Formulation of Ready-to-Use Broccoli Extracts Rich in Polyphenols and Glucosinolates Using Natural Deep Eutectic Solvents
Abstract
1. Introduction
2. Results and Discussion
2.1. In Silico Pre-Selection of NADES
2.2. Preparation and Characterization of NADES
2.3. Characterization of Extracts
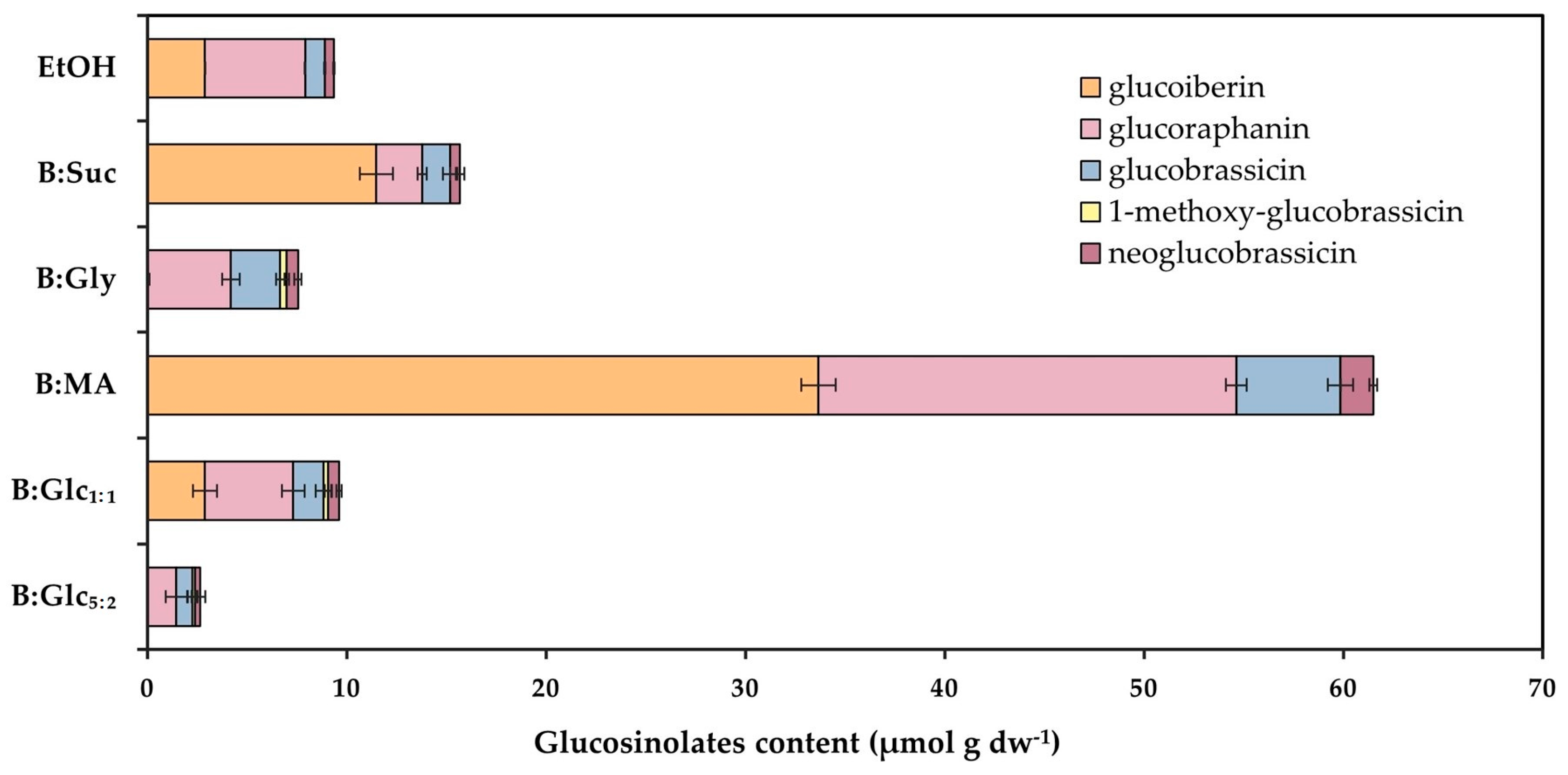
2.3.1. Content of Bioactives
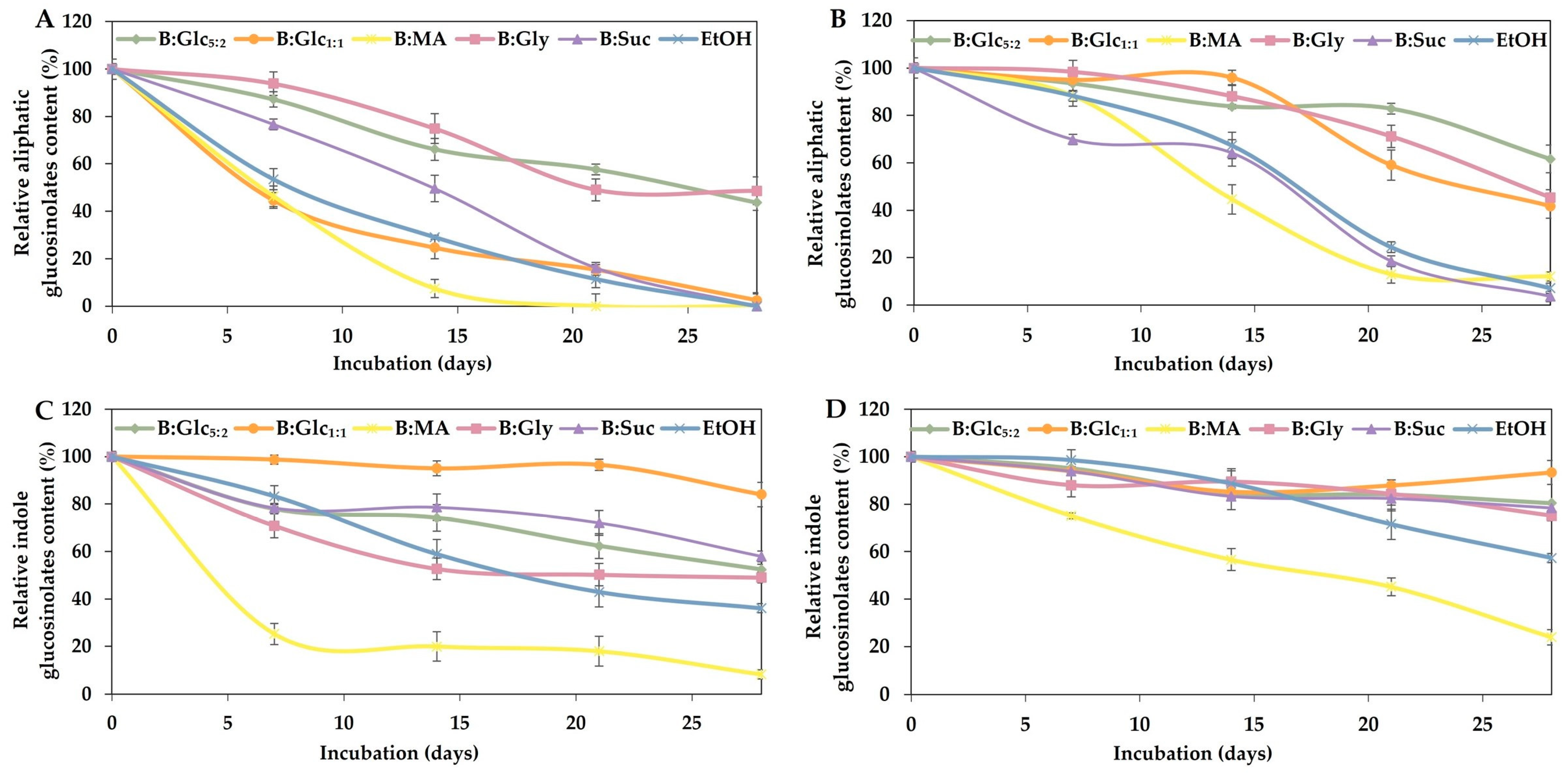
2.3.2. Stability of Bioactives
2.3.3. Antioxidative Activity
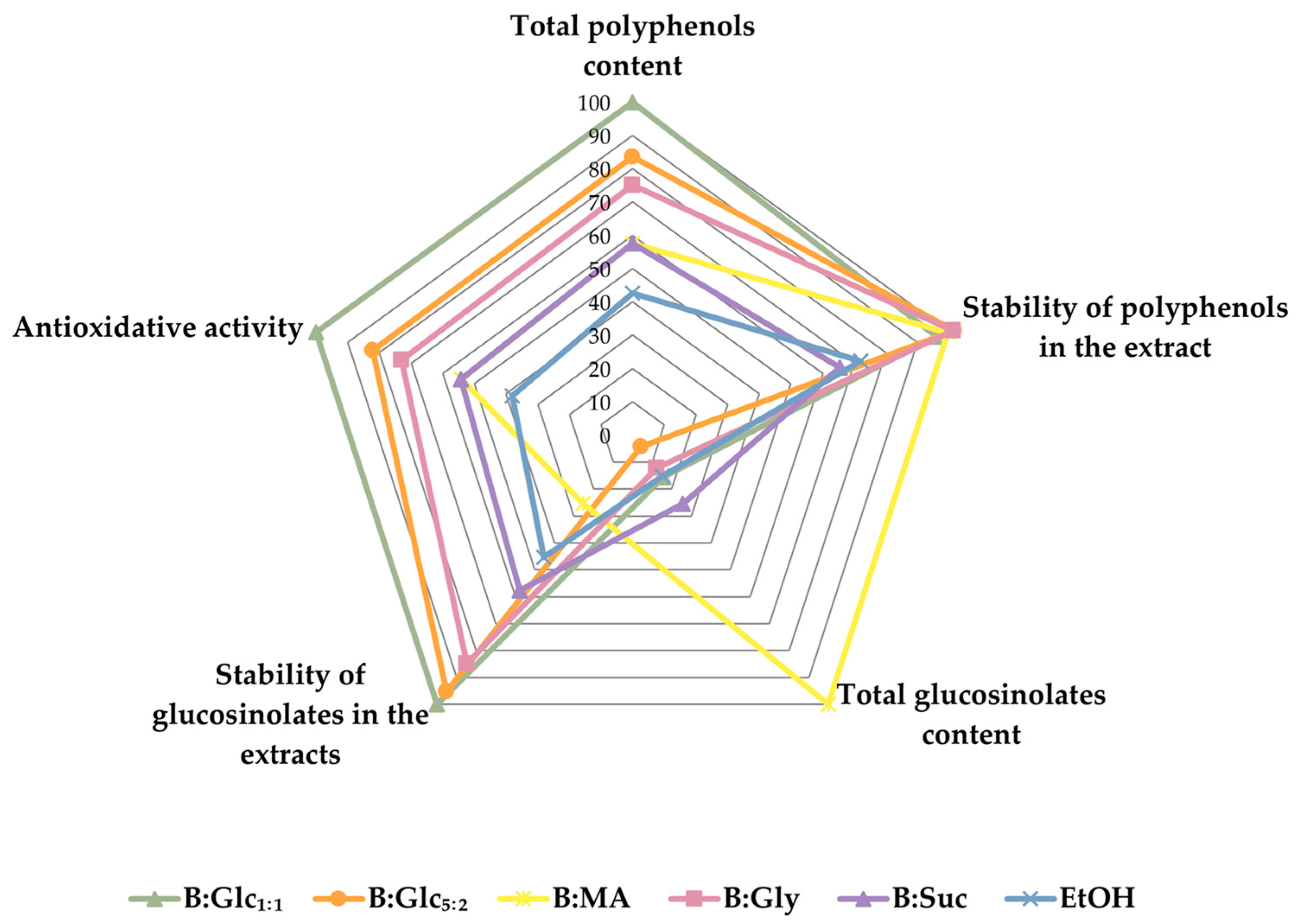
2.3.4. Trades-Off Between the Extracts with Respect to Target Properties
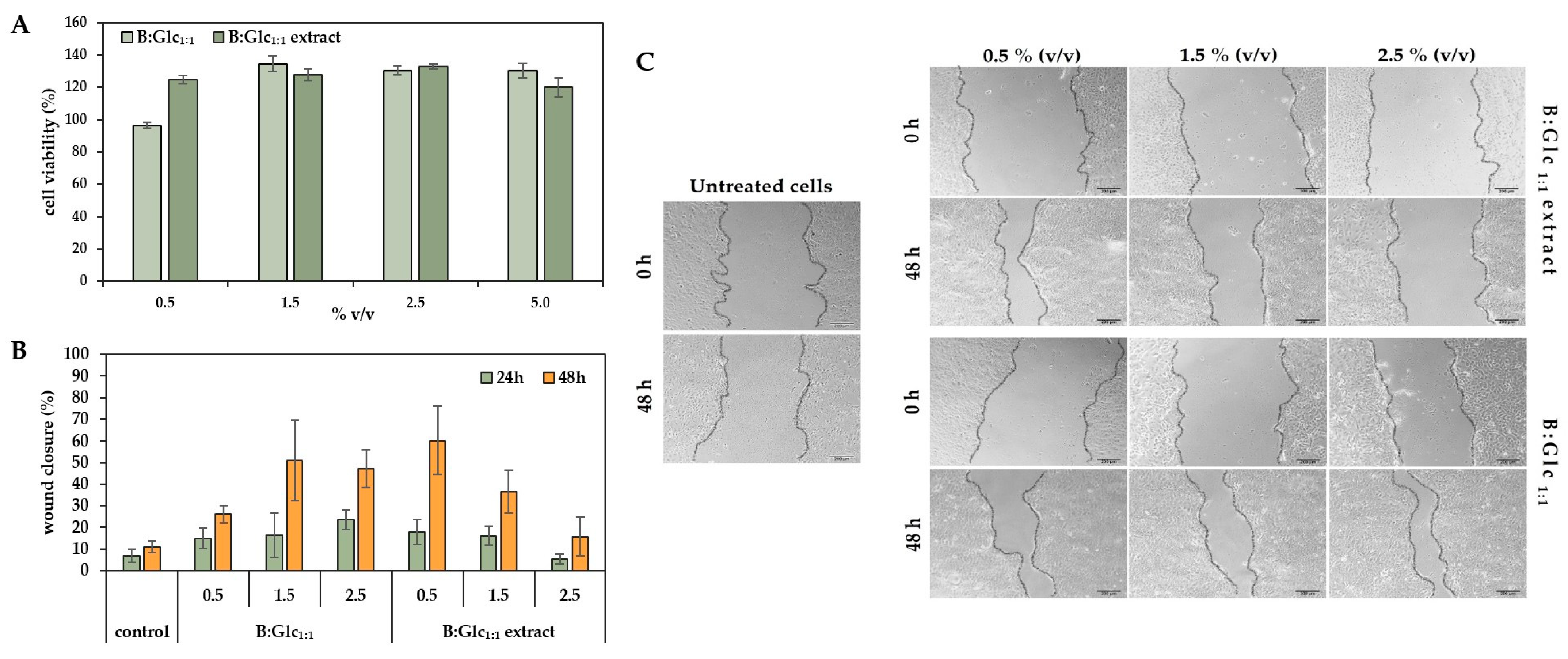
2.3.5. Evaluation of Keratinocyte Response to B:Glc1:1-Based Broccoli Extract
2.4. Sustainability Assessment of the B:Glc1:1 Broccoli Extract Preparation Method
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Computational Methods
3.3. NADES Preparation and Characterisation
3.4. Solid–Liquid Extractions
3.5. Determination of Total Phenolic Content
3.6. Determination of Individual Aliphatic and Indole Glucosinolates
3.6.1. Desulfation of Prepared Glucosinolate-Rich Extracts Using the Enzyme Sulfatase
3.6.2. High-Performance Liquid Chromatography of Desulfo-Glucosinolates
3.7. Oxygen Radical Absorbance Capacity Assay (ORAC)
3.8. Determination of Biological Activity
3.9. Scratch Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The Role of Sulforaphane in Cancer Chemoprevention and Health Benefits: A Mini-Review. J. Cell Commun. Signal. 2017, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of Industrial Broccoli Discards (Brassica oleracea var. Italica) for Their Glucosinolate, Polyphenol and Flavonoid Contents Using UPLC MS/MS and Spectrophotometric Methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Bachiega, P.; Salgado, J.M.; De Carvalho, J.E.; Ruiz, A.L.T.G.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and Antiproliferative Activities in Different Maturation Stages of Broccoli (Brassica oleracea Italica) Biofortified with Selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.; Healy, Z.; Wehage, S.; Benedict, A.; Min, C.; Dinkova-Kostova, A.; Talalay, P. Broccoli Sprout-Derived Extract Protects against Ultraviolet Radiation. Cancer Biol. Ther. 2007, 6, 1675. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Radojčić Redovniković, I.; Vidović, S.; Radošević, K.; Andreou, T.; Mourtzinos, I.; Cvjetko Bubalo, M. Coupling Deep Eutectic Solvents with Innovative Extraction Techniques towards Plant Derived Bioactive Compositions. RSC Sustain. 2024, 2, 1675–1691. [Google Scholar] [CrossRef]
- Abranches, D.O.; Coutinho, J.A.P. Everything You Wanted to Know about Deep Eutectic Solvents but Were Afraid to Be Told. Annu. Rev. Chem. Biomol. Eng. 2023, 14, 141–163. [Google Scholar] [CrossRef]
- Rente, D.; Cvjetko Bubalo, M.; Panić, M.; Paiva, A.; Caprin, B.; Radojčić Redovniković, I.; Duarte, A.R.C. Review of Deep Eutectic Systems from Laboratory to Industry, Taking the Application in the Cosmetics Industry as an Example. J. Clean. Prod. 2022, 380, 135147. [Google Scholar] [CrossRef]
- Ferreira, C.; Sarraguça, M. A Comprehensive Review on Deep Eutectic Solvents and Its Use to Extract Bioactive Compounds of Pharmaceutical Interest. Pharmaceuticals 2024, 17, 124. [Google Scholar] [CrossRef]
- Panić, M.; Radić Stojković, M.; Kraljić, K.; Škevin, D.; Radojčić Redovniković, I.; Gaurina Srček, V.; Radošević, K. Ready-to-Use Green Polyphenolic Extracts from Food by-Products. Food Chem. 2019, 283, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, Cytotoxic and Antioxidative Evaluation of Natural Deep Eutectic Solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced Extraction of Bioactive Natural Products Using Tailor-Made Deep Eutectic Solvents: Application to Flavonoid Extraction from Flos Sophorae. Green. Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Gunjević, V.; Radošević, K.; Bubalo, M.C.; Ganić, K.K.; Redovniković, I.R. COSMOtherm as an Effective Tool for Selection of Deep Eutectic Solvents Based Ready-To-Use Extracts from Graševina Grape Pomace. Molecules 2021, 26, 4722. [Google Scholar] [CrossRef]
- Klamt, A.; Jonas, V.; Bürger, T.; Lohrenz, J.C.W. Refinement and Parametrization of COSMO-RS. J. Phys. Chem. A 1998, 102, 5074–5085. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Ferreira, A.M.; Abranches, D.O.; Mafra, M.R.; Coutinho, J.A.P. Using COSMO-RS in the Design of Deep Eutectic Solvents for the Extraction of Antioxidants from Rosemary. ACS Sustain. Chem. Eng. 2020, 8, 12132–12141. [Google Scholar] [CrossRef]
- Lazović, M.; Cvijetić, I.; Jankov, M.; Milojković-Opsenica, D.; Trifković, J.; Ristivojević, P. COSMO-RS in Prescreening of Natural Eutectic Solvents for Phenolic Extraction from Teucrium Chamaedrys. J. Mol. Liq. 2023, 387, 122649. [Google Scholar] [CrossRef]
- Šalić, A.; Bajo, M.; Cvjetko Bubalo, M.; Radović, M.; Jurinjak Tušek, A.; Zelić, B.; Šamec, D. Extraction of Polyphenolic Compounds from Ginkgo Leaves Using Deep Eutectic Solvents: A Potential Solution for the Sustainable and Environmentally Friendly Isolation of Biflavonoids. Ind. Crops Prod. 2024, 219, 119068. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Z.; Dong, C.; Ni, W.; Xin, K.; Yu, Q.; Han, L. Green Ultrasound-Assisted Natural Deep Eutectic Solvent Extraction of Phenolic Compounds from Waste Broccoli Leaves: Optimization, Identification, Biological Activity, and Structural Characterization. LWT 2023, 190, 115407. [Google Scholar] [CrossRef]
- Dai, Y.; Row, K.H. Application of Natural Deep Eutectic Solvents in the Extraction of Quercetin from Vegetables. Molecules 2019, 24, 2300. [Google Scholar] [CrossRef]
- Wang, B.; Chen, P.; Zhang, H.; Chen, L. Deep Eutectic Solvents Extraction of Polyphenol from the Stem of Broccoli: Optimization, Components, and Antioxidant Activity. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Development of a Method Based on Natural Deep Eutectic Solvents for Extraction of Flavonoids from Food Samples. Food Anal. Methods 2018, 11, 1330–1344. [Google Scholar] [CrossRef]
- Cicco, L.; Dilauro, G.; Perna, F.M.; Vitale, P.; Capriati, V. Advances in Deep Eutectic Solvents and Water: Applications in Metal- and Biocatalyzed Processes, in the Synthesis of APIs, and Other Biologically Active Compounds. Org. Biomol. Chem. 2021, 19, 2558–2577. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Sakulsataporn, N.; Chiu, C.H.; Hsieh, P.C. Polyphenolic Profile and Varied Bioactivities of Processed Taiwanese Grown Broccoli: A Comparative Study of Edible and Non-Edible Parts. Pharmaceuticals 2020, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Oliveira, F.S.; Kurnia, K.A.; Marrucho, I.M. Deep Eutectic Solvents as Azeotrope Breakers: Liquid-Liquid Extraction and COSMO-RS Prediction. ACS Sustain. Chem. Eng. 2016, 4, 5640–5650. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of Natural Deep Eutectic Solvents to the Extraction of Anthocyanins from Catharanthus Roseus with High Extractability and Stability Replacing Conventional Organic Solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of PH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Zhao, S.J.; Ruan, S.Y.; Xie, Z.H.; Dong, Y.; Yu, L. Anthocyanin and Glucosinolate Occurrences in the Roots of Chinese Red Radish (Raphanus sativus L.), and Their Stability to Heat and PH. Food Chem. 2012, 133, 1569–1576. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Martí-Quijal, F.J.; Cilla, A.; Munekata, P.E.S.; Lorenzo, J.M.; Remize, F.; Barba, F.J. Influence of Temperature, Solvent and PH on the Selective Extraction of Phenolic Compounds from Tiger Nuts by-Products: Triple-TOF-LC-MS-MS Characterization. Molecules 2019, 24, 797. [Google Scholar] [CrossRef]
- Sun, H.N.; Mu, T.H.; Xi, L.S. Effect of PH, Heat, and Light Treatments on the Antioxidant Activity of Sweet Potato Leaf Polyphenols. Int. J. Food Prop. 2017, 20, 318–332. [Google Scholar] [CrossRef]
- Damjanović, A.; Logarušić, M.; Tumir, L.M.; Andreou, T.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Enhancing Protein Stability under Stress: Osmolyte-Based Deep Eutectic Solvents as a Biocompatible and Robust Stabilizing Medium for Lysozyme under Heat and Cold Shock. Phys. Chem. Chem. Phys. 2024, 26, 21040–21051. [Google Scholar] [CrossRef]
- Radović, M.; Hok, L.; Panić, M.; Cvjetko Bubalo, M.; Vianello, R.; Vinković, M.; Radojčić Redovniković, I. Deep Eutectic Solvents as a Stabilising Medium for NAD Coenzyme: Unravelling the Mechanism behind Coenzyme Stabilisation Effect. Green. Chem. 2022, 24, 7661–7674. [Google Scholar] [CrossRef]
- Radünz, M.; Hackbart, H.C.D.S.; Bona, N.P.; Pedra, N.S.; Hoffmann, J.F.; Stefanello, F.M.; Da Rosa Zavareze, E. Glucosinolates and Phenolic Compounds Rich Broccoli Extract: Encapsulation by Electrospraying and Antitumor Activity against Glial Tumor Cells. Colloids Surf. B Biointerfaces 2020, 192, 111020. [Google Scholar] [CrossRef] [PubMed]
- Ianni, F.; Scandar, S.; Mangiapelo, L.; Blasi, F.; Marcotullio, M.C.; Cossignani, L. NADES-Assisted Extraction of Polyphenols from Coriander Seeds: A Systematic Optimization Study. Antioxidants 2023, 12, 2048. [Google Scholar] [CrossRef]
- Zhuang, B.; Dou, L.L.; Li, P.; Liu, E.H. Deep Eutectic Solvents as Green Media for Extraction of Flavonoid Glycosides and Aglycones from Platycladi Cacumen. J. Pharm. Biomed. Anal. 2017, 134, 214–219. [Google Scholar] [CrossRef]
- Yoo, D.E.; Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep Eutectic Solvent-Based Valorization of Spent Coffee Grounds. Food Chem. 2018, 255, 357–364. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Li, H. Green Extraction of Polyphenols via Deep Eutectic Solvents and Assisted Technologies from Agri-Food By-Products. Molecules 2023, 28, 6852. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, X.; Liu, P.; Huang, J.; Wang, C.; Pan, M.; Kuang, Z. Enhanced Phenolic Compounds Extraction from Morus alba L. Leaves by Deep Eutectic Solvents Combined with Ultrasonic-Assisted Extraction. Ind. Crops Prod. 2018, 120, 147–154. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Ayumi Shiwaku, I.; Maximo, G.J.; Caldas Batista, E.A. Choline Chloride-Based Deep Eutectic Solvents as Potential Solvent for Extraction of Phenolic Compounds from Olive Leaves: Extraction Optimization and Solvent Characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Wang, J.; Cai, C.; Chang, J.; Zhao, Y.; Wang, Q. Accumulation of Glucosinolates in Broccoli. In Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2017; pp. 133–162. [Google Scholar] [CrossRef]
- Kushad, M.M.; Brown, A.F.; Kurilich, A.C.; Juvik, J.A.; Klein, B.P.; Wallig, M.A.; Jeffery, E.H. Variation of Glucosinolates in Vegetable Crops of Brassica Oleracea. J. Agric. Food Chem. 1999, 47, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Mitar, A.; Kučić Grgić, D.; Prlić Kardum, J. Extraction and Stability of Olive Pomace Polyphenols in Natural Deep Eutectic Solvents. Kem. Ind. Cas. Kem. Kem. Inž. Hrvat. 2019, 68, 407–414. [Google Scholar] [CrossRef]
- Dai, Y.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents Providing Enhanced Stability of Natural Colorants from Safflower (Carthamus Tinctorius). Food Chem. 2014, 159, 116–121. [Google Scholar] [CrossRef]
- Rebocho, S.; Mano, F.; Cassel, E.; Anacleto, B.; Bronze, M.d.R.; Paiva, A.; Duarte, A.R.C. Fractionated Extraction of Polyphenols from Mate Tea Leaves Using a Combination of Hydrophobic/ Hydrophilic NADES. Curr. Res. Food Sci. 2022, 5, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Logarušić, M.; Šubar, K.; Nikolić, M.; Tušek, A.J.; Damjanović, A.; Radović, M.; Redovniković, I.R.; Žnidaršič-Plazl, P.; Kroutil, W.; Bubalo, M.C.; et al. Harnessing the Potential of Deep Eutectic Solvents in Biocatalysis: Design Strategies Using CO2 to Formate Reduction as a Case Study. Front. Chem. 2024, 12, 1467810. [Google Scholar] [CrossRef]
- Sakurai, S.; Kawakami, Y.; Kuroki, M.; Gotoh, H. Structure–Antioxidant Activity (Oxygen Radical Absorbance Capacity) Relationships of Phenolic Compounds. Struct. Chem. 2022, 33, 1055–1062. [Google Scholar] [CrossRef]
- Borowski, J.; Szajdek, A.; Borowska, E.J.; Ciska, E.; Zieliński, H. Content of Selected Bioactive Components and Antioxidant Properties of Broccoli (Brassica oleracea L.). Eur. Food Res. Technol. 2008, 226, 459–465. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Zhang, X. Topical Insulin Application Improves Healing by Regulating the Wound Inflammatory Response. Wound Repair. Regen. 2012, 20, 425–434. [Google Scholar] [CrossRef]
- Mahibalan, S.; Stephen, M.; Nethran, R.T.; Khan, R.; Begum, S. Dermal Wound Healing Potency of Single Alkaloid (Betaine) versus Standardized Crude Alkaloid Enriched-Ointment of Evolvulus Alsinoides. Pharm. Biol. 2016, 54, 2851–2856. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of Deep Eutectic Solvents in Biotechnology and Bioengineering—Promises and Challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-Functioning Deep Eutectic Solvents as Extraction and Storage Media for Bioactive Natural Products That Are Readily Applicable to Cosmetic Products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- ISO 9167:2019; Determination of the Glucosinolate Content—Method Using High-Performance Liquid Chromatography (HPLC). International Organization for Standardization: Geneva, Switzerland, 2019.
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nature Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]



| Water Content (%, w/w) | Water Content (%, w/w) | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 30 | 50 | 10 | 30 | 50 | 10 | 30 | 50 | 10 | 30 | 50 | 10 | 30 | 50 | 10 | 30 | 50 | ||||||
| NADES | Molar Ratio | Quercetin | Ferulic Acid | Glucoraphanin | NADES | Molar Ratio | Quercetin | Ferulic Acid | Glucoraphanin | ||||||||||||||
| B:CA | 1:1 | ChCl:Sol | 2:3 | ||||||||||||||||||||
| B:Glc | 5:2 | ChCl:Sor | 1:1 | ||||||||||||||||||||
| B:Glc | 1:1 | ChCl:U | 1:2 | ||||||||||||||||||||
| B:Gly | 1:2 | ChCl:U:Gly | 1:2:2 | ||||||||||||||||||||
| B:OxA:Gly | 1:2:1 | ChCl:Xyl | 2:1 | ||||||||||||||||||||
| B:Ma | 1:1 | ChCl:Xyol | 5:2 | ||||||||||||||||||||
| B:Ma:Glc | 1:1:1 | CA:Fru | 1:1 | ||||||||||||||||||||
| B:Ma:Pro | 1:1:1 | CA:Glc | 1:1 | ||||||||||||||||||||
| B:Arg | 1:1 | CA:Glc:Gly | 1:1:1 | ||||||||||||||||||||
| B:His | 1:1 | CA:Sor | 2:3 | ||||||||||||||||||||
| B:Lys | 1:1 | CA:Suc | 1:1 | ||||||||||||||||||||
| B:Xyl | 1:1 | Fru:Glc:U | 1:1:2 | ||||||||||||||||||||
| B:Suc | 4:1 | Glc:EG | 1:2 | ||||||||||||||||||||
| ChCl:CA | 2:1 | Glc:Fru | 1:1 | ||||||||||||||||||||
| ChCl:CA | 1:1 | Gly:Glc | 2:1 | ||||||||||||||||||||
| ChCl:Fru | 1:1 | Ma:Fru | 2:1 | ||||||||||||||||||||
| ChCl:Glc | 2:1 | Ma:Fru:Gly | 1:1 | ||||||||||||||||||||
| ChCl:Glc | 1:1 | Ma:Glc | 1:1:1 | ||||||||||||||||||||
| ChCl:Gly | 1:2 | Ma:Glc:Gly | 1:1 | Legend | ln(γ) | ||||||||||||||||||
| ChCh:Ma | 1:1 | Ma:Sor:Gly | 1:1:1 | >2 | |||||||||||||||||||
| ChCh:Mal | 4:1 | Ma:Suc | 1:1:2 | 0–2 | |||||||||||||||||||
| ChCl:OxA | 1:1 | Pro:Fru:Gly | 2:1 | −3–0 | |||||||||||||||||||
| ChCl:Pro:Ma | 1:1:1 | Pro:Ma | 1:1:1 | −6–(−3) | |||||||||||||||||||
| ChCl:Suc | 2:1 | Suc:Glc:Fru | 1:2 | −10–(−6) | |||||||||||||||||||
| ChCl:Sol | 1:1 | Suc:Glc:U | 1:1:1 | <−10 | |||||||||||||||||||
| NADES | Molar Ratio | Viscosity [mPa·s] | Density [g cm−3] | pH | Polarity [kcal mol−1] | HaCaT EC50 [mg L−1] |
|---|---|---|---|---|---|---|
| B:Glc1:1 | 1:1 | 71.49 | 1.20 | 7.24 | 49.21 | >2000 |
| B:Glc5:2 | 5:2 | 53.45 | 1.20 | 7.85 | 49.90 | >2000 |
| B:Suc | 4:1 | 54.13 | 1.19 | 7.71 | 49.90 | >2000 |
| B:Gly | 1:2 | 25.70 | 1.17 | 7.03 | 49.72 | >2000 |
| B:MA | 1:1 | 61.18 | 1.24 | 3.24 | 49.21 | >2000 |
| Extraction Procedure | Yield | E-Factor A (kg kg−1) | EQ B (kg kg−1) | PMI C (kg kg−1) | Solvent Cost D (EUR kg−1) | |
|---|---|---|---|---|---|---|
| Polyphenols (mg g−1dw) | Glucosinolates (mmol kg−1dw) | |||||
| Conventional extraction | 1.9 | 9.3 | 800 | 800 | 2000 | 160 |
| NADES-assisted extraction (ready-to-use extract) | 4.9 | 9.6 | 0 | 0 | 1100 | 140 |
 | STRENGTHS |
| Flexibility: possibility of the adjustment of NADES properties according to specific requirements, such as solubility and stability of various target components, toxicity, and biodegradability. Biocompatibility: NADES often originate from natural sources and consist of biocompatible components, making them safe for human use and the environment. “Active” Solvent: Since NADES components often possess biological activity (e.g., antioxidant and antimicrobial properties), they can provide added value to the products they are included in. Unique Phytochemical Profiles: The use of NADES enables the preparation of extracts with unique phytochemical profiles. | |
 | WEAKNESSES |
| Complexity of Formulation: Balancing components and water in NADES formulation requires precise control and understanding of chemical interactions, which can be challenging. Limited Stability: Some NADES formulations may exhibit limited stability under certain storage conditions, potentially resulting in phase separation or degradation of active compounds over time, such as the negative impact of low pH in acidic NADES. Challenges in Scale-Up: Transitioning the preparation and use of NADES from laboratory to industrial scale, due to high viscosities and densities of NADES, can pose challenges in maintaining the consistency and quality of the final product. | |
 | OPPORTUNITIES |
| Expanding Applications: Collaboration between the academic community and institutions across various research fields could lead to broader applications of NADES in production and industrial development. Regulatory Approvals: Obtaining regulatory approvals could enhance the commercialization of these natural solvents, creating opportunities to open new markets. Enhanced Characterization: Increased application of NADES could potentially allow for more detailed characterization of known plant metabolites and the discovery of new plant properties. | |
 | THREATS |
| Increasing Competition: A more competitive research landscape due to rivalry among investors. Regulatory Barriers: Strict safety, quality, and efficacy requirements for NADES before potential market entry. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaula, I.; Vasung, E.; Damjanović, A.; Panić, M.; Radović, M.; Radošević, K.; Bagović Kolić, M.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Formulation of Ready-to-Use Broccoli Extracts Rich in Polyphenols and Glucosinolates Using Natural Deep Eutectic Solvents. Molecules 2024, 29, 5794. https://doi.org/10.3390/molecules29235794
Karaula I, Vasung E, Damjanović A, Panić M, Radović M, Radošević K, Bagović Kolić M, Cvjetko Bubalo M, Radojčić Redovniković I. Formulation of Ready-to-Use Broccoli Extracts Rich in Polyphenols and Glucosinolates Using Natural Deep Eutectic Solvents. Molecules. 2024; 29(23):5794. https://doi.org/10.3390/molecules29235794
Chicago/Turabian StyleKaraula, Ivona, Emma Vasung, Anja Damjanović, Manuela Panić, Mia Radović, Kristina Radošević, Martina Bagović Kolić, Marina Cvjetko Bubalo, and Ivana Radojčić Redovniković. 2024. "Formulation of Ready-to-Use Broccoli Extracts Rich in Polyphenols and Glucosinolates Using Natural Deep Eutectic Solvents" Molecules 29, no. 23: 5794. https://doi.org/10.3390/molecules29235794
APA StyleKaraula, I., Vasung, E., Damjanović, A., Panić, M., Radović, M., Radošević, K., Bagović Kolić, M., Cvjetko Bubalo, M., & Radojčić Redovniković, I. (2024). Formulation of Ready-to-Use Broccoli Extracts Rich in Polyphenols and Glucosinolates Using Natural Deep Eutectic Solvents. Molecules, 29(23), 5794. https://doi.org/10.3390/molecules29235794








