Development and Validation of a UPLC-MS/MS Method for Therapeutic Drug Monitoring, Pharmacokinetic and Stability Studies of First-Line Antituberculosis Drugs in Urine
Abstract
1. Introduction
2. Results
2.1. Method Validation
2.1.1. Selectivity
2.1.2. Calibration Curves
2.1.3. LLOQ, Precision, and Accuracy
2.1.4. Carry-Over
2.1.5. Matrix Effect
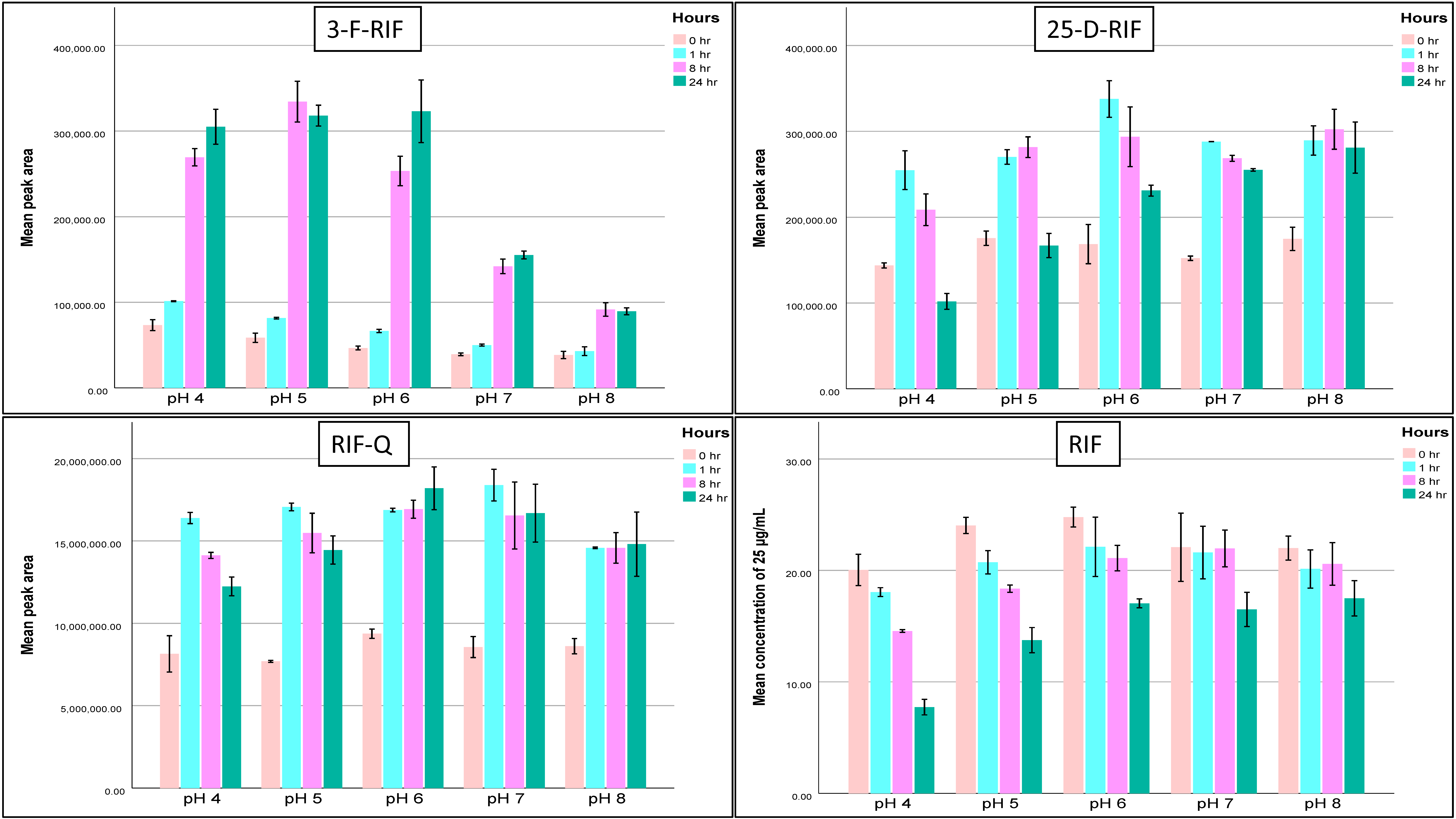
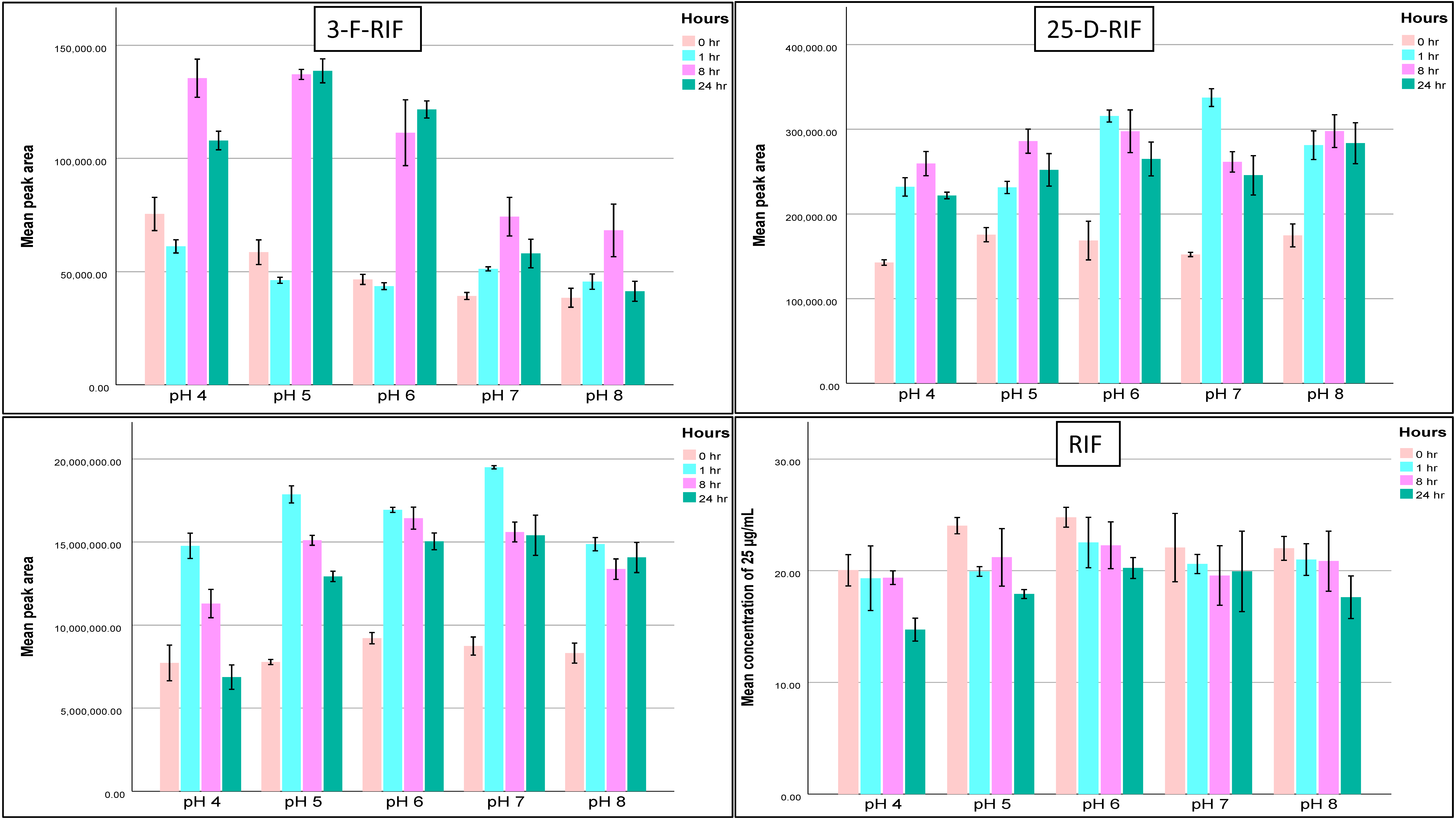
2.1.6. Stability
2.1.7. Dilution Integrity
2.1.8. Application to Clinical Samples
3. Discussion
4. Materials and Methods
4.1. Standards and Reagents
4.2. Instrumentation and Chromatographic Conditions
4.3. Mass Spectrometer Settings
4.4. Stock and Standard Solutions
- RIF, ETH, INH, and PZA: 5.0, 10.0, 25.0, 50.0, 100.0, 250.0, 500.0, and 1000.0 µg/mL.
- 25-D-RIF, RIF-Q, and 3-F-RIF: 1.0, 2.5, 5.0, 10.0, 25.0, 50.0, 100.0, and 200.0 µg/mL.
- ETH-IS and RIF-IS: 0.5 µg/mL.
- PZA-IS: 5 µg/mL.
4.5. Preparation of Calibrators, Quality Control Samples and Biological Samples
- RIF, ETH, INH, and PZA: 0.5, 1.0, 2.5, 5.0, 10.0, 25.0, 50.0, and 100.0 µg/mL.
- 25-D-RIF, RIF-Q, and 3-F-RIF: 0.1, 0.25, 0.5, 1.0, 2.5, 5.0, 10.0, and 20.0 µg/mL.
4.6. Validation
4.6.1. Selectivity
4.6.2. Calibration Curves
4.6.3. LLOQ, Precision, and Accuracy
4.6.4. Carry-Over and Dilution Integrity
4.6.5. Matrix Effect
4.6.6. Stability
4.6.7. Application to Clinical Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Tuberculosis Programme. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-006172-9. [Google Scholar]
- Tuberculosis (TB)—Treatment for TB Disease. Available online: https://www.cdc.gov/tb/topic/treatment/tbdisease.htm (accessed on 14 November 2022).
- Alsultan, A.; Peloquin, C.A. Therapeutic Drug Monitoring in the Treatment of Tuberculosis: An Update. Drugs 2014, 74, 839–854. [Google Scholar] [CrossRef] [PubMed]
- Peloquin, C.A. Therapeutic Drug Monitoring in the Treatment of Tuberculosis. Drugs 2002, 62, 2169–2183. [Google Scholar] [CrossRef] [PubMed]
- Zuur, M.A.; Bolhuis, M.S.; Anthony, R.; den Hertog, A.; van der Laan, T.; Wilffert, B.; de Lange, W.; van Soolingen, D.; Alffenaar, J.-W.C. Current Status and Opportunities for Therapeutic Drug Monitoring in the Treatment of Tuberculosis. Expert Opin. Drug Metab. Toxicol. 2016, 12, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Jeong, B.-H.; Koh, W.-J.; Lee, S.-Y. Recommendations for Optimizing Tuberculosis Treatment: Therapeutic Drug Monitoring, Pharmacogenetics, and Nutritional Status Considerations. Ann. Lab. Med. 2017, 37, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.; Peloquin, C.; Burman, W.; Luo, C.-C.; Engle, M.; Prihoda, T.J.; Mac Kenzie, W.R.; Bliven-Sizemore, E.; Johnson, J.L.; Vernon, A. Effects of Tuberculosis, Race, and Human Gene SLCO1B1 Polymorphisms on Rifampin Concentrations. Antimicrob. Agents Chemother. 2010, 54, 4192–4200. [Google Scholar] [CrossRef] [PubMed]
- Azuma, J.; Ohno, M.; Kubota, R.; Yokota, S.; Nagai, T.; Tsuyuguchi, K.; Okuda, Y.; Takashima, T.; Kamimura, S.; Fujio, Y.; et al. NAT2 Genotype Guided Regimen Reduces Isoniazid-Induced Liver Injury and Early Treatment Failure in the 6-Month Four-Drug Standard Treatment of Tuberculosis: A Randomized Controlled Trial for Pharmacogenetics-Based Therapy. Eur. J. Clin. Pharmacol. 2013, 69, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.G.J.; Akkerman, O.W.; Alffenaar, J.W.C. Role of Therapeutic Drug Monitoring in Treatment Optimization in Tuberculosis and Diabetes Mellitus Comorbidity. Antimicrob. Agents Chemother. 2019, 63, e02074-18. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.J.; Chae, J.-W.; Yun, H.-Y.; Lee, J.I.; Choi, H.D.; Kim, J.; Park, J.S.; Cho, Y.-J.; Yoon, H.I.; Lee, C.-T.; et al. Effects of Type 2 Diabetes Mellitus on the Population Pharmacokinetics of Rifampin in Tuberculosis Patients. Tuberc. Edinb. Scotl. 2015, 95, 54–59. [Google Scholar] [CrossRef]
- Märtson, A.-G.; Burch, G.; Ghimire, S.; Alffenaar, J.-W.C.; Peloquin, C.A. Therapeutic Drug Monitoring in Patients with Tuberculosis and Concurrent Medical Problems. Expert Opin. Drug Metab. Toxicol. 2021, 17, 23–39. [Google Scholar] [CrossRef]
- Rao, P.S.; Modi, N.; Nguyen, N.-T.T.; Vu, D.H.; Xie, Y.L.; Gandhi, M.; Gerona, R.; Metcalfe, J.; Heysell, S.K.; Alffenaar, J.-W.C. Alternative Methods for Therapeutic Drug Monitoring and Dose Adjustment of Tuberculosis Treatment in Clinical Settings: A Systematic Review. Clin. Pharmacokinet. 2023, 62, 375–398. [Google Scholar] [CrossRef]
- Rao, S.P.; Reed, K.; Modi, N.; Handler, D.; de Guex, K.P.; Yu, S.; Kagan, L.; Reiss, R.; Narayanan, N.; Peloquuin, C.A.; et al. Isoniazid Urine Spectrophotometry for Prediction of Serum Pharmacokinetics in Adults with Tuberculosis. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2023. accepted. [Google Scholar]
- Thomas, T.A.; Lukumay, S.; Yu, S.; Rao, P.; Siemiątkowska, A.; Kagan, L.; Augustino, D.; Mejan, P.; Mosha, R.; Handler, D.; et al. Rifampin Urinary Excretion to Predict Serum Targets in Children with Tuberculosis: A Prospective Diagnostic Accuracy Study. Arch. Dis. Child. 2023, 108, 616–621. [Google Scholar] [CrossRef]
- Xie, Y.L.; Modi, N.; Handler, D.; Yu, S.; Rao, P.; Kagan, L.; Petros de Guex, K.; Reiss, R.; Siemiątkowska, A.; Narang, A.; et al. Simplified Urine-Based Method to Detect Rifampin Underexposure in Adults with Tuberculosis: A Prospective Diagnostic Accuracy Study. Antimicrob. Agents Chemother. 2023, 67, e0093223. [Google Scholar] [CrossRef] [PubMed]
- Meissner, P.E.; Musoke, P.; Okwera, A.; Bunn, J.E.G.; Coulter, J.B.S. The Value of Urine Testing for Verifying Adherence to Anti-Tuberculosis Chemotherapy in Children and Adults in Uganda. Int. J. Tuberc. Lung Dis. 2002, 6, 903–908. [Google Scholar] [PubMed]
- Zentner, I.; Modongo, C.; Zetola, N.M.; Pasipanodya, J.G.; Srivastava, S.; Heysell, S.K.; Mpagama, S.; Schlect, H.P.; Gumbo, T.; Bisson, G.P.; et al. Urine Colorimetry for Therapeutic Drug Monitoring of Pyrazinamide during Tuberculosis Treatment. Int. J. Infect. Dis. 2018, 68, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Palanduz, A.; Gültekin, D.; Erdem, E.; Kayaalp, N. Low Level of Compliance with Tuberculosis Treatment in Children: Monitoring by Urine Tests. Ann. Trop. Paediatr. 2003, 23, 47–50. [Google Scholar] [CrossRef]
- Ji, A.J.; Jiang, Z.; Livson, Y.; Davis, J.A.; Chu, J.X.; Weng, N. Challenges in Urine Bioanalytical Assays: Overcoming Nonspecific Binding. Bioanalysis 2010, 2, 1573–1586. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Cheng, Y.; Chen, J.; Zhao, H.; Ren, X. Urine Analysis Has a Very Broad Prospect in the Future. Front. Anal. Sci. 2022, 1, 812301. [Google Scholar] [CrossRef]
- Burman, W.J.; Gallicano, K.; Peloquin, C. Comparative Pharmacokinetics and Pharmacodynamics of the Rifamycin Antibacterials. Clin. Pharmacokinet. 2001, 40, 327–341. [Google Scholar] [CrossRef]
- Thummel, K.E.; Shen, D.D.; Isoherranen, N. Design and Optimization of Dosage Regimens: Pharmacokinetic Data. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics; Brunton, L.L., Chabner, B.A., Knollmann, B.C., Eds.; McGraw-Hill Education: New York, NY, USA, 2015. [Google Scholar]
- Lacroix, C.; Hoang, T.P.; Nouveau, J.; Guyonnaud, C.; Laine, G.; Duwoos, H.; Lafont, O. Pharmacokinetics of Pyrazinamide and Its Metabolites in Healthy Subjects. Eur. J. Clin. Pharmacol. 1989, 36, 395–400. [Google Scholar] [CrossRef]
- Holdiness, M.R. Clinical Pharmacokinetics of the Antituberculosis Drugs. Clin. Pharmacokinet. 1984, 9, 511–544. [Google Scholar] [CrossRef] [PubMed]
- Kuhlin, J.; Sturkenboom, M.G.G.; Ghimire, S.; Margineanu, I.; van den Elsen, S.H.J.; Simbar, N.; Akkerman, O.W.; Jongedijk, E.M.; Koster, R.A.; Bruchfeld, J.; et al. Mass Spectrometry for Therapeutic Drug Monitoring of Anti-Tuberculosis Drugs. Clin. Mass Spectrom. 2019, 14, 34–45. [Google Scholar] [CrossRef]
- Breda, M.; Marrari, P.; Pianezzola, E.; Strolin Benedetti, M. Determination of Ethambutol in Human Plasma and Urine by High-Performance Liquid Chromatography with Fluorescence Detection. J. Chromatogr. A 1996, 729, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Panchagnula, R.; Sood, A.; Sharda, N.; Kaur, K.; Kaul, C.L. Determination of Rifampicin and Its Main Metabolite in Plasma and Urine in Presence of Pyrazinamide and Isoniazid by HPLC Method. J. Pharm. Biomed. Anal. 1999, 18, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Opheim, K.E.; Smith, A.L.; Wong, K. High-Pressure Liquid Chromatographic Quantitation of Rifampin and Its Two Major Metabolites in Urine and Serum. Rev. Infect. Dis. 1983, 5, S433–S439. [Google Scholar] [CrossRef]
- Mishra, P.; Albiol-Chiva, J.; Bose, D.; Durgbanshi, A.; Peris-Vicente, J.; Carda-Broch, S.; Esteve-Romero, J. Optimization and Validation of a Chromatographic Method for the Quantification of Isoniazid in Urine of Tuberculosis Patients According to the European Medicines Agency Guideline. Antibiotics 2018, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Pawar, R.-P.; Bose, D.; Durgbanshi, A.; Albiol-Chiva, J.; Peris-Vicente, J.; Esteve-Romero, J.; Jain, A. Stability Studies of Rifampicin in Plasma and Urine of Tuberculosis Patients According to the European Medicines Agency Guidelines. Bioanalysis 2019, 11, 713–726. [Google Scholar] [CrossRef]
- Sutradhar, I.; Zaman, M.H. Evaluation of the Effect of Temperature on the Stability and Antimicrobial Activity of Rifampicin Quinone. J. Pharm. Biomed. Anal. 2021, 197, 113941. [Google Scholar] [CrossRef]
- Prasad, B.; Singh, S. In Vitro and in Vivo Investigation of Metabolic Fate of Rifampicin Using an Optimized Sample Preparation Approach and Modern Tools of Liquid Chromatography–Mass Spectrometry. J. Pharm. Biomed. Anal. 2009, 50, 475–490. [Google Scholar] [CrossRef]
- Mwila, C.; Walker, R.B. Improved Stability of Rifampicin in the Presence of Gastric-Resistant Isoniazid Microspheres in Acidic Media. Pharmaceutics 2020, 12, 234. [Google Scholar] [CrossRef]
- Shishoo, C.J.; Shah, S.A.; Rathod, I.S.; Savale, S.S.; Kotecha, J.S.; Shah, P.B. Stability of Rifampicin in Dissolution Medium in Presence of Isoniazid. Int. J. Pharm. 1999, 190, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Abulfathi, A.A.; Decloedt, E.H.; Svensson, E.M.; Diacon, A.H.; Donald, P.; Reuter, H. Clinical Pharmacokinetics and Pharmacodynamics of Rifampicin in Human Tuberculosis. Clin. Pharmacokinet. 2019, 58, 1103–1129. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mariappan, T.T.; Shankar, R.; Sarda, N.; Singh, B. A Critical Review of the Probable Reasons for the Poor Variable Bioavailability of Rifampicin from Anti-Tubercular Fixed-Dose Combination (FDC) Products, and the Likely Solutions to the Problem. Int. J. Pharm. 2001, 228, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Karaźniewicz-Łada, M.; Kosicka-Noworzyń, K.; Rao, P.; Modi, N.; Xie, Y.L.; Heysell, S.K.; Kagan, L. New Approach to Rifampicin Stability and First-Line Anti-Tubercular Drug Pharmacokinetics by UPLC-MS/MS. J. Pharm. Biomed. Anal. 2023, 235, 115650. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration Bioanalytical Method Validation. In Guidance for Industry; FDA: Silver Spring, MD, USA, 2018.
- Hemanth Kumar, A.K.; Sudha, V. Geetha Ramachandran Simple and Rapid Method for Simultaneous Determination of Isoniazid and Acetyl Isoniazid in Urine by HPLC. Asian J. Biomed. Pharm. Sci. 2014, 4, 46–50. [Google Scholar] [CrossRef]
- Hashiguchi, M.; Ohno, K.; Sakuma, A.; Hino, F.; Tanaka, T.; Ohtsuji, M.; Matsumoto, N.; Yanase, K.; Urae, A.; Hosogai, Y.; et al. A Simplified Method for Detecting Isoniazid Compliance in Patients Receiving Antituberculosis Chemotherapy. J. Clin. Pharmacol. 2002, 42, 151–156. [Google Scholar] [CrossRef]
- Amlabu, V.; Mulligan, C.; Jele, N.; Evans, A.; Gray, D.; Zar, H.J.; McIlleron, H.; Smith, P. Isoniazid/Acetylisoniazid Urine Concentrations: Markers of Adherence to Isoniazid Preventive Therapy in Children. Int. J. Tuberc. Lung Dis. 2014, 18, 528–530. [Google Scholar] [CrossRef]
- Jindani, A.; Atwine, D.; Grint, D.; Bah, B.; Adams, J.; Ticona, E.R.; Shrestha, B.; Agizew, T.; Hamid, S.; Jamil, B.; et al. Four-Month High-Dose Rifampicin Regimens for Pulmonary Tuberculosis. NEJM Evid. 2023, 2, EVIDoa2300054. [Google Scholar] [CrossRef]
- Espinosa-Pereiro, J.; Ghimire, S.; Sturkenboom, M.G.G.; Alffenaar, J.-W.C.; Tavares, M.; Aguirre, S.; Battaglia, A.; Molinas, G.; Tórtola, T.; Akkerman, O.W.; et al. Safety of Rifampicin at High Dose for Difficult-to-Treat Tuberculosis: Protocol for RIAlta Phase 2b/c Trial. Pharmaceutics 2022, 15, 9. [Google Scholar] [CrossRef]
- Fregonese, F.; Apriani, L.; Barss, L.; Benedetti, A.; Cook, V.; Fisher, D.; Fox, G.J.; Johnston, J.; Long, R.; Nguyen, T.A.; et al. High Dose Rifampin for 2 Months vs Standard Dose Rifampin for 4 Months, to Treat TB Infection: Protocol of a 3-Arm Randomized Trial (2R2). PLoS ONE 2023, 18, e0278087. [Google Scholar] [CrossRef]
- Pršo, K.; Žideková, N.; Porvazník, I.; Solovič, I.; Mokrý, J.; Kertys, M. A High-Throughput LC–MS/MS Method for Simultaneous Determination of Isoniazid, Ethambutol and Pyrazinamide in Human Plasma. Rapid Commun. Mass Spectrom. 2023, 37, e9425. [Google Scholar] [CrossRef] [PubMed]
- Kivrane, A.; Grinberga, S.; Sevostjanovs, E.; Igumnova, V.; Pole, I.; Viksna, A.; Bandere, D.; Krams, A.; Cirule, A.; Pugovics, O.; et al. LC-MS/MS Method for Simultaneous Quantification of the First-Line Anti-Tuberculosis Drugs and Six Primary Metabolites in Patient Plasma: Implications for Therapeutic Drug Monitoring. J. Chromatogr. B 2021, 1185, 122986. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Li, X.; Pan, H.; Ding, L. NQO1 and CYP450 Reductase Decrease the Systemic Exposure of Rifampicin-Quinone and Mediate Its Redox Cycle in Rats. J. Pharm. Biomed. Anal. 2017, 132, 17–23. [Google Scholar] [CrossRef] [PubMed]
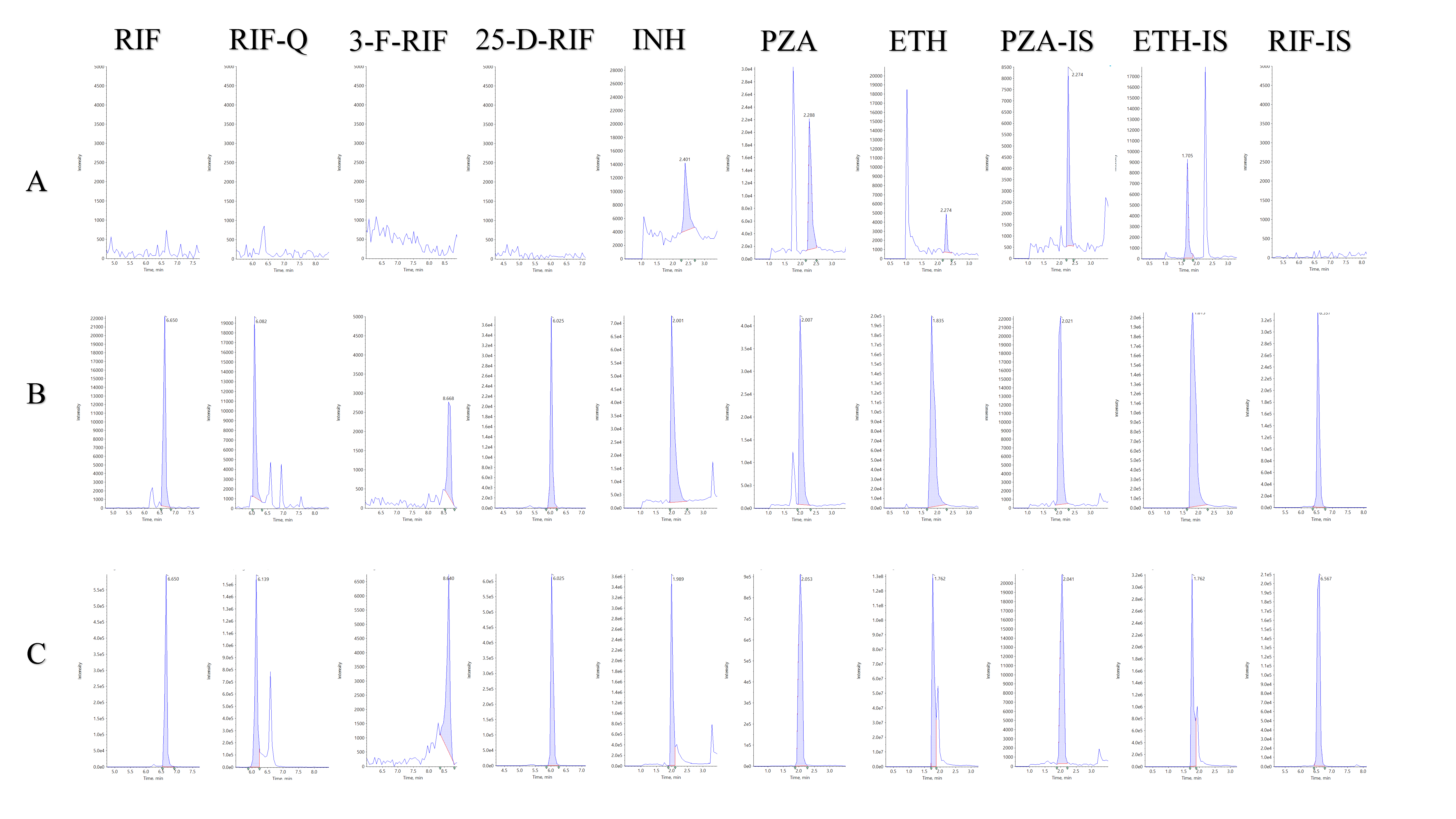
| Compound | QC Level | Conc. (µg/mL) | Precision (%RSD) | Accuracy (%) | ||
|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | |||
| n = 5 | n = 5 | n = 5 | n = 5 | |||
| RIF | LLOQ | 0.5 | 19.4 | 4.7 | 105.9 | 97.3 |
| Low | 1 | 11.3 | 8.7 | 108.0 | 91.8 | |
| Medium | 10 | 13.2 | 7.2 | 98.5 | 93.0 | |
| High | 100 | 10.0 | 11.9 | 99.3 | 111.7 | |
| RIF-Q | LLOQ | 0.1 | 15.5 | 12.9 | 115.7 | 99.4 |
| Low | 0.25 | 4.2 | 9.4 | 104.9 | 91.0 | |
| Medium | 2.5 | 9.2 | 9.2 | 93.2 | 94.5 | |
| High | 20 | 6.9 | 4.3 | 107.4 | 114.0 | |
| 3-F-RIF | LLOQ | 0.1 | 8.9 | 10.5 | 110.5 | 102.2 |
| Low | 0.25 | 7.2 | 11.1 | 108.6 | 102.0 | |
| Medium | 2.5 | 13.3 | 8.8 | 87.7 | 90.0 | |
| High | 20 | 8.3 | 8.9 | 108.6 | 95.8 | |
| 25-D-RIF | LLOQ | 0.1 | 6.2 | 5.0 | 94.3 | 106.2 |
| Low | 0.25 | 12.3 | 6.7 | 101.7 | 99.9 | |
| Medium | 2.5 | 9.7 | 6.0 | 101.9 | 104.3 | |
| High | 20 | 7.4 | 4.2 | 99.6 | 107.8 | |
| INH | LLOQ | 0.5 | 8.2 | 6.7 | 98.1 | 86.4 |
| Low | 1 | 10.0 | 8.8 | 104.3 | 97.0 | |
| Medium | 10 | 5.5 | 4.5 | 98.8 | 93.8 | |
| High | 100 | 3.9 | 2.5 | 107.8 | 100.1 | |
| PZA | LLOQ | 0.5 | 18.1 | 8.3 | 95.7 | 87.4 |
| Low | 1 | 6.0 | 8.5 | 101.5 | 103.3 | |
| Medium | 10 | 6.5 | 1.4 | 97.6 | 104.2 | |
| High | 100 | 4.3 | 4.5 | 101.7 | 109.0 | |
| ETH | LLOQ | 0.5 | 16.3 | 11.3 | 98.6 | 96.2 |
| Low | 1 | 7.2 | 8.8 | 107.9 | 108.9 | |
| Medium | 10 | 9.4 | 14.3 | 91.2 | 106.2 | |
| High | 100 | 3.9 | 7.1 | 104.7 | 109.1 | |
| Compound | QC Level | Conc. [mg/L] | IS-Normalized MF (n = 6) | %RSD | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| RIF | Low | 1 | 1.27 | 0.06 | 5.0 |
| High | 100 | 1.06 | 0.14 | 13.1 | |
| RIF-Q | Low | 0.25 | 0.43 | 0.19 | 45.2 |
| High | 20 | 0.62 | 0.32 | 51.9 | |
| 3-F-RIF | Low | 0.25 | 0.51 | 0.06 | 11.1 |
| High | 20 | 0.66 | 0.10 | 14.5 | |
| 25-D-RIF | Low | 0.25 | 1.74 | 0.16 | 9.2 |
| High | 20 | 1.55 | 0.20 | 12.6 | |
| INH | Low | 1 | 0.37 | 0.05 | 12.6 |
| High | 100 | 0.45 | 0.08 | 18.3 | |
| PZA | Low | 1 | 1.16 | 0.19 | 16.1 |
| High | 100 | 0.70 | 0.15 | 21.7 | |
| ETH | Low | 1 | 0.85 | 0.11 | 12.8 |
| High | 100 | 0.83 | 0.06 | 7.2 | |
| Compound | QC Level | Bench-Top Stability at RT | Autosampler (12 h, 15 °C) | Autosampler (24 h, 15 °C) | Working Solution Stability (−20 °C) | Short-Term Stability (24 h, −20 °C) | Long-Term Stability (30 Days, −20 °C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 h | 4 h | 1 Month | 8 Months | pH 4 | pH 5 | pH 6 | pH 7 | pH 8 | pH 4 | pH 5 | pH 6 | pH 7 | pH 8 | ||||
| RIF | Low | 98 | 90 | 90 | 92 | 103 | 95 | 74 | 81 | 87 | 86 | 90 | 83 | 105 | 115 | 110 | 101 |
| High | 101 | 96 | 99 | 100 | 101 | 94 | 85 | 87 | 90 | 88 | 89 | 96 | 103 | 100 | 95 | 100 | |
| RIF-Q | Low | 69 | 39 | 84 | 39 | 101 | 127 | 80 | 81 | 92 | 76 | 90 | 55 | 55 | 64 | 54 | 46 |
| High | 87 | 79 | 92 | 96 | 98 | 88 | 119 | 97 | 98 | 119 | 126 | 55 | 27 | 29 | 50 | 80 | |
| 3-F-RIF | Low | 97 | 61 | 63 | 30 | 97 | 61 | 60 | 58 | 54 | 50 | 64 | 24 | 39 | 70 | 83 | 77 |
| High | 91 | 88 | 97 | 92 | 100 | 78 | 119 | 97 | 98 | 119 | 126 | 11 | 20 | 84 | 116 | 104 | |
| 25-D-RIF | Low | 111 | 119 | 120 | 123 | 104 | 182 | 102 | 112 | 120 | 117 | 114 | 92 | 90 | 87 | 101 | 98 |
| High | 95 | 111 | 106 | 123 | 102 | 168 | 103 | 112 | 108 | 115 | 107 | 90 | 110 | 110 | 142 | 137 | |
| INH | Low | 99 | 99 | 98 | 91 | 110 | 124 | 75 | 92 | 96 | 97 | 85 | 44 | 47 | 85 | 86 | 77 |
| High | 94 | 94 | 102 | 100 | 109 | 115 | 93 | 103 | 107 | 114 | 105 | 88 | 96 | 98 | 114 | 95 | |
| PZA | Low | 111 | 104 | 105 | 98 | 105 | 107 | 98 | 90 | 94 | 100 | 79 | 102 | 95 | 102 | 110 | 91 |
| High | 102 | 106 | 109 | 109 | 99 | 112 | 106 | 103 | 112 | 110 | 133 | 88 | 78 | 87 | 94 | 111 | |
| ETH | Low | 112 | 108 | 115 | 114 | 104 | - | 111 | 108 | 113 | 114 | 102 | 105 | 107 | 105 | 104 | 101 |
| High | 112 | 114 | 111 | 105 | 98 | - | 110 | 104 | 108 | 97 | 104 | 99 | 111 | 101 | 114 | 103 | |
| Compound | Initial Conc. [mg/L] | 1 h | 8 h | 24 h | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 4 | pH 5 | pH 6 | pH 7 | pH 8 | pH 4 | pH 5 | pH 6 | pH 7 | pH 8 | pH 4 | pH 5 | pH 6 | pH 7 | pH 8 | ||
| After incubation at 37.5 °C | ||||||||||||||||
| RIF | 25 | 82 | 94 | 96 | 88 | 88 | 57 | 75 | 82 | 86 | 80 | 30 | 55 | 66 | 67 | 68 |
| INH | 50 | 88 | 89 | 92 | 90 | 87 | 79 | 84 | 92 | 94 | 81 | 79 | 78 | 86 | 86 | 76 |
| PZA | 50 | 86 | 90 | 93 | 90 | 88 | 92 | 92 | 93 | 94 | 86 | 92 | 90 | 91 | 89 | 86 |
| ETH | 50 | 88 | 85 | 92 | 100 | 90 | 89 | 93 | 94 | 87 | 99 | 89 | 97 | 98 | 92 | 92 |
| After incubation at 20.5 °C | ||||||||||||||||
| RIF | 25 | 79 | 87 | 99 | 89 | 87 | 76 | 82 | 86 | 81 | 81 | 58 | 71 | 78 | 77 | 71 |
| INH | 50 | 86 | 86 | 90 | 91 | 87 | 79 | 77 | 92 | 88 | 91 | 79 | 74 | 87 | 90 | 79 |
| PZA | 50 | 95 | 101 | 99 | 103 | 103 | 90 | 85 | 88 | 87 | 85 | 92 | 87 | 85 | 93 | 85 |
| ETH | 50 | 86 | 98 | 87 | 98 | 99 | 97 | 87 | 87 | 92 | 94 | 91 | 88 | 96 | 104 | 91 |
| Compound | Conc. (µg/mL) (Mean ± SD) |
|---|---|
| RIF | 17.9 ± 7.8 |
| RIF-Q | 1.9 ± 0.8 |
| 3-F-RIF | 1.3 ± 1.3 |
| 25-D-RIF | 4.1 ± 2.5 |
| INH | 48.7 ± 22.7 |
| PZA | 54.3 ± 19.5 |
| ETH | 391.7 ± 200.3 |
| Analyte | Precursor Ion (m/z) | Fragment Ion (m/z) | Collision Energy (V) |
|---|---|---|---|
| RIF | 824.3 | 792.2 | 25 |
| 398.7 | 37 | ||
| RIF-Q | 822.2 | 790.2 | 25 |
| 397.6 | 37 | ||
| 3-F-RIF | 727.2 | 667.1 | 17 |
| 641.2 | 71 | ||
| 25-D-RIF | 782.3 | 750.2 | 17 |
| 399.7 | 33 | ||
| INH | 138.0 | 121.0 | 19 |
| 79.0 | 39 | ||
| PZA | 124.0 | 64.0 | 7 |
| 81.0 | 23 | ||
| ETH | 205.1 | 116.1 | 21 |
| 145 | 11 | ||
| RIF-IS | 827.3 | 795.3 | 25 |
| 151.1 | 37 | ||
| PZA-IS | 128.0 | 84.0 | 25 |
| 99.9 | 9 | ||
| ETH-IS | 209.2 | 120.0 | 21 |
| 149.2 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abouzid, M.; Kosicka-Noworzyń, K.; Karaźniewicz-Łada, M.; Rao, P.; Modi, N.; Xie, Y.L.; Heysell, S.K.; Główka, A.; Kagan, L. Development and Validation of a UPLC-MS/MS Method for Therapeutic Drug Monitoring, Pharmacokinetic and Stability Studies of First-Line Antituberculosis Drugs in Urine. Molecules 2024, 29, 337. https://doi.org/10.3390/molecules29020337
Abouzid M, Kosicka-Noworzyń K, Karaźniewicz-Łada M, Rao P, Modi N, Xie YL, Heysell SK, Główka A, Kagan L. Development and Validation of a UPLC-MS/MS Method for Therapeutic Drug Monitoring, Pharmacokinetic and Stability Studies of First-Line Antituberculosis Drugs in Urine. Molecules. 2024; 29(2):337. https://doi.org/10.3390/molecules29020337
Chicago/Turabian StyleAbouzid, Mohamed, Katarzyna Kosicka-Noworzyń, Marta Karaźniewicz-Łada, Prakruti Rao, Nisha Modi, Yingda L. Xie, Scott K. Heysell, Anna Główka, and Leonid Kagan. 2024. "Development and Validation of a UPLC-MS/MS Method for Therapeutic Drug Monitoring, Pharmacokinetic and Stability Studies of First-Line Antituberculosis Drugs in Urine" Molecules 29, no. 2: 337. https://doi.org/10.3390/molecules29020337
APA StyleAbouzid, M., Kosicka-Noworzyń, K., Karaźniewicz-Łada, M., Rao, P., Modi, N., Xie, Y. L., Heysell, S. K., Główka, A., & Kagan, L. (2024). Development and Validation of a UPLC-MS/MS Method for Therapeutic Drug Monitoring, Pharmacokinetic and Stability Studies of First-Line Antituberculosis Drugs in Urine. Molecules, 29(2), 337. https://doi.org/10.3390/molecules29020337









