A Novel Label-Free Electrochemical Immunosensor for the Detection of Thyroid Transcription Factor 1 Using Ribbon-like Tungsten Disulfide-Reduced Graphene Oxide Nanohybrids and Gold Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
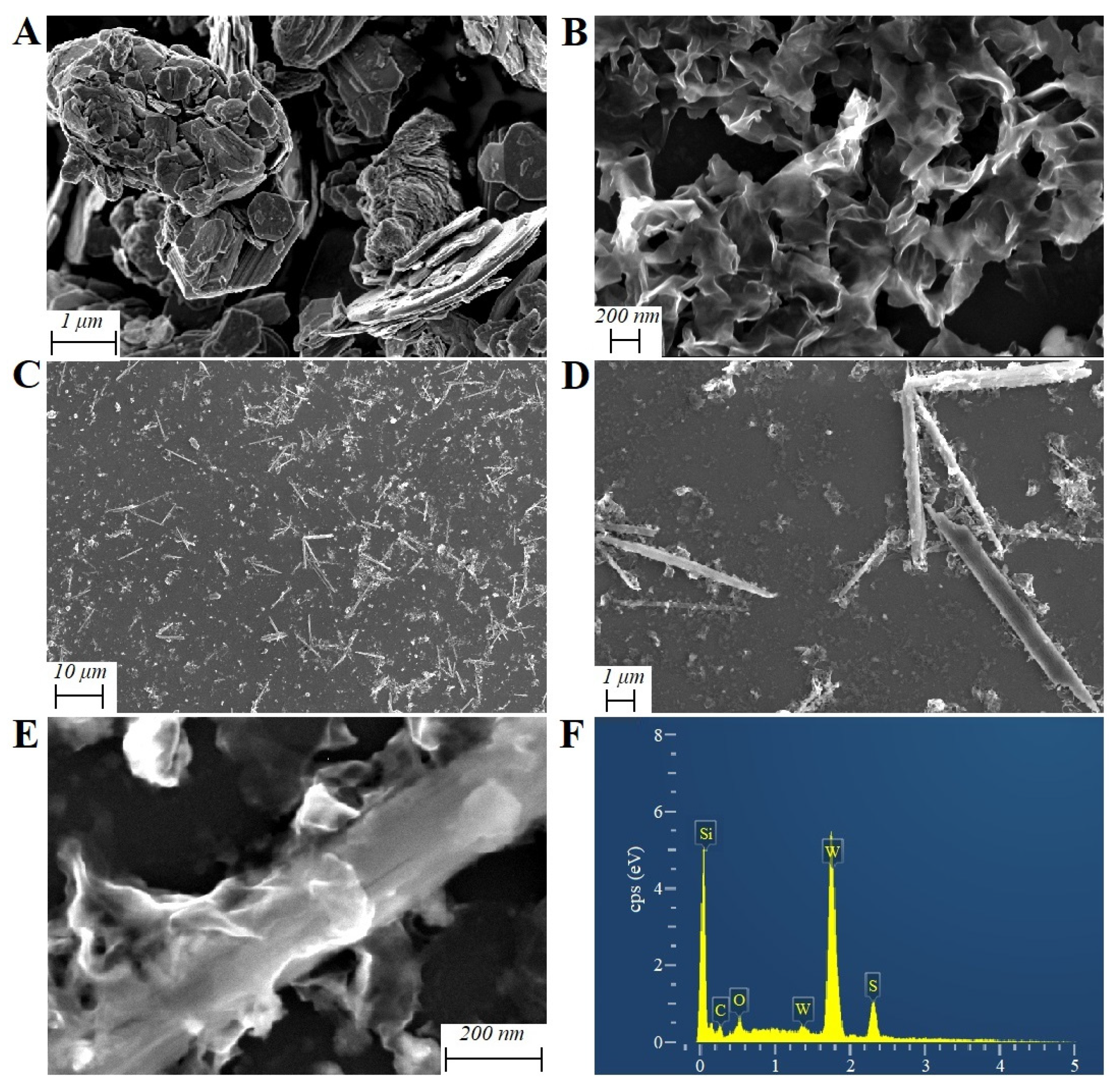
2.1. Characterization of the WS2-rGO Nanohybrids
2.2. Electrochemical Behavior of the Immunosensor
2.3. Optimization of Detection Conditions
2.4. Electrochemical Detection of TTF1 Antigens
2.5. Specificity, Reproducibility and Stability of the Immunosensor
2.6. Determination of TTF1 in Human Serum Sample
3. Materials and Methods
3.1. Apparatus and Reagents
3.2. Synthesis of Ribbon-like WS2-rGO Nanohybrids
3.3. Preparation of Electrochemical Immunosensor
3.4. Electrochemical Measurements of TTF1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, J.M.; Han, J.; Ahn, J.S.; Park, K.; Ahn, M.J. Significance of Thymidylate Synthase and Thyroid Transcription Factor 1 Expression in Patients with Nonsquamous Non-small Cell Lung Cancer Treated with Pemetrexed-Based Chemotherapy. J. Thorac. Oncol. 2011, 6, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- La Salvia, A.; Siciliani, A.; Rinzivillo, M.; Verrico, M.; Baldelli, R.; Puliani, G.; Modica, R.; Zanata, I.; Persano, I.; Fanciulli, G.; et al. Thyroid transcription factor-1 expression in lung neuroendocrine tumours: A gender-related biomarker? Endocrine 2023. [Google Scholar] [CrossRef] [PubMed]
- Schilsky, J.B.; Ni, A.; Ahn, L.; Datta, S.; Travis, W.D.; Kris, M.G.; Chaft, J.E.; Rekhtman, N.; Hellmann, M.D. Prognostic impact of TTF-1 expression in patients with stage IV lung adenocarcinomas. Lung Cancer 2017, 108, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Katzenstein, A.L.A. Comparison of Monoclonal Napsin A, Polyclonal Napsin A, and TTF-1 for Determining Lung Origin in Metastatic Adenocarcinomas. Am. J. Clin. Pathol. 2012, 138, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Porcel, J.M.; Palma, R.; Bielsa, S.; Esquerda, A.; Gatius, S.; Matias-Guiu, X.; Salud, A. TTF-1 and napsin A on cell blocks and supernatants of pleural fluids for labeling malignant effusions. Respirology 2015, 20, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Gao, Y.; Zhang, X.L.; Wang, H.M.; Xia, T.; Bian, C.C.; Liang, S.; Tang, X.Z.; Wang, X. Electrochemical immunosensor for HBe antigen detection based on a signal amplification strategy: The co-catalysis of horseradish peroxidase and nanoporous gold. Sens. Actuator B-Chem. 2019, 284, 296–304. [Google Scholar] [CrossRef]
- Kiio, L.K.; Onyatta, J.O.; Ndangili, P.M.; Oloo, F.; Santamaria, C.; Montuenga, L.M.; Mbui, D.N. Ultrasensitive immunosensor for multiplex detection of cancer biomarkers carcinoembryonic antigen (CEA) and yamaguchi sarcoma viral oncogene homolog 1 (YES1) based on eco-friendly synthesized gold nanoparticles. Talanta 2024, 266, 124934. [Google Scholar] [CrossRef]
- Braz, B.A.; Hospinal-Santiani, M.; Martins, G.; Gogola, J.L.; Valenga, M.G.P.; Beirao, B.C.B.; Bergamini, M.F.; Marcolino-Junior, L.H.; Thomaz-Soccol, V.; Soccol, C.R. Gold-binding peptide as a selective layer for electrochemical detection of SARS-CoV-2 antibodies. Talanta 2023, 257, 124348. [Google Scholar] [CrossRef]
- Zhu, X.D.; Shan, J.K.; Dai, L.; Shi, F.F.; Wang, J.S.; Wang, H.; Li, Y.Y.; Wu, D.; Ma, H.M.; Wei, Q.; et al. PB@PDA nanocomposites as nanolabels and signal reporters for separate-type cathodic photoelectrochemical immunosensors in the detection of carcinoembryonic antigens. Talanta 2023, 254, 124134. [Google Scholar] [CrossRef]
- Cancelliere, R.; Di Tinno, A.; Di Lellis, A.M.; Contini, G.; Micheli, L.; Signori, E. Cost-effective and disposable label-free voltammetric immunosensor for sensitive detection of interleukin-6. Biosens. Bioelectron. 2022, 213, 114467. [Google Scholar] [CrossRef]
- Yukird, J.; Chailapakul, O.; Rodthongkum, N. Label-free anti-Mullerian hormone sensor based on polyaniline micellar modified electrode. Talanta 2021, 222, 121561. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Electrochemical immunosensors for the detection of cytokine tumor necrosis factor alpha: A review. Talanta 2020, 211, 120758. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Rasheed, M.A.; Abbas, S.; Rehman, K.-U.; Shah, A.; Ullah, K.; Khan, Y.; Bibi, M.; Ahmad, M.; Ali, G. Electrochemical sensing of H2O2 using cobalt oxide modified TiO2 nanotubes. Curr. Appl. Phys. 2022, 38, 40–48. [Google Scholar] [CrossRef]
- Zribi, R.; Ferlazzo, A.; Fazio, E.; Condorelli, M.; D’Urso, L.; Neri, G.; Corsaro, C.; Neri, F.; Compagnini, G.; Neri, G. Ag Nanoplates Modified-Screen Printed Carbon Electrode to Improve Electrochemical Performances Toward a Selective H2O2 Detection. IEEE Trans. Instrum. Meas. 2023, 72, 1–8. [Google Scholar] [CrossRef]
- Dong, Y.F.; Qin, X.L.; Wang, M.H.; Gu, C.Y.; Zhu, Z.W.; Yang, D.; Shao, Y.H. Electrochemiluminescent Detection of Proteins Based on Fullerenols Modified Gold Nanoparticles and Triple Amplification Approaches. Anal. Chem. 2020, 92, 1890–1897. [Google Scholar] [CrossRef]
- Yan, Q.; Cao, L.L.; Dong, H.; Tan, Z.L.; Hu, Y.T.; Liu, Q.; Liu, H.; Zhao, P.P.; Chen, L.; Liu, Y.Y.; et al. Label-free immunosensors based on a novel multi-amplification signal strategy of TiO2-NGO/Au@Pd hetero-nanostructures. Biosens. Bioelectron. 2019, 127, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Suwannachat, J.; Saenchoopa, A.; Tun, W.S.T.; Patramanon, R.; Daduang, S.; Daduang, J.; Kulchat, S. An electrochemical AChE-based biosensor for organophosphate pesticides using a modified CuNWs/rGO nanocomposite on a screen-printed carbon electrode. Food Chem. 2024, 434, 137431. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Cao, B.Y.; An, X.W.; Yu, Z.L.; Zhao, W.D.; Su, F.C.; Guan, G.Q.; Zhang, Y.H.; Xie, Z.K.; Ye, B.X. Fabrication of nitrogen-carbon mediated γ-Mo2N nanocomposite based electrochemical sensor for rapid and sensitive determination of antioxidant 6-PPD in the environment. Talanta 2024, 266, 125072. [Google Scholar] [CrossRef] [PubMed]
- Sweety; Kumar, D. Electrochemical immunosensor based on titanium dioxide grafted MXene for EpCAM antigen detection. J. Colloid Interface Sci. 2023, 652, 549–556. [Google Scholar] [CrossRef]
- Zhu, J.; He, B.; Liu, Y.; Wang, Y.; Wang, J.; Liang, Y.; Jin, H.; Wei, M.; Ren, W.; Suo, Z.; et al. A novel magneto-mediated electrochemical biosensor integrated DNAzyme motor and hollow nanobox-like Pt@Ni-Co electrocatalyst as dual signal amplifiers for vanilla detection. Biosens. Bioelectron. 2023, 241, 115690. [Google Scholar] [CrossRef]
- Yan, J.H.; Wang, K.D.; Liu, H.J.; Wang, L.W.; Li, Y.X.; Zhang, G.Q.; Deng, L. Construction of electrochemical biosensors based on MoSe2@1T-MoS2 heterojunction for the sensitive and rapid detection of miRNA-155 biomarker in breast cancer. Bioelectrochemistry 2023, 154, 108541. [Google Scholar] [CrossRef]
- Hua, H.F.; Chen, B.B.; Ji, Z.H.; Wang, J. Sulfur-vacancy-enriched MoS2-CNTs with highly dispersed Au particles for sensitive dopamine detection. Appl. Surf. Sci. 2023, 639, 158244. [Google Scholar] [CrossRef]
- Yeh, Y.S.; Yen, Y.K.; Shanmugam, R. Tungsten disulfide nanotubes enhanced nanocomposite paper-based aptasensor for label-free electrochemical detection of interferon-gamma. Microchem. J. 2023, 193, 109081. [Google Scholar] [CrossRef]
- Kumar, S.R.S.; Rakhi, R.B.; Haritha, V.S. WS2-Nanosheet-Modified Electrodes as an Efficient Electrochemical Sensing Platform for the Nonenzymatic Detection of the Insecticide Imidacloprid. ACS Omega 2023, 8, 8695–8702. [Google Scholar]
- Zhou, L.Y.; Yan, S.C.; Lin, Z.X.; Shi, Y. In situ reduction of WS2 nanosheets for WS2/reduced graphene oxide composite with superior Li-ion storage. Mater. Chem. Phys. 2016, 171, 16–21. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Liu, Z.L.; Peng, W.X.; Parsaee, Z. Design, preparation and evaluation of a high performance sensor for formaldehyde based on a novel hybride nonocomposite ZnWO3/rGO. Anal. Chim. Acta 2019, 1051, 120–128. [Google Scholar] [CrossRef]
- Yildirim, M.; Boelukbas, O.S.; Ozer, Z.P.; Polat, I.; Atar, N.; Yola, M.L. L-Phenylalanine-Imprinted Electrochemical Sensor Based on WS2 Nanoflowers on N,B-Doped Graphene and Its Application to Milk Samples. Ind. Eng. Chem. Res. 2022, 62, 4587–4594. [Google Scholar]
- Chen, T.-W.; Rajaji, U.; Chen, S.-M.; Chinnapaiyan, S.; Ramalingam, R.J. Facile synthesis of mesoporous WS2 nanorods decorated N-doped RGO network modified electrode as portable electrochemical sensing platform for sensitive detection of toxic antibiotic in biological and pharmaceutical samples. Ultrason. Sonochem. 2019, 56, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Hong, J.B.; Zhong, W.X.; Wang, C.X.; Li, Z.F.; Dmytro, S. Auxiliary ball milling to prepare WS2/graphene nanosheets composite for lithium-ion battery anode materials. Tungsten 2023, 1–10. [Google Scholar] [CrossRef]
- Su, D.W.; Dou, S.X.; Wang, G.X. WS2@graphene nanocomposites as anode materials for Na-ion batteries with enhanced electrochemical performances. Chem. Commun. 2014, 50, 4192–4195. [Google Scholar] [CrossRef] [PubMed]
- Shelke, N.T.; Karche, B.R. Hydrothermal synthesis of WS2/RGO sheet and their application in UV photodetector. J. Alloys Compd. 2015, 653, 298–303. [Google Scholar] [CrossRef]
- Hussain, S.; Vikraman, D.; Sheikh, Z.A.; Mehran, M.T.; Shahzad, F.; Batoo, K.M.; Kim, H.S.; Kim, D.K.; Ali, M.; Jung, J.W. WS2-embedded MXene/GO hybrid nanosheets as electrodes for asymmetric supercapacitors and hydrogen evolution reactions. Chem. Eng. J. 2023, 452, 139523. [Google Scholar] [CrossRef]
- Ho, W.K.; Yu, J.C.; Lin, J.; Yu, J.G.; Li, P.S. Preparation and photocatalytic behavior of MoS2 and WS2 nanocluster sensitized TiO2. Langmuir 2004, 20, 5865–5869. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.X.; Liu, T.M.; Zeng, W.; Hussain, S.; Peng, X.H.; Pan, F.S. Synthesis and characterization of flower-like WS2 nanospheres via a facile hydrothermal route. J. Mater. Sci.-Mater. Electron. 2014, 25, 4300–4305. [Google Scholar] [CrossRef]
- Guillon, J.; Petit, C.; Moreau, M.; Toutain, B.; Henry, C.; Roche, H.; Bonichon-Lamichhane, N.; Salmon, J.P.; Lemonnier, J.; Campone, M.; et al. Regulation of senescence escape by TSP1 and CD47 following chemotherapy treatment. Cell Death Dis. 2019, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Carrascosa, L.G.; Wuethrich, A.; Mainwaring, P.; Trau, M. An exosomal- and interfacial-biosensing based strategy for remote monitoring of aberrantly phosphorylated proteins in lung cancer cells. Biomater. Sci. 2018, 6, 2336. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Mo, X. Expression and clinical significance of TTF-1 in the serum of lung adenocarcinoma patients. China Trop. Med. 2013, 13, 1525. [Google Scholar]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Wang, W.; Tang, H.; Wu, Y.; Zhang, Y.; Li, Z. Highly electrocatalytic biosensor based on Hemin@AuNPs/reduced graphene oxide/chitosan nanohybrids for non-enzymatic ultrasensitive detection of hydrogen peroxide in living cells. Biosens. Bioelectron. 2019, 132, 217–223. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Tang, H.; Zhou, L.; Li, Z. A Novel Label-Free Electrochemical Immunosensor for the Detection of Thyroid Transcription Factor 1 Using Ribbon-like Tungsten Disulfide-Reduced Graphene Oxide Nanohybrids and Gold Nanoparticles. Molecules 2024, 29, 552. https://doi.org/10.3390/molecules29020552
Wang W, Tang H, Zhou L, Li Z. A Novel Label-Free Electrochemical Immunosensor for the Detection of Thyroid Transcription Factor 1 Using Ribbon-like Tungsten Disulfide-Reduced Graphene Oxide Nanohybrids and Gold Nanoparticles. Molecules. 2024; 29(2):552. https://doi.org/10.3390/molecules29020552
Chicago/Turabian StyleWang, Wenjing, Huabiao Tang, Leiji Zhou, and Zhaohui Li. 2024. "A Novel Label-Free Electrochemical Immunosensor for the Detection of Thyroid Transcription Factor 1 Using Ribbon-like Tungsten Disulfide-Reduced Graphene Oxide Nanohybrids and Gold Nanoparticles" Molecules 29, no. 2: 552. https://doi.org/10.3390/molecules29020552
APA StyleWang, W., Tang, H., Zhou, L., & Li, Z. (2024). A Novel Label-Free Electrochemical Immunosensor for the Detection of Thyroid Transcription Factor 1 Using Ribbon-like Tungsten Disulfide-Reduced Graphene Oxide Nanohybrids and Gold Nanoparticles. Molecules, 29(2), 552. https://doi.org/10.3390/molecules29020552





