RuO2 Catalysts for Electrocatalytic Oxygen Evolution in Acidic Media: Mechanism, Activity Promotion Strategy and Research Progress
Abstract
1. Introduction
2. Reaction Mechanism
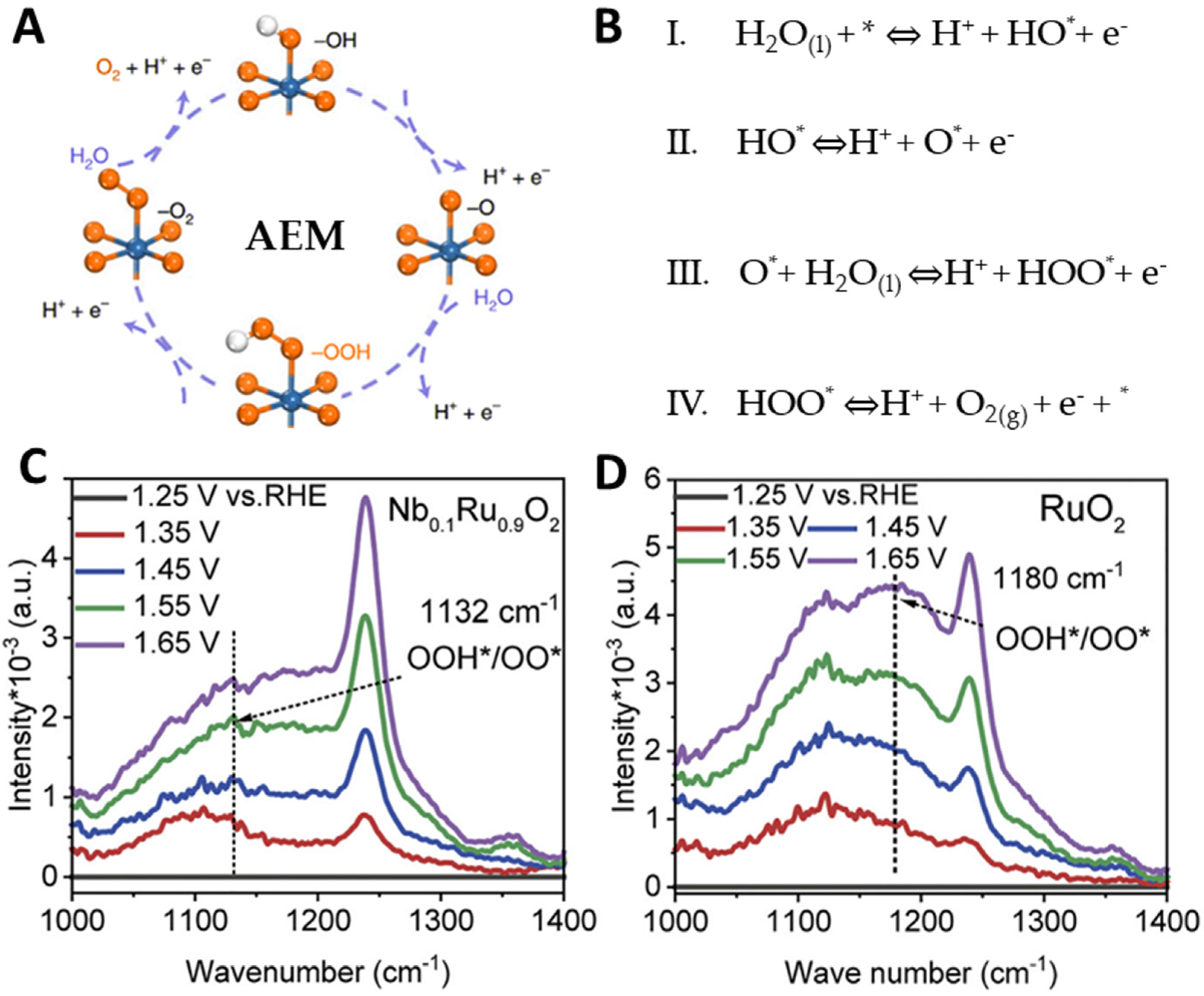
2.1. Adsorbate Evolution Mechanism (AEM)
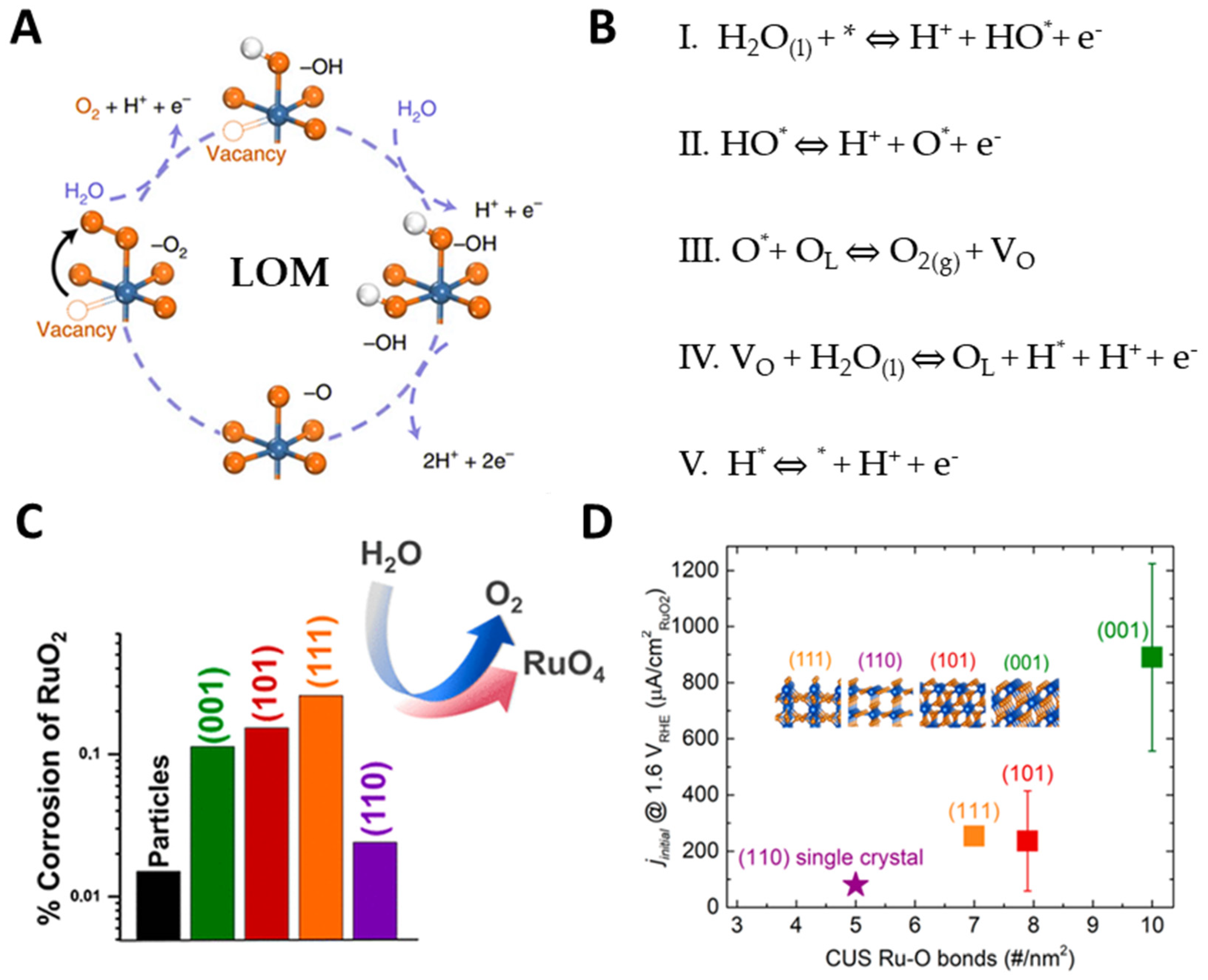
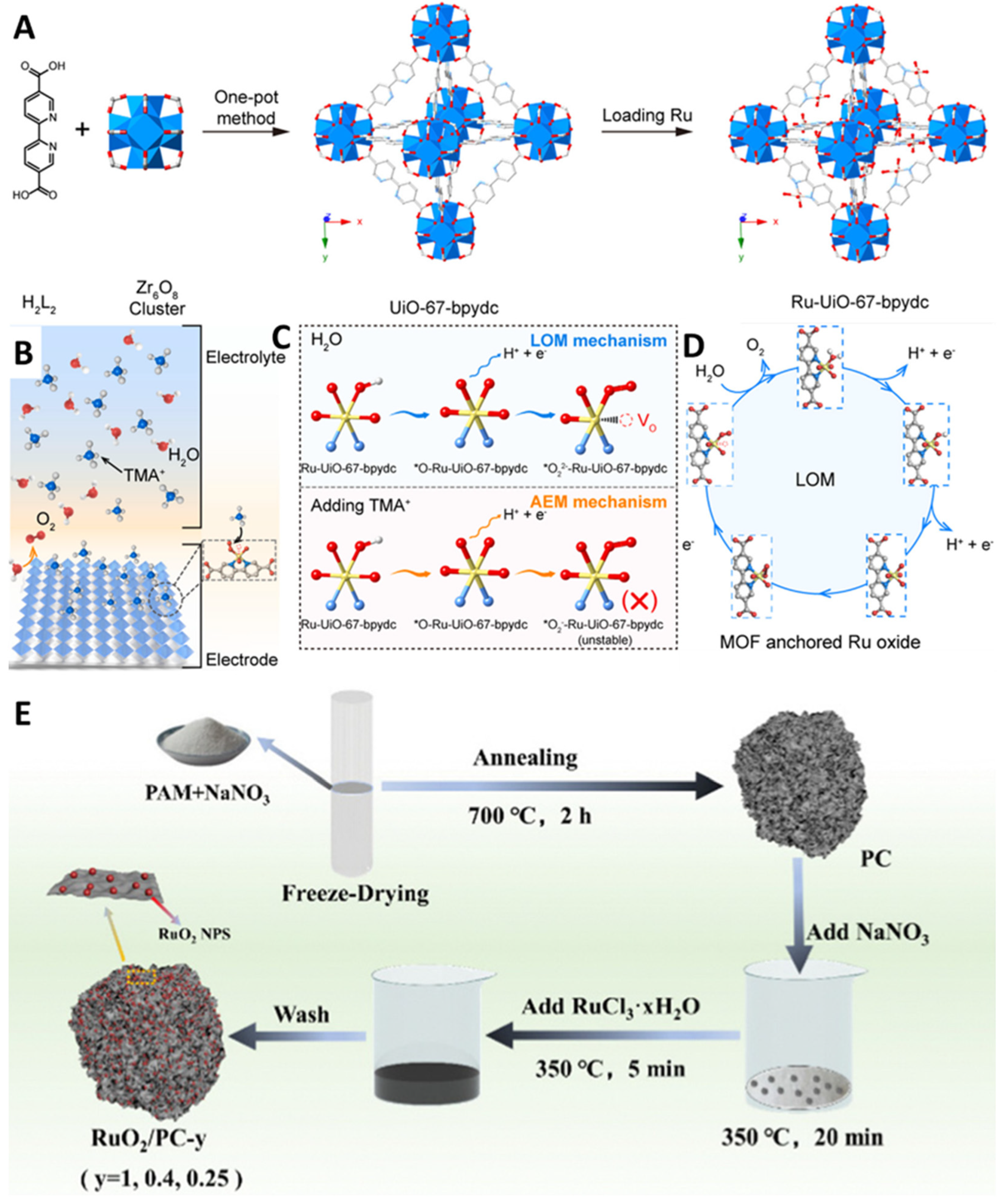
2.2. Lattice Oxygen Oxidation Mechanism (LOM)
3. Activity and Stability Enhancement Strategies
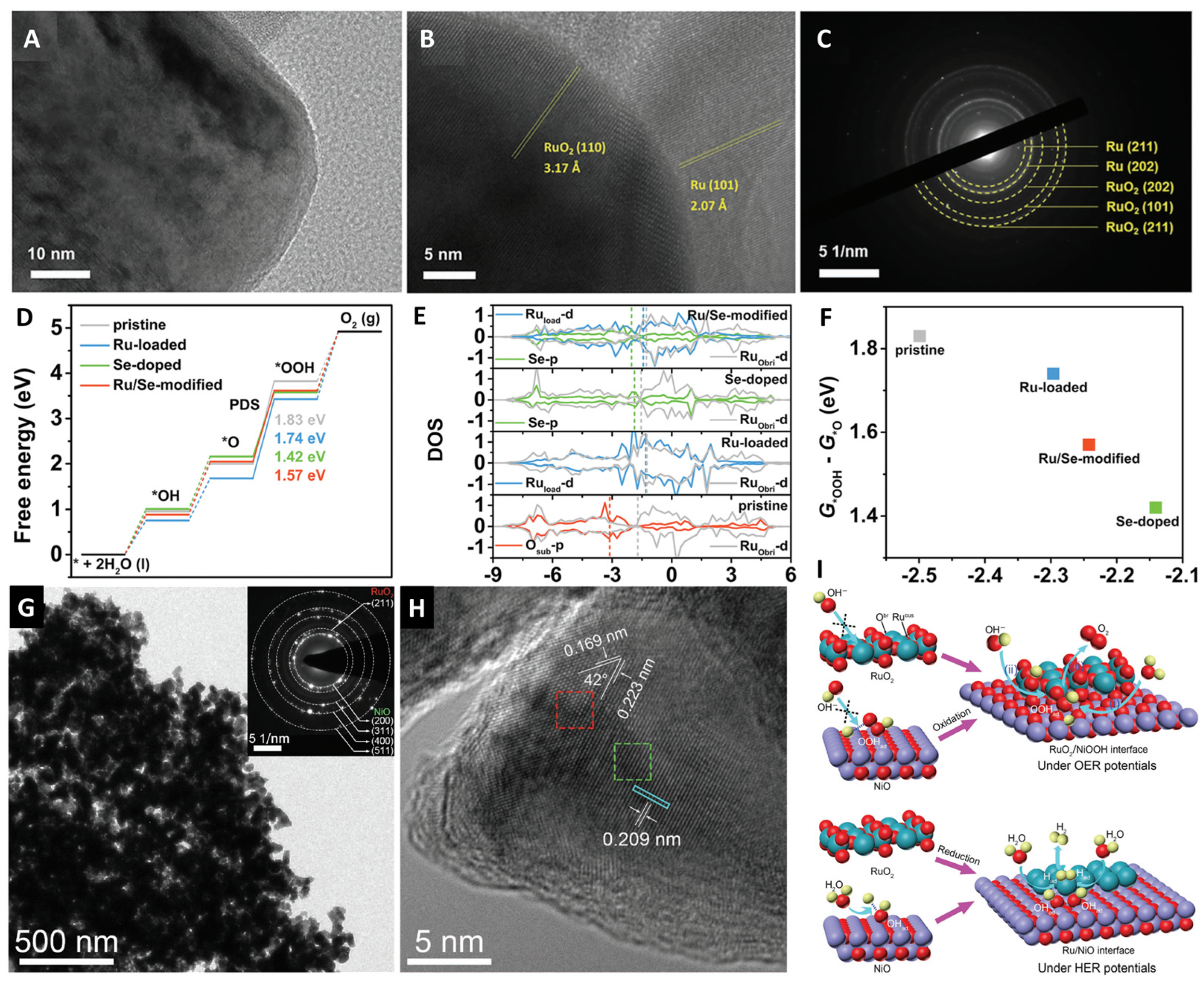
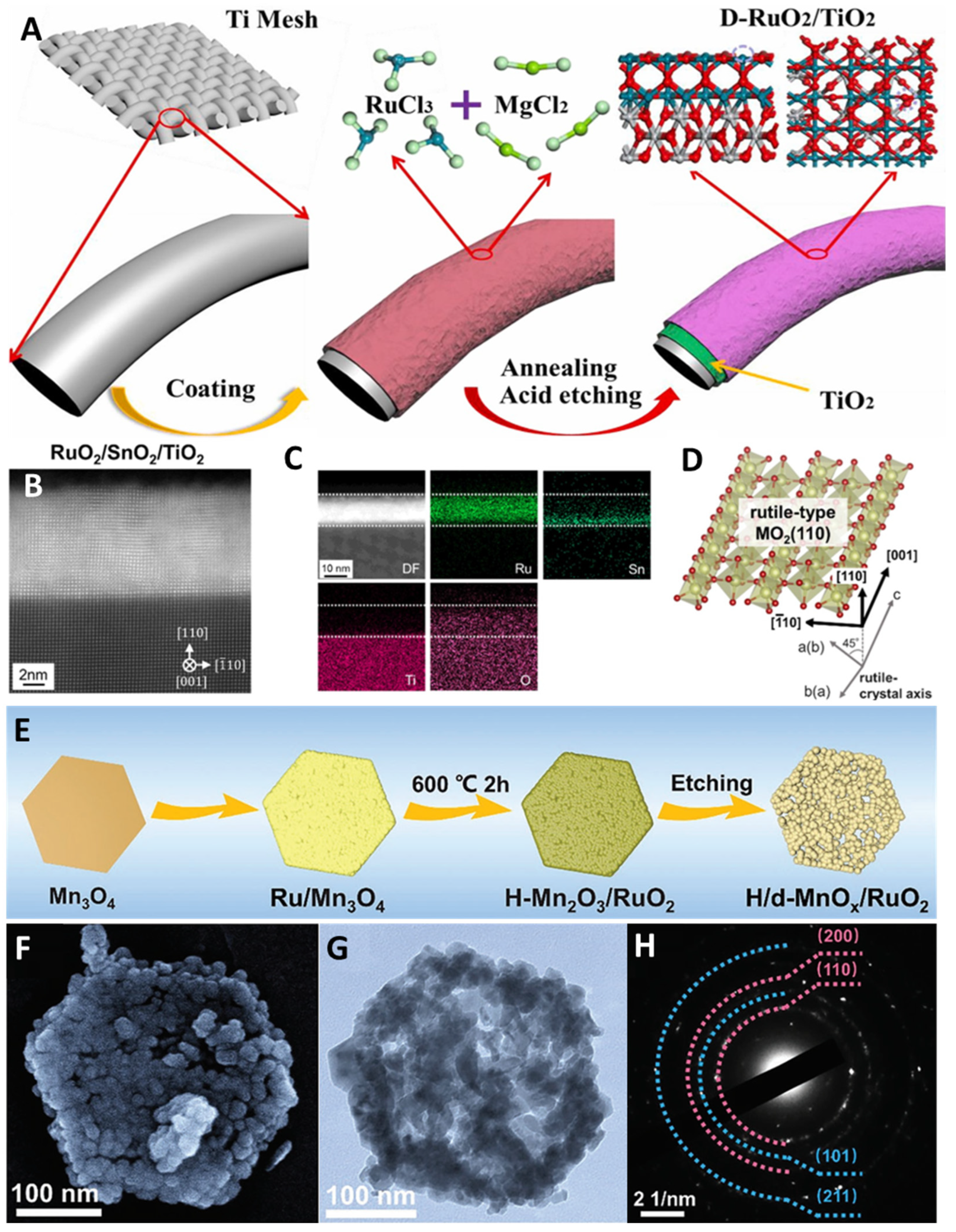
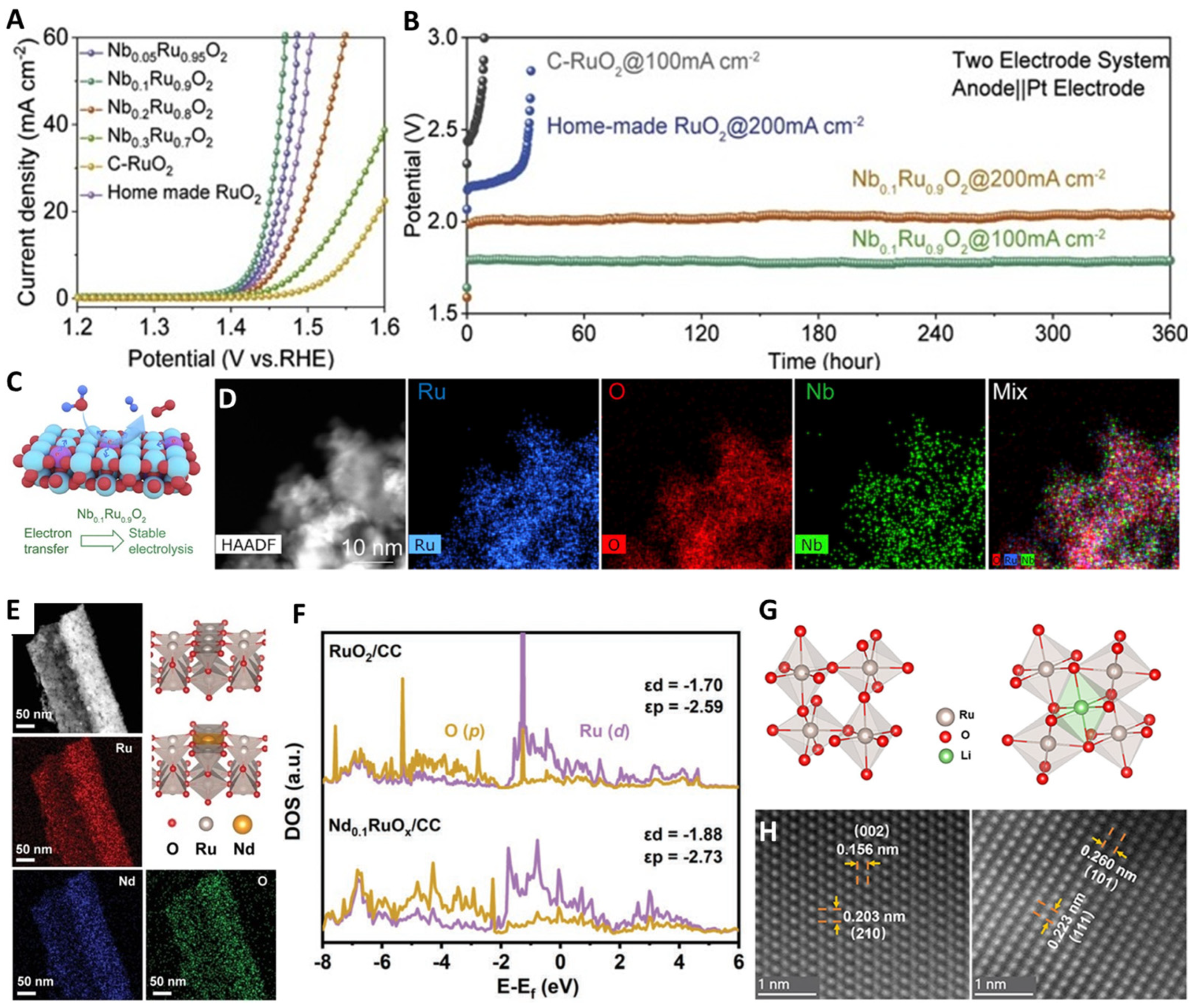
3.1. Heterostructure Construction
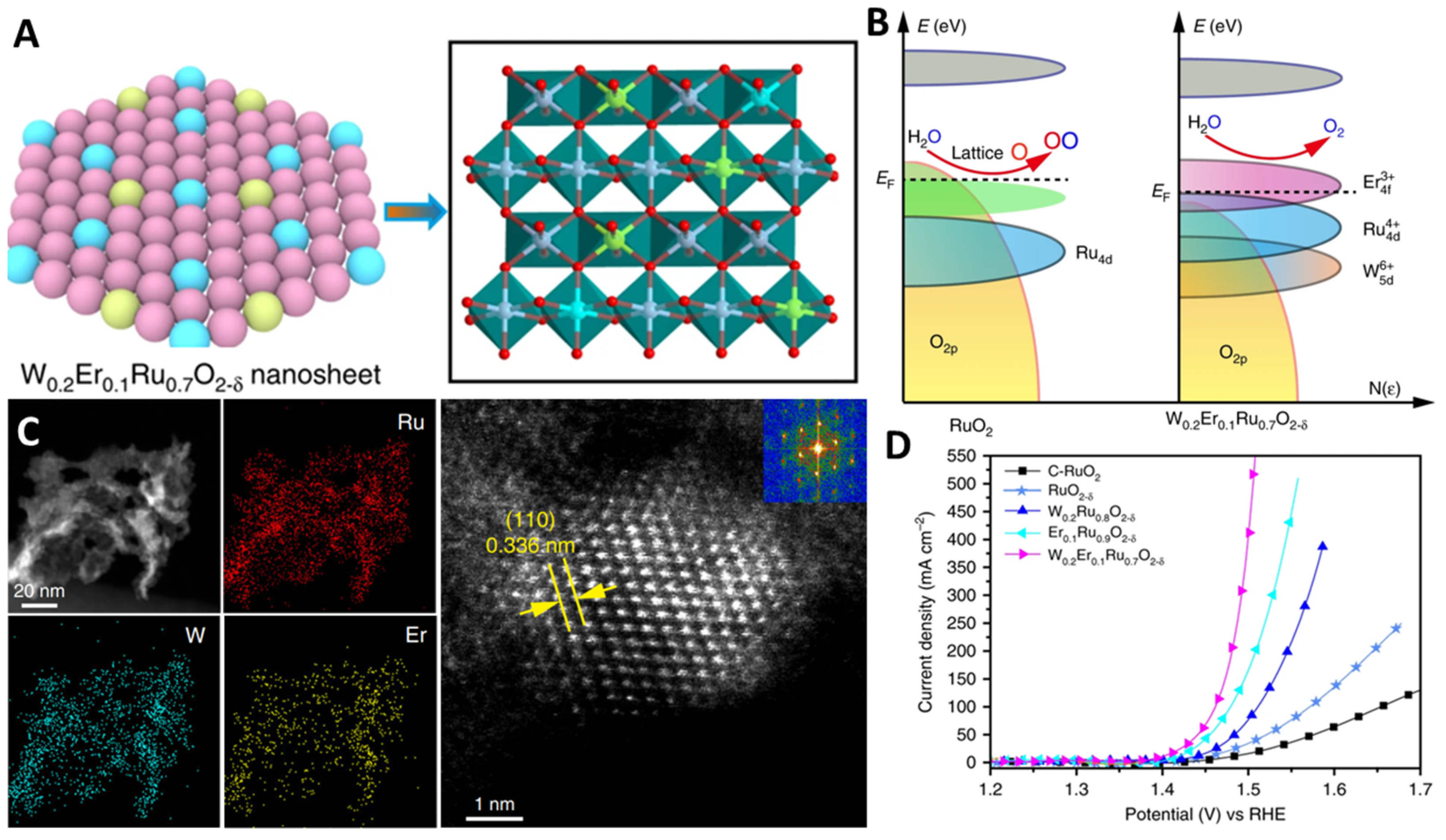
3.2. Heteroatom Doping
3.3. Defect Engineering
3.4. Morphology Engineering
4. Summary and Outlook
- (1)
- Reaction mechanism. Understanding the correlation between the activity/stability and local structure is central to the design of efficient RuO2-based OER catalysts. The deactivation and dissolution of RuO2-based catalyst was studied by advanced technical means to provide guidance for the synthesis of more efficient OER electrocatalyst and the in-depth understanding of catalytic mechanism. Although in situ Raman and Operando XAS techniques can be used to record the oxidation states, geometry, electronic structures, and interfaces of catalysts, the diversity and complexity of active stages make it difficult to interpret the factors controlling and influencing catalytic reactions. In addition, the existing operando techniques can only capture quasi-stable active sites, while reaction intermediates typically have picosecond lifetime. Therefore, the development of more advanced techniques to study the electrocatalytic reaction process combined with theoretical simulation to better reveal the true and accurate electrochemical process will be of great help in improving the understanding of the acidic OER mechanism of RuO2-based catalyst.
- (2)
- Activity and durability. Laboratories primarily use CV, CA, and CP to evaluate the stability of RuO2-based OER catalysts, but these methods ignore mass transport, electrode spacing, and fluid flow effects. Ru dissolution caused by over-oxidation in OER process is generally considered to be the main cause of deactivation of RuO2-based materials. And the stability degradation found in these validation methods can be caused not only by catalyst degradation, but also by catalyst interface separation or active site coverage. The development of accelerated deactivation test systems is necessary to provide information on long-term stability performance at high current densities and high temperatures that will be more useful for practical applications [124]. As applications are often required to operate at high current densities and high temperatures, accelerated deactivation test systems are required to rapidly assess catalyst degradation under these conditions. By accelerating the catalyst deactivation process, test times can be reduced, and the harsh environments of practical applications can be simulated, allowing catalyst stability data to be obtained more quickly, which can help researchers better understand the mechanisms of catalyst degradation and provide guidance on how to improve catalyst stability. In addition, the Accelerated Deactivation Test of RuO2-based catalyst can take into account important factors in practical applications, such as mass transfer, electrode spacing and fluid flow.
- (3)
- Industrial applications. The small-scale durability under laboratory conditions cannot meet the requirements of industrial electrolytes. For large-scale PEM electrolysis applications, the catalyst shall be manufactured using scalable and industrially acceptable methods. Precise computer-aided 3D printing techniques can help construct complex structures, accelerating mass/charge/ion transport rates and enhancing activity and stability. Conductive substrates play a key role in delivering activity and stability, but common substrates are not stable in acids. By using acid oxidized and/or doped substrates, we can improve the corrosion resistance of the substrate, or an alternative substrate with excellent corrosion and oxidation resistance such as Ti or Ta foam could be an effective solution. Depositing conductive layers can inhibit the formation of insulating TiO2 layers to further enhance stability. The selection of appropriate OER substrate electrodes significantly impacts catalyst passivation/detachment, substrate-catalyst interactions, and stability performance. Testing in three-electrode configurations significantly differs from industrial applications in terms of operation conditions. To bridge the gap between material development and industrial applications, it is crucial to perform membrane electrode assembly (MEA) testing under relevant industrial conditions as early as possible and further understand the operational conditions and other components essential for designing an optimal working environment for MEAs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.-Y.; Chen, F.-Y.; Li, B.; Yu, S.-W.; Finfrock, Y.Z.; Meira, D.M.; Yan, Q.-Q.; Zhu, P.; Chen, M.-X.; Song, T.-W.; et al. Non-iridium-based electrocatalyst for durable acidic oxygen evolution reaction in proton exchange membrane water electrolysis. Nat. Mater. 2022, 22, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xi, S.; Huang, P.; Du, Y.; Zhong, H.; Wang, Q.; Borgna, A.; Zhang, Y.-W.; Wang, Z.; Wang, H.; et al. Pivotal role of reversible NiO6 geometric conversion in oxygen evolution. Nature 2022, 611, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, H.; Xiang, M.; Yu, C.; Hui, J.; Dong, S. Coral reef structured cobalt-doped vanadate oxometalate nanoparticle for a high-performance electrocatalyst in water splitting. Int. J. Hydrogen Energy 2022, 47, 31566–31574. [Google Scholar] [CrossRef]
- Sun, S.-C.; Jiang, H.; Chen, Z.-Y.; Chen, Q.; Ma, M.-Y.; Zhen, L.; Song, B.; Xu, C.-Y. Bifunctional WC-Supported RuO2 Nanoparticles for Robust Water Splitting in Acidic Media. Angew. Chem. Int. Ed. 2022, 61, e202202519. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, X.; Kim, H.; Shao, Z.; Jung, W. Advanced electrocatalysts with unusual active sites for electrochemical water splitting. InfoMat 2023, e12494. [Google Scholar] [CrossRef]
- Liu, R.T.; Xu, Z.L.; Li, F.M.; Chen, F.Y.; Yu, J.Y.; Yan, Y.; Chen, Y.; Xia, B.Y. Recent advances in proton exchange membrane water electrolysis. Chem. Soc. Rev. 2023, 52, 5652–5683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H.; et al. Status and perspectives of key materials for PEM electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, C.; Cai, X.; An, L.; Shen, S.; Yan, X.; Zhang, J. Bubble evolution and transport in PEM water electrolysis: Mechanism, impact, and management. Prog. Energy Combust. Sci. 2023, 96, 101075. [Google Scholar] [CrossRef]
- Seitz, L.C.; Dickens, C.F.; Nishio, K.; Hikita, Y.; Montoya, J.; Doyle, A.; Kirk, C.; Vojvodic, A.; Hwang, H.Y.; Norskov, J.K.; et al. A highly active and stable IrOx/SrIrO3 catalyst for the oxygen evolution reaction. Science 2016, 353, 1011–1014. [Google Scholar] [CrossRef]
- Wan, G.; Freeland, J.W.; Kloppenburg, J.; Petretto, G.; Nelson, J.N.; Kuo, D.-Y.; Sun, C.-J.; Wen, J.; Diulus, J.T.; Herman, G.S.; et al. Amorphization mechanism of SrIrO3 electrocatalyst: How oxygen redox initiates ionic diffusion and structural reorganization. Sci. Adv. 2021, 7, eabc7323. [Google Scholar] [CrossRef]
- Lončar, A.; Escalera-López, D.; Cherevko, S.; Hodnik, N. Inter-relationships between Oxygen Evolution and Iridium Dissolution Mechanisms. Angew. Chem. Int. Ed. 2022, 61, e202114437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xue, J.; Zeng, L.; Shang, J.; Lu, S.; Cao, X.; Abrahams, B.F.; Gu, H.; Lang, J. One-pot pyrolysis synthesis of highly active Ru/RuOX nanoclusters for water splitting. Nano Res. 2021, 15, 1020–1026. [Google Scholar] [CrossRef]
- Wang, C.; Jin, L.; Shang, H.; Xu, H.; Shiraishi, Y.; Du, Y. Advances in engineering RuO2 electrocatalysts towards oxygen evolution reaction. Chin. Chem. Lett. 2021, 32, 2108–2116. [Google Scholar] [CrossRef]
- Bai, J.; Cheng, L.; Liu, S.; Zhang, H.; Lian, Y.; Deng, Y.; Zhou, Q.; Tang, Y.; Su, Y. Unravel the mechanism of d-orbital modulation and oxygen vacancy in cerium-doped RuO2 catalysts for acidic oxygen evolution reaction. Appl. Surf. Sci. 2024, 642, 158613. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Cheng, M.; Feng, Y.; Han, X.; Qian, Q.; Zhu, Y.; Zhang, G. Arming Ru with Oxygen-Vacancy-Enriched RuO2 Sub-Nanometer Skin Activates Superior Bifunctionality for pH-Universal Overall Water Splitting. Adv. Mater. 2023, 35, e2206351. [Google Scholar] [CrossRef]
- Wang, T.; Li, Z.; Jang, H.; Kim, M.G.; Qin, Q.; Liu, X. Interface Engineering of Oxygen Vacancy-Enriched Ru/RuO2–Co3O4 Heterojunction for Efficient Oxygen Evolution Reaction in Acidic Media. ACS Sustain. Chem. Eng. 2023, 11, 5155–5163. [Google Scholar] [CrossRef]
- Rao, R.-R.; Kolb, M.J.; Halck, N.B.; Pedersen, A.F.; Mehta, A.; You, H.; Stoerzinger, K.A.; Feng, Z.; Hansen, H.A.; Zhou, H.; et al. Towards identifying the active sites on RuO2(110) in catalyzing oxygen evolution. Energy Environ. Sci. 2017, 10, 2626–2637. [Google Scholar] [CrossRef]
- Lin, Y.; Dong, Y.; Wang, X.; Chen, L. Electrocatalysts for the Oxygen Evolution Reaction in Acidic Media. Adv. Mater. 2022, 35, e2210565. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, P.; Wang, L.; Li, S.; Wang, Z.; Abed, J.; Mao, X.; Min, Y.; Dinh, C.T.; Luna, P.D.; et al. Stabilizing Highly Active Ru Sites by Suppressing Lattice Oxygen Participation in Acidic Water Oxidation. J. Am. Chem. Soc. 2021, 143, 6482–6490. [Google Scholar] [CrossRef]
- Shi, Z.; Li, J.; Wang, Y.; Liu, S.; Zhu, J.; Yang, J.; Wang, X.; Ni, J.; Jiang, Z.; Zhang, L.; et al. Customized reaction route for ruthenium oxide towards stabilized water oxidation in high-performance PEM electrolyzers. Nat. Commun. 2023, 14, 843. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Fang, J.; Li, M.; Sendeku, M.G.; Wang, X.; Wu, H.; Li, Y.; Ge, J.; Zhuang, Z.; et al. Eliminating over-oxidation of ruthenium oxides by niobium for highly stable electrocatalytic oxygen evolution in acidic media. Joule 2023, 7, 558–573. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Yang, B.; Li, Z.; Qin, X.; Zhang, Q.; Lei, L.; Qiu, M.; Wu, G.; Hou, Y. Highly active ruthenium sites stabilized by modulating electron-feeding for sustainable acidic oxygen-evolution electrocatalysis. Energy Environ. Sci. 2022, 15, 2356–2365. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.-F.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.-J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tahini, H.A.; Hu, Z.; Yin, Y.; Lin, Q.; Sun, H.; Zhong, Y.; Chen, Y.; Zhang, F.; Lin, H.-J.; et al. Boosting oxygen evolution reaction by activation of lattice-oxygen sites in layered Ruddlesden-Popper oxide. EcoMat 2020, 2, e12021. [Google Scholar] [CrossRef]
- Shan, J.; Zheng, Y.; Shi, B.; Davey, K.; Qiao, S.-Z. Regulating Electrocatalysts via Surface and Interface Engineering for Acidic Water Electrooxidation. ACS Energy Lett. 2019, 4, 2719–2730. [Google Scholar] [CrossRef]
- Vojvodic, A.; Nørskov, J.K. Optimizing Perovskites for the Water-Splitting Reaction. Science 2011, 334, 1355–1356. [Google Scholar] [CrossRef]
- Hong, W.-T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.-Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Wang, C.; Schechter, A.; Feng, L. Iridium-based catalysts for oxygen evolution reaction in acidic media: Mechanism, catalytic promotion effects and recent progress. Nano Res. Energy 2023, 2, e9120056. [Google Scholar] [CrossRef]
- Damjanovic, A.; Jovanovic, B. Anodic Oxide Films as Barriers to Charge Transfer in O2 Evolution at Pt in Acid Solutions. J. Electrochem. Soc. 1976, 123, 374. [Google Scholar] [CrossRef]
- Grimaud, A.; Diaz-Morales, O.; Han, B.; Hong, W.-T.; Lee, Y.-L.; Giordano, L.; Stoerzinger, K.A.; Koper, M.T.M.; Shao-Horn, Y. Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 2017, 9, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Willsau, J.; Wolter, O.; Heitbaum, J. Does the oxide layer take part in the oxygen evolution reaction on platinum?: A DEMS study. J. Electroanal. Chem. 1985, 195, 299–306. [Google Scholar] [CrossRef]
- Wohlfahrt-Mehrens, M.; Heitbaum, J. Oxygen evolution on Ru and RuO2 electrodes studied using isotope labelling and on-line mass spectrometry. J. Electroanal. Chem. 1987, 237, 251–260. [Google Scholar] [CrossRef]
- Kötz, R.; Stucki, S.; Scherson, D.; Kolb, D.M. In-situ identification of RuO4 as the corrosion product during oxygen evolution on ruthenium in acid media. J. Electroanal. Chem. 1984, 172, 211–219. [Google Scholar] [CrossRef]
- Roy, C.; Rao, R.-R.; Stoerzinger, K.A.; Hwang, J.; Rossmeisl, J.; Chorkendorff, I.; Shao-Horn, Y.; Stephens, I.E.L. Trends in Activity and Dissolution on RuO2 under Oxygen Evolution Conditions: Particles versus Well-Defined Extended Surfaces. ACS Energy Lett. 2018, 3, 2045–2051. [Google Scholar] [CrossRef]
- Cherevko, S.; Zeradjanin, A.R.; Topalov, A.A.; Kulyk, N.; Katsounaros, I.; Mayrhofer, K.J.J. Dissolution of Noble Metals during Oxygen Evolution in Acidic Media. ChemCatChem 2014, 6, 2219–2223. [Google Scholar] [CrossRef]
- Zagalskaya, A.; Alexandrov, V. Role of Defects in the Interplay between Adsorbate Evolving and Lattice Oxygen Mechanisms of the Oxygen Evolution Reaction in RuO2 and IrO2. ACS Catal. 2020, 10, 3650–3657. [Google Scholar] [CrossRef]
- Jana, J.; Bhamu, K.C.; Ngo, Y.-L.T.; Kang, S.G.; Chung, J.S.; Hur, S.H. Designing a bimetallic transition metal oxide/hydroxide composite for effective electrocatalytic oxygen evolution reaction. Appl. Surf. Sci. 2021, 562, 150253. [Google Scholar] [CrossRef]
- Chen, M.; Kitiphatpiboon, N.; Feng, C.; Abudula, A.; Ma, Y.; Guan, G. Recent progress in transition-metal-oxide-based electrocatalysts for the oxygen evolution reaction in natural seawater splitting: A critical review. eScience 2023, 3, 100111. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, H.; Wang, X.; Hu, C.; Jing, H.; Cheng, J. Role of transition metals in catalyst designs for oxygen evolution reaction: A comprehensive review. Int. J. Hydrogen Energy 2022, 47, 17946–17970. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Liu, J.-N.; Wang, J.; Ren, D.; Li, B.-Q.; Zhang, Q. Recent advances of noble-metal-free bifunctional oxygen reduction and evolution electrocatalysts. Chem. Soc. Rev. 2021, 50, 7745–7778. [Google Scholar] [CrossRef]
- Liao, F.; Yin, K.; Ji, Y.; Zhu, W.; Fan, Z.; Li, Y.; Zhong, J.; Shao, M.; Kang, Z.; Shao, Q. Iridium oxide nanoribbons with metastable monoclinic phase for highly efficient electrocatalytic oxygen evolution. Nat. Commun. 2023, 14, 1248. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, G.; Xu, J.; He, H.; Wang, B.; Yang, Z.; Yang, S. Optimizing the Electronic Structure of Ruthenium Oxide by Neodymium Doping for Enhanced Acidic Oxygen Evolution Catalysis. Adv. Funct. Mater. 2023, 33, 2213304. [Google Scholar] [CrossRef]
- Hao, S.; Liu, M.; Pan, J.; Liu, X.; Tan, X.; Xu, N.; He, Y.; Lei, L.; Zhang, X. Dopants fixation of Ruthenium for boosting acidic oxygen evolution stability and activity. Nat. Commun. 2020, 11, 5368. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, H.; Li, F.; Li, L.; Wu, J.; Liu, S.; Cheng, T.; Xu, Y.; Shao, Q.; Huang, X. Single-site Pt-doped RuO2 hollow nanospheres with interstitial C for high-performance acidic overall water splitting. Sci. Adv. 2022, 8, eabl9271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lei, X.; Zhang, B.; Bai, B.; Das, P.; Azam, T.; Xiao, J.; Wu, Z.S. Breaking the Ru-O-Ru Symmetry of a RuO2 Catalyst for Sustainable Acidic Water Oxidation. Angew. Chem. Int. Ed. Engl. 2023, 63, e202316903. [Google Scholar] [CrossRef]
- Wu, L.; Liang, Q.; Zhao, J.; Zhu, J.; Jia, H.; Zhang, W.; Cai, P.; Luo, W. A Bi-doped RuO2 catalyst for efficient and durable acidic water oxidation. Chin. J. Catal. 2023, 55, 182–190. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, R.; Zhao, Q.; Li, J.; Liu, G. La-RuO2 nanocrystals with efficient electrocatalytic activity for overall water splitting in acidic media: Synergistic effect of La doping and oxygen vacancy. Chem. Eng. J. 2022, 439, 135699. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; An, P.; Tang, C.; Yu, H.; Zhang, Q.; Peng, H.J.; Gu, L.; Zheng, Y.; Song, T.; et al. Dynamic rhenium dopant boosts ruthenium oxide for durable oxygen evolution. Nat. Commun. 2023, 14, 354. [Google Scholar] [CrossRef]
- Liu, L.; Ji, Y.; You, W.; Liu, S.; Shao, Q.; Kong, Q.; Hu, Z.; Tao, H.; Bu, L.; Huang, X. Trace Lattice S Inserted RuO2 Flexible Nanosheets for Efficient and Long-Term Acidic Oxygen Evolution Catalysis. Small 2023, 19, e2208202. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, J.; Liu, Q.; Ma, C.; Tang, Y.; Sun, H. Competitive adsorption of oxygen-containing intermediates on ruthenium–tin solid-solution oxides for alkaline oxygen evolution. J. Mater. Chem. A 2023, 11, 23489–23497. [Google Scholar] [CrossRef]
- Du, K.; Zhang, L.; Shan, J.; Guo, J.; Mao, J.; Yang, C.C.; Wang, C.H.; Hu, Z.; Ling, T. Interface engineering breaks both stability and activity limits of RuO2 for sustainable water oxidation. Nat. Commun. 2022, 13, 5448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jang, H.; Liu, H.; Kim, M.G.; Yang, D.; Liu, S.; Liu, X.; Cho, J. Sodium-Decorated Amorphous/Crystalline RuO2 with Rich Oxygen Vacancies: A Robust pH-Universal Oxygen Evolution Electrocatalyst. Angew. Chem. Int. Ed. Engl. 2021, 60, 18821–18829. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zou, J.; Liang, T.; Song, X.; Li, Z.; Wen, J.; Peng, M.; Zeng, X.; Huang, H.; Wu, H. MOF-derived ultrasmall Ru@RuO2 heterostructures as bifunctional and pH-universal electrocatalysts for 0.79 V asymmetric amphoteric overall water splitting. Chem. Eng. J. 2023, 460, 141672. [Google Scholar] [CrossRef]
- Li, W.; Zhao, L.; Wang, C.; Lu, X.; Chen, W. Interface Engineering of Heterogeneous CeO2-CoO Nanofibers with Rich Oxygen Vacancies for Enhanced Electrocatalytic Oxygen Evolution Performance. ACS Appl. Mater. Interfaces 2021, 13, 46998–47009. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Mao, G.; Zhu, Y.; Li, R.; Zhou, Q.; Xiao, F.; Tang, R. Accelerated oxygen evolution enabled by encapsulating hybrid CoOx/RuO2 nanoparticle with nanoporous carbon. Appl. Surf. Sci. 2022, 589, 152958. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Liu, S.; Xu, W.; Wu, L.; Hsieh, Y.C.; Liu, P.; Zhu, Y.; Sasaki, K.; Renner, J.N.; et al. Reaction mechanism for oxygen evolution on RuO2, IrO2, and RuO2@IrO2 core-shell nanocatalysts. Electroanal. Chem. 2018, 819, 296–305. [Google Scholar] [CrossRef]
- Lin, C.; Li, J.-L.; Li, X.; Yang, S.; Luo, W.; Zhang, Y.; Kim, S.H.; Kim, D.H.; Shinde, S.S.; Li, Y.-F.; et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat. Catal. 2021, 4, 1012–1023. [Google Scholar] [CrossRef]
- Wang, J.; Kim, H.; Lee, H.; Ko, Y.J.; Han, M.-H.; Kim, W.; Baik, J.M.; Choi, J.Y.; Oh, H.S.; Lee, W.H. Sb incorporated into oxides enhances stability in acid during the oxygen evolution reaction by inhibiting structural distortion. Nano Energy 2023, 110, 108355. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Y.; Zhang, L.; Tang, T.; Xiao, H.; Chen, W.; Zhao, M.; Jia, J.; Zhu, H. Facile synthesis of PdO-doped Co3O4 nanoparticles as an efficient bifunctional oxygen electrocatalyst. Appl. Catal. B—Environ. 2019, 243, 175–182. [Google Scholar] [CrossRef]
- Hou, L.; Li, Z.; Jang, H.; Wang, Y.; Cui, X.; Gu, X.; Kim, M.G.; Feng, L.; Liu, S.; Liu, X. Electronic and Lattice Engineering of Ruthenium Oxide towards Highly Active and Stable Water Splitting. Adv. Energy Mater. 2023, 13, 2300177. [Google Scholar] [CrossRef]
- Xu, J.; Chen, C.; Kong, X. Ru-O-Cu center constructed by catalytic growth of Ru for efficient hydrogen evolution. Nano Energy 2023, 111, 108403. [Google Scholar] [CrossRef]
- Lu, X.; Yang, Y.; Yin, Y.; Wang, Z.; Sutrisno, L.; Yan, C.; Schmidt, O.G. Atomic Heterointerface Boosts the Catalytic Activity toward Oxygen Reduction/Evolution Reaction. Adv. Energy Mater. 2021, 11, 2102235. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, S.; Sun, Y.; Xi, S.; Chi, X.; Zhang, Q.; Ren, X.; Wang, J.; Ong, S.J.H.; Du, Y.; et al. Mastering Surface Reconstruction of Metastable Spinel Oxides for Better Water Oxidation. Adv. Mater. 2019, 31, e1807898. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Zang, W.; Li, X.; Chen, D.; Kou, Z.; Mu, S.; Wang, J. Nanoframes of Co3O4–Mo2N Heterointerfaces Enable High-Performance Bifunctionality toward Both Electrocatalytic HER and OER. Adv. Funct. Mater. 2021, 32, 2107382. [Google Scholar] [CrossRef]
- Huang, J.; Sheng, H.; Ross, R.D.; Han, J.; Wang, X.; Song, B.; Jin, S. Modifying redox properties and local bonding of Co3O4 by CeO2 enhances oxygen evolution catalysis in acid. Nat. Commun. 2021, 12, 3036. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, L.; Guan, B.-Y.; Song, S.; Lou, X.W.D. Metal-Organic Framework Hybrid-Assisted Formation of Co3O4/Co-Fe Oxide Double-Shelled Nanoboxes for Enhanced Oxygen Evolution. Adv. Mater. 2018, 30, e1801211. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, C.; Dong, B.; Liu, C.; Chai, Y. High-Voltage-Enabled Stable Cobalt Species Deposition on MnO2 for Water Oxidation in Acid. Adv. Mater. 2023, 35, e2207066. [Google Scholar] [CrossRef]
- Huang, B.; Cui, Y.; Liu, X.; Zheng, C.; Wang, H.; Guan, L. Dense-Packed RuO2 Nanorods with In Situ Generated Metal Vacancies Loaded on SnO2 Nanocubes for Proton Exchange Membrane Water Electrolyzer with Ultra-Low Noble Metal Loading. Small 2023, 19, e2301516. [Google Scholar] [CrossRef]
- Huang, K.; Lin, C.; Yu, G.; Du, P.; Xie, X.; He, X.; Zheng, Z.; Sun, N.; Tang, H.; Li, X.; et al. Ru/Se-RuO2 Composites via Controlled Selenization Strategy for Enhanced Acidic Oxygen Evolution. Adv. Funct. Mater. 2022, 33, 2211102. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y.; Jiao, Y.; Wang, Z.; Lu, Z.; Vasileff, A.; Qiao, S.-Z. NiO as a Bifunctional Promoter for RuO2 toward Superior Overall Water Splitting. Small 2018, 14, e1704073. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Hong, M.; Zhang, L.; Feng, X.; Shi, M.; Hu, W.; Mu, S. Defective RuO2/TiO2 nano-heterostructure advances hydrogen production by electrochemical water splitting. Chem. Eng. J. 2022, 431, 134072. [Google Scholar] [CrossRef]
- Todoroki, N.; Kudo, R.; Hayashi, K.; Yokoi, M.; Naraki, N.; Wadayama, T. Improving the Oxygen Evolution Activity and Stability of Nb-Doped TiO2-Supported RuO2 by a SnO2 Interlayer: A Model Catalyst Study on Single-Crystal Oxide Heterostructures. ACS Catal. 2023, 13, 11433–11440. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Liu, D.; Zhou, B.; Yang, P.; Liu, R.; Xiao, W.; Ma, T.; Wang, J.; Wang, L. Hexagonal Defect-Rich MnOx/RuO2 with Abundant Heterointerface to Modulate Electronic Structure for Acidic Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2307010. [Google Scholar] [CrossRef]
- Banerjee, S.; Debata, S.; Madhuri, R.; Sharma, P.K. Electrocatalytic behavior of transition metal (Ni, Fe, Cr) doped metal oxide nanocomposites for oxygen evolution reaction. Appl. Surf. Sci. 2018, 449, 660–668. [Google Scholar] [CrossRef]
- Al-Naggar, A.H.; Shinde, N.M.; Kim, J.-S.; Mane, R.S. Water splitting performance of metal and non-metal-doped transition metal oxide electrocatalysts. Coord. Chem. Rev. 2023, 474, 214864. [Google Scholar] [CrossRef]
- Wang, N.; Ou, P.; Miao, R.-K.; Chang, Y.; Wang, Z.; Hung, S.-F.; Abed, J.; Ozden, A.; Chen, H.-Y.; Wu, H.-L.; et al. Doping Shortens the Metal/Metal Distance and Promotes OH Coverage in Non-Noble Acidic Oxygen Evolution Reaction Catalysts. J. Am. Chem. Soc. 2023, 145, 7829–7836. [Google Scholar] [CrossRef]
- Peng, Y.; Hajiyani, H.; Pentcheva, R. Influence of Fe and Ni Doping on the OER Performance at the Co3O4(001) Surface: Insights from DFT+U Calculations. ACS Catal. 2021, 11, 5601–5613. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Han, M.-H.; Lim, C.; Yu, S.-H.; Choi, C.H.; Min, B.K.; Choi, J.Y.; Lee, W.H.; Oh, H.-S. Unveiling the role of Ni in Ru-Ni oxide for oxygen evolution: Lattice oxygen participation enhanced by structural distortion. J. Energy Chem. 2023, 77, 54–61. [Google Scholar] [CrossRef]
- Deng, M.; Tang, Y.; Lu, Z.; Wang, Y.; Lin, Y. Self-Supporting Mn-RuO2 Nanoarrays for Stable Oxygen Evolution Reaction in Acid. Molecules 2023, 28, 7727. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Guan, B.-Y.; Lu, X.-F.; Xi, S.; Du, Y.; Lou, X.W.D. Metal Atom-Doped Co3O4 Hierarchical Nanoplates for Electrocatalytic Oxygen Evolution. Adv. Mater. 2020, 32, e2002235. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, M.; Pan, F.; Zhu, Z.; Sun, H.; Tang, Y.; Fu, G. Plasma-induced Mo-doped Co3O4 with enriched oxygen vacancies for electrocatalytic oxygen evolution in water splitting. Carbon Energy 2022, 5, e279. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Koketsu, T.; Kroschel, M.; Chen, J.-M.; Hsu, S.Y.; Henkelman, G.; Hu, Z.; Strasser, P.; Ma, J. Iridium single atoms incorporated in Co3O4 efficiently catalyze the oxygen evolution in acidic conditions. Nat. Commun. 2022, 13, 7754. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ji, Y.; Li, Y.; An, P.; Zhang, J.; Yan, J.; Liu, S.-F. Single-Atom Doping and High-Valence State for Synergistic Enhancement of NiO Electrocatalytic Water Oxidation. Small 2021, 17, e2102448. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, C.; Zhao, S.-N.; Wu, Y.; Liu, C.; Huang, J.; Xue, L.; Sun, J.; Zhang, W.; Wang, X.; et al. A dual-site doping strategy for developing efficient perovskite oxide electrocatalysts towards oxygen evolution reaction. Nano Energy 2022, 99, 107344. [Google Scholar] [CrossRef]
- An, L.; Zhang, H.; Zhu, J.; Xi, S.; Huang, B.; Sun, M.; Peng, Y.; Xi, P.; Yan, C.H. Balancing Activity and Stability in Spinel Cobalt Oxides through Geometrical Sites Occupation towards Efficient Electrocatalytic Oxygen Evolution. Angew. Chem. Int. Ed. 2023, 62, e202214600. [Google Scholar] [CrossRef]
- Li, L.; Cao, X.; Huo, J.; Qu, J.; Chen, W.; Liu, C.; Zhao, Y.; Liu, H.; Wang, G. High valence metals engineering strategies of Fe/Co/Ni-based catalysts for boosted OER electrocatalysis. J. Energy Chem. 2023, 76, 195–213. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Wang, J.; Zhang, J.; Qiu, L.; Chen, Y.; Zhong, C.; Han, X.; Deng, Y.; Hu, W. Regulating metal active sites of atomically-thin nickel-doped spinel cobalt oxide toward enhanced oxygen electrocatalysis. Chem. Eng. J. 2022, 435, 134261. [Google Scholar] [CrossRef]
- Yu, J.; Garces-Pineda, F.A.; Gonzalez-Cobos, J.; Pena-Diaz, M.; Rogero, C.; Gimenez, S.; Spadaro, M.C.; Arbiol, J.; Barja, S.; Galan-Mascaros, J.R. Sustainable oxygen evolution electrocatalysis in aqueous 1 M H2SO4 with earth abundant nanostructured Co3O4. Nat. Commun. 2022, 13, 4341. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, Y.; An, L.; Li, Q.; Yin, J.; Li, J.; Yang, R.; Lu, M.; Zhang, S.; Xi, P.; et al. Surface Activation and Ni-S Stabilization in NiO/NiS2 for Efficient Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202207217. [Google Scholar] [CrossRef]
- Chen, S.; Huang, H.; Jiang, P.; Yang, K.; Diao, J.; Gong, S.; Liu, S.; Huang, M.; Wang, H.; Chen, Q. Mn-Doped RuO2 Nanocrystals as Highly Active Electrocatalysts for Enhanced Oxygen Evolution in Acidic Media. ACS Catal. 2019, 10, 1152–1160. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Ding, Y.; Zhang, B.; Li, H.; Bai, B.; Li, M.; Cui, Y.; Xiao, J.; Wu, Z.S. Unraveling oxygen vacancy site mechanism of Rh-doped RuO2 catalyst for long-lasting acidic water oxidation. Nat. Commun. 2023, 14, 1412. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zheng, G.; He, Y.; Lei, L.; Zhang, X. Ultra-small Sn-RuO2 nanoparticles supported on N-doped carbon polyhedra for highly active and durable oxygen evolution reaction in acidic media. Chem. Eng. J. 2021, 409, 128155. [Google Scholar] [CrossRef]
- Qin, Y.; Yu, T.; Deng, S.; Zhou, X.-Y.; Lin, D.; Zhang, Q.; Jin, Z.; Zhang, D.; He, Y.-B.; Qiu, H.-J.; et al. RuO2 electronic structure and lattice strain dual engineering for enhanced acidic oxygen evolution reaction performance. Nat. Commun. 2022, 13, 3784. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Lu, J.; Yang, B.; San, X.; Wu, Z.-S. 2D intrinsically defective RuO2/Graphene heterostructures as All-pH efficient oxygen evolving electrocatalysts with unprecedented activity. Nano Energy 2020, 78, 105185. [Google Scholar] [CrossRef]
- Ge, R.-X.; Li, L.; Su, J.-W.; Lin, Y.-C.; Tian, Z.-Q.; Chen, L. Ultrafine Defective RuO2 Electrocatayst Integrated on Carbon Cloth for Robust Water Oxidation in Acidic Media. Adv. Energy Mater. 2019, 9, 1901313. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Maxakato, N.W. Porous metal oxide electrocatalytic nanomaterials for energy conversion: Oxygen defects and selection techniques. Coord. Chem. Rev. 2022, 457, 214389. [Google Scholar] [CrossRef]
- Zhang, R.; Pan, L.; Guo, B.; Huang, Z.-F.; Chen, Z.; Wang, L.; Zhang, X.; Guo, Z.; Xu, W.; Loh, K.P.; et al. Tracking the Role of Defect Types in Co3O4 Structural Evolution and Active Motifs during Oxygen Evolution Reaction. J. Am. Chem. Soc. 2023, 145, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Béjar, J.; Álvarez-Contreras, L.; Guerra-Balcázar, M.; Ledesma-García, J.; Arriaga, L.G.; Arjona, N. Synthesis of a small-size metal oxide mixture based on MoO and NiO with oxygen vacancies as bifunctional electrocatalyst for oxygen reactions. Appl. Surf. Sci. 2020, 509, 144898. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, B.; Xu, Y.; Li, L.; Shao, Q.; Huang, X. A chemical etching strategy to improve and stabilize RuO2-based nanoassemblies for acidic oxygen evolution. Nano Energy 2021, 84, 105909. [Google Scholar] [CrossRef]
- Xiao, Z.; Huang, Y.C.; Dong, C.L.; Xie, C.; Liu, Z.; Du, S.; Chen, W.; Yan, D.; Tao, L.; Shu, Z.; et al. Operando Identification of the Dynamic Behavior of Oxygen Vacancy-Rich Co3O4 for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2020, 142, 12087–12095. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Oubla, M.h.; Zhong, X.; Alonso-Vante, N.; Du, F.; Xie, Y.; Huang, Y.; Ma, J. Rational defect and anion chemistries in Co3O4 for enhanced oxygen evolution reaction. Appl. Catal. B—Environ. 2021, 281, 119535. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, X.; Xu, L.; Yuan, D.; Dou, Y.; Qiu, J.; Li, H.; Ma, J.; Wang, Y.; Su, D.; et al. Engineering Crystallinity and Oxygen Vacancies of Co(II) Oxide Nanosheets for High Performance and Robust Rechargeable Zn–Air Batteries. Adv. Funct. Mater. 2021, 31, 2101239. [Google Scholar] [CrossRef]
- Chen, J.; Cui, P.; Zhao, G.; Rui, K.; Lao, M.; Chen, Y.; Zheng, X.; Jiang, Y.; Pan, H.; Dou, S.-X.; et al. Low–Coordinate Iridium Oxide Confined on Graphitic Carbon Nitride for Highly Efficient Oxygen Evolution. Angew. Chem. Int. Ed. 2019, 58, 12540–12544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qian, J.; Ran, J.; Xi, P.; Yang, L.; Gao, D. Engineering Lower Coordination Atoms onto NiO/Co3O4 Heterointerfaces for Boosting Oxygen Evolution Reactions. ACS Catal. 2020, 10, 12376–12384. [Google Scholar] [CrossRef]
- Nong, H.-N.; Reier, T.; Oh, H.S.; Gliech, M.; Paciok, P.; Vu, T.H.T.; Teschner, D.; Heggen, M.; Petkov, V.; Schlögl, R.; et al. A unique oxygen ligand environment facilitates water oxidation in hole-doped IrNiOx core–shell electrocatalysts. Nat. Catal. 2018, 1, 841–851. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, T.; Zhu, K.; Xie, W.; Zhu, X.; Yang, W. Interface modulation of perovskite oxides to simultaneously enhance the activity and stability toward oxygen evolution reaction. Chem. Eng. J. 2023, 455, 140829. [Google Scholar] [CrossRef]
- Yan, H.; Jiang, Z.; Deng, B.; Wang, Y.; Jiang, Z.-J. Ultrathin Carbon Coating and Defect Engineering Promote RuO2 as an Efficient Catalyst for Acidic Oxygen Evolution Reaction with Super-High Durability. Adv. Energy Mater. 2023, 13, 2300152. [Google Scholar] [CrossRef]
- Jin, H.; Choi, S.; Bang, G.-J.; Kwon, T.; Kim, H.S.; Lee, S.J.; Hong, Y.; Lee, D.W.; Park, H.S.; Baik, H.; et al. Safeguarding the RuO2 phase against lattice oxygen oxidation during acidic water electrooxidation. Energy Environ. Sci. 2022, 15, 1119–1130. [Google Scholar] [CrossRef]
- Liao, C.; Yang, B.; Zhang, N.; Liu, M.; Chen, G.; Jiang, X.; Chen, G.; Yang, J.; Liu, X.; Chan, T.-S.; et al. Constructing Conductive Interfaces between Nickel Oxide Nanocrystals and Polymer Carbon Nitride for Efficient Electrocatalytic Oxygen Evolution Reaction. Adv. Funct. Mater. 2019, 29, 1904020. [Google Scholar] [CrossRef]
- Ruiz-Cornejo, J.C.; Vivo-Vilches, J.F.; Sebastián, D.; Martínez-Huerta, M.V.; Lázaro, M.J. Carbon nanofiber-supported tantalum oxides as durable catalyst for the oxygen evolution reaction in alkaline media. Renew. Energy 2021, 178, 307–317. [Google Scholar] [CrossRef]
- Chalgin, A.; Song, C.; Tao, P.; Shang, W.; Deng, T.; Wu, J. Effect of supporting materials on the electrocatalytic activity, stability and selectivity of noble metal-based catalysts for oxygen reduction and hydrogen evolution reactions. Prog. Nat. Sci. 2020, 30, 289–297. [Google Scholar] [CrossRef]
- Kumar, P.; Kannimuthu, K.; Zeraati, A.S.; Roy, S.; Wang, X.; Wang, X.; Samanta, S.; Miller, K.A.; Molina, M.; Trivedi, D.; et al. High-Density Cobalt Single-Atom Catalysts for Enhanced Oxygen Evolution Reaction. J. Am. Chem. Soc. 2023, 145, 8052–8063. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, W.; Lu, Y.; Liang, Z.; He, T.; Li, J.; Nan, H.; Luo, S.; Yu, J. Lamellar-stacked cobalt-based nanopiles integrated with nitrogen/sulfur co-doped graphene as a bifunctional electrocatalyst for ultralong-term zinc-air batteries. J. Energy Chem. 2023, 81, 633–641. [Google Scholar] [CrossRef]
- Ruiz-Cornejo, J.C.; Sebastián, D.; Pardo, J.I.; Martínez-Huerta, M.V.; Lázaro, M.J. Sulfur-doped carbon nanofibers as support for tantalum oxides bifunctional catalysts for the oxygen reduction and evolution reactions. J. Power Sources 2022, 546, 231988. [Google Scholar] [CrossRef]
- Browne, M.P.; Tyndall, D.; Nicolosi, V. The potential of MXene materials as a component in the catalyst layer for the Oxygen Evolution Reaction. Curr. Opin. Electrochem. 2022, 34, 101021. [Google Scholar] [CrossRef]
- Yang, X.; Chen, J.; Chen, Y.; Feng, P.; Lai, H.; Li, J.; Luo, X. Novel Co3O4 Nanoparticles/Nitrogen-Doped Carbon Composites with Extraordinary Catalytic Activity for Oxygen Evolution Reaction (OER). Nano-Micro Lett. 2018, 10, 15. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Xiang, D.; Qin, J.; Miao, K.; Wang, X.; Kang, X.; Tian, Y. Yolk-Shell Structured Zinc-Cobalt-Ruthenium Alloy Oxide Assembled with Ultra-Small Nanoparticles: A Superior Cascade Catalyst toward Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2214529. [Google Scholar] [CrossRef]
- Du, J.; Chen, D.; Ding, Y.; Wang, L.; Li, F.; Sun, L. Highly Stable and Efficient Oxygen Evolution Electrocatalyst Based on Co Oxides Decorated with Ultrafine Ru Nanoclusters. Small 2023, 19, e2207611. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, C.; Zhang, Z.-Y.; Song, Y.-F.; Feng, B.-Q.; Na, P.; Wang, Z.-L. Ultrafine NiFe-Based (Oxy)Hydroxide Nanosheet Arrays with Rich Edge Planes and Superhydrophilic-Superaerophobic Characteristics for Oxygen Evolution Reaction. Small 2023, 19, e2301609. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Jia, H.; Zhu, J.; Shi, Z.; Cong, H.; Ge, J.; Luo, W. Atomically dispersed Ru oxide catalyst with lattice oxygen participation for efficient acidic water oxidation. Chem 2023, 9, 1882–1896. [Google Scholar] [CrossRef]
- Yu, B.; Liu, J.-H.; Guo, S.; Huang, G.; Zhang, S.; Chen, S.; Li, X.; Wang, Y.; Lv, L.P. Densely populated tiny RuO2 crystallites supported by hierarchically porous carbon for full acidic water splitting. Mater. Horiz. 2023, 10, 4589–4596. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, X.; Tang, T.; Zhong, Y.; Shao, Z. Perovskite-Based Electrocatalysts for Cost-Effective Ultrahigh-Current-Density Water Splitting in Anion Exchange Membrane Electrolyzer Cell. Small Methods 2022, 6, 2201099. [Google Scholar] [CrossRef]

| Sample | Overpotential @10 mA cm−2 | Tafel Slope | Reference |
|---|---|---|---|
| Ni-RuO2 | 214 | 42.6 | [1] |
| Ru@V-RuO2/C | 176 | 45.6 | [15] |
| Nb0.1Ru0.9O2 | 201 | 47.9 | [21] |
| Mn0.73Ru0.27O2−δ | 208 | 65.3 | [22] |
| Nd0.1RuOx | 211 | 50 | [43] |
| W0.2Er0.1Ru0.7O2−δ | 168 | 66.8 | [44] |
| SS Pt RuO2 HNSs | 228 | 51 | [45] |
| In-RuO2/G | 187 | 46.2 | [46] |
| Bi0.15Ru0.85O2 | 200 | 59.6 | [47] |
| La-RuO2 | 208 | 57.4 | [48] |
| Re0.06Ru0.94O2 | 190 | 45.5 | [49] |
| S-RuO2 | 219 | 54.2 | [50] |
| Ru0.6Sn0.4O2 | 245 | 61.8 | [51] |
| RuO2/CoOx | 240 | 70 | [52] |
| a/c-RuO2 | 205 | 48.6 | [53] |
| Ru@RuO2 | 198 | 42.6 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Zhou, W.; Xu, J.; Zhou, P.; Deng, Y.; Xiang, M.; Xiang, D.; Su, Y. RuO2 Catalysts for Electrocatalytic Oxygen Evolution in Acidic Media: Mechanism, Activity Promotion Strategy and Research Progress. Molecules 2024, 29, 537. https://doi.org/10.3390/molecules29020537
Bai J, Zhou W, Xu J, Zhou P, Deng Y, Xiang M, Xiang D, Su Y. RuO2 Catalysts for Electrocatalytic Oxygen Evolution in Acidic Media: Mechanism, Activity Promotion Strategy and Research Progress. Molecules. 2024; 29(2):537. https://doi.org/10.3390/molecules29020537
Chicago/Turabian StyleBai, Jirong, Wangkai Zhou, Jinnan Xu, Pin Zhou, Yaoyao Deng, Mei Xiang, Dongsheng Xiang, and Yaqiong Su. 2024. "RuO2 Catalysts for Electrocatalytic Oxygen Evolution in Acidic Media: Mechanism, Activity Promotion Strategy and Research Progress" Molecules 29, no. 2: 537. https://doi.org/10.3390/molecules29020537
APA StyleBai, J., Zhou, W., Xu, J., Zhou, P., Deng, Y., Xiang, M., Xiang, D., & Su, Y. (2024). RuO2 Catalysts for Electrocatalytic Oxygen Evolution in Acidic Media: Mechanism, Activity Promotion Strategy and Research Progress. Molecules, 29(2), 537. https://doi.org/10.3390/molecules29020537








