Abstract
Quantum heat engines (QHEs) are established by applying the principles of quantum thermodynamics to small−scale systems, which leverage quantum effects to gain certain advantages. In this study, we investigate the quantum Otto cycle by employing the dipole−dipole coupled polar molecules as the working substance of QHE. Here, the molecules are considered to be trapped within an optical lattice and located in an external electric field. We analyze the work output and the efficiency of the quantum Otto heat engine (QOHE) as a function of various physical parameters, including electric field strength, dipole−dipole interaction and temperatures of heat baths. It is found that by adjusting these physical parameters the performance of the QOHE can be optimized effectively. Moreover, we also examine the influences of the entanglement and relative entropy of coherence for the polar molecules in thermal equilibrium states on the QOHE. Our results demonstrate the potential of polar molecules in achieving QHEs.
1. Introduction
Thermodynamics was initially developed to improve the performance of large−scale machinery; however, it has been extended to the quantum scale in recent years, leading to the emergence of quantum thermodynamics [1,2,3]. As a key topic in quantum thermodynamics, quantum heat engines (QHEs) have attracted widespread attention due to their potential applications in energy production, transportation, and computation [4,5]. The distinguishing feature of QHEs is that they use quantum systems as the working medium, such as quantum dots [6,7], trapped ions [8], atomic ensembles [9,10,11], optomechanical oscillators [12,13], nuclear magnetic resonance setup [14], etc. This enables the exploitation of quantum advantages including superposition and coherence [15,16,17]. Consequently, the QHEs may outperform the equivalent classical heat engines in certain aspects [18,19,20]. As the quantum counterpart of classical combustion engines, the quantum Otto heat engine (QOHE) follows a similar principle with two isochoric and two adiabatic steps, has been widely investigated in recent years. For example, Camati et al. [16] analyzed the role of coherence in the QOHE, establishing a direct correlation between power and efficiency with energy coherence. Gao et al. [21] demonstrated that the maximum power of the coupled−qubit QOHE is greater than that of the single−qubit QOHE. Moreover, in Heisenberg spin system, the influences of different physical parameters (e.g., magnetic field and Dzyaloshinskii−Moriya interaction) on the quantum Otto cycle were studied in Refs. [5,22]. Lately, the experimental realizations of the QOHEs in different quantum systems have been reported [8,9,14,23].
Polar molecules have the coherence times comparable to those of neutral atoms, strong controllable long−range interactions, and rich internal degrees of freedom, playing a key role in quantum computation [24,25,26,27,28], quantum simulation [29,30], precision measurement [31], and controlled chemistry [32]. In 2002, the proposal for a prototype of a polar molecule-based quantum computer was put forward by DeMille [24]. In DeMille’s proposal, the electric dipole moments of lattice−confined polar molecules, which are oriented along or against an external electric field, can serve as qubits. These qubits are coupled through electric dipole−dipole interaction, and could be used to achieve approximately CNOT gates based on ultracold KCs molecules. Inspired by [24], we designed the optimal control pulses that interact with the polar YbF molecules by employing the recently popular machine learning technique [28]. Then, the NOT, CNOT, and Hadamard gates were implemented with high fidelities. Moreover, in recent years, the quantum correlation and coherence for polar molecules have been extensively investigated [33,34,35,36,37]. For example, Wei et al. [33] studied the dependence of entanglement in polar molecules on the strength of the external electric field, dipole−dipole coupling, and ambient temperature. Recently, we have successfully demonstrated the generation of entanglement in polar molecular systems through the utilization of deep reinforcement learning [37]. On the other hand, significant advancements have been made in diverse methodologies and technologies related to the cooling, trapping, and manipulation of cold molecules over the past few decades [38,39,40,41], establishing polar molecules as a highly promising candidate for quantum information processing.
Note that, in some existing studies, the molecules have been considered as a platform for implementing QHEs. For example, Hübner et al. [42] presented the molecular quantum Otto cycle utilizing the Ni2 dimer in the presence of a static magnetic field and discussed the relationship between efficiency and entanglement. It is found that the performance of the cycles is significantly affected by the detailed electronic energy levels. Moreover, Chen et al. [43] considered a generic donor−bridge−acceptor molecular model, and investigated the effects of quantum coherence and dephasing on the performance of molecular heat engines by utilizing nonequilibirum Green function methodology.
In this paper, we consider a qubit−qubit system consisting of two polar molecules coupled by the dipole−dipole interaction as the working substance of the QOHE. The molecules are assumed to be located in a static electric field, resulting in the superposition of molecular rotational modes into pendular qubit states. Our results show that the work output and efficiency of the QOHE can be manipulated by adjusting the electric field strength, the dipole−dipole interaction, and the ambient temperature. Moreover, the effects of thermal entanglement and coherence on the performance of QOHE are discussed. Our results could shed some light on the implementation of QHEs based on polar molecules.
The outline of this paper is organized as follows. In Section 2, we briefly review the model of polar molecules in pendular states and the quantum Otto cycle. In Section 3, the evolution behaviors of the work output and efficiency for the QOHE are numerically analyzed. We summarize our conclusions in Section 4.
2. Theory
2.1. Polar Molecules in Pendular States
The Hamiltonian of a polar diatomic or linear molecule in the presence of an electric field can be expressed by [33]
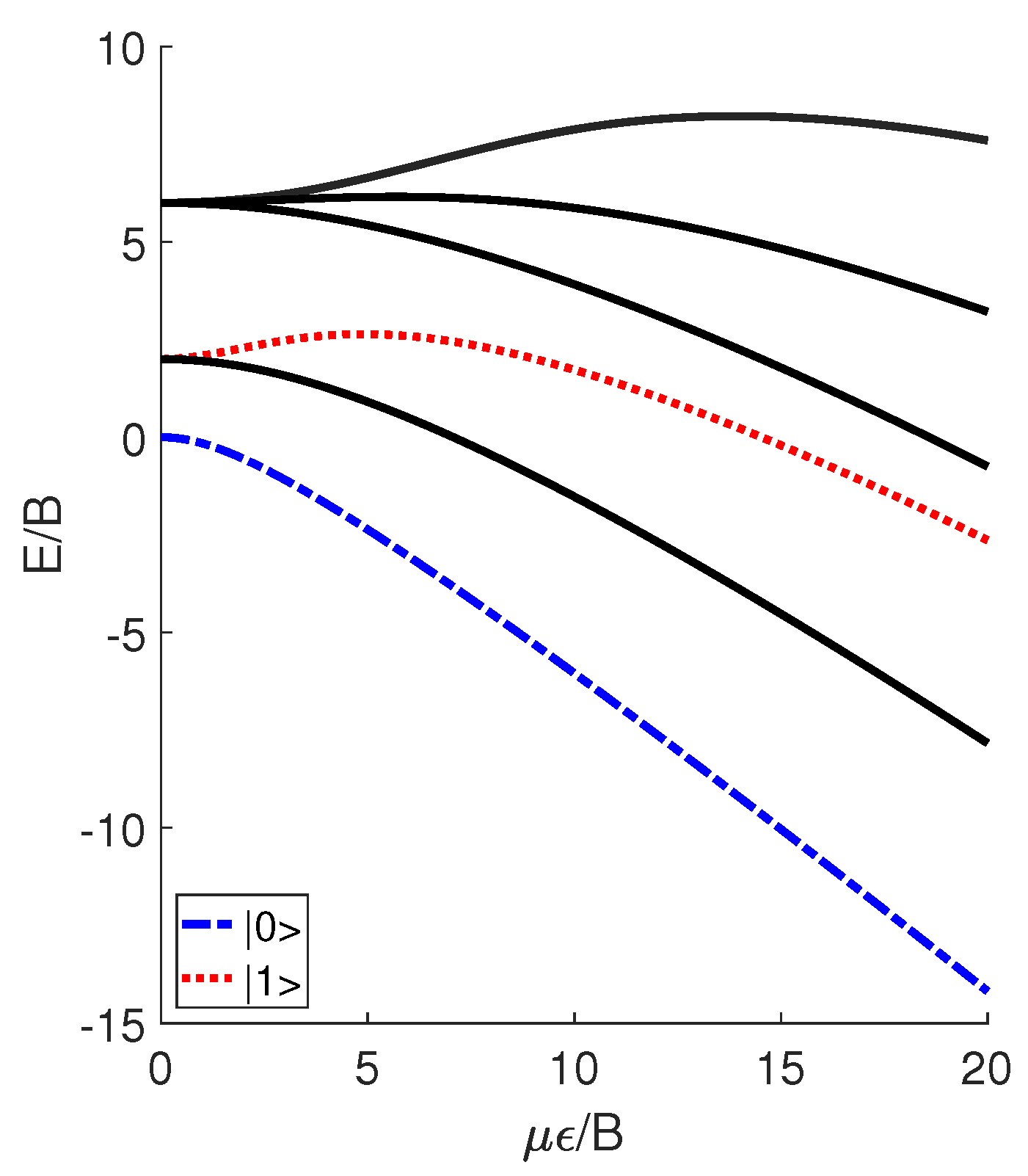
where B denotes the molecular rotational constant, J denotes the angular momentum operator, and denotes the angle between the molecular permanent dipole moment and the electric field . The introduction of the external electric field leads to the generation of pendular states, which are actually the superposition of molecular rotational modes. In Figure 1, we plot the unitless energy levels of the pendular states versus the unitless parameter , where E denotes the eigenenergies of the Hamiltonian H. Note that, in this study, all of the results are presented using the unitless reduced variables, ensuring that the conclusions obtained are applicable to various polar diatomic molecules rather than just a specific one. DeMille [24] utilized the two lowest pendular states with to define qubits and , which can be expressed as linear combinations of spherical harmonics and symbolized by
where spans from 0 to , and and are the coefficients of the sum of the spherical harmonics. Under the pendular qubit states and , the effective dipole moments of the polar molecule are described as and , and the transition dipole moment between the two pendular states can be expressed as .
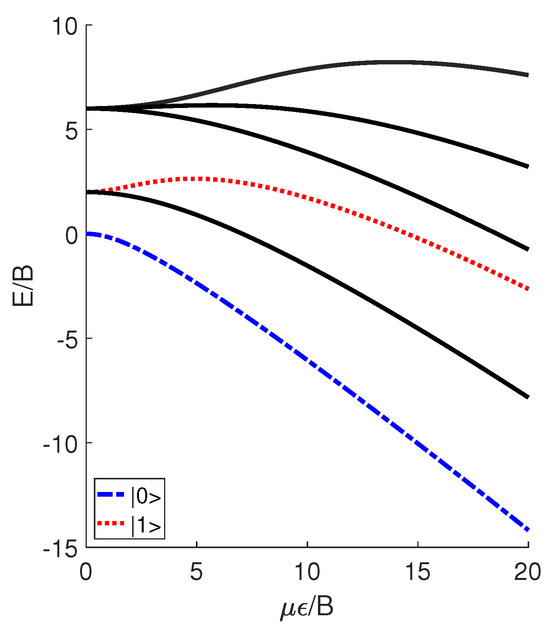
Figure 1.
Several unitless reduced pendular levels of an ultracold polar molecule as a function of . Here, qubits and are encoded in the levels for .
When considering another identical polar molecule in the same static electric field, the dipole−dipole interaction between the two coupled molecules for can be simplified as [28,33]
where can be regarded as the coupling strength, is the intermolecular distance, and is the angle between and the electric field’s direction. As a result, the total Hamiltonian of the dipole−dipole coupled molecular system in an electronic field is given by
Under the basis of the pendular qubits , the Hamiltonian is expanded as
where I denotes a identity matrix, and are the eigenenergies versus the pendular states and for the first molecule. Similarly, the Hamiltonian is
where and are the eigenenergies versus the pendular states and for the second molecule. Additionally, the dipole−dipole interaction term is given by
Here, and () characterize the effective dipole moments for the xth polar molecule, while characterizes the transition dipole moment.
To explore the thermal properties of the QOHE using the coupled polar molecules in an electric fields as the working substance, we consider the thermal equilibrium state of the molecular system at temperature T, for which the density matrix can be written as
Here, represents the ith eigenstate corresponding to the eigenvalue of the total Hamiltonian , denotes Boltzmann’s constant, and is the partition function with the following form
where the trace operation sums over all exponentiated eigenvalues of the Hamiltonian.
We note that, in 2018, the CaF molecule has been laser cooled to a temperature regime of microkelvin [44]. Recently, the entanglement of CaF molecules in an optical tweezer array has been successfully prepared in experiments [45,46]. These achievements may make the CaF molecule a suitable test carrier for QHEs.
2.2. Quantum Otto Cycle
The quantum Otto cycle of coupled bipolar molecules as working substances consists of two quantum isochoric processes and two quantum adiabatic processes [3], which can be described in four distinct stages.
Stage One: Quantum isochoric heating. In this process, the two polar molecules, which are subjected to an electric field , serve as the working substance and come into contact with a hot heat bath at temperature . Here, the dipole−dipole coupling strength between the two molecules is denoted as . After a sufficient period, the molecular system reaches thermal equilibrium with the heat source. The density matrix of the working substance at this point is defined as , where represents the system’s Hamiltonian at this stage, denotes the population probabilities for each eigenstate, and is the partition function. During this process, heat is transferred from the heat source to the working substance.
Stage Two: Quantum adiabatic expansion. During this stage, the system is isolated from the heat source and undergoes an adiabatic expansion. This stage must be executed slowly enough to satisfy the quantum adiabatic theorem, ensuring that the population probabilities of each eigenstate remain unchanged. Note that, in this stage, there is no heat exchange between the system and the environment, and the system’s Hamiltonian transforms to from at the end of evolution.
Stage Three: Quantum isochoric cooling. In this stage, the working substance is brought into contact with a cold bath at , and undergoes another quantum isochoric process, where the electric field and the dipole−dipole coupling strength are represented by and , respectively. At the conclusion of this stage, the system and the low-temperature heat source reach thermal equilibrium. Then, the density matrix is changed to , where , and . During the process of time evolution, a quantity of heat is transferred from the working substance to the low−temperature heat source.
Stage Four: Quantum adiabatic compression. In this final stage, the external electric field recovers to from , and the coupling strength recovers to from . The probabilities remain unchanged. At the end of evolution, the system’s Hamiltonian reverts to .
When the entire cycle is completed, the heat transferred can be quantitatively analyzed based on the quantum form of the first law of thermodynamics as follows [2,22]:
Here, and characterize the processes of the absorption and release of heat, respectively. The net work output W of the QOHE, which is determined by the principles of energy conservation, is given by
When , the engine performs positive work. As a result, the efficiency of the heat engine can be expressed as .
3. Results
3.1. QOHE of Coupled Dipoles in Thermal Equilibrium
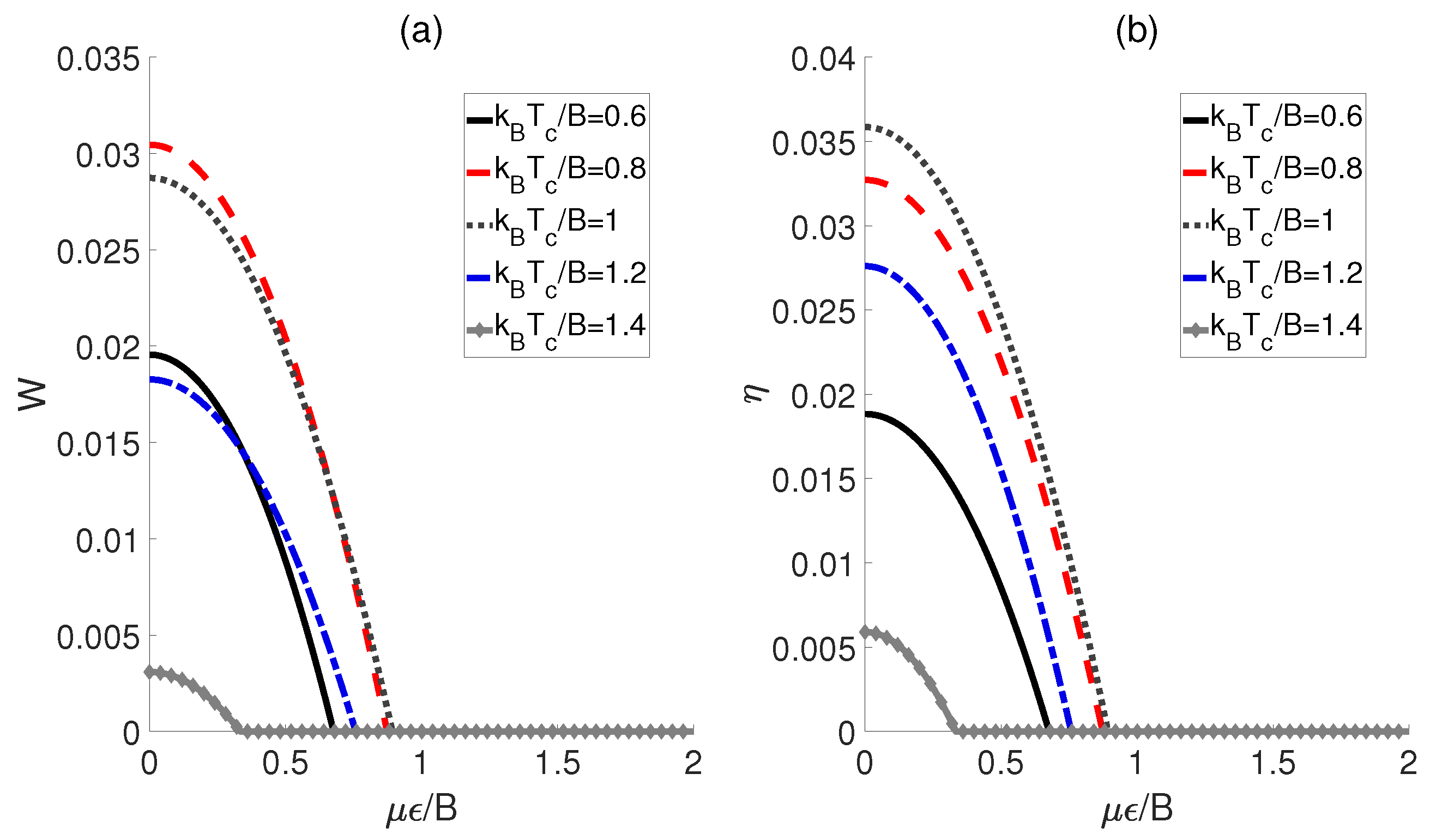
First, the influences of the external electric field and the temperatures of the cold bath on the performance of a QOHE based on polar molecules are explored. Here, we consider the scenario in which the dipole−dipole coupling strength experiences a transformation of , while the external electric fields corresponding to the cold and hot baths remain identical (i.e., ). As shown in Figure 2, both the output work W and the efficiency exhibit similar behaviors with respect to for different temperatures . Concretely speaking, in Figure 2a, we can see that the output work W for each initiates at a non−zero value, and then decreases monotonically to zero as increases. This means that within a limited electric field strength, the QOHE can work normally. However, beyond this range, the positive work cannot be output. Furthermore, we observe that the maximum work output of QOHE is achieved at when is relatively weak. Moreover, the evolution behaviors of the heat engine efficiency are plotted in Figure 2b. For a given , it can be found that the maximum value of occurs at . However, is not the minimum value among the several given but rather a middle value, indicating that the excessive temperature differences between the cold and heat baths might reduce the output efficiency of the QOHE based on the polar molecules.
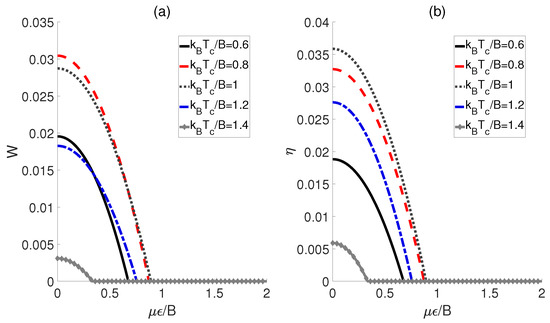
Figure 2.
Work output W (a) and efficiency (b) as functions of the external electric field strength and cold bath temperatures . Here, , , and .
Then, we investigate the scenario where the external electric field varies as the working substance comes into contact with the cold and hot baths, i.e., , while maintaining identical dipole−dipole interactions, i.e., . In this setup, the impact of cold bath temperatures on the performance of QOHE is also considered. For , 0.8 and 1, Figure 3a demonstrates that the work output W initially increases and reaches a peak as is elevated, but then declines with further increases in . This implies the existence of an optimal coupling strength at which the maximum work output can be attained. However, it should be noted that when the temperature is high, e.g., and 1.4, W will decrease monotonously with the increase in . Figure 3b indicates that as increases, the efficiency for all exhibits a single−peak trajectory. Thus, the maximum efficiency of the QOHE can be obtained by precisely controlling . Furthermore, from Figure 3, we can observe that both the output work and efficiency for a given always increase as the temperature difference expands, which contrasts with the case shown in Figure 2.
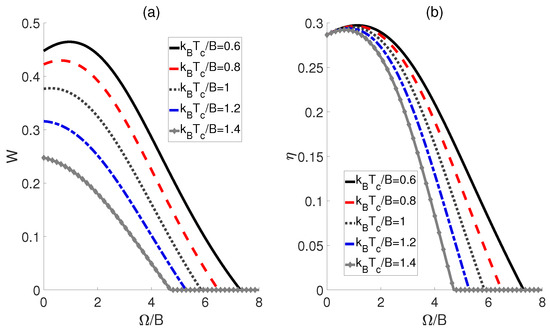
Figure 3.
Work output W (a) and efficiency (b) of the QOHE as functions of dipole−dipole coupling strength and cold bath temperatures . Here, , , and .
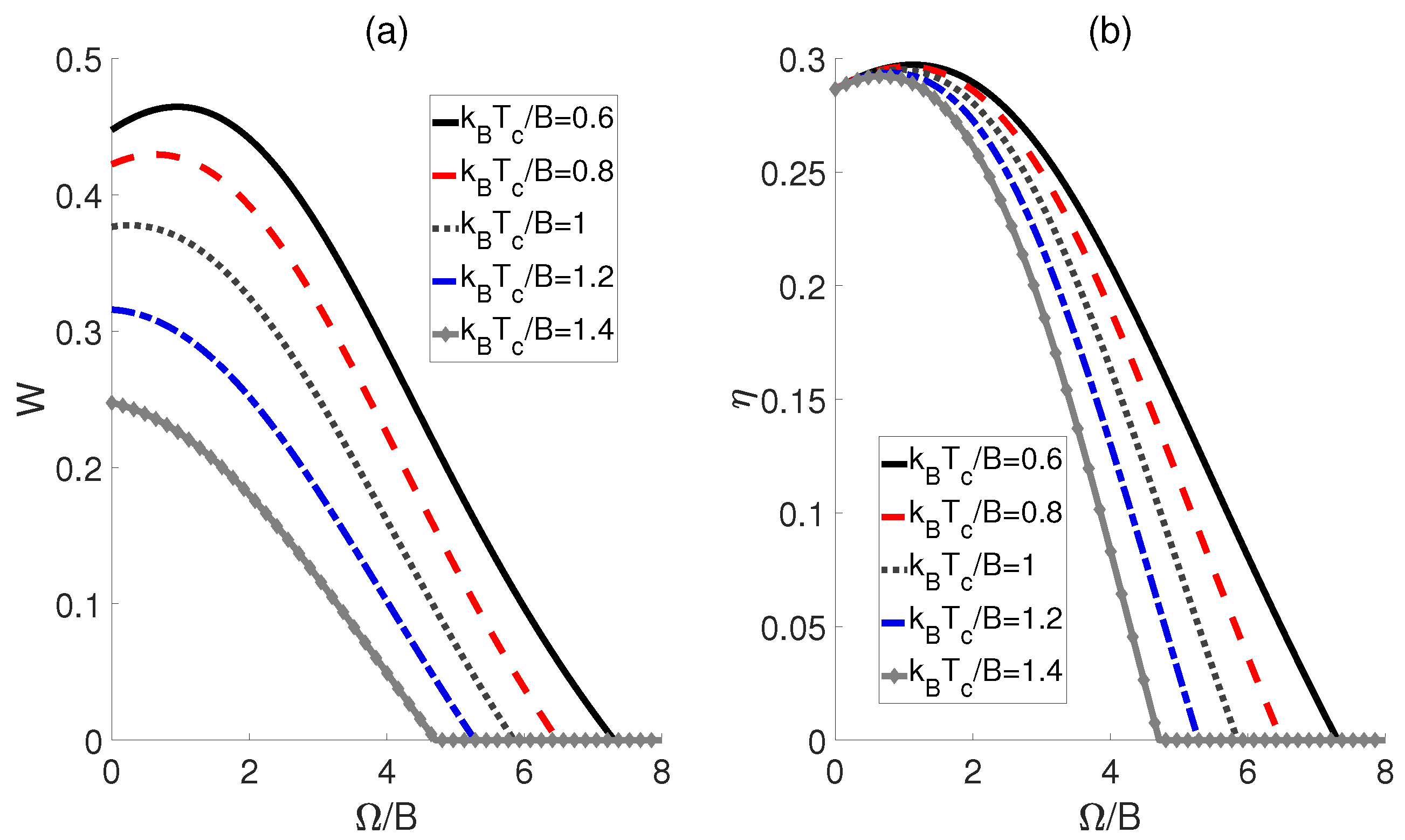
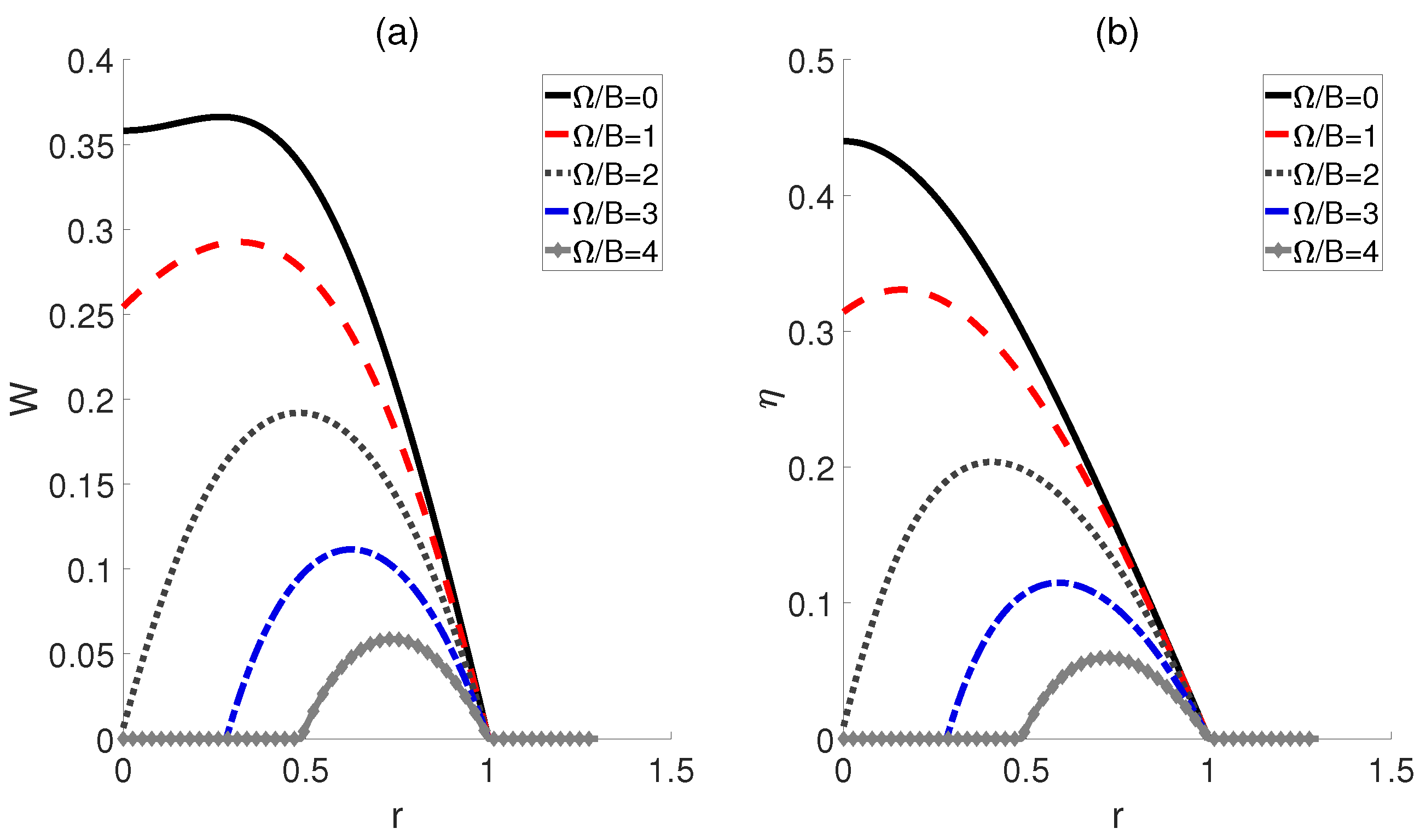
Inspired by Ref. [22], we subsequently proposed an alternative scenario wherein the external electric fields and the dipole−dipole interaction are proportionally linked across adiabatic transitions, denoted as , where r represents a ratio. In Figure 4, we plot the work output W and efficiency as functions of the ratio r. The curves for different temperatures of the cold bath exhibit a peak followed by a subsequent decline as r increases, thereby confirming the existence of the optimal values of r that can improve the performance of the heat engine. Moreover, Figure 4b shows that the highest efficiency is achieved at the lowest temperature for a given ratio r, which is consistent with the prior experience in classical thermodynamic cycles, i.e., a larger temperature difference between the high− and low−temperature heat sources can result in higher efficiency. Furthermore, the peaks of these efficiency curves are found to shift rightward with decreasing temperature differences, indicating that smaller temperature differences require a higher ratio r to achieve maximum work output and efficiency. However, it is worth noting that as the ratio r increases to a critical value, the impact of temperature differences diminishes, leading to the convergence of multiple curves. If , W and for different temperatures tend to 0.
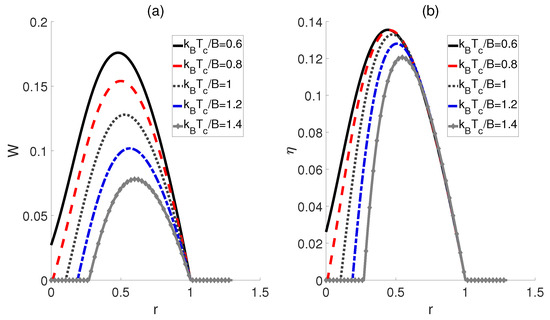
Figure 4.
Impact of the ratio r on the work output W (a) and efficiency (b) of the QOHE. Here, , , and .
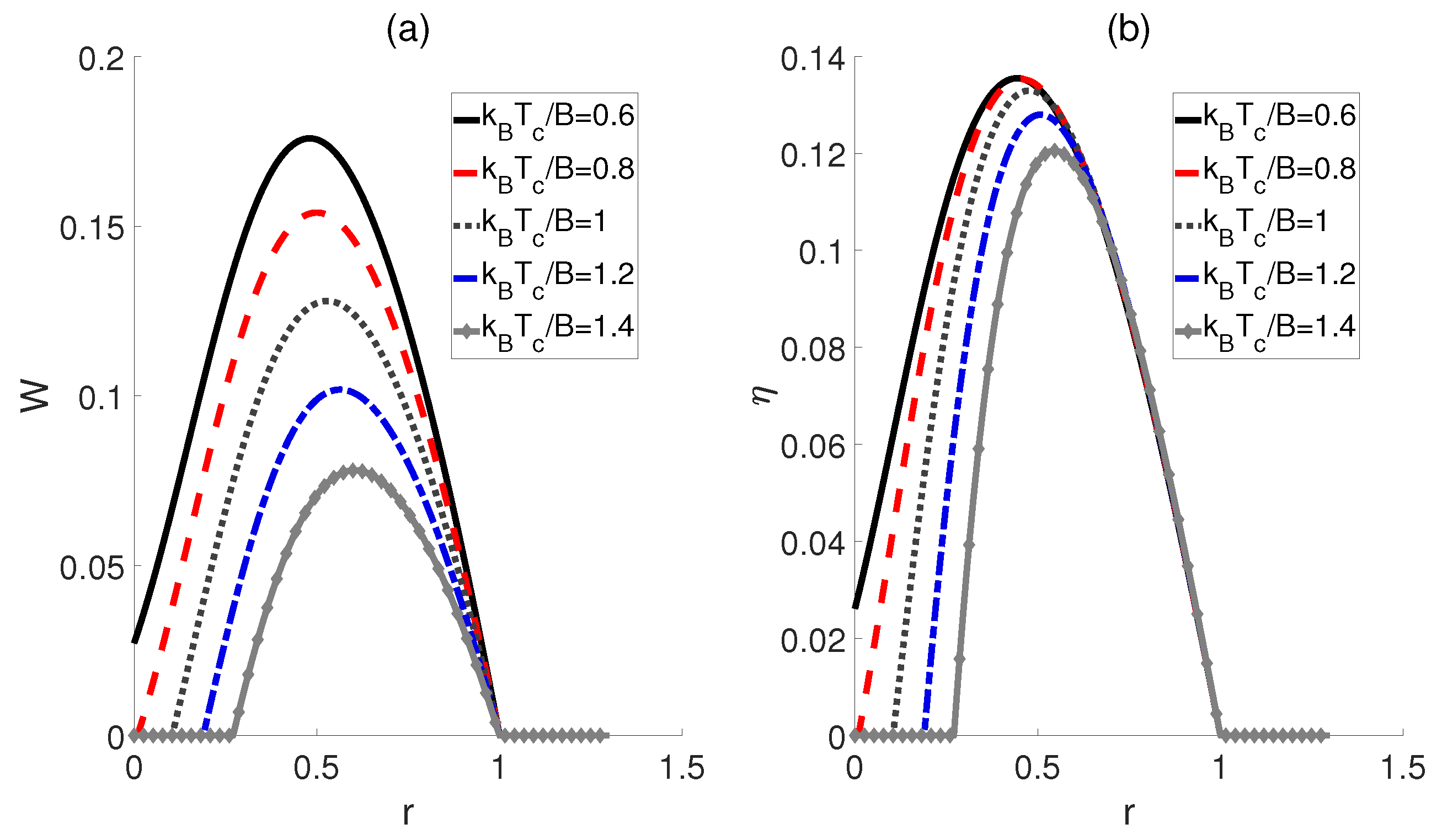
Finally, we consider the scenario where the electric fields corresponding to the hot and cold baths remain proportional, denoted as , while the coupling strengths and are equal, denoted as = = . Here, the temperatures for hot and cold baths are fixed. As shown in Figure 5, for a given ratio r, the smaller the , the larger the work output W and the efficiency become. Moreover, with an increase in , the ratios r corresponding to the peaks of W and exhibit a shift towards larger values. Additionally, it should be noted that when the ratio r exceeds 1, both W and become 0 for all , which is similar to the cases depicted in Figure 4.
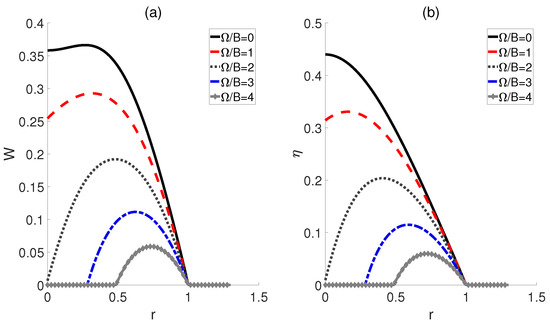
Figure 5.
Work output W (a) and efficiency (b) of a QOHE as functions of the ratio r = and coupling strength . Here, , , and .
3.2. Effects of Entanglement and Coherence on QOHE
In this subsection, we investigate how the thermal entanglement (characterized by concurrence) and coherence (quantified by relative entropy) influence the QOHE. For a qubit−qubit system, the concurrence c is defined as [47]:
Here, with are the eigenvalues of the matrix M, listed in descending order, with
where is the density matrix of an arbitrary two−qubit state, is the complex conjugate of , and represents the Pauli−Y operator. For maximally entangled states, ; for completely separable states, ; for partially entangled states, .
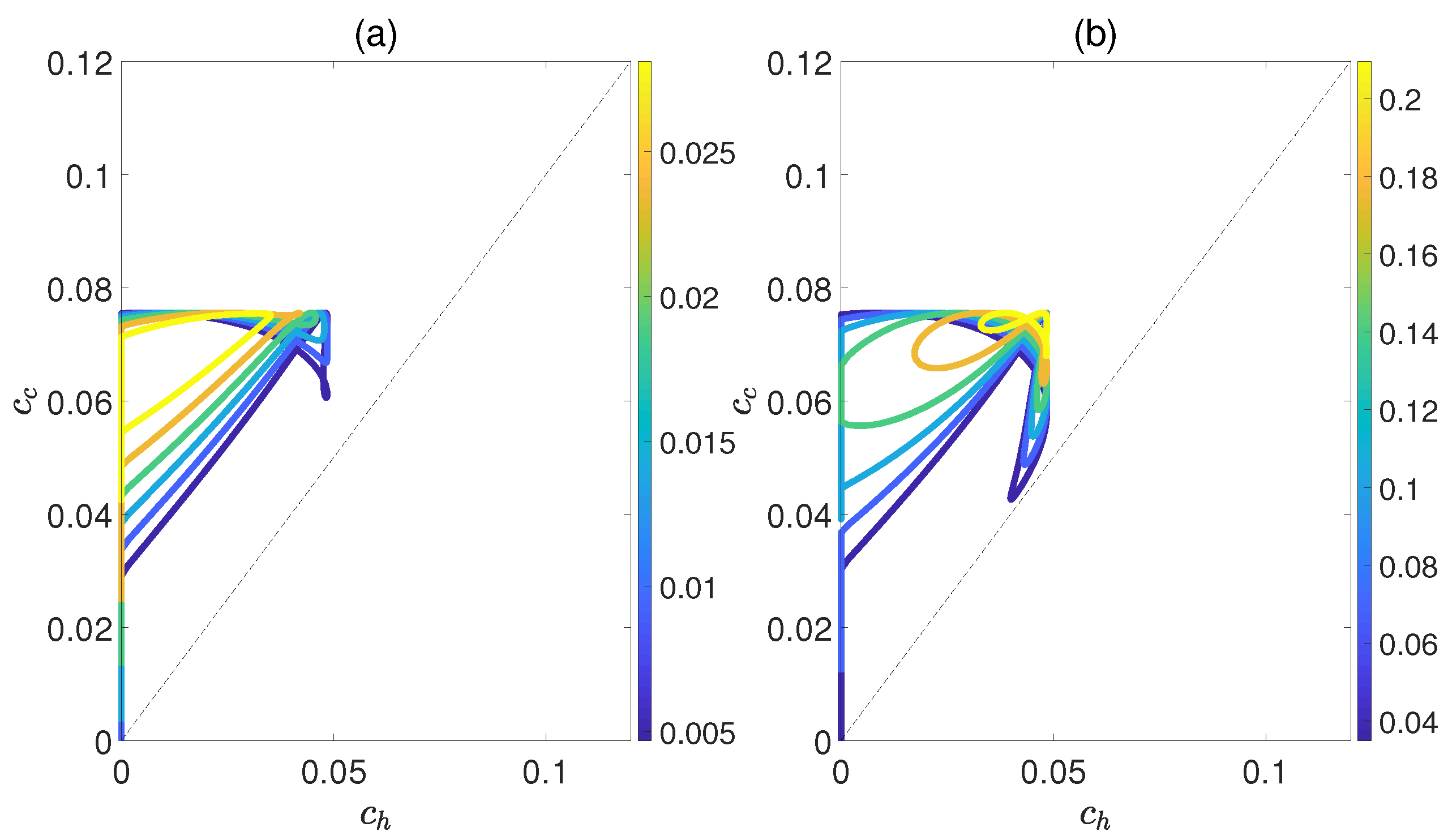
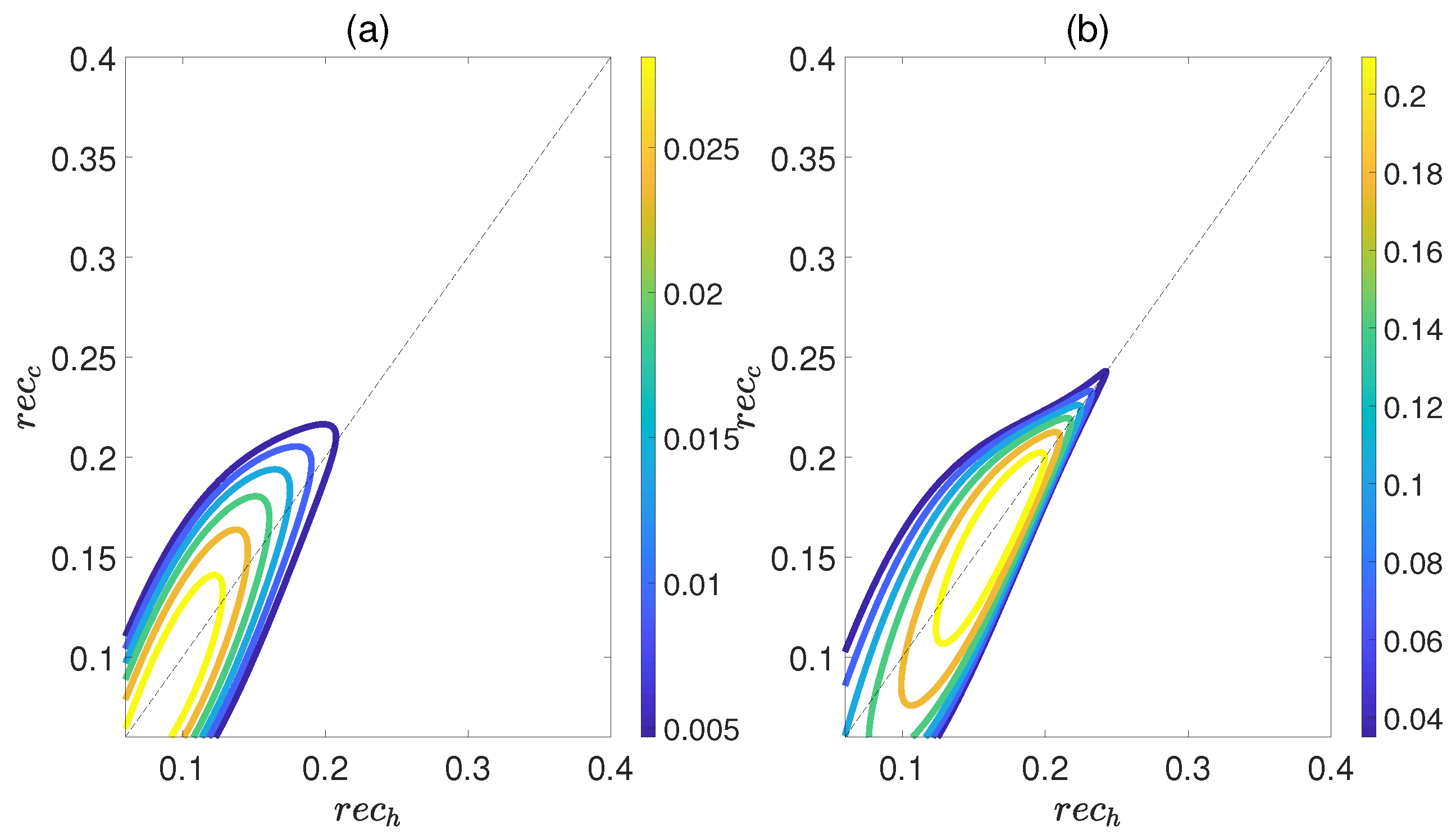
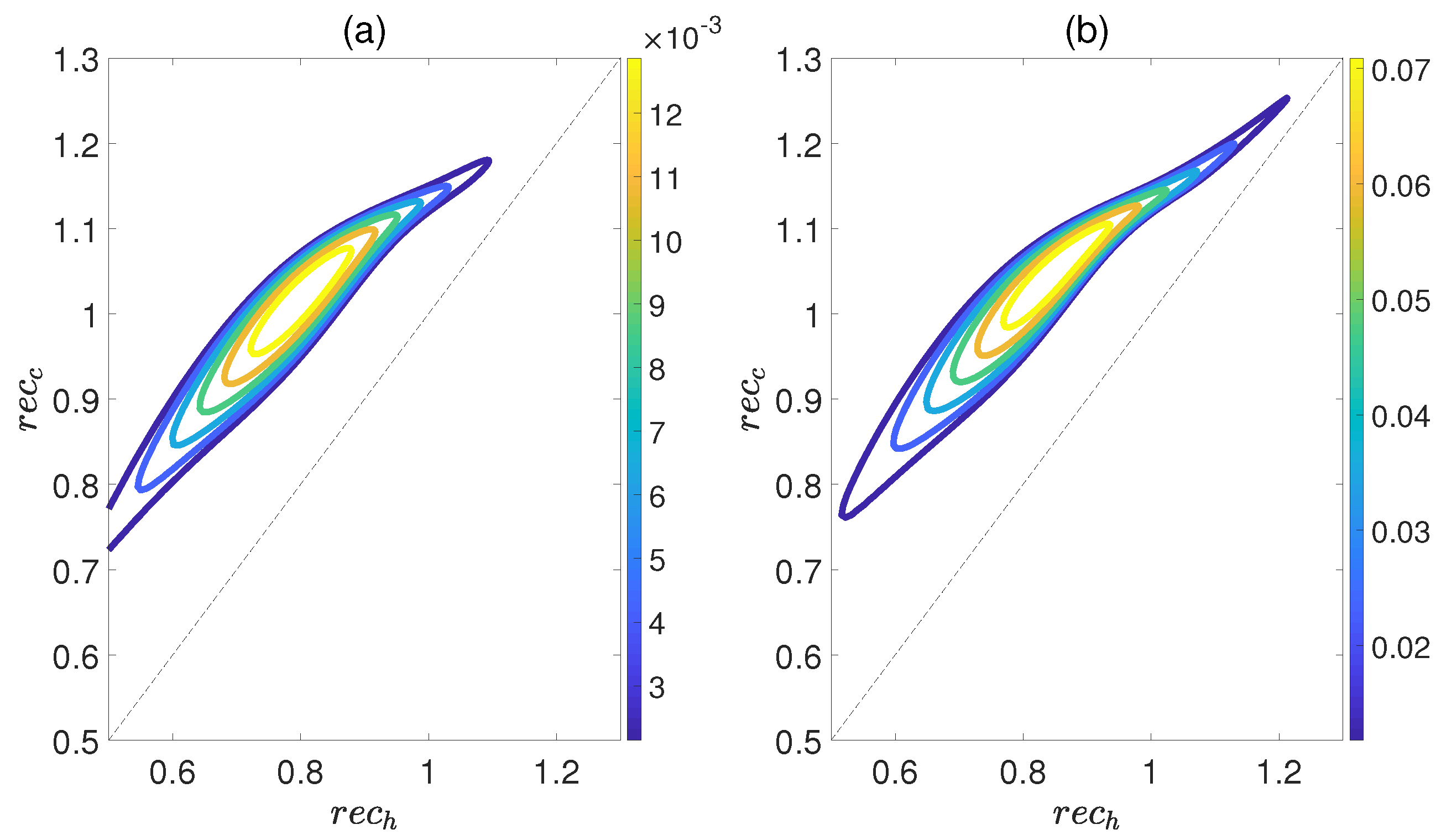
Figure 6 demonstrates the dependence of the work output W and the efficiency on the concurrence and , where and denote the entanglement for the state of the working substance reaching thermal equilibrium with the high− and low−temperature baths, respectively. Here, we introduce a relative coupling strength k, defined as the ratio between the dipole−dipole coupling strength and the coupling strength between dipole moment and electric field , i.e., . In Figure 6, k is set to 0.5. We can observe that both W and exhibit non−monotonic behaviors with respect to , while keeping fixed. This highlights an optimal entanglement range that enables the heat engine to work most efficiently. In addition, the density of contour lines varies within different ranges of and . The denser the contour lines are, the more sensitive W and become to entanglement, and vice versa. Furthermore, we also consider the case of , as shown in Figure 7. The trends of W and exhibit similarities to those depicted in Figure 6, except that their order of magnitude is smaller and the non−monotonicities versus and become more apparent. Moreover, regardless of whether k is 0.5 or 1.5, the QOHE can only generate net work within the range of . If , then . Consequently, it can be inferred that the entanglement of polar molecules in thermal states significantly influences the performance of QOHE.
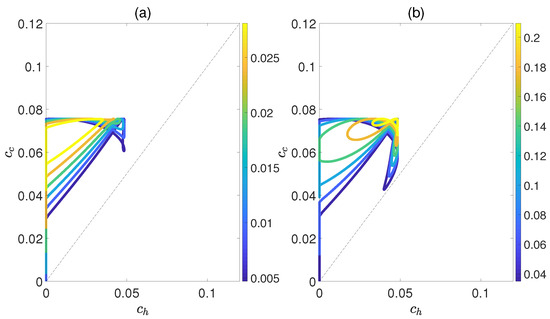
Figure 6.
(Color online) Variations in work output W (a) and the efficiency (b) with the concurrence and in isoline maps. Here, the relative coupling strength , , and .
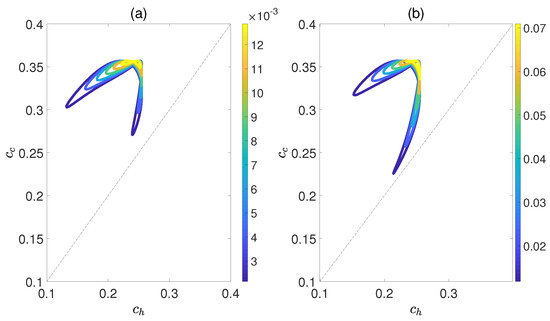
Figure 7.
(Color online) Variations in work output W (a) and the efficiency (b) with the concurrence and in isoline maps. Here, the relative coupling strength , , and .
Quantum coherence is an essential physical resource in quantum information processing, which arises from the superposition of quantum states. Here, we utilize the relative entropy to evaluate the coherence of the molecular system in thermal equilibrium. In Ref. [48], the relative entropy of coherence is given by
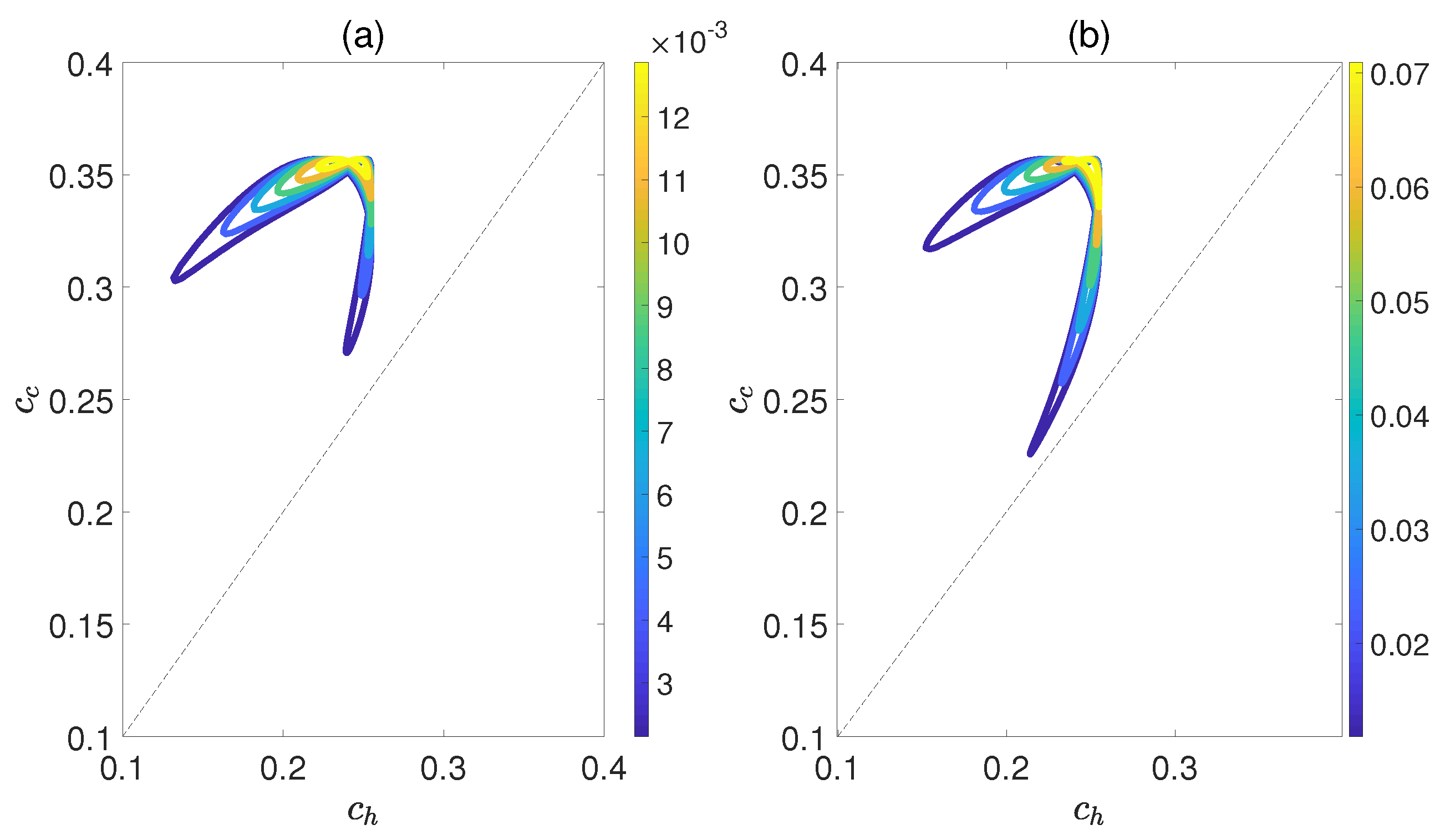
Here, is the density matrix obtained by deleting all the off−diagonal elements of the quantum state , and is the von Neumann entropy. The work output W and efficiency are depicted in Figure 8 as functions of and , while maintaining a relative coupling strength of . Here, and represent the coherence of polar molecules as they reach thermal equilibrium with the cold and hot baths, respectively. It is evident that as is fixed, initially both W and increase with an increase in , but then they decrease. Similarly, when is fixed, both W and exhibit an increasing trend followed by a decreasing one with respect to . This indicates the existence of an optimal region of relative entropy coherence for achieving the best performance of a heat engine. While moderate quantum coherence contributes to the enhancement of W and to some extent, an excessive level of coherence may lead to the disappearance of W and . Furthermore, unlike the cases depicted in Figure 6 and Figure 7, within the region of it is possible for the positive work W and efficiency to exist. However, for the case of , the heat engine can only output net work when , as depicted in Figure 9. These observations imply that the relative strength between molecule−molecule interaction and field−molecule interaction plays a significant role in the QOHE.
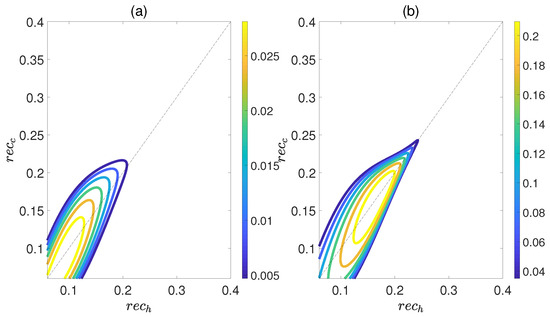
Figure 8.
(Color online) Variations in the work output W (a) and the efficiency (b) with the coherence and in isoline maps. Here, the relative coupling strength , , and .
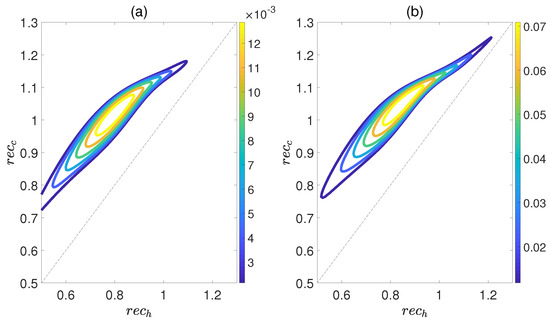
Figure 9.
(Color online) Variations in the work output W (a) and the efficiency (b) with the coherence and in isoline maps. Here, the relative coupling strength , , and .
4. Conclusions
We have studied the properties of QOHE by taking the dipole−dipole coupled polar molecules in an external electric field as the working substance. After numerically calculating, we have found that the work output and efficiency of the QOHE decrease monotonically with the increase in electric field strength under the conditions of and . However, the variation in efficiency with temperature differences between the cold and hot baths is non−monotonic. In the scenario where and , there exist optimal values of that can maximize the efficiency versus different temperatures . For a given , the efficiency is directly proportional to the temperature difference. Moreover, when , it can be observed that both the work output and efficiency show an initial enhancement followed by a subsequent decrease with increasing r, ultimately reaching zero for . For the case of with = = , the work output and efficiency are found to be inversely proportional to . Additionally, we have also discussed the effects of thermal entanglement and coherence on QOHE. It is found that the positive work can be output when . Additionally, in the case of a relative coupling strength of , it is not possible to generate positive work when .
In summary, our results show that the polar molecule−based QOHE can be effectively manipulated by tuning the electric field and dipole−dipole interaction. As a contrast, by adjusting the magnetic field, coupling constant, and Dzyaloshinskii−Moriya interaction, the fine−tuning of work output and efficiency of the QOHE based on a three−qubit Heisenberg XXZ model can also be achieved [22]. It indicates that regulating the external field (e.g., electric field, magnetic field, and optical field) and the interactions between particles is a viable approach for improving the performance of the QHEs.
Although our results are theoretical and conceptual, they still hold significance for the diversification of QHE models and the understanding of quantum thermodynamics. Moreover, the QHEs in molecular systems may have potential applications in molecule-based quantum computing, such as powering quantum computers or serving as cooling agents to avoid thermal noises. We expect that our results could inspire the further studies on quantum computing and thermodynamics in polar molecule systems.
Author Contributions
X.L.: Conceptualization, Methodology, Software, Investigation, Formal analysis, Writing original draft. Z.S., Y.-Y.F., X.-L.H., X.H. and J.-F.L.: Conceptualization, Investigation, Writing review and editing. Z.-Y.Z. and J.-M.L.: Conceptualization, Investigation, Writing review and editing, Funding acquisition, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China under Grant Nos. 62305285, 92265209, and 62301476, the Natural Science Foundation of Chongqing under Grant No. CSTB2024NSCQ-MSX0643, the Shanghai Municipal Science and Technology Major Project under Grant No. 2019SHZDZX01, and the Special Scientific Research Project of Shaanxi Provincial Education Department under Grant No. 23JK0713.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the anonymous referees for their helpful comments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| QHEs | quantum heat engines |
| QOHE | quantum Otto heat engine |
References
- Gemmer, J.; Michel, M.; Mahler, G. Quantum Thermodynamics; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2004. [Google Scholar]
- Kieu, T.D. The second law, Maxwell’s demon, and work derivable from quantum heat engines. Phys. Rev. Lett. 2004, 93, 140403. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.T.; Liu, Y.; Sun, C.P.; Nori, F. Quantum thermodynamic cycles and quantum heat engines. Phys. Rev. E 2007, 76, 031105. [Google Scholar] [CrossRef] [PubMed]
- Kosloff, R.; Levy, A. Quantum heat engines and refrigerators: Continuous devices. Annu. Rev. Phys. Chem. 2014, 65, 365. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabbou, M.Y.; Rahman, A.U.; Yurischev, M.A.; Haddadi, S. Comparative study of quantum Otto and Carnot engines powered by a spin working substance. Phys. Rev. E 2023, 108, 034106. [Google Scholar] [CrossRef]
- Josefsson, M.; Svilans, A.; Burke, A.M.; Hoffmann, E.A.; Fahlvik, S.; Thelander, C.; Leijnse, M.; Linke, H. A quantum-dot heat engine operating close to the thermodynamic efficiency limits. Nat. Nanotechnol. 2018, 13, 920. [Google Scholar] [CrossRef]
- Khlifi, Y.; Abaach, S.; EL Baz, M.; El Allati, A. A quantum Otto heat engine driven by three quantum dots. Phys. Scr. 2024, 99, 075967. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Zhang, J.-Q.; Ding, G.-Y.; Li, J.-C.; Bu, J.-T.; Wang, B.; Yan, L.-L.; Su, S.-L.; Chen, L.; Nori, F.; et al. Dynamical control of quantum heat engines using exceptional points. Nat. Commun. 2022, 13, 6225. [Google Scholar] [CrossRef]
- Bouton, Q.; Nettersheim, J.; Burgardt, S.; Adam, D.; Lutz, E.; Widera, A. A quantum heat engine driven by atomic collisions. Nat. Commun. 2021, 12, 2063. [Google Scholar] [CrossRef]
- Macovei, M.A. Performance of the collective three-level quantum thermal engine. Phys. Rev. A 2022, 105, 043708. [Google Scholar] [CrossRef]
- Feyisa, C.G.; Jen, H.H. A photonic engine fueled by entangled two atoms. New J. Phys. 2024, 26, 033038. [Google Scholar]
- Zhang, K.; Bariani, F.; Meystre, P. Quantum optomechanical heat engine. Phys. Rev. Lett. 2014, 112, 150602. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.; Macrŕ, V.; Wilhelm, F.K.; Nori, F.; Bruschi, D.E. Quantum field heat engine powered by phonon-photon interactions. Phys. Rev. Res. 2023, 5, 043274. [Google Scholar] [CrossRef]
- Peterson, J.P.S.; Batalhão, T.B.; Herrera, M.; Souza, A.M.; Sarthour, R.S.; Oliveira, I.S.; Serra, R.M. Experimental characterization of a spin quantum heat engine. Phys. Rev. Lett. 2019, 123, 240601. [Google Scholar] [CrossRef] [PubMed]
- Aimet, S.; Kwon, H. Engineering a heat engine purely driven by quantum coherence. Phys. Rev. A 2023, 107, 012221. [Google Scholar] [CrossRef]
- Camati, P.A.; Santos, J.F.G.; Serra, R.M. Coherence effects in the performance of the quantum Otto heat engine. Phys. Rev. A 2019, 99, 062103. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; He, J. Thermal entanglement in two-atom cavity QED and the entangled quantum Otto engine. Phys. Rev. A 2009, 79, 041113. [Google Scholar] [CrossRef]
- Klatzow, J.; Becker, J.N.; Ledingham, P.M.; Weinzetl, C.; Kaczmarek, K.T.; Saunders, D.J.; Nunn, J.; Walmsley, I.A.; Uzdin, R.; Poem, E. Experimental demonstration of quantum effects in the operation of microscopic heat engines. Phys. Rev. Lett. 2019, 122, 110601. [Google Scholar] [CrossRef]
- Funo, K.; Lambert, N.; Karimi, B.; Pekola, J.P.; Masuyama, Y.; Nori, F. Speeding up a quantum refrigerator via counterdiabatic driving. Phys. Rev. B 2019, 100, 035407. [Google Scholar] [CrossRef]
- Denzler, T.; Lutz, E. Efficiency fluctuations of a quantum heat engine. Phys. Rev. Res. 2020, 2, 032062. [Google Scholar] [CrossRef]
- Gao, J.; Hatano, N. Maximum power of coupled-qubit Otto engines. Phys. Rev. Res. 2024, 6, 023172. [Google Scholar] [CrossRef]
- Huang, X.L.; Sun, Q.; Guo, D.Y.; Yu, Q. Quantum Otto heat engine with three-qubit XXZ model as working substance. Physica A 2018, 491, 604. [Google Scholar] [CrossRef]
- Ono, K.; Shevchenko, S.N.; Mori, T.; Moriyama, S.; Nori, F. Analog of a quantum heat engine using a single-spin qubit. Phys. Rev. Lett. 2020, 125, 166802. [Google Scholar] [CrossRef] [PubMed]
- DeMille, D. Quantum computation with trapped polar molecules. Phys. Rev. Lett. 2002, 88, 067901. [Google Scholar] [CrossRef] [PubMed]
- Yelin, S.F.; Kirby, K.; Côté, R. Schemes for robust quantum computation with polar molecules. Phys. Rev. A 2006, 74, 050301. [Google Scholar] [CrossRef]
- Yu, P.; Cheuk, L.W.; Kozyryev, I.; Doyle, J.M. A scalable quantum computing platform using symmetric-top molecules. New J. Phys. 2019, 21, 093049. [Google Scholar] [CrossRef]
- Gregory, P.D.; Blackmore, J.A.; Bromley, S.L.; Hutson, J.M.; Cornish, S.L. Robust storage qubits in ultracold polar molecules. Nat. Phys. 2021, 17, 1149. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Hu, J.-R.; Fang, Y.-Y.; Li, J.-F.; Liu, J.-M.; Huang, X.; Sun, Z. Quantum gate control of polar molecules with machine learning. J. Chem. Phys. 2024, 161, 034102. [Google Scholar] [CrossRef]
- Gorshkov, A.V.; Hazzard, K.R.A.; Rey, A.M. Kitaev honeycomb and other exotic spin models with polar molecules. Mol. Phys. 2013, 111, 1908. [Google Scholar] [CrossRef]
- Rosson, P.; Kiffner, M.; Mur-Petit, J.; Jaksch, D. Characterizing the phase diagram of finite-size dipolar Bose-Hubbard systems. Phys. Rev. A 2020, 101, 013616. [Google Scholar] [CrossRef]
- ACME Collaboration. Improved limit on the electric dipole moment of the electron. Nature 2018, 562, 355. [Google Scholar]
- Ospelkaus, S.; Ni, K.-K.; Wang, D.; de Miranda, M.H.G.; Neyenhuis, B.; Quéméner, G.; Julienne, P.S.; Bohn, J.L.; Jin, D.S.; Ye, J. Quantum-state controlled chemical reactions of ultracold potassium-rubidium molecules. Science 2010, 327, 853. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Kais, S.; Friedrich, B.; Herschbach, D. Entanglement of polar molecules in pendular states. J. Chem. Phys. 2011, 134, 124107. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Frye, M.D.; Sawant, R.; Bhole, G.; Jones, J.A.; Cornish, S.L.; Tarbutt, M.R.; Hutson, J.M.; Jaksch, D.; Mur-Petit, J. Robust entangling gate for polar molecules using magnetic and microwave fields. Phys. Rev. A 2020, 101, 062308. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Liu, J.-M. Creation of high-dimensional entanglement of polar molecules via optimal control fields. Phys. Rev. A 2022, 105, 023113. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Fang, Y.-Y.; Li, J.-F.; Hu, J.-R.; Liu, J.-M.; Sun, Z.; Huang, X. Entropic uncertainty relation and entanglement of molecular dipoles in an electric field. Chaos Solitons Fractals 2024, 186, 115220. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Sun, Z.; Duan, T.; Ding, Y.-K.; Huang, X.; Liu, J.-M. Entanglement generation of polar molecules via deep reinforcement learning. J. Chem. Theory Comput. 2024, 20, 1811. [Google Scholar] [CrossRef]
- Anderegg, L.; Augenbraun, B.L.; Chae, E.; Hemmerling, B.; Hutzler, N.R.; Ravi, A.; Collopy, A.; Ye, J.; Ketterle, W.; Doyle, J.M. Radio frequency magneto-optical trapping of CaF with high density. Phys. Rev. Lett. 2017, 119, 103201. [Google Scholar] [CrossRef]
- Lim, J.; Almond, J.R.; Trigatzis, M.A.; Devlin, J.A.; Fitch, N.J.; Sauer, B.E.; Tarbutt, M.R.; Hinds, E.A. Laser cooled YbF molecules for measuring the electron’s electric dipole moment. Phys. Rev. Lett. 2018, 120, 123201. [Google Scholar] [CrossRef]
- Mitra, D.; Vilas, N.B.; Hallas, C.; Anderegg, L.; Augenbraun, B.L.; Baum, L.; Miller, C.; Raval, S.; Doyle, J.M. Direct laser cooling of a symmetric top molecule. Science 2020, 369, 1366. [Google Scholar] [CrossRef]
- Wu, Y.; Burau, J.J.; Mehling, K.; Ye, J.; Ding, S. High phase-space density of laser-cooled molecules in an optical lattice. Phys. Rev. Lett. 2021, 127, 263201. [Google Scholar] [CrossRef]
- Hübner, W.; Lefkidis, G.; Dong, C.D.; Chaudhuri, D. Spin-dependent Otto quantum heat engine based on a molecular substance. Phys. Rev. B 2014, 90, 024401. [Google Scholar] [CrossRef]
- Chen, F.; Gao, Y.; Galperin, M. Molecular heat engines: Quantum coherence effects. Entropy 2017, 19, 472. [Google Scholar] [CrossRef]
- Anderegg, L.; Augenbraun, B.L.; Bao, Y.; Burchesky, S.; Cheuk, L.W.; Ketterle, W.; Doyle, J.M. Laser cooling of optically trapped molecules. Nat. Phys. 2018, 14, 890–893. [Google Scholar] [CrossRef]
- Bao, Y.C.; Yu, S.S.; Anderegg, L.; Chae, E.; Ketterle, W.; Ni, K.K.; Doyle, J.M. Dipolar spin-exchange and entanglement between molecules in an optical tweezer array. Science 2023, 382, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.M.; Lu, Y.K.; Cheuk, L.W. On-demand entanglement of molecules in a reconfigurable optical tweezer array. Science 2023, 382, 1143–1147. [Google Scholar] [CrossRef]
- Wootters, W.K. Entanglement of formation of an arbitrary state of two qubits. Phys. Rev. Lett. 1998, 80, 2245. [Google Scholar] [CrossRef]
- Baumgratz, T.; Cramer, M.; Plenio, M.B. Quantifying coherence. Phys. Rev. Lett. 2014, 113, 140401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).