Antidiabetic Potential of Black Elderberry Cultivars Flower Extracts: Phytochemical Profile and Enzyme Inhibition
Abstract
1. Introduction
2. Results and Discussion
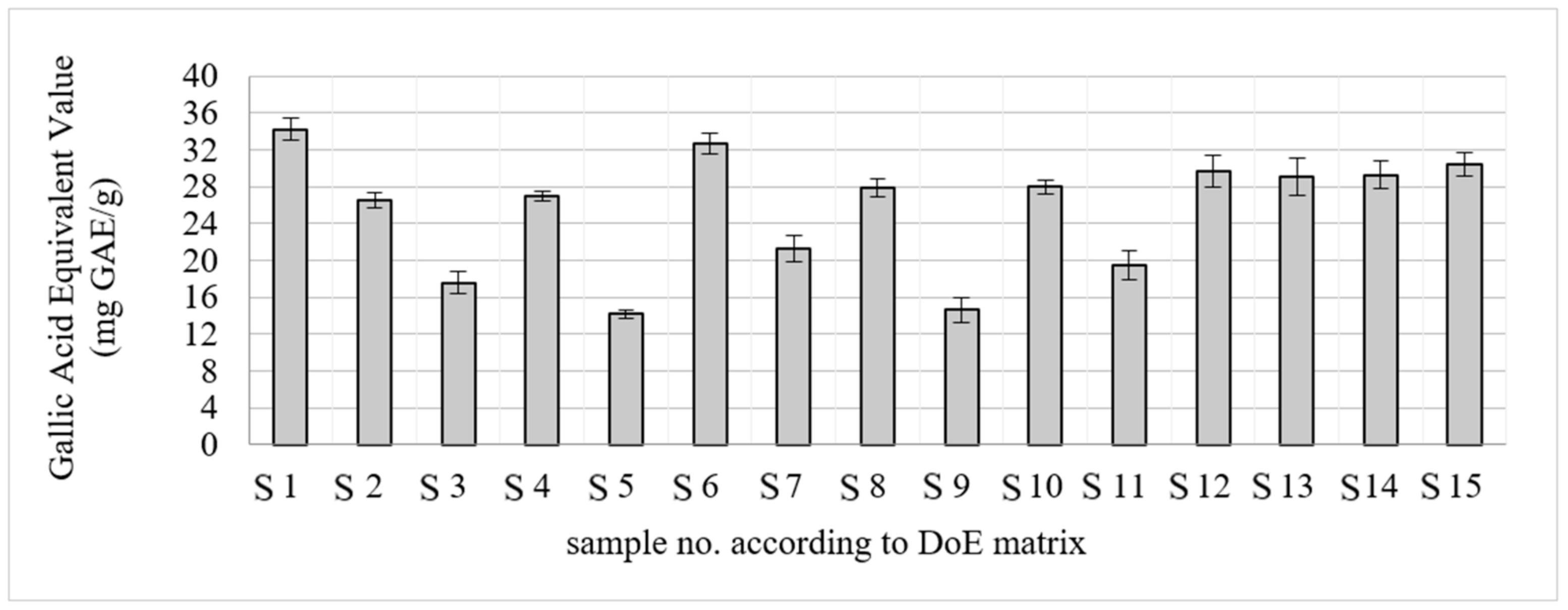
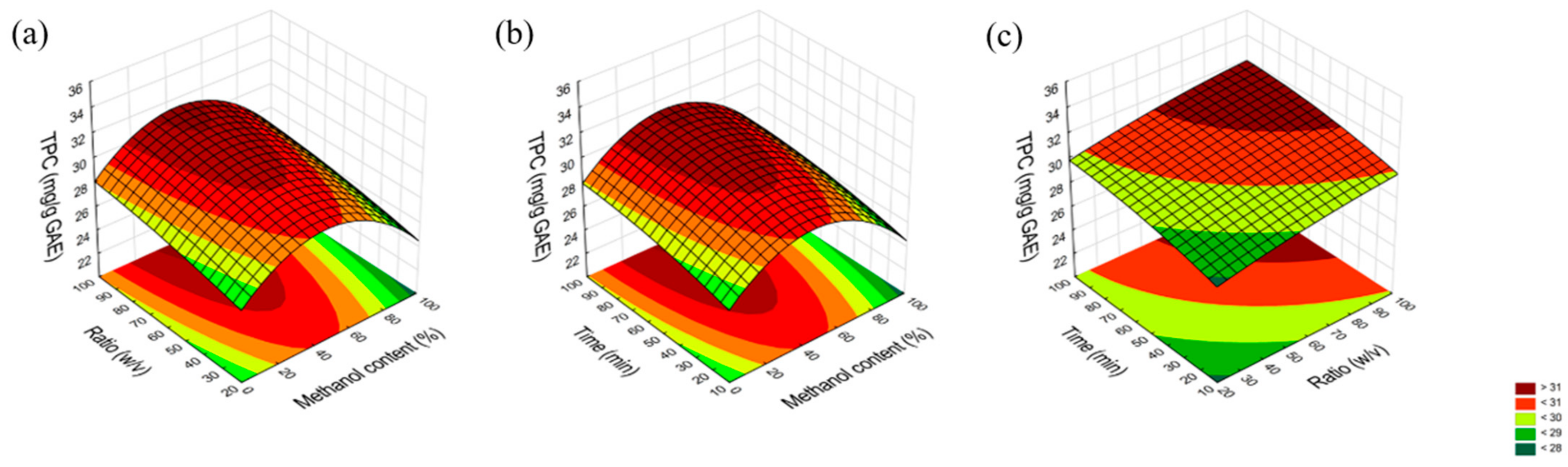
2.1. Optimization of the Extraction Process
2.2. Phytochemical Studies of the Extracts
2.3. Studies of Biological Activity of Extracts
2.3.1. Extracts from the Flowers of Cultivars of Black Elderberry Can Protect Against Free Radicals
2.3.2. Extracts from the Flowers of Cultivars of Black Elderberry Inhibit α-Glucosidase More Strongly than α-Amylase
2.3.3. Extracts from the Flowers of Black Beauty and Black Tower Cultivars Reveal the Higher Anti-Inflammatory Potential
3. Materials and Methods
3.1. Chemical Reagents
3.2. Plant Material
3.3. Optimization of the Extraction Process
3.3.1. Determination of Total Polyphenol Content (TPC) Using the Folin–Ciocalteu Reagent
3.4. Extraction and Preparation of Extracts for Testing
- (1)
- Plant material weight: 500 mg;
- (2)
- Extraction temperature: 50 °C;
- (3)
- Methanol concentration: 45%;
- (4)
- Process time: 15 min;
- (5)
- Solvent volume: 10 mL;
- (6)
- Number of extraction repetitions: 5.
3.5. Phytochemical Studies of the Extracts
3.5.1. Chemical Analysis of the Extract Using High-Performance Liquid Chromatography (HPLC)
3.5.2. Determination of Total Polyphenol Content (TPC)
3.5.3. Determination of Total Flavonoid Content (TFC) Using Aluminum Chloride
3.6. Studies of Biological Activity of Extracts
3.6.1. Antioxidant Activity Assay
DPPH Assay
CUPRAC Assay
3.6.2. α-Glucosidase Inhibition Assay
3.6.3. α-Amylase Inhibition Assay
3.6.4. Anti-Inflammatory Activity Assay
Hyaluronidase Inhibition Assay
Determination of Anti-Inflammatory Potential in RAW 264.7 Model
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32 (Suppl. S1), S62. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 Diabetes Mellitus, Oxidative Stress and Inflammation: Examining the Links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar] [PubMed]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Sugandh, F.N.U.; Chandio, M.; Raveena, F.N.U.; Kumar, L.; Karishma, F.N.U.; Khuwaja, S.; Memon, U.A.; Bai, K.; Kashif, M.; Varrassi, G.; et al. Advances in the Management of Diabetes Mellitus: A Focus on Personalized Medicine. Cureus 2023, 15, e43697. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, V.; Nayak, S.K.; Wadhwa, P.; Kaur, P.; Sahu, S.K. Alpha-Amylase as Molecular Target for Treatment of Diabetes Mellitus: A Comprehensive Review. Chem. Biol. Drug Des. 2021, 98, 539–560. [Google Scholar] [CrossRef]
- Kashtoh, H.; Baek, K.-H. New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects. Plants 2023, 12, 2944. [Google Scholar] [CrossRef]
- Przeor, M. Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals 2022, 15, 65. [Google Scholar] [CrossRef]
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary Polyphenols as Natural Inhibitors of α-Amylase and α-Glucosidase. Life 2022, 12, 1692. [Google Scholar] [CrossRef]
- Viapiana, A.; Wesolowski, M. The Phenolic Contents and Antioxidant Activities of Infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef]
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Health-Promoting Properties: Anti-Inflammatory and Anticancer Properties of Sambucus nigra L. Flowers and Fruits. Molecules 2023, 28, 6235. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders. Molecules 2023, 28, 3148. [Google Scholar] [CrossRef] [PubMed]
- Kielak, O. From the Name to the Popular Image of the Plant: The Polish Names for the Black Elder (Sambucus nigra). J. Ethnobiol. Ethnomed. 2024, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Harokopakis, E.; Albzreh, M.H.; Haase, E.M.; Scannapieco, F.A.; Hajishengallis, G. Inhibition of Proinflammatory Activities of Major Periodontal Pathogens by Aqueous Extracts From Elder Flower (Sambucus nigra). J. Periodontol. 2006, 77, 271–279. [Google Scholar] [CrossRef]
- Laurutis, A.; Liobikas, J.; Stanciauskaite, M.; Marksa, M.; Ramanauskiene, K.; Majiene, D. Comparison of the Formulation, Stability and Biological Effects of Hydrophilic Extracts from Black Elder Flowers (Sambucus nigra L.). Pharmaceutics 2022, 14, 2831. [Google Scholar] [CrossRef]
- Porter, R.S.; Bode, R.F. A Review of the Antiviral Properties of Black Elder (Sambucus nigra L.) Products. Phytother. Res. 2017, 31, 533–554. [Google Scholar] [CrossRef]
- Festa, J.; Singh, H.; Hussain, A.; Da Boit, M. Elderberries as a Potential Supplement to Improve Vascular Function in a SARS-CoV-2 Environment. J. Food Biochem. 2022, 46, e14091. [Google Scholar] [CrossRef]
- Asgary, S.; Pouramini, A. The Pros and Cons of Using Elderberry (Sambucus nigra) for Prevention and Treatment of COVID-19. Adv. Biomed. Res. 2022, 11, 96. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Kolniak-Ostek, J.; Zubaidi, M.A.; Maksimowski, D.; Brandova, P.; Oziembłowski, M. Elderflowers (Sambuci flos L.): A Potential Source of Health-Promoting Components. Foods 2024, 13, 2560. [Google Scholar] [CrossRef]
- Boroduske, A.; Jekabsons, K.; Riekstina, U.; Muceniece, R.; Rostoks, N.; Nakurte, I. Wild Sambucus nigra L. from North-East Edge of the Species Range: A Valuable Germplasm with Inhibitory Capacity against SARS-CoV2 S-Protein RBD and hACE2 Binding In Vitro. Ind. Crops Prod. 2021, 165, 113438. [Google Scholar] [CrossRef]
- Seymenska, D.; Shishkova, K.; Hinkov, A.; Benbassat, N.; Teneva, D.; Denev, P. Comparative Study on Phytochemical Composition, Antioxidant, and Anti-HSV-2 Activities of Sambucus nigra L. and Sambucus ebulus L. Extracts. Appl. Sci. 2023, 13, 12593. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European Elderberry (Sambucus nigra L.) Rich in Sugars, Organic Acids, Anthocyanins and Selected Polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Paczkowska-Walendowska, M.; Woźna, Z.; Plech, T.; Szulc, P.; Cielecka-Piontek, J. Elderberry Leaves with Antioxidant and Anti-Inflammatory Properties as a Valuable Plant Material for Wound Healing. Pharmaceuticals 2024, 17, 618. [Google Scholar] [CrossRef] [PubMed]
- Marțiș (Petruț), G.S.; Mureșan, V.; Marc (Vlaic), R.M.; Mureșan, C.C.; Pop, C.R.; Buzgău, G.; Mureșan, A.E.; Ungur, R.A.; Muste, S. The Physicochemical and Antioxidant Properties of Sambucus nigra L. and Sambucus nigra Haschberg during Growth Phases: From Buds to Ripening. Antioxidants 2021, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef]
- Pascariu, O.-E.; Israel-Roming, F. Bioactive Compounds from Elderberry: Extraction, Health Benefits, and Food Applications. Processes 2022, 10, 2288. [Google Scholar] [CrossRef]
- Vujanović, M.; Majkić, T.; Zengin, G.; Beara, I.; Tomović, V.; Šojić, B.; Đurović, S.; Radojković, M. Elderberry (Sambucus nigra L.) Juice as a Novel Functional Product Rich in Health-Promoting Compounds. RSC Adv. 2020, 10, 44805–44814. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhang, M.; Sun, B. Novel Approach for Extraction of Grape Skin Antioxidants by Accelerated Solvent Extraction: Box–Behnken Design Optimization. J. Food Sci. Technol. 2019, 56, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Hosni, S.; Gani, S.S.A.; Orsat, V.; Hassan, M.; Abdullah, S. Ultrasound-Assisted Extraction of Antioxidants from Melastoma Malabathricum Linn.: Modeling and Optimization Using Box–Behnken Design. Molecules 2023, 28, 487. [Google Scholar] [CrossRef]
- Gościniak, A.; Bazan-Woźniak, A.; Pietrzak, R.; Cielecka-Piontek, J. Pomegranate Flower Extract—The Health-Promoting Properties Optimized by Application of the Box–Behnken Design. Molecules 2022, 27, 6616. [Google Scholar] [CrossRef]
- Tai, N.V.; Linh, M.N.; Thuy, N.M. Optimization of Extraction Conditions of Phytochemical Compounds in “Xiem” Banana Peel Powder Using Response Surface Methodology. J. Appl. Biol. Biotechnol. 2021, 9, 56–62. [Google Scholar] [CrossRef]
- Oziembłowski, M.; Nawirska-Olszańska, A.; Maksimowski, D. Optimization of Chlorogenic Acid in Ethanol Extracts from Elderberry Flowers (Sambucus nigra L.) under Different Conditions: Response Surface Methodology. Appl. Sci. 2023, 13, 3201. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as Potential Source of Antioxidants. Characterization, Optimization of Extraction Parameters and Bioactive Properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef] [PubMed]
- Zawiślak, A.; Francik, R.; Francik, S.; Knapczyk, A. Impact of Drying Conditions on Antioxidant Activity of Red Clover (Trifolium pratense), Sweet Violet (Viola odorata) and Elderberry Flowers (Sambucus nigra). Materials 2022, 15, 3317. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O. Anthocyanins as Natural Food Colorings: The Chemistry Behind and Challenges Still Ahead. J. Agric. Food Chem. 2024, 72, 12356–12372. [Google Scholar] [CrossRef]
- Matłok, N.; Kapusta, I.; Piechowiak, T.; Zardzewiały, M.; Gorzelany, J.; Balawejder, M. Characterisation of Some Phytochemicals Extracted from Black Elder (Sambucus nigra L.) Flowers Subjected to Ozone Treatment. Molecules 2021, 26, 5548. [Google Scholar] [CrossRef]
- Tundis, R.; Ursino, C.; Bonesi, M.; Loizzo, M.R.; Sicari, V.; Pellicanò, T.; Manfredi, I.L.; Figoli, A.; Cassano, A. Flower and Leaf Extracts of Sambucus nigra L.: Application of Membrane Processes to Obtain Fractions with Antioxidant and Antityrosinase Properties. Membranes 2019, 9, 127. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Chen, X.; Xie, N.; Feng, L.; Huang, Y.; Wu, Y.; Zhu, H.; Tang, J.; Zhang, Y. Oxidative Stress in Diabetes Mellitus and Its Complications: From Pathophysiology to Therapeutic Strategies. Chin. Med. J. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzymes Inhibitors from Natural Sources with Antidiabetic Activity: A Review. Phytother. Res. 2019, 33, 41–54. [Google Scholar] [CrossRef]
- Assefa, S.T.; Yang, E.-Y.; Chae, S.-Y.; Song, M.; Lee, J.; Cho, M.-C.; Jang, S. Alpha Glucosidase Inhibitory Activities of Plants with Focus on Common Vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and Chlorogenic Acids Inhibit Key Enzymes Linked to Type 2 Diabetes (In Vitro): A Comparative Study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Bljajić, K.; Brajković, A.; Čačić, A.; Vujić, L.; Jablan, J.; Saraiva de Carvalho, I.; Zovko Končić, M. Chemical Composition, Antioxidant, and α-Glucosidase-Inhibiting Activity of Aqueous and Hydroethanolic Extracts of Traditional Antidiabetics from Croatian Ethnomedicine. Horticulturae 2021, 7, 15. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of Harvesting Year and Elderberry Cultivar on the Chemical Composition and Potential Bioactivity: A Three-Year Study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of Porcine Pancreatic α-Amylase Activity by Chlorogenic Acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Dubey, S.; Ganeshpurkar, A.; Ganeshpurkar, A.; Bansal, D.; Dubey, N. Glycolytic Enzyme Inhibitory and Antiglycation Potential of Rutin. Future J. Pharm. Sci. 2017, 3, 158–162. [Google Scholar] [CrossRef]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation–Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef]
- Jung, H. Hyaluronidase: An Overview of Its Properties, Applications, and Side Effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef]
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Kim, Y.-C.; Chung, S.-K. Identification and in Vitro Biological Activities of Flavonols in Garlic Leaf and Shoot: Inhibition of Soybean Lipoxygenase and Hyaluronidase Activities and Scavenging of Free Radicals. J. Sci. Food Agric. 2005, 85, 633–640. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, G.-H. Evaluation of Antioxidant and Inhibitory Activities for Different Subclasses Flavonoids on Enzymes for Rheumatoid Arthritis. J. Food Sci. 2010, 75, H212–H217. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.T.T.; Wangensteen, H.; Barsett, H. Elderberry and Elderflower Extracts, Phenolic Compounds, and Metabolites and Their Effect on Complement, RAW 264.7 Macrophages and Dendritic Cells. Int. J. Mol. Sci. 2017, 18, 584. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Galanty, A.; Gościniak, A.; Wieczorek, M.; Kłaput, M.; Dudek-Makuch, M.; Cielecka-Piontek, J. Herbal Infusions as a Valuable Functional Food. Nutrients 2021, 13, 4051. [Google Scholar] [CrossRef]
- Galanty, A.; Juncewicz, P.; Podolak, I.; Grabowska, K.; Służały, P.; Paśko, P. Comparative Analysis of Polyphenolic Profile and Chemopreventive Potential of Hemp Sprouts, Leaves, and Flowers of the Sofia Variety. Plants 2024, 13, 2023. [Google Scholar] [CrossRef]




| Cultivar | Content [mg of Standard/g of Plant Material] | TPC [mg GAE/g] | TFC [mg QE/g] | ||
|---|---|---|---|---|---|
| Chlorogenic Acid | Rutin | Isoquercetin | |||
| Samyl | 19.35 ± 0.16 c | 25.70 ± 0.11 c | 0.16 ± 0.01 h | 33.00 ± 0.49 e | 12.52 ± 0.23 g |
| Samyl 1 | 19.40 ± 0.07 c | 14.35 ± 0.22 g | 2.13 ± 0.04 c | 36.00 ± 0.77 d | 13.90 ± 0.48 e |
| Obelisk | 21.03 ± 0.00 b | 30.12 ± 0.18 b | 1.91 ± 0.11 d | 36.48 ± 1.09 d | 18.65 ± 0.25 b |
| Sambo | 19.46 ± 0.00 c | 10.63 ± 0.06 k | 4.69 ± 0.00 a | 38.47 ± 1.75 c | 13.44 ± 0.31 f |
| Golden Hybrid | 16.00 ± 0.02 g | 4.01 ± 0.01 l | 0.53 ± 0.02 g | 32.14 ± 1.38 e,f | 16.68 ± 0.35 c,d |
| Bez koralowy | 9.39 ± 0.05 j | 22.41 ± 0.27 d | 0.22 ± 0.01 h | 25.05 ± 1.12 h | 13.15 ± 0.14 f |
| Haschberg | 18.57 ± 0.44 d | 38.37 ± 0.01 a | 0.09 ± 0.03 h | 30.80 ± 0.89 f | 20.29 ± 0.20 a |
| Sampo | 16.78 ± 0.05 f | 11.33 ± 0.15 j | 1.78 ± 0.11 d,e | 32.40 ± 0.67 e,f | 11.68 ± 0.17 h |
| Black Tower | 17.30 ± 0.02 e | 16.58 ± 0.03 f | 1.72 ± 0.00 e | 40.48 ± 1.04 b | 16.28 ± 0.09 d |
| Black Beauty | 23.73 ± 0.01 a | 12.75 ± 0.01 i | 1.10 ± 0.21 f | 44.97 ± 1.15 a | 14.27 ± 0.18 e |
| Haschberg 1 | 18.46 ± 0.06 d | 10.51 ± 0.00 k | 4.46 ± 0.05 b | 34.95 ± 1.23 d | 12.72 ± 0.12 g |
| Bez dwubarwny | 14.78 ± 0.15 i | 20.09 ± 0.30 e | 1.84 ± 0.12 d,e | 32.09 ± 0.61 e,f | 16.84 ± 0.23 c |
| Wild elderberry | 15.39 ± 0.10 h | 13.33 ± 0.15 h | 1.76 ± 0.09 d,e | 28.57 ± 0.77 g | 11.46 ± 0.23 h |
| Cultivar | DPPH [mg TE/g] | CUPRAC [mg TE/g] |
|---|---|---|
| Samyl | 38.30 ± 4.64 c,d | 55.10 ± 0.92 g |
| Samyl 1 | 39.61 ± 1.80 c,d | 70.32 ± 0.70 b |
| Obelisk | 49.92 ± 2.40 a | 68.25 ± 1.00 c |
| Sambo | 40.09 ± 1.82 c | 67.80 ± 2.06 c |
| Golden Hybrid | 33.22 ± 1.03 e,f | 62.46 ± 1.73 e |
| Bez koralowy | 19.23 ± 0.64 h | 42.32 ± 0.99 j |
| Haschberg | 45.01 ± 1.12 b | 65.71 ± 0.80 d |
| Sampo | 31.57 ± 1.29 f,g | 59.16 ± 1.41 f |
| Black Tower | 38.18 ± 2.16 c,d | 67.71 ± 1.09 c |
| Black Beauty | 53.15 ± 0.85 a | 77.19 ± 1.21 a |
| Haschberg 1 | 41.16 ± 2.41 c | 62.71 ± 1.00 e |
| Bez dwubarwny | 35.92 ± 2.00 d,e | 52.70 ± 1.11 h |
| Wild elderberry | 29.34 ± 2.47 g | 50.47 ± 0.21 i |
| Chlorogenic acid | 0.150 ± 0.011 IC50 [mg/mL] | 25.70 ± 0.52 IC0.5 [mg/mL] |
| Rutin | 0.148 ± 0.000 IC50 [mg/mL] | 39.86 ± 0.21 IC0.5 [mg/mL] |
| Cultivar | α-Glucosidase Inhibition [%] | α-Amylase Inhibition [%] |
|---|---|---|
| Samyl | 42.31 ± 1.67 e | nd |
| Samyl 1 | 50.96 ± 1.79 b | nd |
| Obelisk | 49.61 ± 1.36 b,c | 33.95 ± 3.48 a |
| Sambo | 48.34 ± 1.70 b,c,d | 13.48 ± 1.45 c |
| Golden Hybrid | 46.72 ± 1.11 c,d | 22.89 ± 2.77 b |
| Bez koralowy | 34.30 ± 3.52 f | nd |
| Haschberg | 55.58 ± 2.42 a | nd |
| Sampo | 45.25 ± 1.25 d,e | nd |
| Black Tower | 51.39 ± 0.83 b | nd |
| Black Beauty | 55.89 ± 1.51 a | nd |
| Haschberg 1 | 46.83 ± 1.51 c,d | 13.70 ± 2.28 c |
| Bez dwubarwny | 42.24 ± 1.51 e | 22.70 ± 2.34 b |
| Wild elderberry | 29.74 ± 1.21 g | nt |
| Chlorogenic acid | 0.509 ± 0.021 IC50 [mg/mL] | nt |
| Rutin | 0.390 ± 0.011 IC50 [mg/mL] | nt |
| Acarbose 3.125 | 27.85 ± 1.70 | nt |
| Acarbose | 8.24 ± 0.55 IC50 [mg/mL] | 0.011 ± 0.001 IC50 [mg/mL] |
| Lp. | Methanol Content [%] | Time [min] | Solvent Volume [mL] |
|---|---|---|---|
| S1 | 50 | 15 | 50 |
| S2 | 100 | 52.5 | 10 |
| S3 | 0 | 52.5 | 50 |
| S4 | 100 | 52.5 | 50 |
| S5 | 0 | 52.5 | 10 |
| S6 | 50 | 52.5 | 30 |
| S7 | 100 | 15 | 30 |
| S8 | 50 | 15 | 10 |
| S9 | 0 | 15 | 30 |
| S10 | 100 | 90 | 30 |
| S11 | 0 | 90 | 30 |
| S12 | 50 | 90 | 50 |
| S13 | 50 | 90 | 10 |
| S14 | 50 | 52.5 | 30 |
| S15 | 50 | 52.5 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studzińska-Sroka, E.; Paczkowska-Walendowska, M.; Kledzik, J.; Galanty, A.; Gościniak, A.; Szulc, P.; Korybalska, K.; Cielecka-Piontek, J. Antidiabetic Potential of Black Elderberry Cultivars Flower Extracts: Phytochemical Profile and Enzyme Inhibition. Molecules 2024, 29, 5775. https://doi.org/10.3390/molecules29235775
Studzińska-Sroka E, Paczkowska-Walendowska M, Kledzik J, Galanty A, Gościniak A, Szulc P, Korybalska K, Cielecka-Piontek J. Antidiabetic Potential of Black Elderberry Cultivars Flower Extracts: Phytochemical Profile and Enzyme Inhibition. Molecules. 2024; 29(23):5775. https://doi.org/10.3390/molecules29235775
Chicago/Turabian StyleStudzińska-Sroka, Elżbieta, Magdalena Paczkowska-Walendowska, Justyna Kledzik, Agnieszka Galanty, Anna Gościniak, Piotr Szulc, Katarzyna Korybalska, and Judyta Cielecka-Piontek. 2024. "Antidiabetic Potential of Black Elderberry Cultivars Flower Extracts: Phytochemical Profile and Enzyme Inhibition" Molecules 29, no. 23: 5775. https://doi.org/10.3390/molecules29235775
APA StyleStudzińska-Sroka, E., Paczkowska-Walendowska, M., Kledzik, J., Galanty, A., Gościniak, A., Szulc, P., Korybalska, K., & Cielecka-Piontek, J. (2024). Antidiabetic Potential of Black Elderberry Cultivars Flower Extracts: Phytochemical Profile and Enzyme Inhibition. Molecules, 29(23), 5775. https://doi.org/10.3390/molecules29235775












