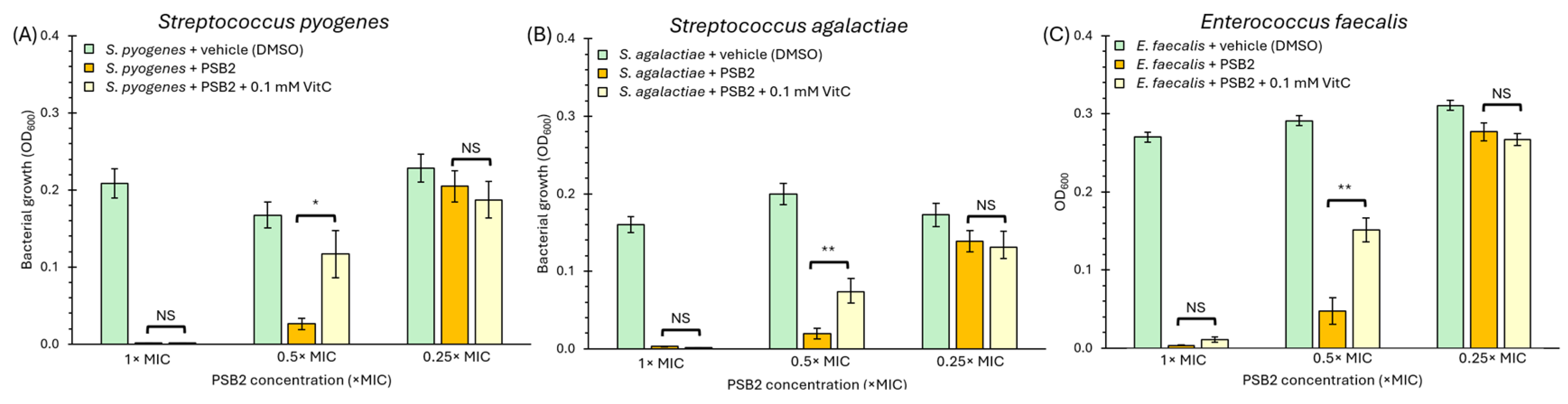
2.4. Antimicrobial Activity of Pyridine Schiff Bases
3. Experimental and Theoretical Details
3.1. Materials and Instruments
3.2. Procedure for Preparing Pyridine Schiff (PSB) Bases for This Study
3.2.1. Synthesis of (E)-2-(((4-Aminopyridin-3-yl)imino)methyl)-4,6-dibromophenol (PSB1)
3.2.2. Synthesis of (E)-2-(((4-Aminopyridin-3-yl)imino)methyl)-4,6-diiodophenol (PSB2)
3.2.3. Synthesis of (E)-2-(((4-Aminopyridin-3-yl)imino)methyl)-4-iodophenol (PSB3)
3.2.4. Synthesis of (E)-2-(((4-Aminopyridin-3-yl)imino)methyl)-4-chloro-6-iodophenol (PSB4)
3.3. DFT Calculations
3.4. Antimicrobial Activity
3.5. Determination of Intracellular Reactive Oxygen Species (ROS)
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases-Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Jiang, P.; Leng, Y.; Feng, S.; Lu, M.; Chen, S.; Sheng, X. Synthesis of zinc Schiff base complex and its application as highly efficient thermal stabilizer for flexible poly (vinyl chloride). Polym. Degrad. Stab. 2023, 211, 110317. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Kadhom, M.; Hadi, A.G.; Bufaroosha, M.; Salih, N.; Al-Dahhan, W.H.; Yousif, E. Tetra Schiff Bases as Polyvinyl Chloride Thermal Stabilizers. Chemistry 2021, 3, 288–295. [Google Scholar] [CrossRef]
- Senthil Kumar, R.; Archana, S.; Archana, T.; Divya, R.; Praveen, S.; Shridharshini, K. Synthetic approaches of medicinally important Schiff bases: An updated Review. World J. Adv. Res. Rev. 2022, 16, 838–852. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Heidarian, M.; Alizadeh, M. Pd-Isatin-Schiff base complex supported on graphene oxide as a catalyst for the Sonogashira cross-coupling reaction. J. Organomet. Chem. 2024, 1009, 123079. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Yang, W.-L.; Liu, Y.-Z.; Shang, S.-J.; Deng, W.-P. A copper(i)-catalyzed asymmetric Mannich reaction of glycine Schiff bases with isatin-derived ketimines: Enantioselective synthesis of 3-substituted 3-aminooxindoles. Org. Chem. Front. 2018, 5, 70–74. [Google Scholar] [CrossRef]
- Marcantoni, E.; Palmieri, A.; Petrini, M. Recent synthetic applications of α-amido sulfones as precursors of N-acylimino derivatives. Org. Chem. Front. 2019, 6, 2142–2182. [Google Scholar] [CrossRef]
- Ballini, R.; Palmieri, A.; Petrini, M. Catalysts’ evolution in the asymmetric conjugate addition of nitroalkanes to electron-poor alkenes. Org. Chem. Front. 2022, 9, 6077–6113. [Google Scholar] [CrossRef]
- Huang, W.S.; Chen, L.; Zheng, Z.J.; Yang, K.F.; Xu, Z.; Cui, Y.M.; Xu, L.W. Catalytic asymmetric bromochlorination of aromatic allylic alcohols promoted by multifunctional Schiff base ligands. Org. Biomol. Chem. 2016, 14, 7927–7932. [Google Scholar] [CrossRef]
- Anand, V.; Sadhasivam, B.; Dhamodharan, R. Facile synthesis of triphenylamine and phenothiazine-based Schiff bases for aggregation-induced enhanced emission, white light generation, and highly selective and sensitive copper(ii) sensing. New J. Chem. 2018, 42, 18979–18990. [Google Scholar] [CrossRef]
- Barot, Y.B.; Anand, V.; Mishra, R. Phenothiazine and triphenylamine-based fluorescent Schiff bases for the dual application of white light generation and H2O2 sensing. New J. Chem. 2022, 46, 15666–15677. [Google Scholar] [CrossRef]
- Berrones-Reyes, J.C.; Muñoz-Flores, B.M.; Cantón-Diáz, A.M.; Treto-Suárez, M.A.; Páez-Hernández, D.; Schott, E.; Zarate, X.; Jiménez-Pérez, V.M. Quantum chemical elucidation of the turn-on luminescence mechanism in two new Schiff bases as selective chemosensors of Zn2+: Synthesis, theory and bioimaging applications. RSC Adv. 2019, 9, 30778–30789. [Google Scholar] [CrossRef] [PubMed]
- Maruthesh, H.; Katagi, M.S.; Samuel, J.; Nandeshwarappa, B.P. Design, Synthesis, and Computational Characterization of Interesting Schiff Base Scaffolds as Antibacterial, Antimycobacterial, and Antifungal Agents. Russ. J. Org. Chem. 2024, 59, 1783–1796. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Burmistrova, D.A.; Arsenyev, M.V.; Almyasheva, N.R.; Ivanova, E.S.; Smolyaninova, S.A.; Pashchenko, K.P.; Poddel’sky, A.I.; Berberova, N.T. Catechol- and Phenol-Containing Thio-Schiff Bases: Synthesis, Electrochemical Properties and Biological Evaluation. ChemistrySelect 2021, 6, 10609–10618. [Google Scholar] [CrossRef]
- Hamdan, I.A.A.; Hamdan, A.A.A.; Aljabawi, R.A.A. Synthesis, Spectral Identification of Some New Schiff Bases, and Evaluation of Their Antibacterial Activity and Inhibitory Effect on Proinflammatory Cytokines (IL-1β, TNF), and DNA Ligase. Russ. J. Bioorganic Chem. 2024, 50, 138–146. [Google Scholar] [CrossRef]
- Meeran, I.S.; Raja, T.W.; Dusthakeer, V.N.A.; Ali, M.M.N.; Tajudeen, S.S.; Shabeer, T.K. An insight into antimycobacterial and antioxidant potentials of INH-Schiff base complexes and in silico targeting of MtKasB receptor of M. tuberculosis. New J. Chem. 2022, 46, 4620–4633. [Google Scholar] [CrossRef]
- Abdel-Kader, N.S.; Moustafa, H.; El-Ansary, A.L.; Sherif, O.E.; Farghaly, A.M. A coumarin Schiff base and its Ag(i) and Cu(ii) complexes: Synthesis, characterization, DFT calculations and biological applications. New J. Chem. 2021, 45, 7714–7730. [Google Scholar] [CrossRef]
- Gacitúa, M.; Carreño, A.; Morales-Guevara, R.; Páez-Hernández, D.; Martínez-Araya, J.I.; Araya, E.; Preite, M.; Otero, C.; Rivera-Zaldívar, M.M.; Silva, A.; et al. Physicochemical and Theoretical Characterization of a New Small Non-Metal Schiff Base with a Differential Antimicrobial Effect against Gram-Positive Bacteria. Int. J. Mol. Sci. 2022, 23, 2553. [Google Scholar] [CrossRef]
- Panghal, M.; Kumari, S.; Goel, N.; Rani, I. Synthesis, Spectroscopic Characterization, Antioxidant Properties and Antimicrobial Activity of Transition Metal Complexes from Novel Schiff Base. Curr. J. Appl. Sci. Technol. 2023, 42, 40–47. [Google Scholar] [CrossRef]
- Guo, Y.N.; Hu, X.B.; Zhang, H.G.; Han, Y.F.; Wang, H. Synthesis, Crystal Structure, Photophysical Properties, and Antibacterial Activities of the Copper(II) Complex Derived from 4-Chloro-2-{[(2,6-Dimethylphenyl)Imino]Methyl}Phenol. J. Struct. Chem. 2024, 65, 868–881. [Google Scholar] [CrossRef]
- Mohd Alwi, N.S.; Hamali, M.A.; Rosnizam, A.N.; Muhammad Low, A.L.; El Hassane, A.; Nassar, A.A.; Mohd Tajuddin, A. Crystallographic, antibacterial, and in silico studies of Ni(II) and Cu(II) Schiff base complexes derived from 2-acetylpyridine. Inorganica Chim. Acta 2023, 558, 121742. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Oladipo, S.D.; Zamisa, S.J.; Sanusi, I.A.; Omondi, B. DNA/BSA binding studies and in vitro anticancer and antibacterial studies of isoelectronic Cu(I)- and Ag(I)-pyridinyl Schiff base complexes incorporating triphenylphosphine as co-ligands. Inorganica Chim. Acta 2023, 558, 121760. [Google Scholar] [CrossRef]
- Santos Oliveira, I.; Marrote Manzano, C.; Hideki Nakahata, D.; Brentini Santiago, M.; Bernadelli Sousa Silva, N.; Henrique Gomes Martins, C.; Pimentel Respíndula, F.; Henrique Pereira, D.; Paulo Corbi, P. Antibacterial and antifungal activities in vitro of a novel silver(I) complex with sulfadoxine-salicylaldehyde Schiff base. Polyhedron 2022, 225, 116073. [Google Scholar] [CrossRef]
- Vhanale, B.; Kadam, D.; Shinde, A. Synthesis, spectral studies, antioxidant and antibacterial evaluation of aromatic nitro and halogenated tetradentate Schiff bases. Heliyon 2022, 8, e09650. [Google Scholar] [CrossRef]
- Coanda, M.; Limban, C.; Nuta, D.C. Small Schiff Base Molecules-A Possible Strategy to Combat Biofilm-Related Infections. Antibiotics 2024, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Ergüden, B.; Lüleci, H.B.; Ünver, Y. Benzothiophene Schiff Bases Disrupt Cytoplasmic Membrane Integrity of Gram-Positive and -Negative Bacteria Cells. Russ. J. Bioorganic Chem. 2024, 50, 128–137. [Google Scholar] [CrossRef]
- Vaikosen, E.N.; Bunu, S.J.; Miediegha, O.; Chilaka, U.P.; Echendu, C.E.; Usifoh, C.O. Synthesis and Antimicrobial Evaluation of Some Schiff Base Derivatives. Pharm. Drug Dev. 2024, 3, 1–8. [Google Scholar]
- Abdullahi, B.L.; Siraj, I.T. Synthesis, characterization and antimicrobial activities of Schiff base complexes derived from Salicylaldehyde and amino acid (isoleucine). Bayero J. Pure Appl. Sci. 2022, 15, 113–118. [Google Scholar] [CrossRef]
- Singh, P.; Yadav, P.; Kaur Sodhi, K.; Tomer, A.; Bali Mehta, S. Advancement in the synthesis of metal complexes with special emphasis on Schiff base ligands and their important biological aspects. Results Chem. 2024, 7, 101222. [Google Scholar] [CrossRef]
- Nagar, S.; Raizada, S.; Tripathee, N. A review on various green methods for synthesis of Schiff base ligands and their metal complexes. Results Chem. 2023, 6, 101153. [Google Scholar] [CrossRef]
- Carreno, A.; Rodriguez, L.; Paez-Hernandez, D.; Martin-Trasanco, R.; Zuniga, C.; Oyarzun, D.P.; Gacitua, M.; Schott, E.; Arratia-Perez, R.; Fuentes, J.A. Two New Fluorinated Phenol Derivatives Pyridine Schiff Bases: Synthesis, Spectral, Theoretical Characterization, Inclusion in Epichlorohydrin-beta-Cyclodextrin Polymer, and Antifungal Effect. Front. Chem. 2018, 6, 312. [Google Scholar] [CrossRef]
- Carreño, A.; Zúñiga, C.; Páez-Hernández, D.; Gacitúa, M.; Polanco, R.; Otero, C.; Arratia-Pérez, R.; Fuentes, J.A. Study of the structure–bioactivity relationship of three new pyridine Schiff bases: Synthesis, spectral characterization, DFT calculations and biological assays. New J. Chem. 2018, 42, 8851–8863. [Google Scholar] [CrossRef]
- Grdadolnik, J. ATR-FTIR Spectroscopy: Its advantages and limitations. Acta Chim. Slov. 2002, 49, 631–642. [Google Scholar]
- Issa, R.M.; Hassanein, A.A.; El-Mehasseb, I.M.; El-Wadoud, R.I. UV-vis, IR and 1H NMR spectroscopic studies of some 6-chloro,2-pyridyl hydrazones. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 65, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Issa, Y.M.; Hassib, H.B.; Abdelaal, H.E.; Kenawi, I.M. Spectral investigation of the intramolecular charge-transfer in some aminotriazole Schiff bases. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 1364–1374. [Google Scholar] [CrossRef]
- Carreno, A.; Gacitua, M.; Paez-Hernandez, D.; Polanco, R.; Preite, M.; Fuentes, J.A.; Mora, G.C.; Chavez, I.; Arratia-Perez, R. Spectral, theoretical characterization and antifungal properties of two phenol derivative Schiff bases with an intramolecular hydrogen bond. New J. Chem. 2015, 39, 7822–7831. [Google Scholar] [CrossRef]
- Carreno, A.; Gacitua, M.; Schott, E.; Zarate, X.; Manriquez, J.M.; Preite, M.; Ladeira, S.; Castel, A.; Pizarro, N.; Vega, A.; et al. Experimental and theoretical studies of the ancillary ligand (E)-2-((3-amino-pyridin-4-ylimino)-methyl)-4,6-di-tert-butylphenol in the rhenium(I) core. New J. Chem. 2015, 39, 5725–5734. [Google Scholar] [CrossRef]
- Carreno, A.; Vega, A.; Zarate, X.; Schott, E.; Gacitua, M.; Valenzuela, N.; Preite, M.; Manriquez, J.M.; Chavez, I. Synthesis, Characterization and Computational Studies of (E)-2-{[(2-Aminopyridin-3-Yl)Imino]-Methyl}-4,6-Di-Tert-Butylphenol. Quim. Nova 2014, 37, 584–588. [Google Scholar] [CrossRef]
- Kochem, A.; Orio, M.; Jarjayes, O.; Neese, F.; Thomas, F. Unsymmetrical one-electron oxidized Ni(II)-bis(salicylidene) complexes: A protonation-induced shift of the oxidation site. Chem. Commun. 2010, 46, 6765–6767. [Google Scholar] [CrossRef]
- Martinez Belmonte, M.; Wezenberg, S.J.; Haak, R.M.; Anselmo, D.; Escudero-Adan, E.C.; Benet-Buchholz, J.; Kleij, A.W. Self-assembly of Zn(salphen) complexes: Steric regulation, stability studies and crystallographic analysis revealing an unexpected dimeric 3,3’-t-Bu-substituted Zn(salphen) complex. Dalton Trans. 2010, 39, 4541–4550. [Google Scholar] [CrossRef]
- Hunt, A.D.; Dion, I.; Das Neves, N.; Taing, S.; Beauchemin, A.M. Synthesis of azomethine imines using an intramolecular alkyne hydrohydrazination approach. J. Org. Chem. 2013, 78, 8847–8852. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.Y.; Wang, C.S.; Zheng, J.; Shi, F.; Tu, S.J. Organocatalytic asymmetric inverse-electron-demand 1,3-dipolar cycloaddition of N,N’-cyclic azomethine imines. J. Org. Chem. 2014, 79, 9305–9312. [Google Scholar] [CrossRef]
- Płowaś, I.; Świergiel, J.; Jadżyn, J. Relative Static Permittivity of Dimethyl Sulfoxide + Water Mixtures. J. Chem. Eng. Data 2013, 58, 1741–1746. [Google Scholar] [CrossRef]
- Liu, J.-P.; Wilding, W.V.; Giles, N.F.; Rowley, R.L. A Quantitative Structure Property Relation Correlation of the Dielectric Constant for Organic Chemicals. J. Chem. Eng. Data 2009, 55, 41–45. [Google Scholar] [CrossRef]
- Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Ligation of Substituted Pyridines to Metallosalphen Complexes—Crystallographic Characterization of an Unexpected Four-Component Supramolecular Assembly Comprising a Sterically Demanding Ligand. Eur. J. Inorg. Chem. 2009, 2009, 3562–3568. [Google Scholar] [CrossRef]
- Wezenberg, S.J.; Metselaar, G.A.; Escudero-Adán, E.C.; Benet-Buchholz, J.; Kleij, A.W. Access to hybrid supramolecular salen–porphyrin assemblies via a selective in situ transmetalation–metalation self-assembly sequence. Inorganica Chim. Acta 2009, 362, 1053–1057. [Google Scholar] [CrossRef]
- Drozdzak, R.; Allaert, B.; Ledoux, N.; Dragutan, I.; Dragutan, V.; Verpoort, F. Ruthenium complexes bearing bidentate Schiff base ligands as efficient catalysts for organic and polymer syntheses. Coord. Chem. Rev. 2005, 249, 3055–3074. [Google Scholar] [CrossRef]
- Karatsu, T.; Kitamura, A.; Zeng, H.; Arai, T.; Sakuragi, H.; Tokumaru, K. Photoisomerization and Photocyclization Reactions of 1-Styrylanthracene. Bull. Chem. Soc. Jpn. 1995, 68, 920–928. [Google Scholar] [CrossRef]
- Gopal, V.R.; Reddy, A.M.; Rao, V.J. Wavelength Dependent Trans to Cis and Quantum Chain Isomerizations of Anthrylethylene Derivatives. J. Org. Chem. 2002, 60, 7966–7973. [Google Scholar] [CrossRef]
- Boeré, R.T.; Roemmele, T.L. Electrochemistry of redox-active Group 15/16 heterocyles. Coord. Chem. Rev. 2000, 210, 369–445. [Google Scholar] [CrossRef]
- Dokic, J.; Gothe, M.; Wirth, J.; Peters, M.V.; Schwarz, J.; Hecht, S.; Saalfrank, P. Quantum chemical investigation of thermal cis-to-trans isomerization of azobenzene derivatives: Substituent effects, solvent effects, and comparison to experimental data. J. Phys. Chem. A 2009, 113, 6763–6773. [Google Scholar] [CrossRef] [PubMed]
- Leyssner, F.; Hagen, S.; Óvári, L.; Dokić, J.; Saalfrank, P.; Peters, M.V.; Hecht, S.; Klamroth, T.; Tegeder, P. Photoisomerization Ability of Molecular Switches Adsorbed on Au(111): Comparison between Azobenzene and Stilbene Derivatives. J. Phys. Chem. C 2009, 114, 1231–1239. [Google Scholar] [CrossRef]
- Garcia-Amoros, J.; Sanchez-Ferrer, A.; Massad, W.A.; Nonell, S.; Velasco, D. Kinetic study of the fast thermal cis-to-trans isomerisation of para-, ortho- and polyhydroxyazobenzenes. Phys. Chem. Chem. Phys. 2010, 12, 13238–13242. [Google Scholar] [CrossRef]
- Lewiński, J.; Zachara, J.; Justyniak, I.; Dranka, M. Hydrogen-bond supramolecular structure of group 13 Schiff base complexes. Coord. Chem. Rev. 2005, 249, 1185–1199. [Google Scholar] [CrossRef]
- Mills, J.P.; Marchaim, D. Multidrug-Resistant Gram-Negative Bacteria: Infection Prevention and Control Update. Infect. Dis. Clin. N. Am. 2021, 35, 969–994. [Google Scholar] [CrossRef] [PubMed]
- Dougan, G.; Baker, S. Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu. Rev. Microbiol. 2014, 68, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Fabrega, A.; Vila, J. Salmonella enterica serovar Typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef]
- Chong, Y.; Shimoda, S.; Shimono, N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2018, 61, 185–188. [Google Scholar]
- Liu, H.; Zhu, J.; Hu, Q.; Rao, X. Morganella morganii, a non-negligent opportunistic pathogen. Int. J. Infect. Dis. 2016, 50, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Nevermann, J.; Silva, A.; Otero, C.; Oyarzun, D.P.; Barrera, B.; Gil, F.; Calderon, I.L.; Fuentes, J.A. Identification of Genes Involved in Biogenesis of Outer Membrane Vesicles (OMVs) in Salmonella enterica Serovar Typhi. Front. Microbiol. 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Z.; Fei, X.; Tian, Y.; Zhou, G.; Hu, Y.; Wang, S.; Shi, H. The role of TolA, TolB, and TolR in cell morphology, OMVs production, and virulence of Salmonella Choleraesuis. AMB Express 2022, 12, 5. [Google Scholar] [CrossRef]
- Franco Melendez, K.; Crenshaw, K.; Barrila, J.; Yang, J.; Gangaraju, S.; Davis, R.R.; Forsyth, R.J.; Ott, C.M.; Kader, R.; Curtiss, R., 3rd; et al. Role of RpoS in Regulating Stationary Phase Salmonella Typhimurium Pathogenesis-Related Stress Responses under Physiological Low Fluid Shear Force Conditions. mSphere 2022, 7, e0021022. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.A.; Jofre, M.R.; Villagra, N.A.; Mora, G.C. RpoS- and Crp-dependent transcriptional control of Salmonella Typhi taiA and hlyE genes: Role of environmental conditions. Res. Microbiol. 2009, 160, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Jofre, M.R.; Rodriguez, L.M.; Villagra, N.A.; Hidalgo, A.A.; Mora, G.C.; Fuentes, J.A. RpoS integrates CRP, Fis, and PhoP signaling pathways to control Salmonella Typhi hlyE expression. BMC Microbiol. 2014, 14, 139. [Google Scholar] [CrossRef]
- Rapp, R.P.; Record, K.E. Gram-Negative Bacteria. In Management of Antimicrobials in Infectious Diseases: Impact of Antibiotic Resistance; Mainous, A.G., Pomeroy, C., Eds.; Humana Press: Totowa, NJ, USA, 2001; pp. 43–59. [Google Scholar]
- Hammond, S.M.; Lambert, P.A.; Rycroft, A.N. The Envelope of Gram-Negative Bacteria. In The Bacterial Cell Surface; Springer: Dordrecht, The Netherlands, 1984; pp. 57–118. [Google Scholar]
- Rojas, E.R.; Billings, G.; Odermatt, P.D.; Auer, G.K.; Zhu, L.; Miguel, A.; Chang, F.; Weibel, D.B.; Theriot, J.A.; Huang, K.C. The outer membrane is an essential load-bearing element in Gram-negative bacteria. Nature 2018, 559, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Begum, F.; Rabaan, A.A.; Aljeldah, M.; Al Shammari, B.R.; Alawfi, A.; Alshengeti, A.; Sulaiman, T.; Khan, A. Classification and Multifaceted Potential of Secondary Metabolites Produced by Bacillus subtilis Group: A Comprehensive Review. Molecules 2023, 28, 927. [Google Scholar] [CrossRef]
- Raabe, V.N.; Shane, A.L. Group B Streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, M.G.; Lacey, J.A.; Tong, S.Y.C.; Davies, M.R. Global genomic epidemiology of Streptococcus pyogenes. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2020, 86, 104609. [Google Scholar] [CrossRef] [PubMed]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A Review of Combination Antimicrobial Therapy for Enterococcus faecalis Bloodstream Infections and Infective Endocarditis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 67, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Eltwisy, H.O.; Twisy, H.O.; Hafez, M.H.; Sayed, I.M.; El-Mokhtar, M.A. Clinical Infections, Antibiotic Resistance, and Pathogenesis of Staphylococcus haemolyticus. Microorganisms 2022, 10, 1130. [Google Scholar] [CrossRef] [PubMed]
- Akhova, A.V.; Tkachenko, A.G. Role of Secondary Oxidative Stress in the Bactericidal Action of Antibiotics. Mosc. Univ. Biol. Sci. Bull. 2021, 75, 218–223. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Roman, E.; Sanchez-Fresneda, R.; Casas, C.; Herrero, E.; Arguelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef]
- Alvarez, R.; Neumann, G.; Fravega, J.; Diaz, F.; Tejias, C.; Collao, B.; Fuentes, J.A.; Paredes-Sabja, D.; Calderon, I.L.; Gil, F. CysB-dependent upregulation of the Salmonella Typhimurium cysJIH operon in response to antimicrobial compounds that induce oxidative stress. Biochem. Biophys. Res. Commun. 2015, 458, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Fravega, J.; Alvarez, R.; Diaz, F.; Inostroza, O.; Tejias, C.; Rodas, P.I.; Paredes-Sabja, D.; Fuentes, J.A.; Calderon, I.L.; Gil, F. Salmonella Typhimurium exhibits fluoroquinolone resistance mediated by the accumulation of the antioxidant molecule H2S in a CysK-dependent manner. J. Antimicrob. Chemother. 2016, 71, 3409–3415. [Google Scholar] [CrossRef]
- Aribisala, J.O.; Sabiu, S. Redox Impact on Bacterial Macromolecule: A Promising Avenue for Discovery and Development of Novel Antibacterials. Biomolecules 2022, 12, 1545. [Google Scholar] [CrossRef]
- Ameziane-El-Hassani, R.; Dupuy, C. Detection of Intracellular Reactive Oxygen Species (CM-H2DCFDA). Bio-Protocol 2013, 3. [Google Scholar] [CrossRef]
- Ortega, A.P.; Villagra, N.A.; Urrutia, I.M.; Valenzuela, L.M.; Talamilla-Espinoza, A.; Hidalgo, A.A.; Rodas, P.I.; Gil, F.; Calderon, I.L.; Paredes-Sabja, D.; et al. Lose to win: marT pseudogenization in Salmonella enterica serovar Typhi contributed to the surV-dependent survival to H2O2, and inside human macrophage-like cells. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2016, 45, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Chautrand, T.; Souak, D.; Chevalier, S.; Duclairoir-Poc, C. Gram-Negative Bacterial Envelope Homeostasis under Oxidative and Nitrosative Stress. Microorganisms 2022, 10, 924. [Google Scholar] [CrossRef]
- Borisov, V.B.; Siletsky, S.A.; Nastasi, M.R.; Forte, E. ROS Defense Systems and Terminal Oxidases in Bacteria. Antioxidants 2021, 10, 839. [Google Scholar] [CrossRef]
- Hebrard, M.; Viala, J.P.; Meresse, S.; Barras, F.; Aussel, L. Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J. Bacteriol. 2009, 191, 4605–4614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, C.; Yin, J.; Sui, Y.; Wang, Y.; Zhai, G. RpoS Affects Gene Expression in Salmonella enterica serovar Typhi Under Early Hyperosmotic Stress. Curr. Microbiol. 2017, 74, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Nizet, V.; Liu, G.Y. Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae. J. Bacteriol. 2008, 190, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L.; Liu, J.; Ji, T.; Gao, Y.; Yang, D.; Zhao, M.; Zhai, Y.; Cao, Z. Serotype distribution, antimicrobial resistance, and molecular characterization of group B Streptococcus isolates from Chinese pregnant woman. J. Matern. Fetal Neonatal Med. 2024, 37, 2295805. [Google Scholar] [CrossRef]
- Carreno, A.; Paez-Hernandez, D.; Cantero-Lopez, P.; Zuniga, C.; Nevermann, J.; Ramirez-Osorio, A.; Gacitua, M.; Oyarzun, P.; Saez-Cortez, F.; Polanco, R.; et al. Structural Characterization, DFT Calculation, NCI, Scan-Rate Analysis and Antifungal Activity against Botrytis cinerea of (E)-2-{[(2-Aminopyridin-2-yl)imino]-methyl}-4,6-di-tert-butylphenol (Pyridine Schiff Base). Molecules 2020, 25, 2741. [Google Scholar] [CrossRef] [PubMed]
- Carreño, A.; Fernández, K.; Sáez-Cortez, F.; Otero, C.; Arratia-Pérez, R.; Fuentes, J.A.; Polanco, R. Confocal Microscopy Studies of Living Fungal Hyphae and Conidia Using Rhenium (I) Tricarbonyl Complexes as Fluorescent Dyes. J. Chil. Chem. Soc. 2019, 64, 4428–4431. [Google Scholar] [CrossRef]
- Otero, C.; Carreno, A.; Polanco, R.; Llancalahuen, F.M.; Arratia-Perez, R.; Gacitua, M.; Fuentes, J.A. Rhenium (I) Complexes as Probes for Prokaryotic and Fungal Cells by Fluorescence Microscopy: Do Ligands Matter? Front Chem 2019, 7, 454. [Google Scholar] [CrossRef]
- Carreño, A.; Páez-Hernández, D.; Zúñiga, C.; Ramírez-Osorio, A.; Pizarro, N.; Vega, A.; Solis-Céspedes, E.; Rivera-Zaldívar, M.M.; Silva, A.; Fuentes, J.A. Exploring rhenium (I) complexes as potential fluorophores for walled-cells (yeasts and bacteria): Photophysics, biocompatibility, and confocal microscopy. Dye. Pigment. 2021, 184, 108876. [Google Scholar] [CrossRef]
- Morales-Guevara, R.; Fuentes, J.A.; Paez-Hernández, D.; Carreño, A. The role of substituted pyridine Schiff bases as ancillary ligands in the optical properties of a new series of fac-rhenium(i) tricarbonyl complexes: A theoretical view. RSC Adv. 2021, 11, 37181–37193. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guevara, R.; Fuentes, J.A.; Paez-Hernandez, D.; Carreno, A. Intramolecular Hydrogen Bond in Pyridine Schiff Bases as Ancillary Ligands of Re(I) Complexes Is a Switcher between Visible and NIR Emissions: A Relativistic Quantum Chemistry Study. J. Phys. Chem. A 2022, 126, 8997–9007. [Google Scholar] [CrossRef] [PubMed]


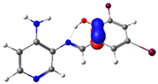
| Pyridine Schiff Base | Donor | Acceptor | ΔE (kcal/mol) |
|---|---|---|---|
| PSB1 | 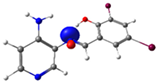 N4 (LP) | 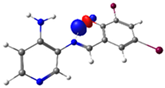 O1-H2 (BD*) | 10.3 (IHB) |
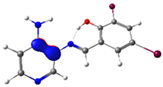 C7-C8 (BD) | 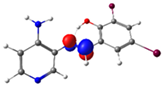 N4-C13 (BD*) | 11.6 | |
 C14-C15 (BD) | 21.6 | ||
| PSB2 | 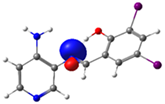 N4 (LP) | 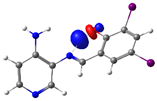 O1-H2 (BD*) | 10.0 (IHB) |
 C7-C8 (BD) | 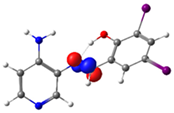 N4-C13 (BD*) | 10.1 | |
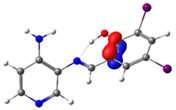 C14-C15 (BD) | 21.7 | ||
| PSB3 | 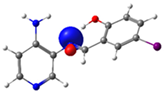 N4 (LP) | 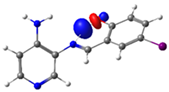 O1-H2 (BD*) | 8.0 (IHB) |
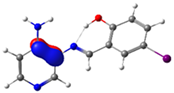 C7-C8 (BD) | 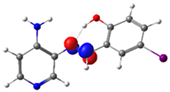 N4-C13 (BD*) | 9.2 | |
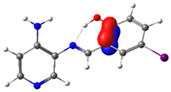 C14-C15 (BD) | 22.3 | ||
| PSB4 | 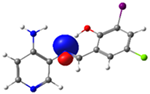 N4 (LP) | 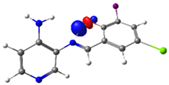 O1-H2 (BD*) | 10.0 (IHB) |
 C7-C8 (BD) | 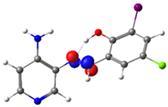 N4-C13 (BD*) | 10.4 | |
 C14-C15 (BD) | 21.7 |
| Species | Precursor 1 1 | MIC Precursor 1 (µM) | Precursor 2 2 | MIC Precursor 2 (µM) | Pyridine Schiff Base | MIC Pyridine Schiff Base (µM) |
|---|---|---|---|---|---|---|
| Bacillus subtilis | 3,5-dibromo-2-hydroxybenzaldehyde | No effect | 3,4-diaminopyridine | No effect | PSB1 | 25.3 ± 0.0 |
| 2-hydroxy-3,5-diiodobenzaldehyde | 6.3 ± 0.0 | 3,4-diaminopyridine | No effect | PSB2 | 5.7 ± 0.5 | |
| 5-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | No effect | PSB3 | No effect | |
| 5-chloro-3-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | No effect | PSB4 | No effect | |
| Streptococcus agalactiae | 3,5-dibromo-2-hydroxybenzaldehyde | 17.3 ± 2.3 | 3,4-diaminopyridine | No effect | PSB1 | 11.8 ± 0.7 |
| 2-hydroxy-3,5-diiodobenzaldehyde | 3.9 ± 0.5 | 3,4-diaminopyridine | No effect | PSB2 | 2.6 ± 0.2 | |
| 5-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | No effect | PSB3 | 25.3 ± 0.0 | |
| 5-chloro-3-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | No effect | PSB4 | 11.8 ± 0.7 | |
| Streptococcus pyogenes | 3,5-dibromo-2-hydroxybenzaldehyde | 9.4 ± 1.1 | 3,4-diaminopyridine | 25.3 ± 0.0 | PSB1 | 11.8 ± 0.7 |
| 2-hydroxy-3,5-diiodobenzaldehyde | 3.4 ± 0.7 | 3,4-diaminopyridine | 25.3 ± 0.0 | PSB2 | 1.3 ± 0.2 | |
| 5-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | 25.3 ± 0.0 | PSB3 | 25.3 ± 0.0 | |
| 5-chloro-3-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | 25.3 ± 0.0 | PSB4 | 6.3 ± 0.0 | |
| Enterococcus faecalis | 3,5-dibromo-2-hydroxybenzaldehyde | 50.5 ± 0.0 | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB1 | 50.5 ± 0.0 |
| 2-hydroxy-3,5-diiodobenzaldehyde | 10.2 ± 1.1 | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB2 | 5.3 ± 0.3 | |
| 5-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB3 | 50.5 ± 0.0 | |
| 5-chloro-3-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB4 | 50.5 ± 0.0 | |
| Staphylococcus aureus strain 2 | 3,5-dibromo-2-hydroxybenzaldehyde | 12.6 ± 0.0 | 3,4-diaminopyridine | No effect | PSB1 | 12.6 ± 0.0 |
| 2-hydroxy-3,5-diiodobenzaldehyde | 6.3 ± 0.0 | 3,4-diaminopyridine | No effect | PSB2 | 3.2 ± 0.0 | |
| 5-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | No effect | PSB3 | No effect | |
| 5-chloro-3-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | No effect | PSB4 | 11.8 ± 0.7 | |
| Staphylococcus aureus strain 6 | 3,5-dibromo-2-hydroxybenzaldehyde | 11.8 ± 0.7 | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB1 | 12.6 ± 0.0 |
| 2-hydroxy-3,5-diiodobenzaldehyde | 6.3 ± 0.0 | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB2 | 3.5 ± 0.3 | |
| 5-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB3 | 6.3 ± 0.0 | |
| 5-chloro-3-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB4 | 11.0 ± 1.0 | |
| Staphylococcus aureus strain 7 | 3,5-dibromo-2-hydroxybenzaldehyde | 12.6 ± 0.0 | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB1 | 12.6 ± 0.0 |
| 2-hydroxy-3,5-diiodobenzaldehyde | 6.3 ± 0.0 | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB2 | 3.2 ± 0.0 | |
| 5-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB3 | 50.5 ± 0.0 | |
| 5-chloro-3-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | 50.5 ± 0.0 | PSB4 | 11.8 ± 0.7 | |
| Staphylococcus haemolyticus | 3,5-dibromo-2-hydroxybenzaldehyde | No effect | 3,4-diaminopyridine | No effect | PSB1 | No effect |
| 2-hydroxy-3,5-diiodobenzaldehyde | 12.6 ± 0.0 | 3,4-diaminopyridine | No effect | PSB2 | 6.3 ± 0.0 | |
| 5-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | No effect | PSB3 | No effect | |
| 5-chloro-3-iodosalicylaldehyde | Not determined | 3,4-diaminopyridine | No effect | PSB4 | 25.3 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreño, A.; Morales-Guevara, R.; Cepeda-Plaza, M.; Páez-Hernández, D.; Preite, M.; Polanco, R.; Barrera, B.; Fuentes, I.; Marchant, P.; Fuentes, J.A. Synthesis, Physicochemical Characterization, and Antimicrobial Evaluation of Halogen-Substituted Non-Metal Pyridine Schiff Bases. Molecules 2024, 29, 4726. https://doi.org/10.3390/molecules29194726
Carreño A, Morales-Guevara R, Cepeda-Plaza M, Páez-Hernández D, Preite M, Polanco R, Barrera B, Fuentes I, Marchant P, Fuentes JA. Synthesis, Physicochemical Characterization, and Antimicrobial Evaluation of Halogen-Substituted Non-Metal Pyridine Schiff Bases. Molecules. 2024; 29(19):4726. https://doi.org/10.3390/molecules29194726
Chicago/Turabian StyleCarreño, Alexander, Rosaly Morales-Guevara, Marjorie Cepeda-Plaza, Dayán Páez-Hernández, Marcelo Preite, Rubén Polanco, Boris Barrera, Ignacio Fuentes, Pedro Marchant, and Juan A. Fuentes. 2024. "Synthesis, Physicochemical Characterization, and Antimicrobial Evaluation of Halogen-Substituted Non-Metal Pyridine Schiff Bases" Molecules 29, no. 19: 4726. https://doi.org/10.3390/molecules29194726
APA StyleCarreño, A., Morales-Guevara, R., Cepeda-Plaza, M., Páez-Hernández, D., Preite, M., Polanco, R., Barrera, B., Fuentes, I., Marchant, P., & Fuentes, J. A. (2024). Synthesis, Physicochemical Characterization, and Antimicrobial Evaluation of Halogen-Substituted Non-Metal Pyridine Schiff Bases. Molecules, 29(19), 4726. https://doi.org/10.3390/molecules29194726






