Efficient Degradation of Methylene Blue in Industrial Wastewater and High Cycling Stability of Nano ZnO
Abstract
1. Introduction
2. Results and Discussion
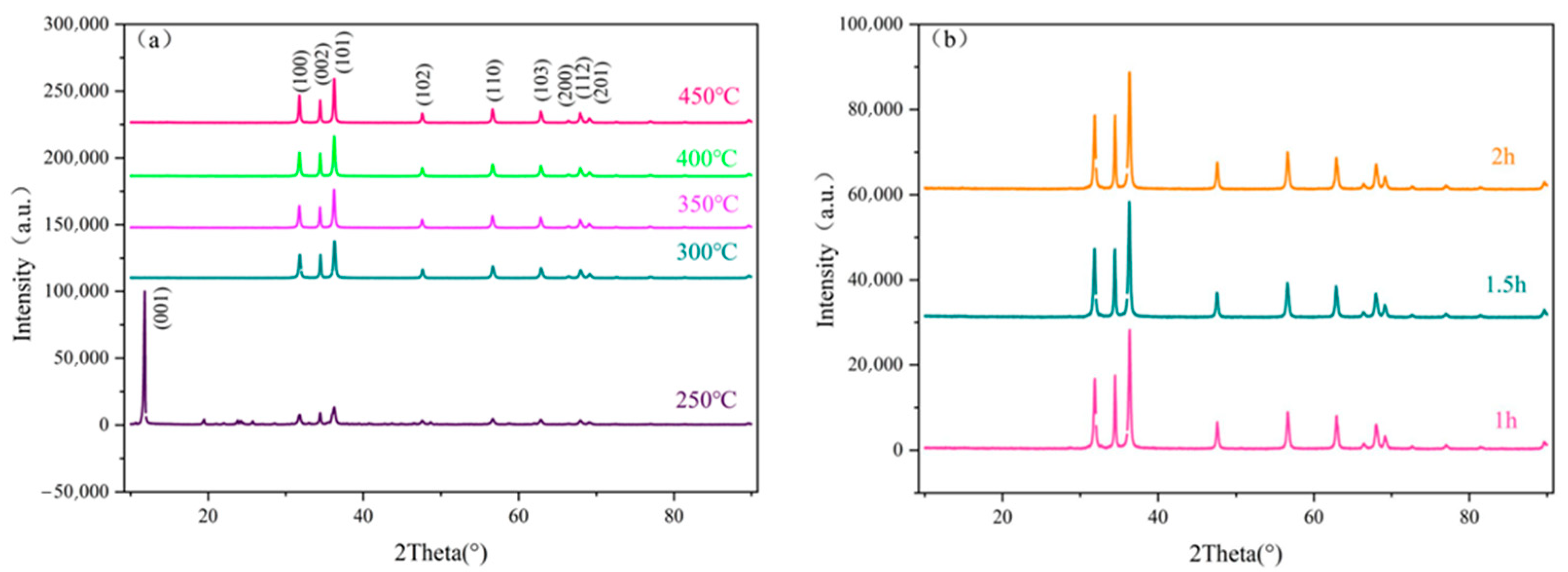
2.1. XRD Analysis
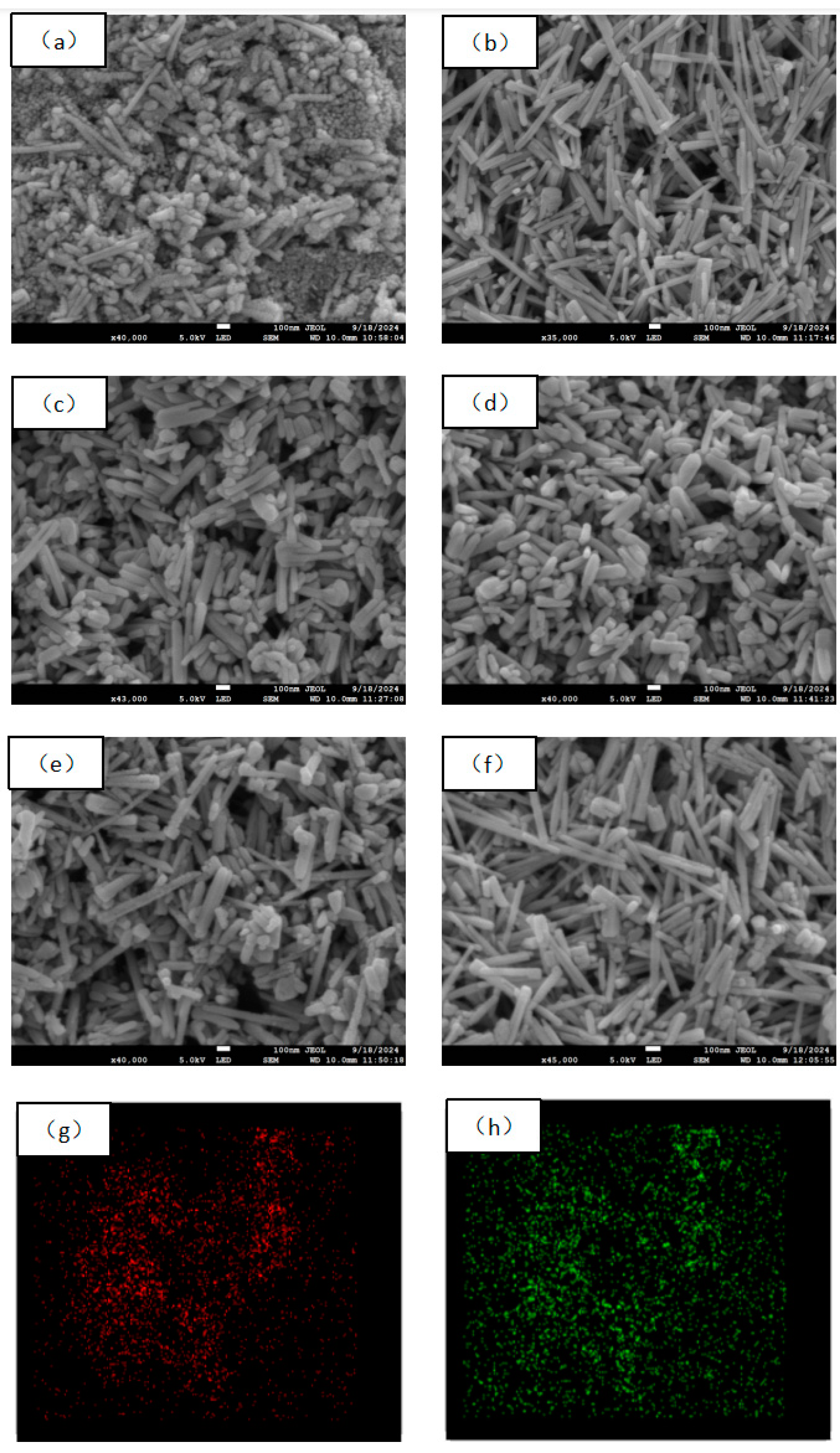
2.2. SEM and Mapping Analysis
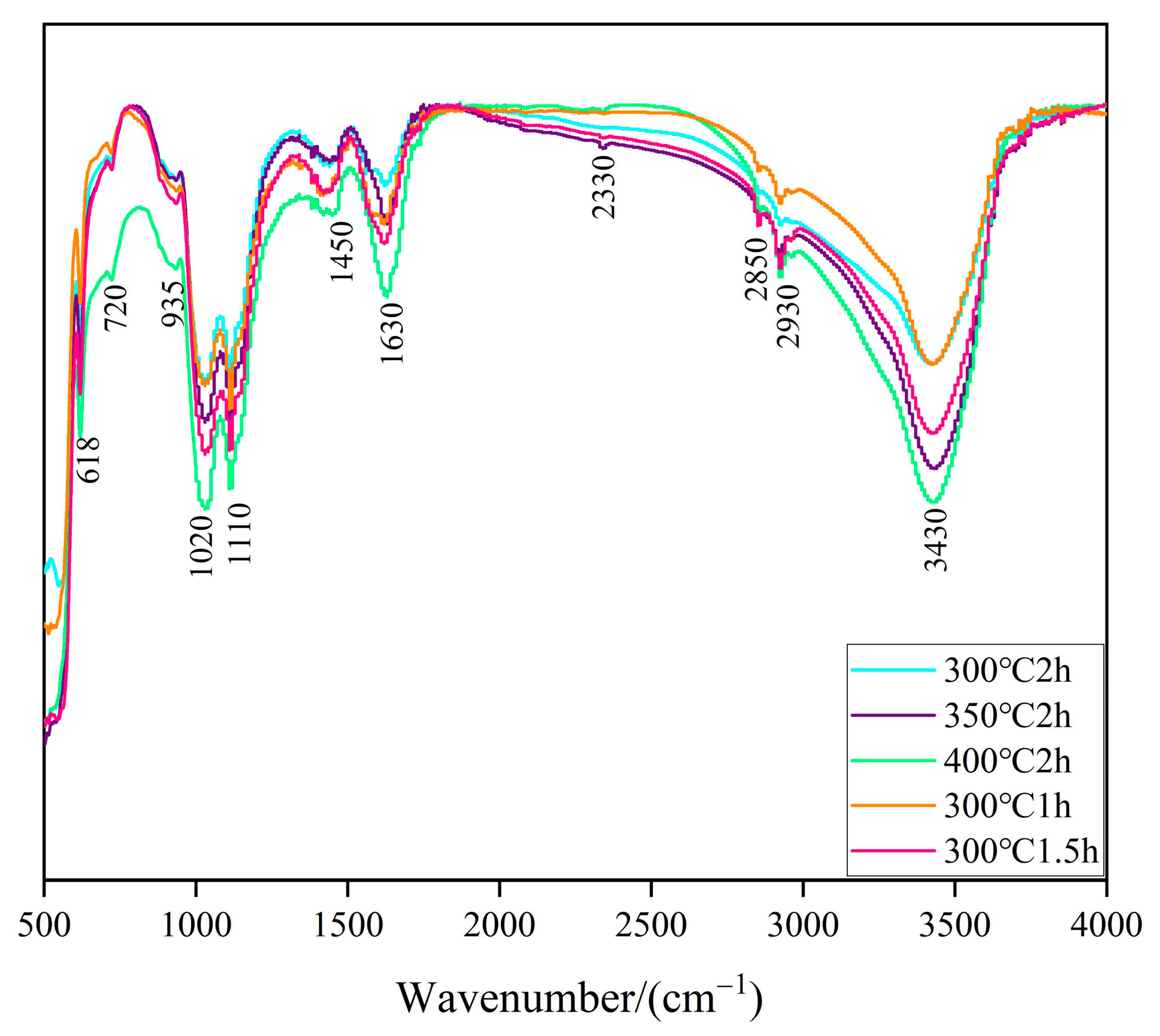
2.3. FTIR Analysis
2.4. Light Absorption Performance and Bandgap Analysis
2.5. XPS Analysis
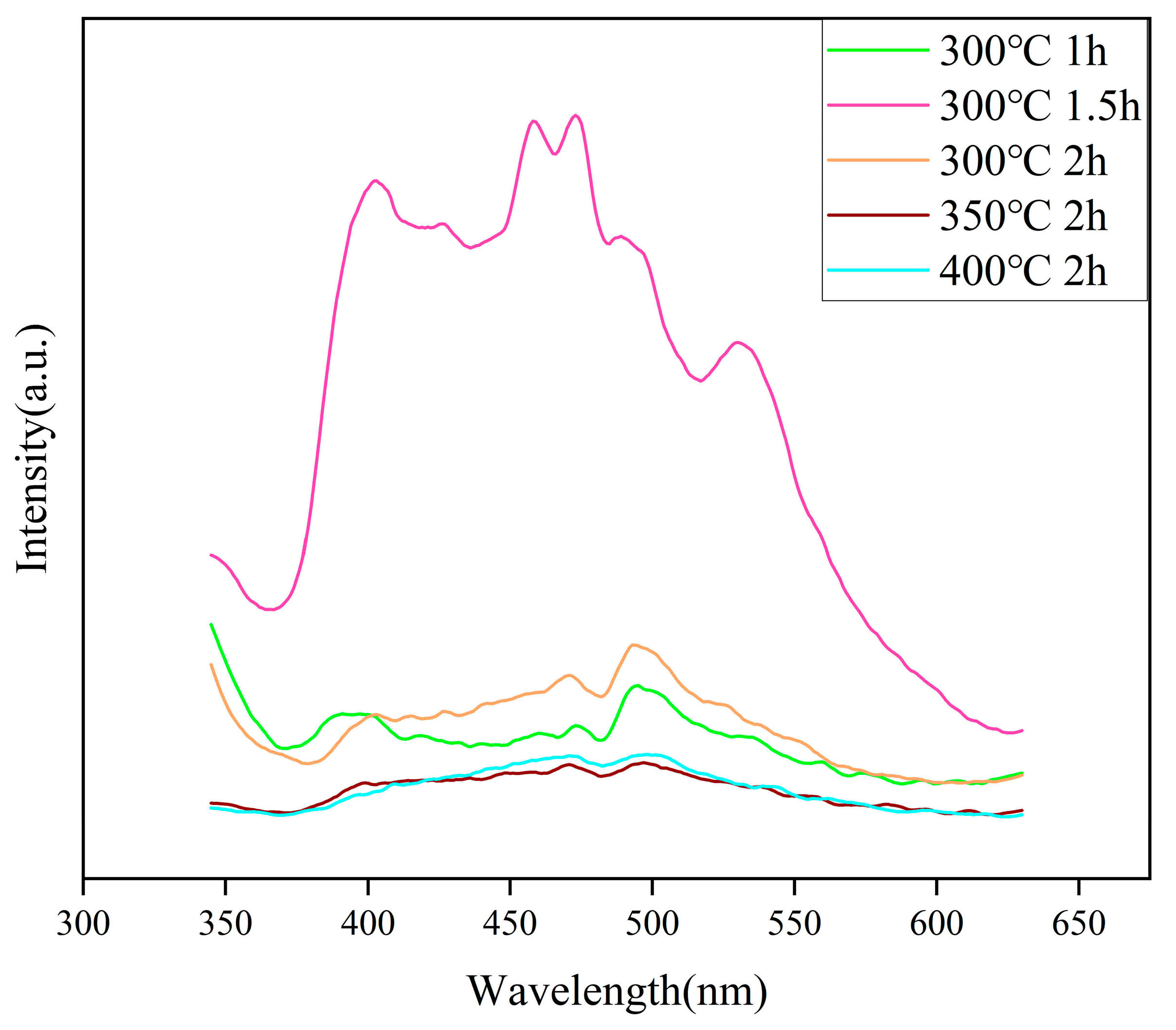
2.6. PL Analysis
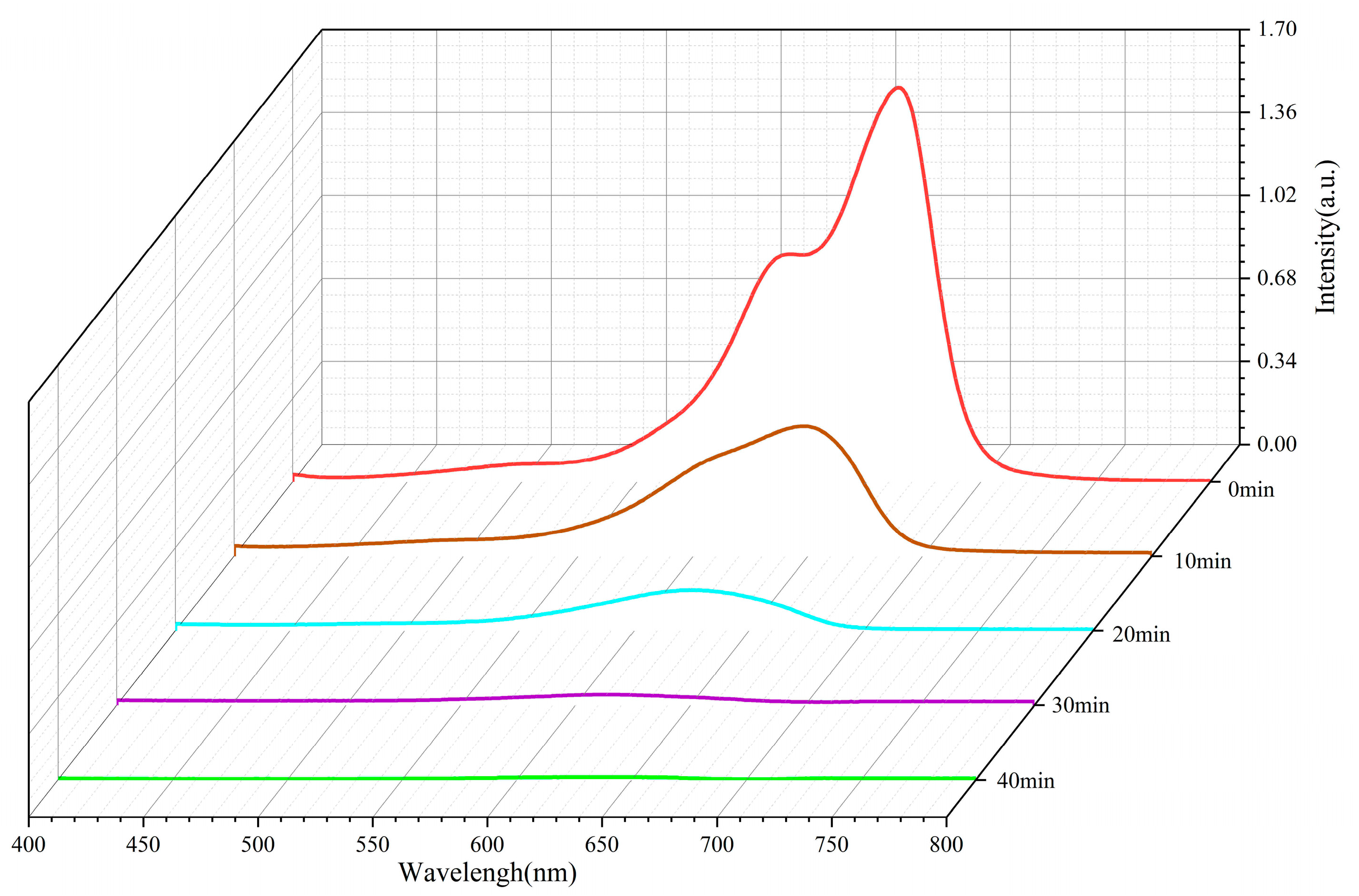
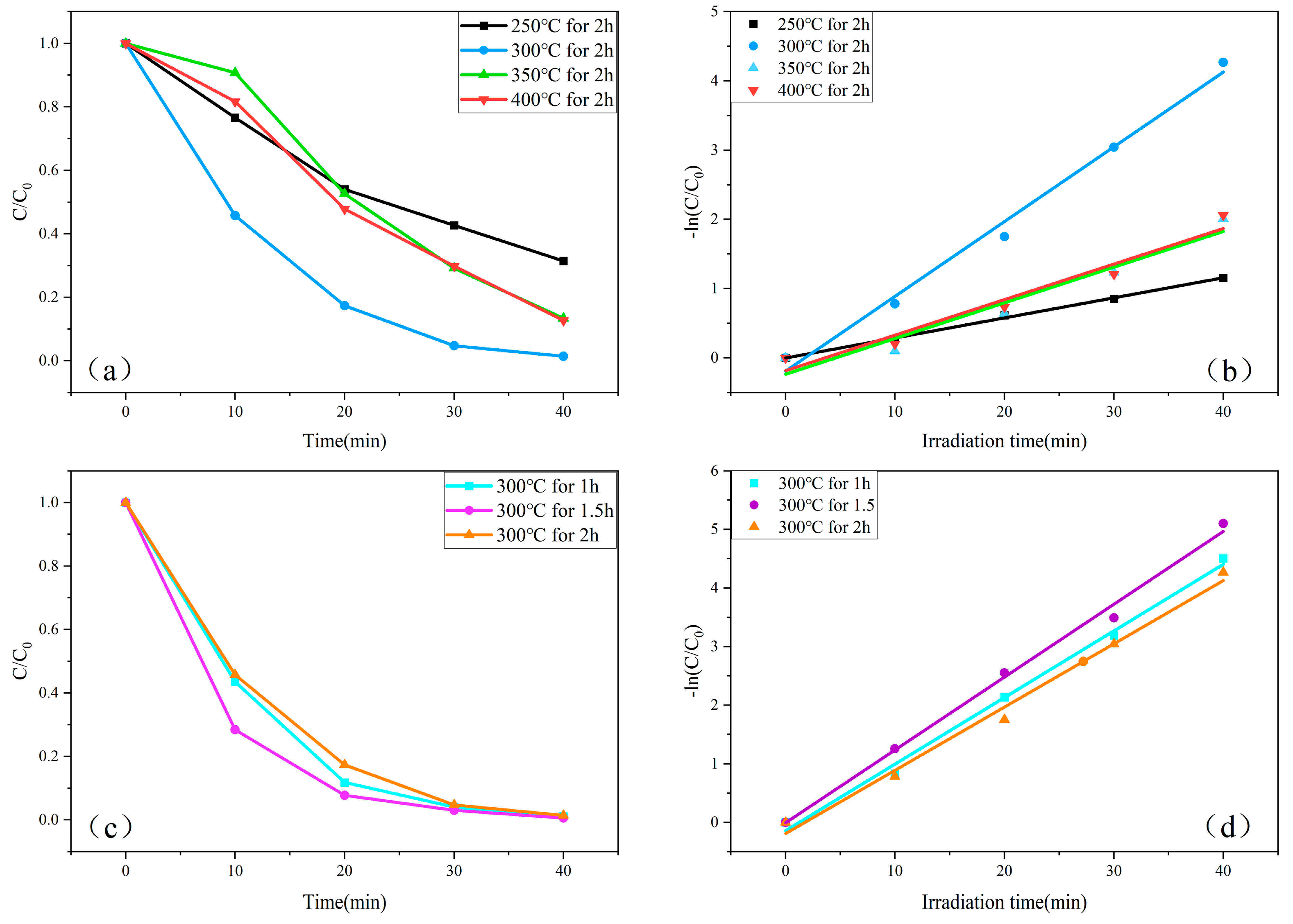
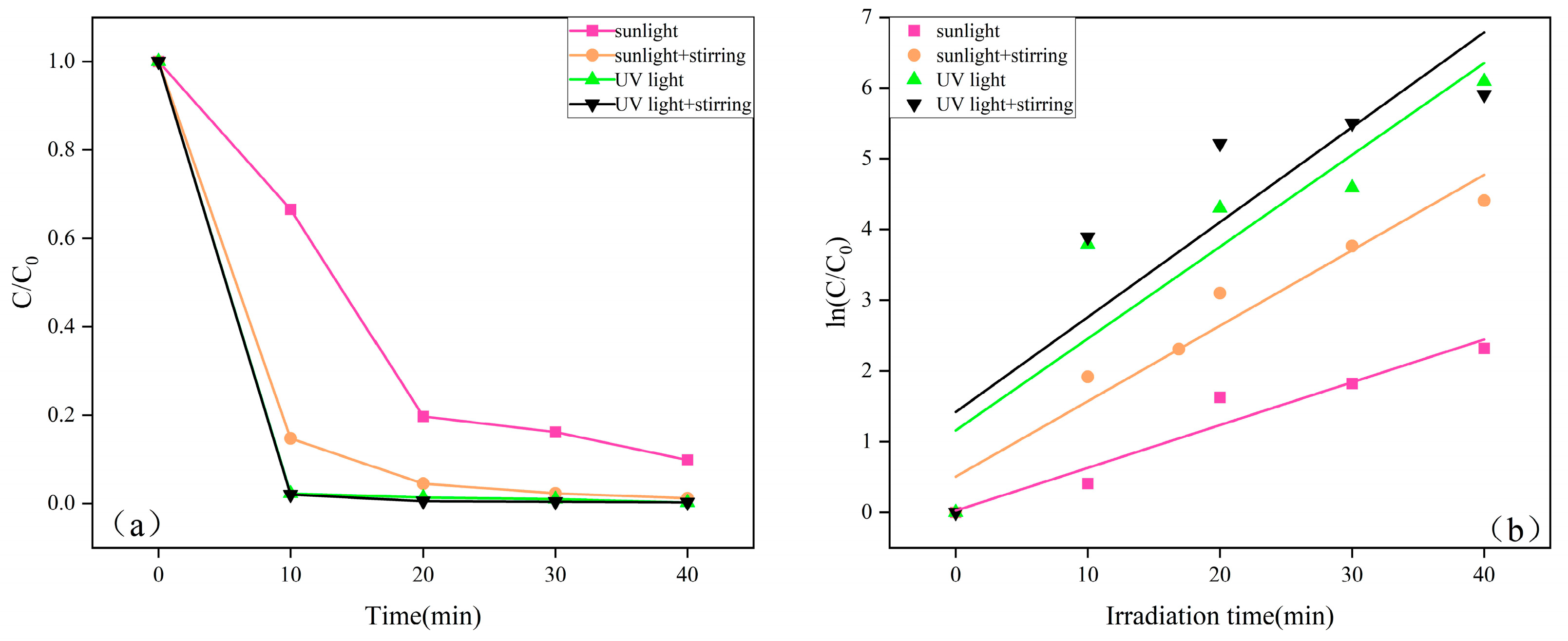
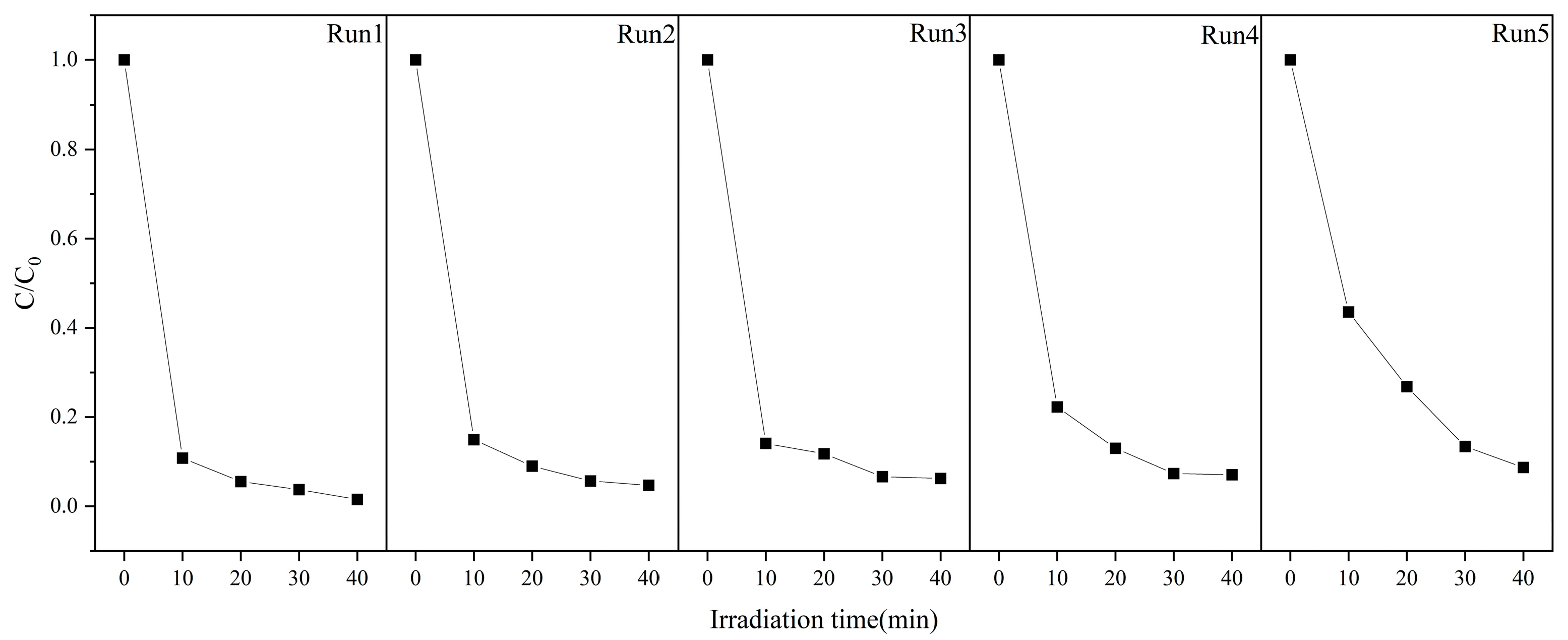
2.7. ZnO Photodegradable MB Performance
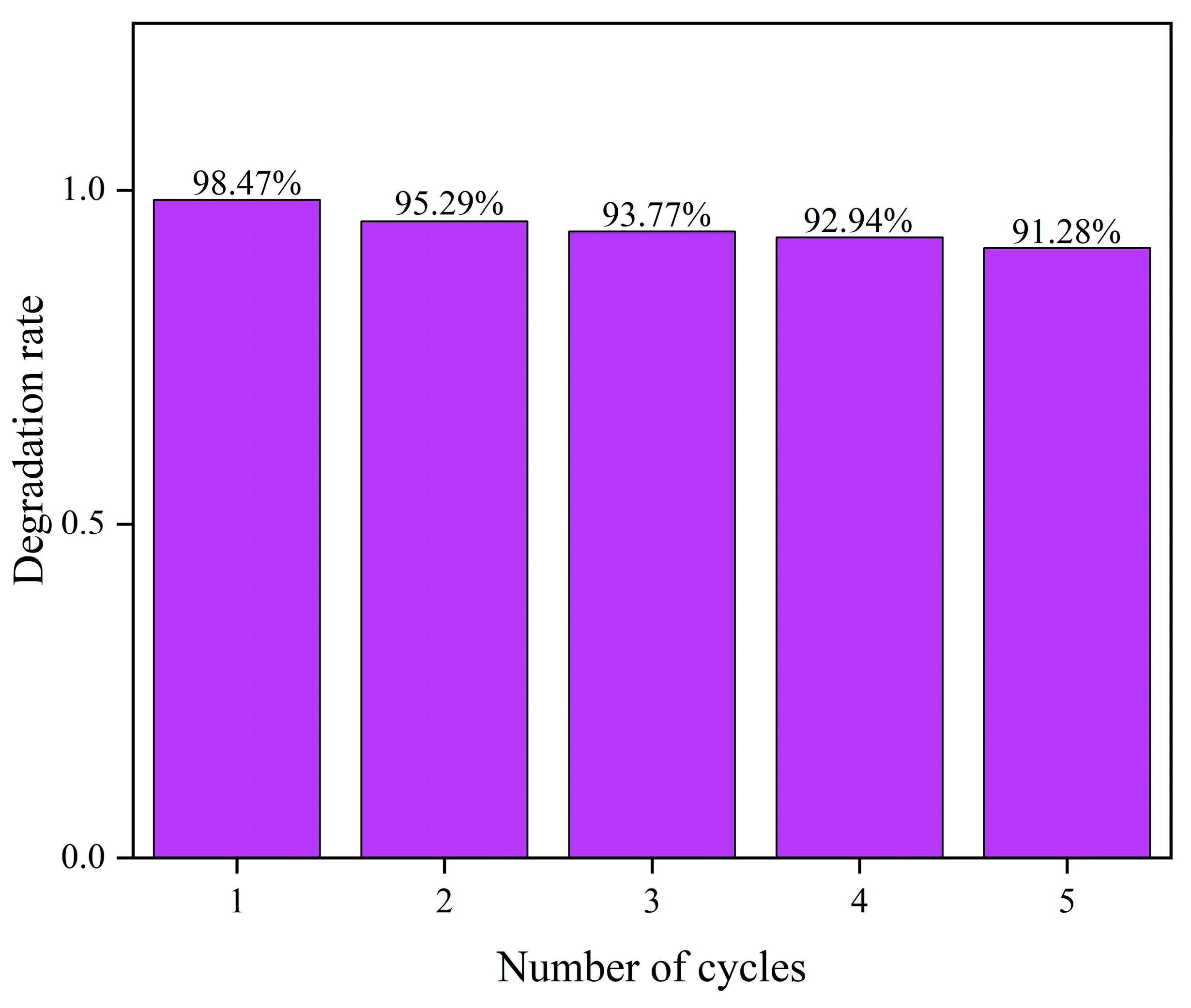
2.8. Catalyst Recycling and Stability Testing
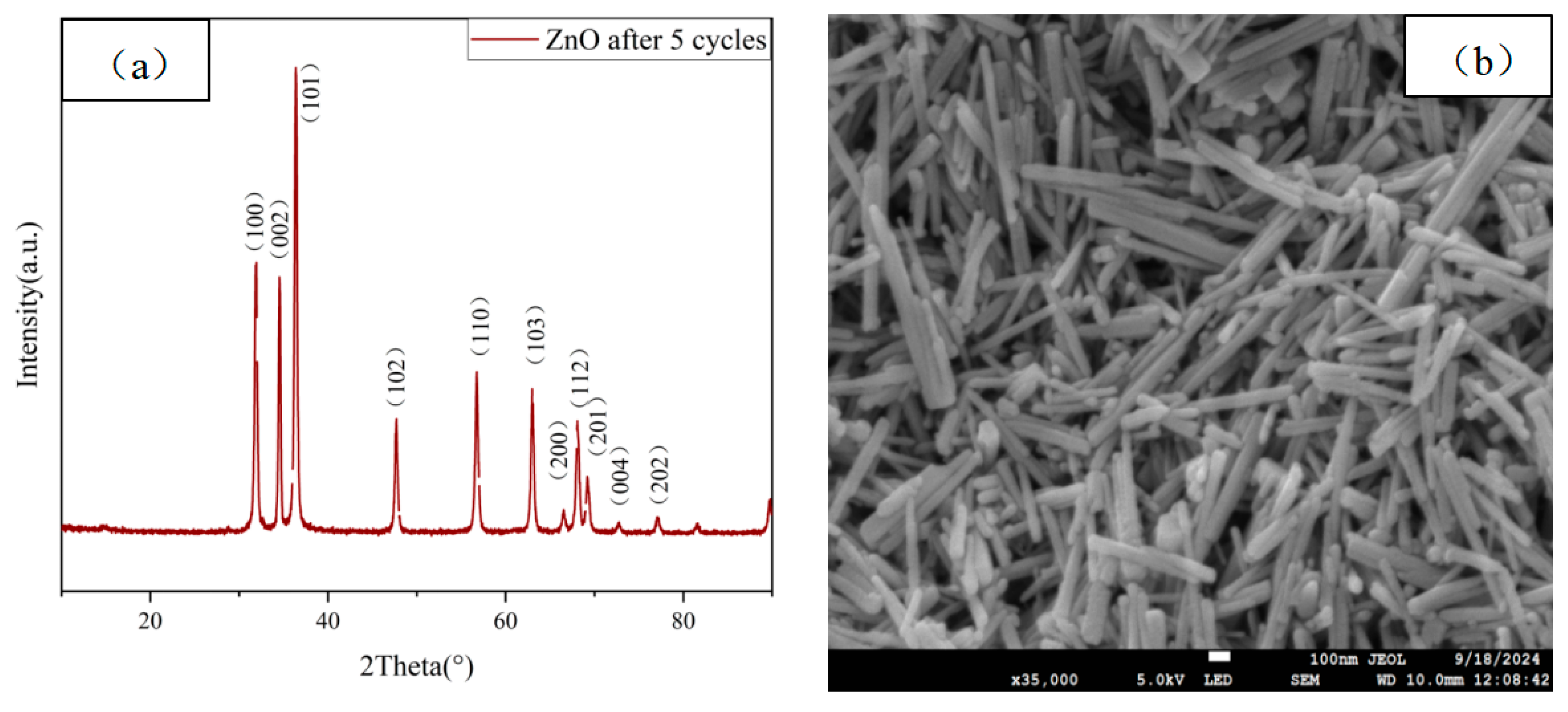
2.9. SEM and XRD After Recycling
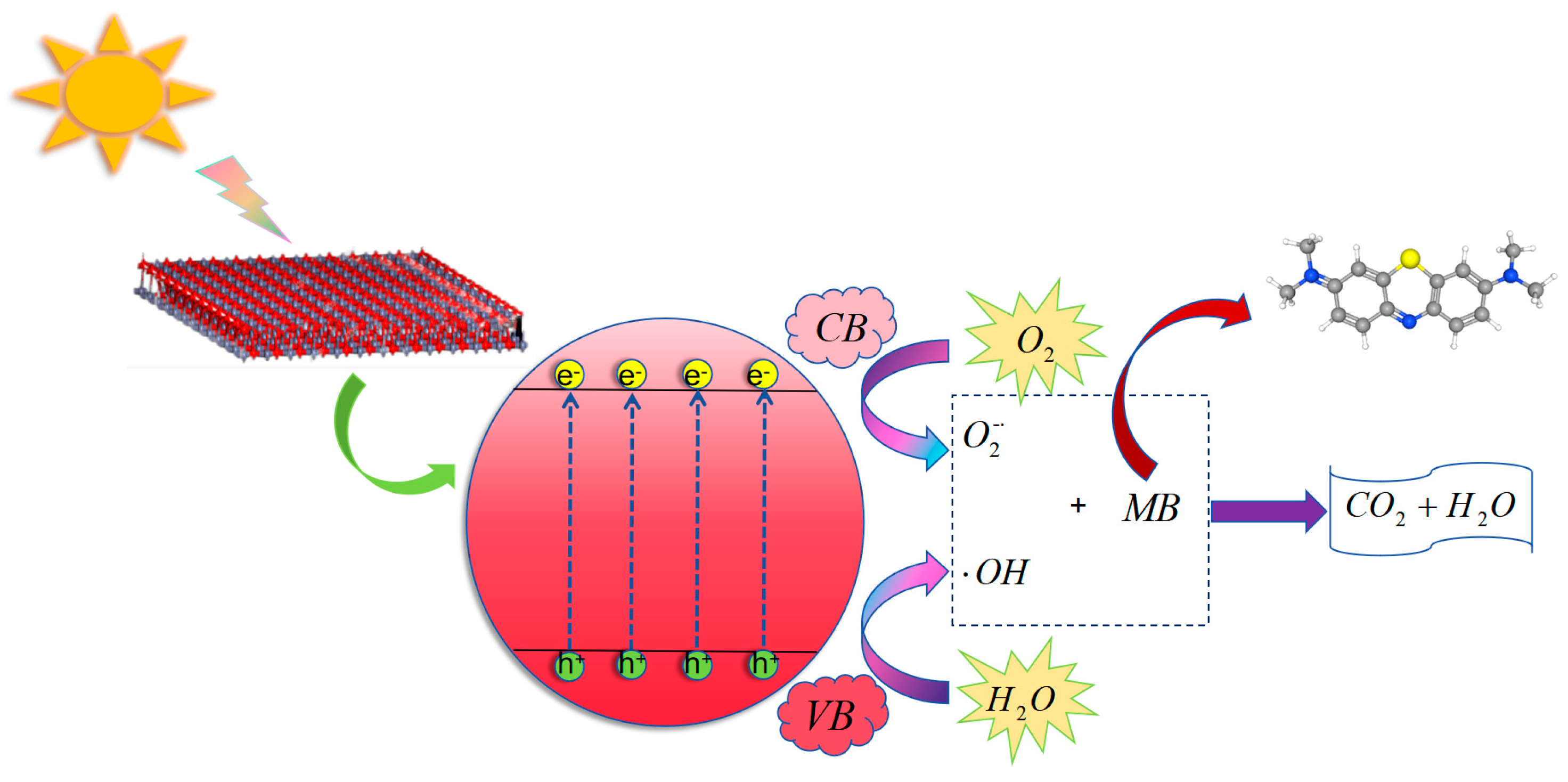
2.10. ZnO Photocatalytic Mechanism Diagram
3. Materials and Methods
3.1. Preparation of Zinc Oxide
3.2. Photocatalytic Degradation of Dyes
3.3. Catalyst Recycling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Gunten, U.V.; Wehrli, B. Global Water Pollution and Human Health. Soc. Sci. Electron. Publ. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Getnet, M.A.; Mekonnen, M.Y.; Yimam, H.M.; Berihun, A.M.; Malede, B.A. Histopathology based study of Nile tilapia fish (Oreochromis niloticus) as a biomarker for water pollution evaluation in the southern gulf of Lake Tana, Ethiopia. BMC Vet. Res. 2024, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Badawy, N.M.; Naguib, D.M. Polyphenol Oxidase from Agricultural Wastes for Dye Removal from Wastewater. Water Air Soil Pollut. 2024, 235, 331. [Google Scholar] [CrossRef]
- Khannyra, S.; Gil, M.L.A.; Addou, M.; Mosquera, M. Dye decomposition and air de-pollution performance of TiO2/SiO2 and N-TiO2/SiO2 photocatalysts coated on Portland cement mortar substates. Environ. Sci. Pollut. Res. Int. 2022, 29, 63112–63125. [Google Scholar] [CrossRef]
- He, L.; Michailidou, F.; Gahlon, H.L.; Zeng, W. Hair Dye Ingredients and Potential Health Risks from Exposure to Hair Dyeing. Chem. Res. Toxicol. 2022, 6, 35. [Google Scholar] [CrossRef]
- Dafale, N.; Agrawal, L.; Kapley, A.; Meshram, S.; Purohit, H.; Wate, S. Selection of indicator bacteria based on screening of 16S rDNA metagenomic library from a two-stage anoxic–oxic bioreactor system degrading azo dyes. Bioresour. Technol. 2010, 101, 476–484. [Google Scholar] [CrossRef]
- Abbas, M. Removal of methylene blue pollutant from the textile industry by adsorption onto Zeolithe: Kinetic and thermodynamic study. J. Eng. Fibers Fabr. 2022, 17, 1558925021993692. [Google Scholar] [CrossRef]
- Fito, J.; Abrham, S.; Angassa, K. Adsorption of Methylene Blue from Textile Industrial Wastewater onto Activated Carbon of Parthenium hysterophorus. Int. J. Environ. Res. Public Health 2020, 14, 501–511. [Google Scholar] [CrossRef]
- Mulushewa, Z.; Dinbore, W.T.; Ayele, Y. Removal of methylene blue from textile waste water using kaolin and zeolite-x synthesized from Ethiopian kaolin. Environ. Anal. Health Toxicol. 2021, 36, e2021007. [Google Scholar] [CrossRef]
- Kotkar, S.N.; Gadekar, G.P.; Singh, R.P.; Rewatkar, S.B. Solar light-driven photocatalytic decontamination of MB using Co and Cu doped ZnO with excellent antibacterial activity. Inorg. Chem. Commun. 2023, 156, 111197. [Google Scholar] [CrossRef]
- Ahmed, A.B.; Benhaliliba, M. Experimental characterization of ZnO properties and the impact of doping and DFT methods. Int. J. Mod. Phys. B 2024, 21, 38. [Google Scholar]
- Zhao, S.; Ma, H.; Wang, L.; Yang, L.; Cui, Y. Synthesis and luminescence properties of ZnO nanoneedle arrays via electrodeposited method. Surf. Rev. Lett. 2010, 17, 425–430. [Google Scholar] [CrossRef]
- Hariharalakshmanan, R.K.; Martinez, J.; Ergul-Yilmaz, B.; Karabacak, T. Suspension of ZnO Nanostructures Synthesized by Hot Water Treatment for Photocatalytic Wastewater Treatment. Water Air Soil Pollut 2023, 234, 209. [Google Scholar] [CrossRef]
- Tandorn, S.; Lamkhao, S.; Thiraphatchotiphum, C.; Rujijanagul, G.; Randorn, C. Fabrication of a bifunctionalized photocatalyst/hydrogel composite for the degradation of particulate matter (PM)-bound polycyclic aromatic hydrocarbons(PAHs). Chem. Eng. J. 2023, 457, 141–190. [Google Scholar] [CrossRef]
- Li, H.; Bi, F.; Li, Y.; Fu, X.; Zhou, B. Research Progress of Modified Zine Oxide Photocatalytic Nanomaterials. Technol. Dev. Chem. Ind. 2022, 51, 51–54. [Google Scholar]
- Wang, Y.; Zhao, X. Research Progress in Photocatalytic Properties of Metal-doped ZnO. Contemp. Chem. Ind. 2023, 52, 1691–1695. [Google Scholar]
- Leprince-Wang, Y. Advancements in ZnO-Based Photocatalysts for Water Treatment: A Comprehensive Review. Crystals 2024, 14, 611. [Google Scholar] [CrossRef]
- Folawewo, A.D.; Bala, M.D. Nanocomposite Zinc Oxide-Based Photocatalysts: Recent Developments in Their Use for the Treatment of Dye-Polluted Wastewater. Water 2022, 14, 3899. [Google Scholar] [CrossRef]
- Abdul, H.S.B.; Jyan, T.S.; Wei, L.C. Photocatalytic Water Oxidation on ZnO: A Review. Catalysts 2017, 7, 93. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Yang, L.; Wang, Z. Synthesis and luminescence properties of ZnO:Tb3+ nanotube arrays via electrodeposited method. Phys. B Condens. Matter 2010, 17, 425–430. [Google Scholar] [CrossRef]
- Wu, Z.; Li, F.; Li, C.; Zhu, W.J.; Fang, M. Preparation and Photocatalytic Properties of Different Morphological ZnO@ PANI Nanocomposites. Inorganica Chim. Acta 2013, 29, 8. [Google Scholar]
- Ali, H.; Guler, A.C.; Masar, M.; Antos, J.; Hanulikova, B.; Urbanek, P.; Yasir, M.; Sopik, T.; Machovsky, M.; Kuritka, I. Structural factors influencing photocatalytic and photoelectrochemical performance of low-dimensional ZnO nanostructures. Catalysis Today. 2025, 445, 115088. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Sugunan, A.; Guduru, V.K.; Uheida, A.; Toprak, M.S.; Muhammed, M. Radially Oriented ZnO Nanowires on Flexible Poly-L-Lactide Nanofibers for Continuous-Flow Photocatalytic Water Purification. J. Am. Ceram. Soc. 2010, 93, 3740–3744. [Google Scholar] [CrossRef]
- Zhao, S.; Shu, F.; Li, Y.; Liu, C.; Shan, W.; Cui, Y.; Yang, L. Synthesis and Luminescence Properties of ZnO:Eu3+ Nano Crystalline via a Facile Solution Method. J. Nanosci. Nanotechnol. 2012, 12, 2607–2611. [Google Scholar] [CrossRef]
- Güell, F.; Galdámez-Martínez, A.; Martínez-Alanis, P.R.; Catto, A.; Silva, L.F.D.; Mastelaro, V.; Santana, G.; Dutt, A. ZnO-based nanomaterials approach for photocatalytic and sensing applications: Recent Progress and Trends. Mater. Adv. 2023, 4, 6092. [Google Scholar] [CrossRef]
- Albiter, E.; Merlano, A.S.; Rojas, E.; José, M. Barrera-Andrade; Valenzuela, M.A. Synthesis, Characterization, and Photocatalytic Performance of ZnO–Graphene Nanocomposites: A Review. J. Compos. Sci. 2020, 5, 4. [Google Scholar] [CrossRef]
- Nakata, A.; Arai, H.; Murayama, H.; Fukuda, K.; Yamane, T.; Hirai, T.; Uchimoto, Y.; Yamaki, J.I.; Ogumi, Z. In situ Zn/ZnO mapping elucidating for “shape change” of zinc electrode. APL Mater. 2018, 6, 04770. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Huang, S. Study on Preparation of Nano-ZnO with Different Sizes and Their Ability to Resist Ultraviolet Ray. Guangdong Chem. Ind. 2018, 45, 3. [Google Scholar]
- Gu, G.; Zhang, Y.; Huang, C.; Sun, L.; Wang, X. Synthesis of ZnO Nanometer Powders Doped with Ce* lonsand the Photocatalytic Degradation of Dying Wastewater. J. Chang. Inst. Technol. 2014, 4, 55–61. [Google Scholar]
- Li, X.; Zhao, R.; Liu, C.; Gao, X.; Feng, J. Salt Cosolvent-Combustion Rapidly Synthesized Small-Size Rod Zinc Oxide. J. Petrochem. Univ. 2012, 25, 4. [Google Scholar]
- Jiang, Z.; Zhang, Y.; Zhang, L.; Cheng, B.; Wang, L. Effect of calcination temperatures on photocatalytic H2O2 -production activity of ZnO nanorods. Chin. J. Catal. 2022, 43, 226–233. [Google Scholar] [CrossRef]
- Yang, W.; Tang, J.; Ou, Q.; Liu, L.; Liu, S.; Chen, H.; Liu, Y. High sensing performance toward acetone vapor using TiO2 flower-like nanomaterials. Nanoscale Res. Lett. 2022, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Xia, Y.; Zhai, H.; Gao, Y.; Li, S.; Lu, C. Preparation by Co-precipitation Method and PhotocatalyticPerformances on the Degradation of Dyes of Ce:-Doped Nano-ZnO. Inorganica Chim. Acta 2020, 36, 13. [Google Scholar]
- Behrens, M.; Lolli, G.; Muratova, N.; Kasatkin, I.; Hävecker, M.; Raoul, N.; Storcheva, O.; Köhler, K.; Muhler, M.; Schlögl, R. The effect of Al-doping on ZnO nanoparticles applied as catalyst support. Phys. Chem. Chem. Phys. 2012, 15, 629. [Google Scholar] [CrossRef]
- Masood, A.; Iqbal, T.; Ashraf, M.; Nazir, A.; Ali, F.; Ranjha, Q.A.; Hussain, M.; Al-Harbi, F.F.; Galal, A.M. Synthesis and Characterization of Cadmium Doped Zinc Oxide Nanoparticles for Visible Light Driven Catalytic Removal of MB and RhB Dye: Experimental and Computational Analysis. J. Inorg. Organomet. Polym. Mater. 2023, 33, 1841–1854. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Jha, S.; Tripathi, S.K.; Shukla, R.; Awasthi, R.R.; Bhardwaj, A.K.; Singh, A.K.; Dikshit, A. Spectroscopic investigations of green synthesized zinc oxide nanoparticles (ZnO NPs): Antioxidant and antibacterial activity. Discov. Appl. Sci. 2024, 6, 8. [Google Scholar] [CrossRef]
- Khan, M.; Nowsherwan, G.A.; Ali, R.; Ahmed, M.; Anwar, N.; Riaz, S.; Faroog, A.; Hussain, S.; Naseem, S.; Choj, J. Investigation of Photoluminescence and Optoelectronics Properties of Transition Metal-Doped ZnO Thin Films. Molecules 2023, 28, 7963. [Google Scholar] [CrossRef]
- Song, T.; Liu, L.; Xu, F.; Pan, Y.T.; Qian, M.; Li, D.; Yang, R. Multi-dimensional characterizations of washing durable ZnO/phosphazene-siloxane coated fabrics via ToF-SIMS and XPS. Polym. Test. 2022, 114, 0142–9418. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Yang, D.; Liu, J.; He, L.; Tang, M.; Feng, W.; Wu, X. Fabrication, characterization and high photocatalytic activity of Ag–ZnO heterojunctions under UV-visible light. RSC Adv. 2021, 11, 27257–27266. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ou, Q.; Li, C.; Cheng, M.; Li, W.; Liu, Y. Ultrasensitive flower-like TiO2/Ag substrate for SERS detection of pigments and melamine. R. Soc. Chem. 2022, 12, 6958–6965. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, X.; Zhou, S.; Liu, S.; Jia, D. Controllable Synthesis and Photocatalytic Mechanism of Spherical and Flower-like ZnO Nanostructures. Inorganica Chim. Acta 2016, 32, 9. [Google Scholar]
- Ilyas, U.; Rawat, R.S.; Roshan, G.; Tan, T.L.; Lee, P.; Springham, S.V.; Chen, R.; Sun, H.D.; Li, F.; Zhang, S. Structural and photoluminescence study of zinc oxide thin films grown by laser induced plasma. In Proceedings of the International Conference on the Frontiers of Plasma Physics and Technology, Singapore, 18–22 April 2011. [Google Scholar]
- Malyutina-Bronskaya, V.; Saad, A.M.; Zalesski, V.; Leonova, T.; Mudryi, A.; Fedorov, V. Morphological, structural and room temperature optical properties of ZnO:Eu layers deposited by RF-Sputtering. Opt. Mater. 2019, 88, 718–722. [Google Scholar] [CrossRef]
- Martins, D.; Santos, D.A.; Macêdo, M.A. Intra-4f transitions-induced red emission in ZnO-Eu2O3 ceramic. Radiat. Phys. Chem. 2021, 183, 109392. [Google Scholar] [CrossRef]
- Kamalian, P.; Khorasani, S.N.; Abdolmaleki, A.; Karevan, M.; Khalili, S.; Shirani, M.; Neisiany, R.E. Toward the development of polyethylene photocatalytic degradation. J. Polym. Eng. 2020, 40, 181–191. [Google Scholar] [CrossRef]
- Mubeen, K.; Safeen, K.; Irshad, A.; Safeen, A.; Ghani, T.; Shah, W.; Khan, R.; Ahmad, K.; Casin, R.; Rashwan, M.; et al. ZnO/CuSe composite-mediated bandgap modulation for enhanced photocatalytic performance against methyl blue dye. Sci. Rep. 2023, 13, 19580. [Google Scholar] [CrossRef]
- He, D.; Tan, Z.; Tian, Q. Characterization, Photocatalytic Property and Kineties of ZnO Nanoparticles Synthesized by One Step Solid State Reaction. Inorganica Chim. Acta 2017, 33, 9. [Google Scholar]
- Zhan, Y.; Liu, T.; Wang, T. Preparation of Nano Ag Loaded Wood Filter Material and Its Catalytic Degradation of Dyes. J. Phys. Conf. Ser. 2023, 2463, 012051. [Google Scholar] [CrossRef]
- Aadnan, I.; Zegaoui, O.; El Mragui, A.; Daou, L.; Moussout, H.; da Esteves, S. Structural, Optical and Photocatalytic Properties of Mn Doped ZnO Nanoparticles Used as Photocatalysts for Azo-Dye Degradation under Visible Light. Catalysts 2022, 12, 1382. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Lou, J.; Huang, Y.; Peng, J.; Li, Y.; Liu, Y. Enhance ZnO Photocatalytic Performance via Radiation Modified g-C3N4. Molecules 2022, 27, 8476. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cai, H.; Meng, J.; Huang, Q. Preparation of ZnO nanorods by thermal deposition of ZF-8 and their photocatalytic properties. Appl. Chem. Ind. 2023, 52, 1395–1398. [Google Scholar]
- Su, B.; Hu, C.; Zuo, X.; Lei, Z. Preparation of ZnO Nanoparticles and Their Catalytic Property under Solar Light. Inorganica Chim. Acta 2010, 26, 96–100. [Google Scholar]
- Wang, Z.; Wang, J.; Wang, C.; You, T.; Jin, X.; Chang, J. Study on Preparation and Photocatalytic Performance of Flower-like Zinc Oxide. Contemp. Chem. Ind. 2022, 51, 2278–2281. [Google Scholar]
- Hu, J.; Ding, J.; Ai, J.; Li, H.; Xu, X. Room temperature growth of ZnO with highly active exposed facets for photocatalytic application. Nanotechnol. Rev. 2021, 10, 919–932. [Google Scholar] [CrossRef]
- Wu, D.; Fan, X.; Dai, J.; Liu, H.; Liu, H.; Zhang, F. Preparation and Photocatalytic Properties of Cu2S/Tetrapod-Like ZnO Whisker Nanocomposites. Chin. J. Catal. 2012, 33, 802–807. [Google Scholar] [CrossRef]
- Yan, X.; Yi, C.; Wang, Y.; Cao, W.; Mao, D.; Ou, Q.; Shen, P.; Wang, H. Multi-catalysis of nano-zinc oxide for bisphenol A degradation in a dielectric barrier discharge plasma system: Effect and mechanism. Sep. Purif. Technol. 2020, 231, 115897. [Google Scholar] [CrossRef]
- Lad, P.; Pathak, V.; Thakka, A.B.; Thakor, P.; Deshpande, M.P.; Pandya, S. ZnO Nanoparticles Synthesized by Precipitation Method for Solar-Driven Photodegradation of Methylene Blue Dye and Its Potential as an Anticancer Agent. Braz. J. Phys. 2023, 53, 63. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; Kalpana, V.N.; Pugazhendhi, A. Enhanced photocatalytic degradationof water pollutants using bio-green synthesis of zinc oxide nanoparticles (ZnONPs). J. Environ. Chem. Eng. 2021, 9, 105772. [Google Scholar] [CrossRef]
- Sadiq, H.; Sher, F.; Sehar, S.; Lima, E.; Zhang, S.; Iqbal, H.; Zafar, F.; Nuhanović, M. Green synthesis of ZnO nanoparticles from Syzygium Cumini leavesextract with robust photocatalysis applications. J. Mol. Liq. 2021, 335, 116567. [Google Scholar] [CrossRef]
- Aldeen, T.S.; Mohamed, H.E.A.; Maaza, M. ZnO nanoparticles prepared via a greensynthesis approach: Physical properties, photocatalytic and antibacterial activity. J. Phys. Chem. Solids 2022, 160, 110313. [Google Scholar] [CrossRef]
- Kamarajan, G.; Anburaj, B.; Porkalai, V.; Muthuvel, A.; Nedunchezhian, G. Green synthesis of ZnO nanoparticles and their photocatalystdegradation and antibacterial activity. J. Water Environ. Nanotechnol. 2022, 7, 180–193. [Google Scholar]
- Wijesinghe, U.; Thiripuranathar, G.; Menaa, F.; Iqbal, H.; Razzaq, A.; Almukhlifi, H. Green synthesis, structural characterization and photocatalytic applications of ZnCnanoconjugates using Heliotropium indicum. Catalysts 2021, 11, 831. [Google Scholar] [CrossRef]
- Osuntokun, J.; Onwudiwe, D.C.; Ebenso, E.E. Green synthesis of ZnO nanoparticlesusing aqueous Brassica oleracea L, var, italica and the photocatalytic activity. Green Chem. Lett. Rev. 2019, 12, 444–457. [Google Scholar] [CrossRef]
- Raja, A.; Ashokkumar, S.; Marthandam, R.P.; Jayachandiran, J.; Khatiwada, C.P.; Kaviyarasu, K.; Ganapathi Raman, R.; Swaminathan, M. Eco-friendly preparation of zincoxide nanoparticles using Tabernaemontana diyaricata and its photocatalytic andantimicrobial activity. J. Photochem. Photobiol. B Biol. 2018, 181, 53–58. [Google Scholar] [CrossRef]
- Venkatesan, S.; Suresh, S.; Ramu, P.; Kandasamy, M.; Arumugam, J.; Thambidurai, S.; Prabu, K.M.; Pugazhenthiran, N. Biosynthesis of zinc oxide nanoparticles usingEuphorbia milii leaf constituents: Characterization and improved photocatalyticdegradation of methylene blue dye under natural sunlight. J. Indian Chem. Soc. 2022, 99, 100436. [Google Scholar] [CrossRef]
- Suresh, S.; Thambidurai, S.; Arumugam, J.; Kandasamy, M.; Pugazhenthiran, N.; Balaji, D.; Al-Asbahi, B.A.; Reddy, N.R.; Arunkumar, A.; Muneeswaran, T. Antibacterial activity and photocatalytic oxidative performance of zinc oxidenanorods biosynthesized using Aerya lanata leaf extract. Inorg. Chem. Commun. 2022, 139, 109398. [Google Scholar] [CrossRef]
- Pai, S.; Sridevi, H.; Varadavenkatesan, T.; Vinayagam, R.; Selvaraj, R. Photocatalyticzinc oxide nanoparticles synthesis using Peltophorum pterocarpum leaf extract andtheir characterization. Optik 2019, 185, 248–255. [Google Scholar] [CrossRef]
- Kahsay, M.H. Synthesis and characterization of ZnO nanoparticles using aqueousextract of Becium grandiforum for antimicrobial activity and adsorption ofmethylene blue. Appl. Water Sci. 2021, 11, 45. [Google Scholar] [CrossRef]
- Soto-Robles, C.A.; Nava, O.; Cornejo, L.; Lugo-Medina, E.; Vilchis-Nestor, A.R.; Castro-Beltran, A.; Luque, P.A. Biosynthesis, characterization and photocatalytic activity of ZnO nanoparticles using extracts of Justicia spicigera for the degradationof methylene blue. J. Mol. Struct. 2021, 1225, 129101. [Google Scholar] [CrossRef]
- Venkatesan, S.; Suresh, S.; Ramu, P.; Arumugam, J.; Thambidurai, S.; Pugazhenthiran, N. Methylene blue dye degradation potential of zinc oxide nanoparticles bioreduced using Solanum trilobatum leaf extract. Results Chem. 2022, 4, 100637. [Google Scholar] [CrossRef]
- Yu, H.; Zou, H.; Lei, S.; Liu, Y.; Ma, L. Preparation of ZnO nanostructure via SDS assistedmicrowave hydrothermal method. J. Bohai Univ. (Nat. Sci. Ed.) 2022, 43, 217–223. [Google Scholar]
- Albiss, B.; Abu-Dalo, M. Photocatalytic Degradation of Methylene Blue Using Zinc Oxide Nanorods Grown on Activated Carbon Fibers. Sustainability 2021, 13, 4729. [Google Scholar] [CrossRef]
- Mohamed, K.M.; Benitto, J.J.; Vijaya, J.J.; Bououdina, M. Recent Advances in ZnO-Based Nanostructures for the Photocatalytic Degradation of Hazardous, Non-Biodegradable Medicines. Crystals 2023, 13, 329. [Google Scholar] [CrossRef]
- Gu, L. Preparation of Ag loaded ZnO composite and its application to photocatalytic degradation of Methylene Blue. China Dye. Finish. 2019, 45, 5. [Google Scholar]
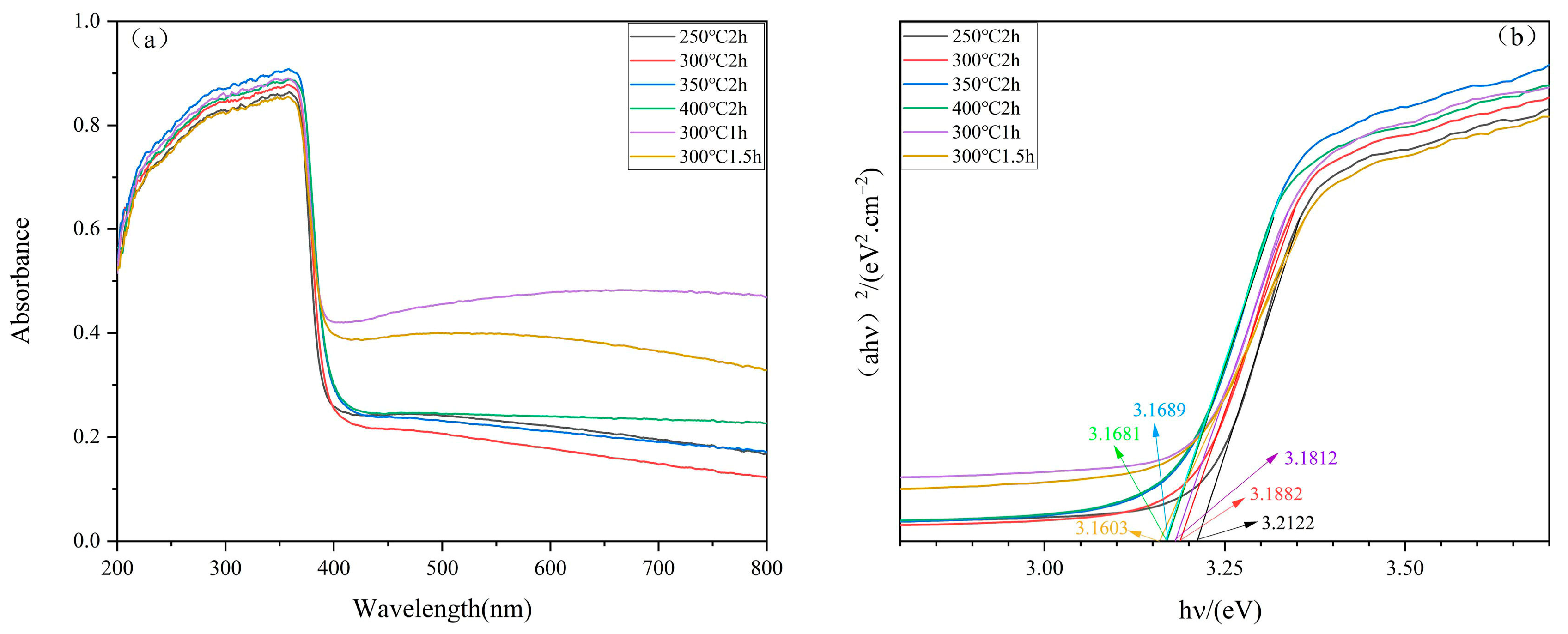


| Terms | Eg/eV |
|---|---|
| 250 °C 2 h | 3.2122 |
| 300 °C 2 h | 3.1882 |
| 350 °C 2 h | 3.1689 |
| 400 °C 2 h | 3.1681 |
| 300 °C 1 h | 3.1812 |
| 300 °C 1.5 h | 3.1603 |
| Terms | Degradation Rate | ka/min−1 | R2 |
|---|---|---|---|
| 250 °C 2 h | 83.15% | 0.05154 | 0.94598 |
| 300 °C 2 h | 99.86% | 0.10793 | 0.99034 |
| 350 °C 2 h | 98.04% | 0.02897 | 0.9974 |
| 400 °C 2 h | 97.86% | 0.0513 | 0.95658 |
| 300 °C 1 h | 99.84% | 0.11365 | 0.99521 |
| 300 °C 1.5 h | 99.88% | 0.12432 | 0.99501 |
| UV light | 90.15% | 0.06051 | 0.94364 |
| UV light + stirring | 98.78% | 0.1067 | 0.9403 |
| sunlight | 99.77% | 0.12986 | 0.81995 |
| sunlight + stirring | 99.72% | 0.13423 | 0.77258 |
| Sr. no. | Catalyst | Morphology | Catalyst Loading | Organic Pollutant | Light Source | Irradiation Time (min) | % Degradation | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | ZnO | Ouasi-spherical/ellipsoidal | 0.1 g/L | 10 mg/L | Solar | 120 | 96.38 | [59] |
| 2 | ZnO NPs | Stick-shaped | 0.2 g/L | 10 mg/L | Sunlight | 150 | 94 | [60] |
| 3 | ZnO NPs | Agglomerated | 0.2 g/L | 20 mg/L | Sunlight | 180 | 91.4 | [61] |
| 4 | ZnO NPs | Spherical | 0.2 g/L | 10 ppm | UV light | 105 | 98 | [62] |
| 5 | ZnO NPs | Spherical | 1.0 g/L | 50 ppm | Sunlight | 90 | 96 | [63] |
| 6 | ZnO NPs | Spherical | 0.4 g/L | 5 ppm | Sunlight | 100 | 95.1 | [64] |
| 7 | ZnO NPs | Spherical | 0.8 g/L | 50 μM | UV light | 180 | 74 | [65] |
| 8 | ZnO NPs | Spherical | 1.0 g/L | 10 μM | Sunlight | 90 | ~100 | [66] |
| 9 | ZnO NPs | Spherical | 0.6 g/L | 10 μM | Sunlight | 50 | 98.17 | [67] |
| 10 | ZnO NRs | Stick-shaped | 0.6 g/L | 10 μM | Sunlight | 60 | 99 | [68] |
| 11 | ZnO NPs | Flower-shaped | 0.5 g/L | 20 ppm | Sunlight | 120 | 95 | [69] |
| 12 | ZnO NPs | Stick-shaped | 0.5 g/L | 100 ppm | UV light | 200 | 69 | [70] |
| 13 | ZnO NPs | Semi-spherical | 1.0 g/L | 15 ppm | Hg lamp | 90 | 92.78 | [71] |
| 14 | ZnO NPs | Spherical | 0.6 g/L | 10 μM | Sunlight | 90 | 94.07 | [72] |
| 15 | ZnO NRs | Stick-shaped | 1.0 g/L | 10 mg/L | UV light | 40 | 90.15 | This work |
| 16 | ZnO NRs | Stick-shaped | 1.0 g/L | 10 mg/L | UV light + stirring | 40 | 98.78 | This work |
| 17 | ZnO NRs | Stick-shaped | 1.0 g/L | 10 mg/L | Sunlight | 40 | 99.77 | This work |
| 18 | ZnO NRs | Stick-shaped | 1.0 g/L | 10 mg/L | Sunlight +stirring | 40 | 99.72 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, P.; Yang, W.; Peng, H.; Zhao, S. Efficient Degradation of Methylene Blue in Industrial Wastewater and High Cycling Stability of Nano ZnO. Molecules 2024, 29, 5584. https://doi.org/10.3390/molecules29235584
Liang P, Yang W, Peng H, Zhao S. Efficient Degradation of Methylene Blue in Industrial Wastewater and High Cycling Stability of Nano ZnO. Molecules. 2024; 29(23):5584. https://doi.org/10.3390/molecules29235584
Chicago/Turabian StyleLiang, Ping, Weiye Yang, Hongyan Peng, and Shihua Zhao. 2024. "Efficient Degradation of Methylene Blue in Industrial Wastewater and High Cycling Stability of Nano ZnO" Molecules 29, no. 23: 5584. https://doi.org/10.3390/molecules29235584
APA StyleLiang, P., Yang, W., Peng, H., & Zhao, S. (2024). Efficient Degradation of Methylene Blue in Industrial Wastewater and High Cycling Stability of Nano ZnO. Molecules, 29(23), 5584. https://doi.org/10.3390/molecules29235584






