Removal of Pesticides from Lemon and Vegetables Using Electrolyzed Water Kitchen Devices
Abstract
:1. Introduction
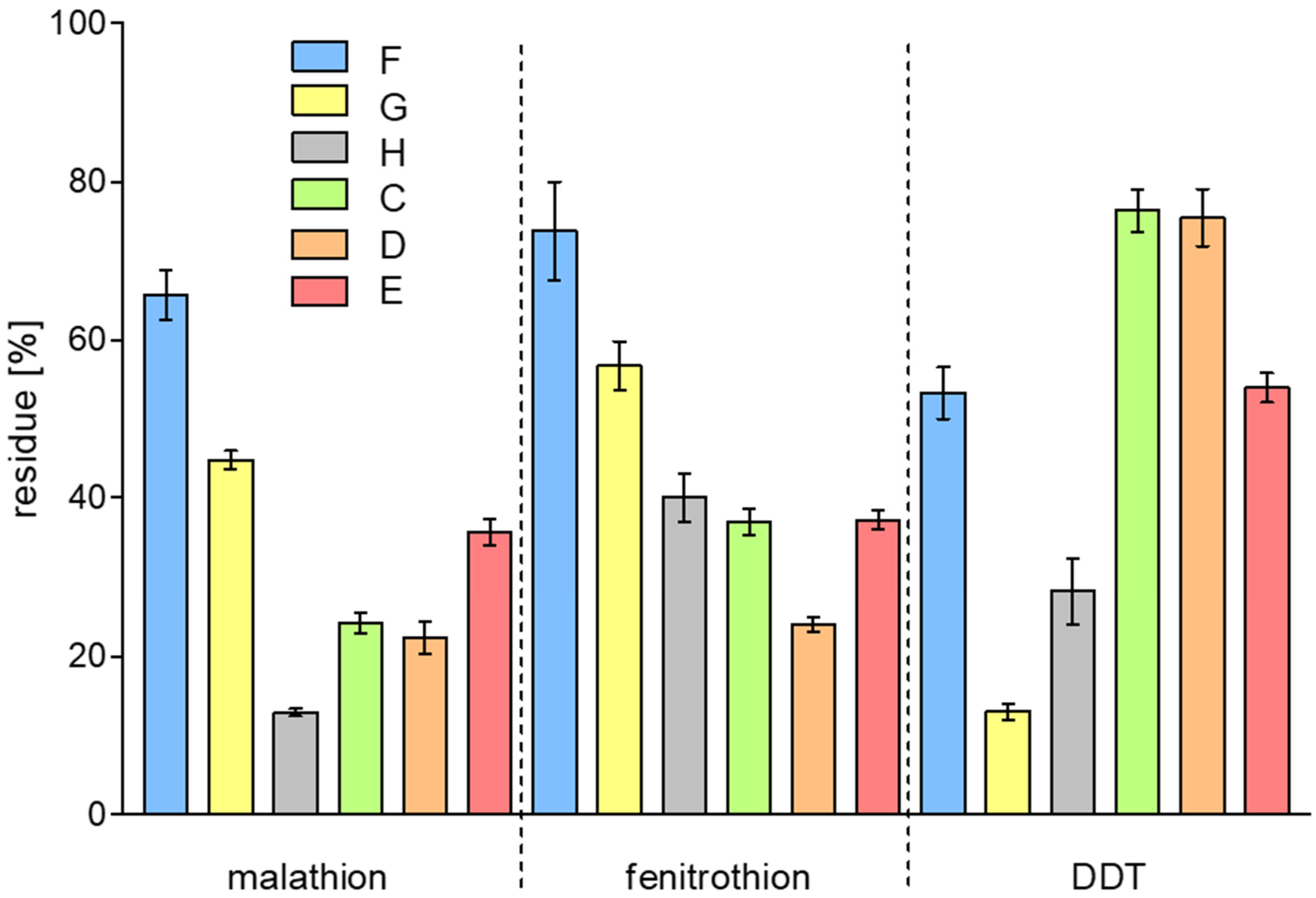
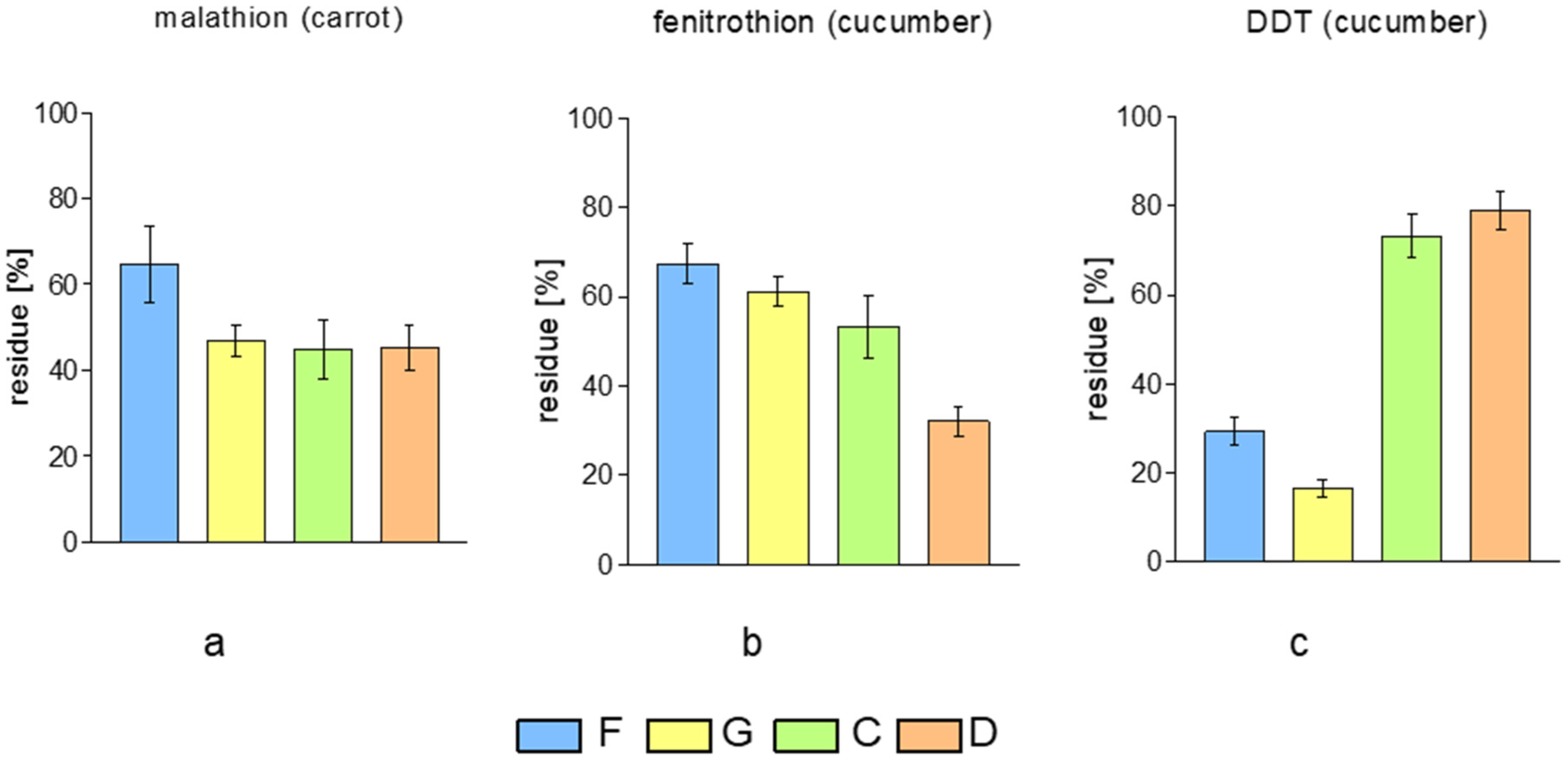
2. Results and Discussion
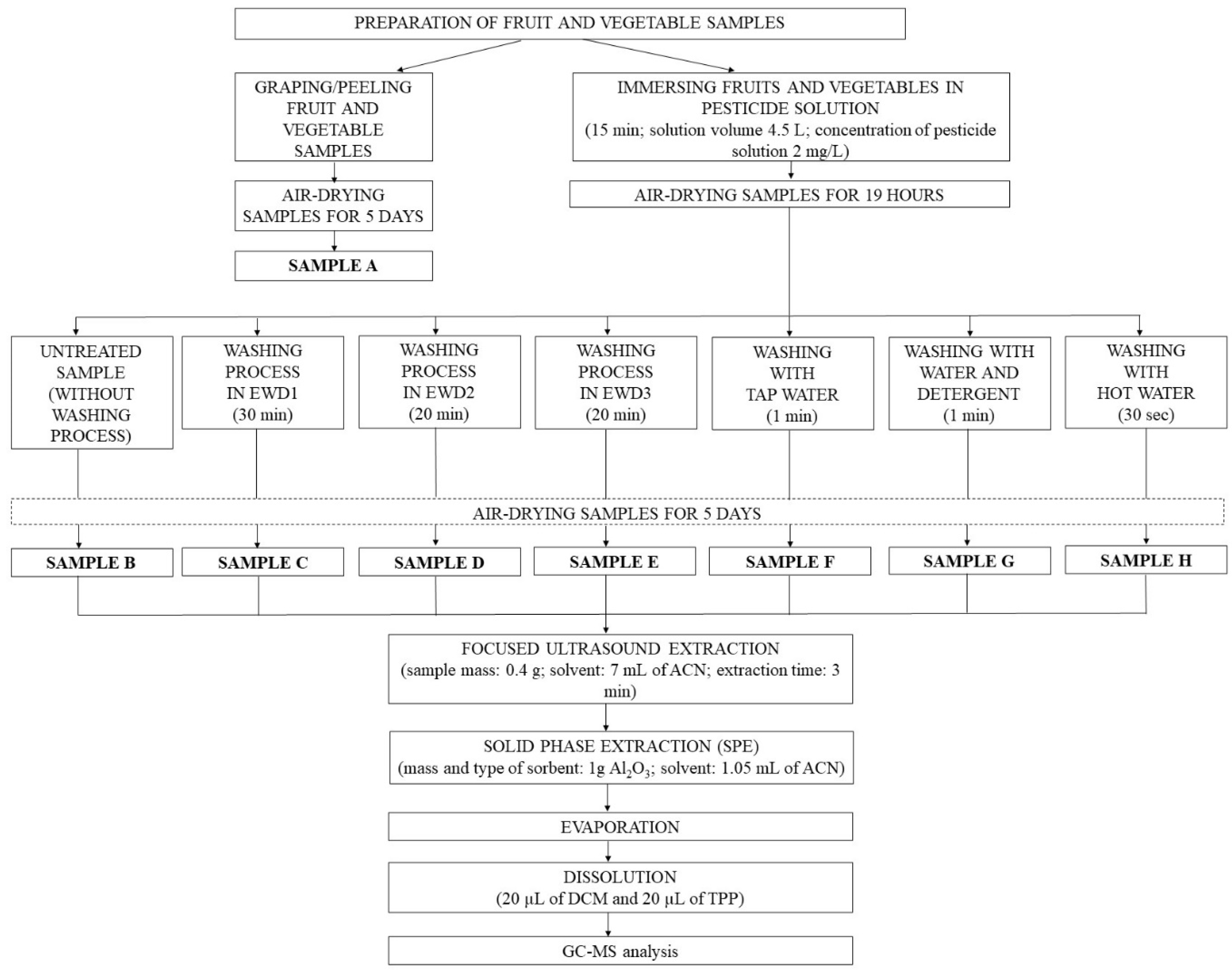
3. Methodology
3.1. Chemical Reagents
3.2. Electrolyzed Water Devices
3.3. Preparation of Samples
3.4. Focused Ultrasound Extraction
3.5. Solid Phase Extraction
3.6. GC/MS Analysis and Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anbarasan, R.; Jaspin, S.; Bhavadharini, B.; Pare, A.; Pandiselvam, R.; Mahendran, R. Chlorpyrifos Pesticide Reduction in Soybean Using Cold Plasma and Ozone Treatments. LWT 2022, 159, 113193. [Google Scholar] [CrossRef]
- Caponio, G.; Vendemia, M.; Mallardi, D.; Marsico, A.D.; Alba, V.; Gentilesco, G.; Forte, G.; Velasco, R.; Coletta, A. Pesticide Residues and Berry Microbiome after Ozonated Water Washing in Table Grape Storage. Foods 2023, 12, 3144. [Google Scholar] [CrossRef] [PubMed]
- Lozowicka, B.; Kaczynski, P.; Paritova, A.Y.; Kuzembekova, G.B.; Abzhalieva, A.B.; Sarsembayeva, N.B.; Alihan, K. Pesticide Residues in Grain from Kazakhstan and Potential Health Risks Associated with Exposure to Detected Pesticides. Food Chem. Toxicol. 2014, 64, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, C.S.; Hjelmsø, M.H. Agricultural Soils, Pesticides and Microbial Diversity. Curr. Opin. Biotechnol. 2014, 27, 15–20. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wang, T.; Li, C.; Wu, Z. Effects of Ozone Treatment on Pesticide Residues in Food: A Review. Int. J. Food Sci. Technol. 2019, 54, 301–312. [Google Scholar] [CrossRef]
- Narenderan, S.T.; Meyyanathan, S.N.; Babu, B. Review of Pesticide Residue Analysis in Fruits and Vegetables. Pre-Treatment, Extraction and Detection Techniques. Food Res. Int. 2020, 133, 109141. [Google Scholar] [CrossRef]
- Han, M.A.; Kim, J.H.; Song, H.S. Persistent Organic Pollutants, Pesticides, and the Risk of Thyroid Cancer: Systematic Review and Meta-Analysis. Eur. J. Cancer Prev. 2019, 28, 344–349. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabbarov, Z.; Arora, N.K.; Wirth, S.; Bellingrath-Kimura, S.D. Biochar Mitigates Effects of Pesticides on Soil Biological Activities. Environ. Sustain. 2021, 4, 335–342. [Google Scholar] [CrossRef]
- Ambrus, Á.; Yang, Y.Z. Global Harmonization of Maximum Residue Limits for Pesticides. J. Agric. Food Chem. 2016, 64, 30–35. [Google Scholar] [CrossRef]
- Bonnechere, A.; Hanot, V.; Bragard, C.; Bedoret, T.; Van Loco, J. Effect of Household and Industrial Processing on the Levels of Pesticide Residues and Degradation Products in Melons. Food Addit. Contam. Part A 2012, 29, 1058–1066. [Google Scholar] [CrossRef]
- Cámara, M.A.; Cermeño, S.; Martínez, G.; Oliva, J. Removal Residues of Pesticides in Apricot, Peach and Orange Processed and Dietary Exposure Assessment. Food Chem. 2020, 325, 126936. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Dong, F.; Liu, X.; Xu, J.; Li, Y.; Han, Y.; Zhu, Y.; Cheng, Y.; Chen, Z.; Tao, Y.; et al. Effect of Household Canning on the Distribution and Reduction of Thiophanate-Methyl and Its Metabolite Carbendazim Residues in Tomato. Food Control 2014, 43, 115–120. [Google Scholar] [CrossRef]
- Chung, S.W.C. How Effective Are Common Household Preparations on Removing Pesticide Residues from Fruit and Vegetables? A Review. J. Sci. Food Agric. 2018, 98, 2857–2870. [Google Scholar] [CrossRef]
- Farha, W.; Abd El-Aty, A.M.; Rahman, M.M.; Jeong, J.H.; Shin, H.C.; Wang, J.; Shin, S.S.; Shim, J.H. Analytical Approach, Dissipation Pattern and Risk Assessment of Pesticide Residue in Green Leafy Vegetables: A Comprehensive Review. Biomed. Chromatogr. 2018, 32, e4134. [Google Scholar] [CrossRef]
- Bae, J.Y.; Lee, D.Y.; Oh, K.Y.; Jeong, D.K.; Lee, D.Y.; Kim, J.H. Photochemical Advanced Oxidative Process Treatment Effect on the Pesticide Residues Reduction and Quality Changes in Dried Red Peppers. Sci. Rep. 2023, 13, 4444. [Google Scholar] [CrossRef]
- Yang, T.; Doherty, J.; Zhao, B.; Kinchla, A.J.; Clark, J.M.; He, L. Effectiveness of Commercial and Homemade Washing Agents in Removing Pesticide Residues on and in Apples. J. Agric. Food Chem. 2017, 65, 9744–9752. [Google Scholar] [CrossRef]
- Yang, S.J.; Mun, S.; Kim, H.J.; Han, S.J.; Kim, D.W.; Cho, B.S.; Kim, A.G.; Park, D.W. Effectiveness of Different Washing Strategies on Pesticide Residue Removal: The First Comparative Study on Leafy Vegetables. Foods 2022, 11, 2916. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Moradi, N. Sonochemical degradation of pesticides in aqueous solution: Investigation on the influence of operating parameters and degradation pathway—A systematic review. RSC Adv. 2020, 10, 7396–7423. [Google Scholar] [CrossRef]
- Fernandes, A.; Gągol, M.; Makoś, P.; Khan, J.A.; Boczkaj, G. Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds. Sep. Purif. Technol. 2019, 224, 1–14. [Google Scholar] [CrossRef]
- Kaushik, V.; Murudkar, S.; Gohil, K.; Ghatkar, S.; Gode, V.; Mhaskar, S. Review on Household Decontamination Technologies for Fruits & Vegetables. Int. J. Food Sci. Nutr. Eng. 2020, 10, 12–36. [Google Scholar]
- Calvo, H.; Redondo, D.; Remón, S.; Venturini, M.E.; Arias, E. Efficacy of Electrolyzed Water, Chlorine Dioxide and Photocatalysis for Disinfection and Removal of Pesticide Residues from Stone Fruit. Postharvest Biol. Technol. 2019, 148, 22–31. [Google Scholar] [CrossRef]
- Lozowicka, B.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Removal of 16 pesticide residues from strawberries by washing with tap and ozone water, ultrasonic cleaning and boiling. Environ. Monit. Assess. 2016, 188, 51. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, W.; Shen, Y.; Liu, Y.; Liu, X.J. Effects of home preparation on organophosphorus pesticide residues in raw cucumber. Food Chem. 2012, 133, 636–640. [Google Scholar] [CrossRef]
- Gracy, T.K.R.; Gupta, V.; Mahendran, R. Influence of low-pressure nonthermal dielectric barrier discharge plasma on chlorpyrifos reduction in tomatoes. J. Food Process Eng. 2019, 42, e13242. [Google Scholar] [CrossRef]
- Phan, K.T.K.; Phan, H.T.; Boonyawan, D.; Intipunya, P.; Brennan, C.S.; Regenstein, J.M.; Phimolsiripol, Y. Non, -thermal plasma for elimination of pesticide residues in mango. Innov. Food Sci. Emerg. Technol. 2018, 48, 164–171. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Yu, F.; Xi, D.; Wang, P.; Li, J.; Wang, X.; Zhang, X.; Bazaka, K.; Ostrikov, K. Removal of organophosphorus pesticide residues from Lycium barbarum by gas phase surface discharge plasma. Chem. Eng. J. 2018, 342, 401–409. [Google Scholar] [CrossRef]
- Sarangapani, C.; O’Toole, G.; Cullen, P.J.; Bourke, P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov. Food Sci. Emerg. Technol. 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Imani, S.; Dorranian, D.; Larijani, K.; Shojaee, M. Effect of cold plasma on degradation of organophosphorus pesticides used on some agricultural products. J. Plant Prot. Res. 2017, 57, 25–35. [Google Scholar] [CrossRef]
- Heo, N.S.; Lee, M.K.; Kim, G.W.; Lee, S.J.; Park, J.Y.; Park, T.J. Microbial inactivation and pesticide removal by remote exposure of atmospheric air plasma in confined environments. J. Biosci. Bioeng. 2014, 117, 81–85. [Google Scholar] [CrossRef]
- Dorraki, N.; Mahdavi, V.; Ghomi, H.; Ghasempour, A. Elimination of diazinon insecticide from cucumber surface by atmospheric pressure air-dielectric barrier discharge plasma. Biointerphases 2016, 11, 041007. [Google Scholar] [CrossRef]
- Misra, N.N.; Pankaj, S.K.; Walsh, T.; O’Regan, F.; Bourke, P.; Cullen, P.J. In-package nonthermal plasma degradation of pesticides on fresh produce. J. Hazard. Mater. 2014, 271, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ciarrocchi, I.R.; Mendes, K.F.; Pimpinato, R.F.; Spoto, M.H.F.; Tornisielo, V.L. The effect of radiation in the degradation of carbendazim and azoxystrobin in strawberry. Radiat. Phys. Chem. 2021, 179, 109269. [Google Scholar] [CrossRef]
- Rodrigues, F.T.; Marchioni, E.; Lordel-Madeleine, S.; Kuntz, F.; Villavicencio, A.L.C.H.; Julien-David, D. Degradation of profenofos in aqueous solution and in vegetable sample by electron beam radiation. Radiat. Phys. Chem. 2020, 166, 108441. [Google Scholar] [CrossRef]
- Chowdhury, M.A.Z.; Jahan, I.; Karim, N.; Alam, M.K.; Rahman, M.A.; Moniruzzaman, M.; Gan, S.H.; Fakhruddin, A.N.M. Determination of Carbamate and Organophosphorus Pesticides in Vegetable Samples and the Efficiency of Gamma-Radiation in Their Removal. Biomed Res. Int. 2014, 145159. [Google Scholar] [CrossRef] [PubMed]
- Basfar, A.A.; Mohamed, K.A.; Al-Saqer, O.A. De-contamination of pesticide residues in food by ionizing radiation. Radiat. Phys. Chem. 2012, 81, 473–478. [Google Scholar] [CrossRef]
- Cengiz, M.F.; Başlar, M.; Basançelebi, O.; Kılıçlı, M. Reduction of pesticide residues from tomatoes by low intensity electrical current and ultrasound applications. Food Chem. 2018, 267, 60–66. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, X.; Chen, G.; Qin, H.; Xu, B.; Luo, Y.; Liao, Y.; Wang, S.; Yan, S.; Zhao, J. Causal inference and mechanism for unraveling the removal of four pesticides from lettuce (Lactuca sativa L.) via ultrasonic processing and various immersion solutions. Ultrason. Sonochem. 2024, 108, 106937. [Google Scholar] [CrossRef]
- Karaca, H. The Effects of Ozone-Enriched Storage Atmosphere on Pesticide Residues and Physicochemical Properties of Table Grapes. Ozone: Sci. Eng. 2018, 41, 404–414. [Google Scholar] [CrossRef]
- de Souza, L.P.; Faroni, L.R.D.; Heleno, F.F.; Pinto, F.G.; Lopes Ribeiro de Queiroz, M.E.; Prates, L.H.F. Ozone treatment for pesticide removal from carrots: Optimization by response surface methodology. Food Chem. 2018, 243, 435–441. [Google Scholar] [CrossRef]
- de Freitas, R.; Faroni, L.R.D.; de Queiroz, M.E.L.R.; Heleno, F.F.; Prates, L.H.F. Degradation kinetics of pirimiphos-methyl residues in maize grains exposed to ozone gas. J. Stored Prod. Res. 2017, 74, 1–5. [Google Scholar] [CrossRef]
- Sadło, S.; Szpyrka, E.; Piechowicz, B.; Antos, P.; Józefczyk, R.; Balawejder, M. Reduction of Captan, Boscalid and Pyraclostrobin Residues on Apples Using Water Only, Gaseous Ozone, and Ozone Aqueous Solution. Ozone: Sci. Eng. 2017, 39, 97–103. [Google Scholar] [CrossRef]
- Savi, G.S.; Piacentini, K.C.; Bortolotto, T.; Scussel, V.M. Degradation of bifenthrin and pirimiphos-methyl residues in stored wheat grains (Triticum aestivum L.) by ozonation. Food Chem. 2016, 203, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Savi, G.D.; Piacentini, K.C.; Scussel, V.M. Reduction in residues of deltamethrin and fenitrothion on stored wheat grains by ozone gas. J. Stored Prod. Res. 2015, 61, 65–69. [Google Scholar] [CrossRef]
- Omeroglu, P.Y.; Acoglu Celik, B.; Koc Alibasoglu, E. The Effect of Household Food Processing on Pesticide Residues in Oranges (Citrus sinensis). Foods 2022, 11, 3918. [Google Scholar] [CrossRef]
- Acoglu, B.; Omeroglu, P.Y. Effectiveness of Different Type of Washing Agents on Reduction of Pesticide Residues in Orange (Citrus sinensis). LWT 2021, 147, 111690. [Google Scholar] [CrossRef]
- Rodrigues, A.A.Z.; de Queiroz, M.E.L.R.; Neves, A.A.; de Oliveira, A.F.; Prates, L.H.F.; de Freitas, J.F.; Heleno, F.F.; Faroni, L.R.D.A. Use of Ozone and Detergent for Removal of Pesticides and Improving Storage Quality of Tomato. Food Res. Int. 2019, 125, 108626. [Google Scholar] [CrossRef]
- Rawn, D.F.K.; Quade, S.C.; Sun, W.F.; Fouguet, A.; Bélanger, A.; Smith, M. Captan Residue Reduction in Apples as a Result of Rinsing and Peeling. Food Chem. 2008, 109, 790–796. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Mir, M.M.; Sofi, S.A.; Shah, M.A.; Sidiq, T.; Sunooj, K.V.; Hamdani, A.M.; Mousavi Khaneghah, A. Current Strategies for the Reduction of Pesticide Residues in Food Products. J. Food Compos. Anal. 2022, 106, 104274. [Google Scholar] [CrossRef]
- Kruve, A.; Lamos, A.; Kirillova, J.; Herodes, K. Pesticide Residues in Commercially Available Oranges and Evaluation of Potential Washing Methods. Proc. Est. Acad. Sci. Chem. 2007, 56, 134–141. [Google Scholar]
- Xiao, H.W.; Pan, Z.; Deng, L.Z.; El-Mashad, H.M.; Yang, X.H.; Mujumdar, A.S.; Gao, Z.J.; Zhang, Q. Recent Developments and Trends in Thermal Blanching—A Comprehensive Review. Inf. Process. Agric. 2017, 4, 101–127. [Google Scholar] [CrossRef]
| EWD1 | EWD2 | EWD3 | |
|---|---|---|---|
| Rated Power | 90 W | 72 W | 85 W |
| Time of the electrolyzed water generation | 30 min | 20 min | 20 min |
| UV LED wavelength | 275 nm | 275 nm | 275 nm |
| Capacity | 12 L | 9 L | 9 L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Studziński, W.; Narloch, I.; Dąbrowski, Ł. Removal of Pesticides from Lemon and Vegetables Using Electrolyzed Water Kitchen Devices. Molecules 2024, 29, 5797. https://doi.org/10.3390/molecules29235797
Studziński W, Narloch I, Dąbrowski Ł. Removal of Pesticides from Lemon and Vegetables Using Electrolyzed Water Kitchen Devices. Molecules. 2024; 29(23):5797. https://doi.org/10.3390/molecules29235797
Chicago/Turabian StyleStudziński, Waldemar, Izabela Narloch, and Łukasz Dąbrowski. 2024. "Removal of Pesticides from Lemon and Vegetables Using Electrolyzed Water Kitchen Devices" Molecules 29, no. 23: 5797. https://doi.org/10.3390/molecules29235797
APA StyleStudziński, W., Narloch, I., & Dąbrowski, Ł. (2024). Removal of Pesticides from Lemon and Vegetables Using Electrolyzed Water Kitchen Devices. Molecules, 29(23), 5797. https://doi.org/10.3390/molecules29235797







