Assembly and Valence Modulation of Ordered Bimetallic MOFs for Highly Efficient Electrocatalytic Water Oxidation
Abstract
1. Introduction
2. Results and Discussion
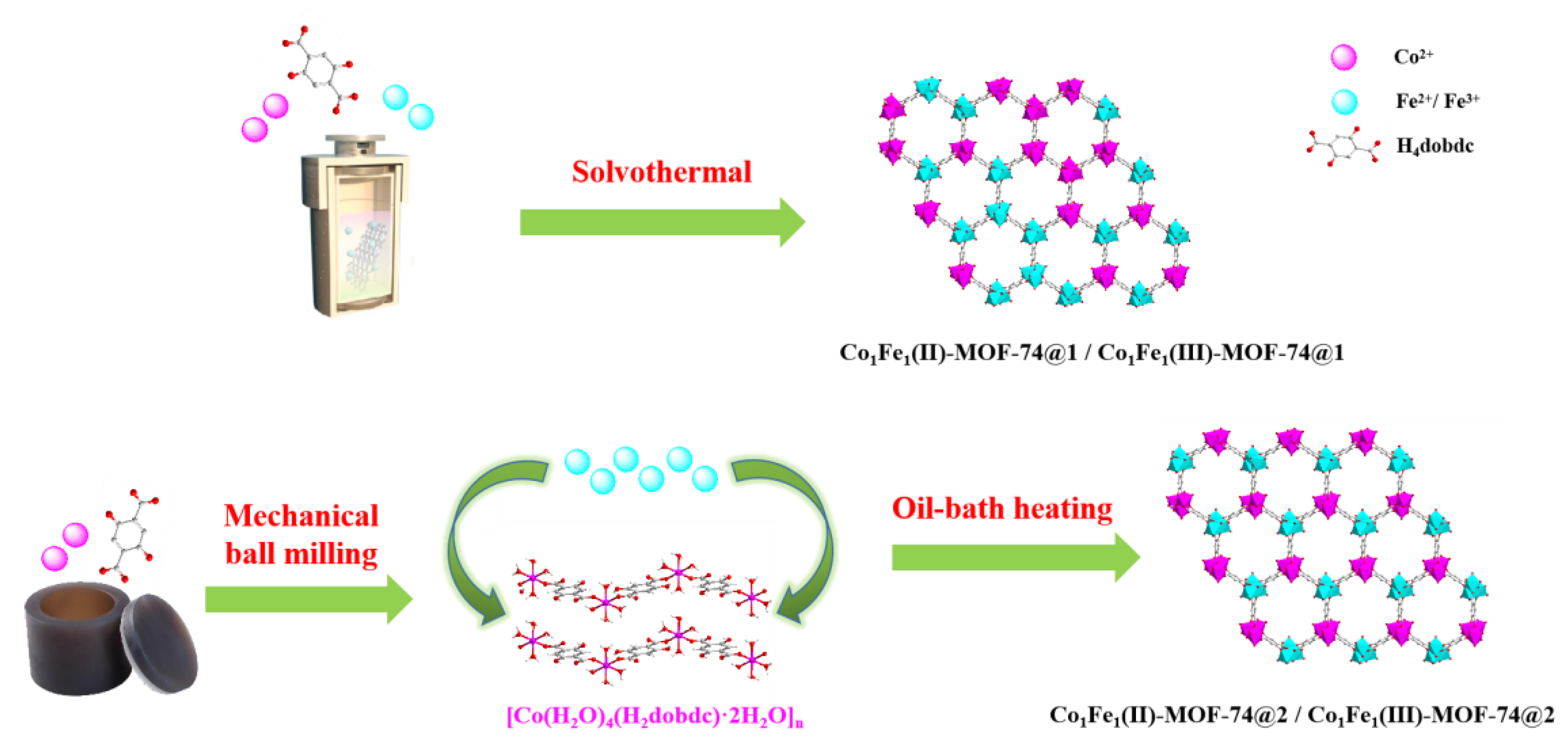
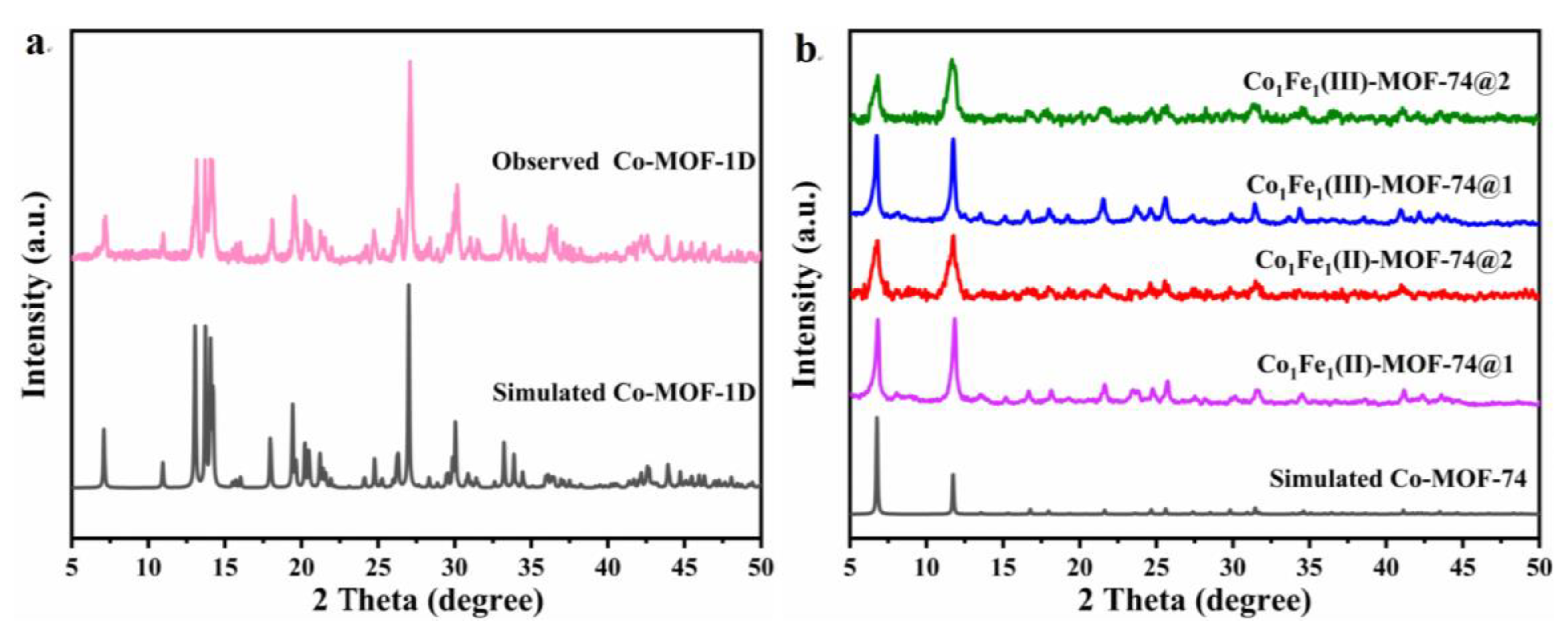
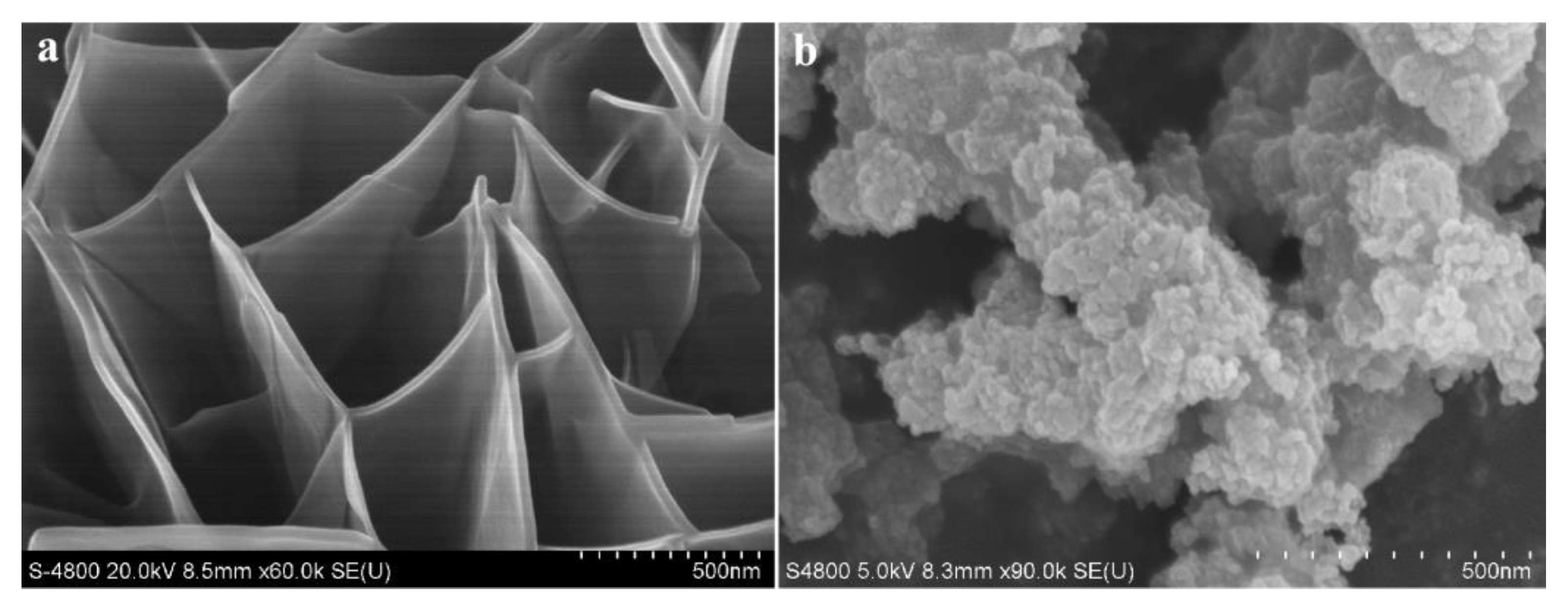
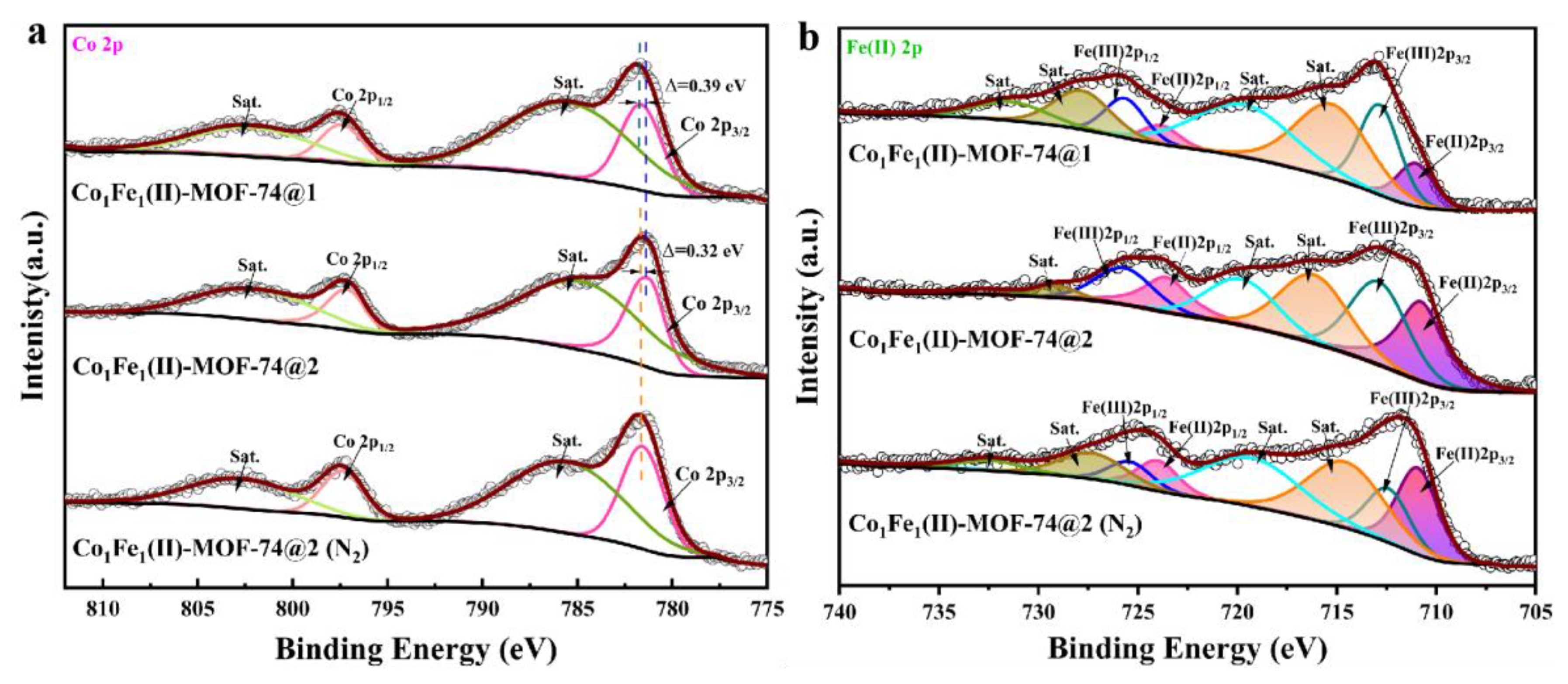
2.1. Structure and Morphology
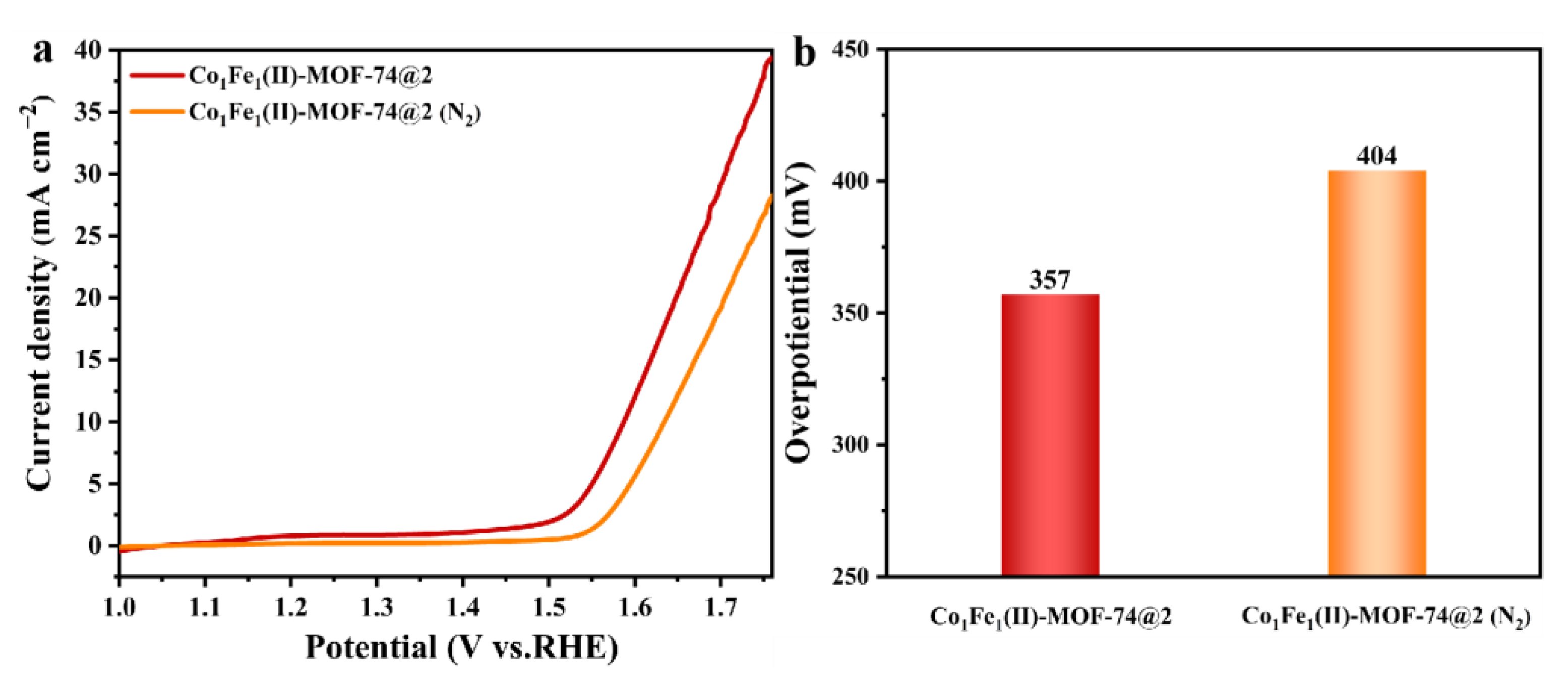
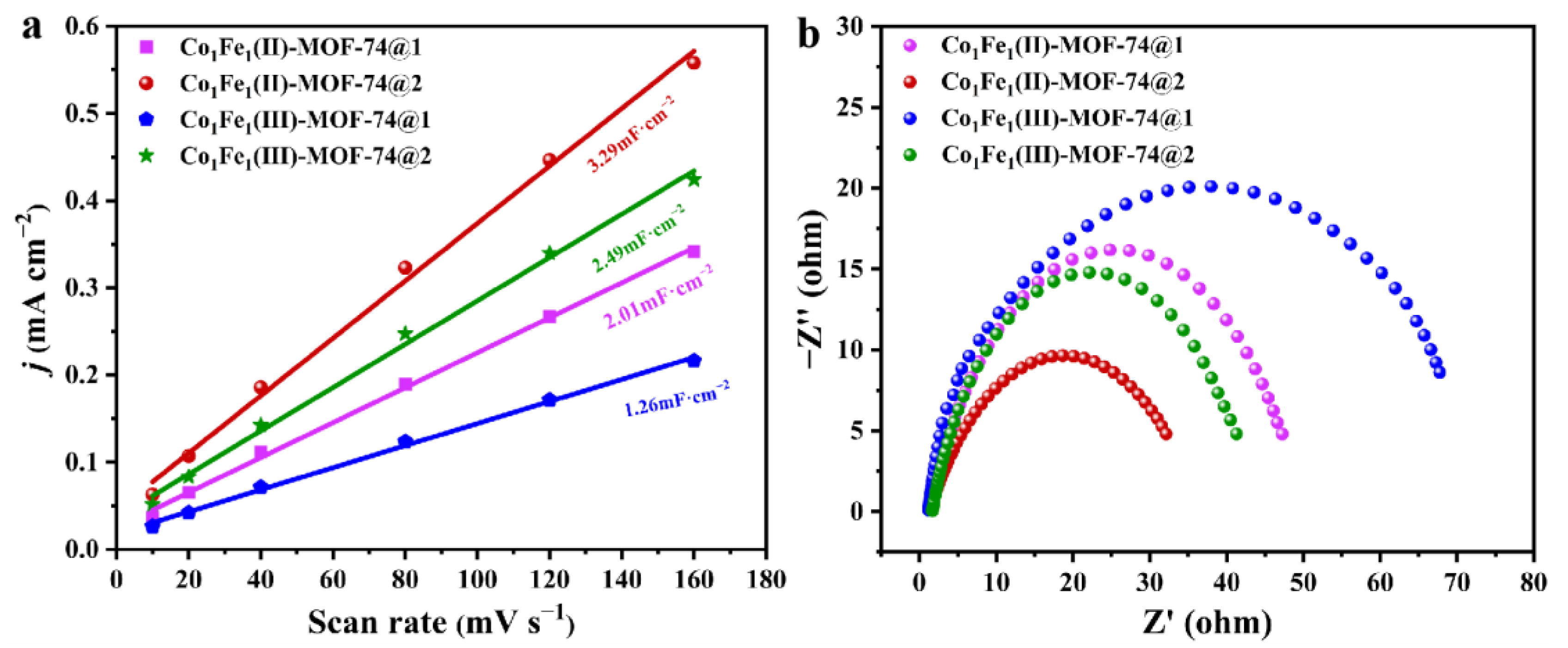
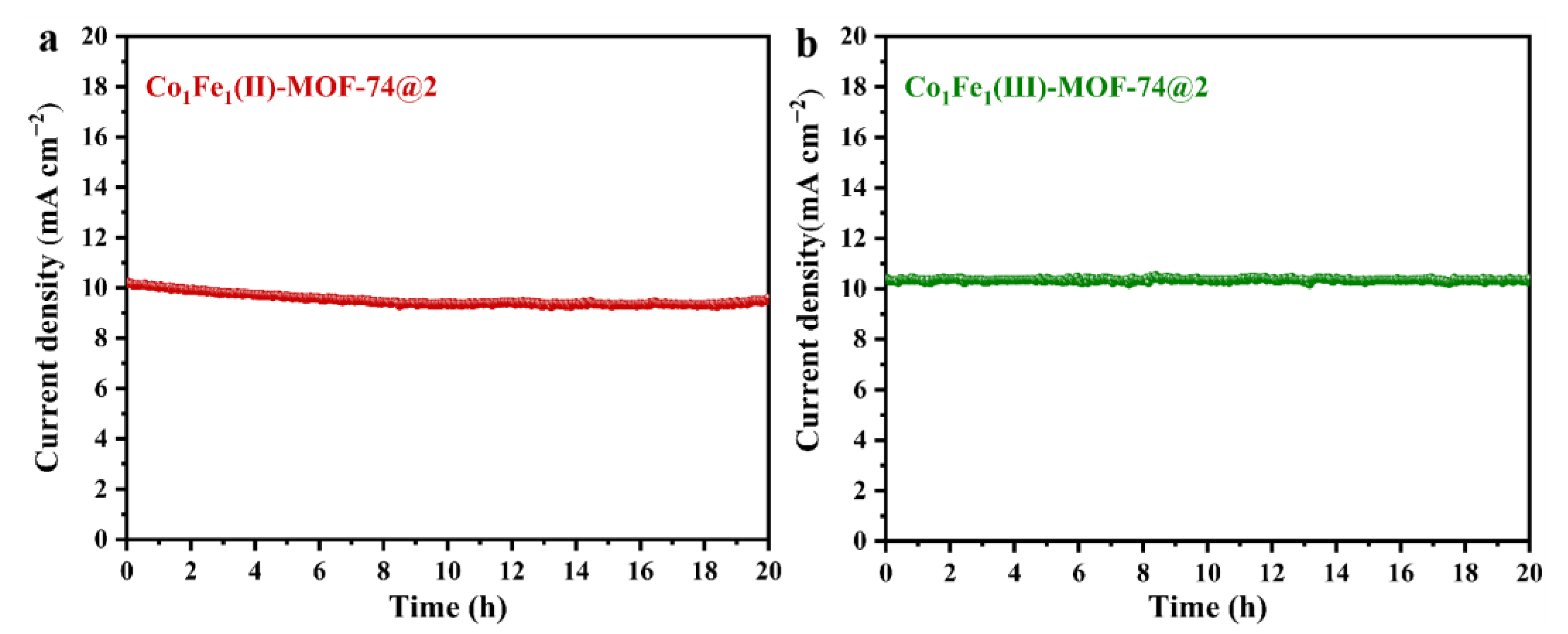
2.2. Electrochemical Performance Testing
3. Materials and Methods
3.1. Materials
3.2. Synthesis Methods
3.3. Characterization of Catalysts
3.4. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawashima, K.; Márquez, R.A.; Smith, L.A.; Vaidyula, R.R.; Carrasco-Jaim, O.A.; Wang, Z.; Son, Y.J.; Cao, C.L.; Mullins, C.B. A Review of Transition Metal Boride, Carbide, Pnictide, and Chalcogenide Water Oxidation Electrocatalysts. Chem. Rev. 2023, 123, 12795–13208. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Louisia, S.; Yu, S.; Jin, J.; Roh, I.; Chen, C.; Fonseca Guzman, M.V.; Feijóo, J.; Chen, P.-C.; Wang, H.; et al. Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 2023, 614, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Cobb, S.J.; Badiani, V.M.; Dharani, A.M. Fast CO2 hydration kinetics impair heterogeneous but improve enzymatic CO2 reduction catalysis. Nat. Chem. 2022, 14, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-B.; Nguyen, T.T.T.; Vaidhyanathan, R.; Burner, J.; Taylor, J.M.; Durekova, H.; Akhtar, F.; Mah, R.K.; Ghaffari-Nik, O.; Marx, S. A Scalable Metal-Organic Framework as a Durable Physisorbent for Carbon Dioxide Capture. Science 2021, 374, 1464–1469. [Google Scholar] [CrossRef]
- Li, H.; Lin, Y.; Duan, J.; Wen, Q.; Liu, Y.; Zhai, T. Stability of electrocatalytic OER: From principle to application. Chem. Soc. Rev. 2024, 53, 10709–10740. [Google Scholar] [CrossRef]
- Quan, L.; Jiang, H.; Mei, G.; Sun, Y.; You, B. Bifunctional Electrocatalysts for Overall and Hybrid Water Splitting. Chem. Rev. 2024, 124, 3694–3812. [Google Scholar] [CrossRef]
- Xie, C.; Chen, W.; Wang, Y.Y.; Yang, Y.H.; Wang, S.Y. Dynamic evolution processes in electrocatalysis: Structure evolution, characterization and regulation. Chem. Soc. Rev. 2024, 53, 10852–10877. [Google Scholar] [CrossRef]
- Zhao, Y.; Adiyeri Saseendran, D.P.; Huang, C.; Triana, C.A.; Marks, W.R.; Chen, H.; Zhao, H.; Patzke, G.R. Oxygen evolution/reduction reaction catalysts: From in situ monitoring and reaction mechanisms to rational design. Chem. Rev. 2023, 123, 6257–6358. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating metal–organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. SusMat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Sun, H.N.; Xu, X.M.; Fei, L.S.; Zhou, W.; Shao, Z.P. Electrochemical Oxidation of Small Molecules for Energy Saving Hydrogen Production. Adv. Energy Mater. 2024, 14, 2401242. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, X.; Lin, X.; Liu, G.; Ling, C.; Xi, S.; Chen, B.; Ge, Y.; Tan, C.; Lai, Z.; et al. Phase-Dependent Growth of Pt on MoS2 for Highly Efficient H2 Evolution. Nature 2023, 621, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Oliveira, N.J.; Kwon, S.; Tyukhtenko, S.; Guo, J.J.; Myrthil, N.; Lopez, S.A.; Kendrick, I.; Mukerjee, S.; Ma, L.; et al. Understanding Hydrogen Electrocatalysis by Probing the Hydrogen-Bond Network of Water at the Electrified Pt-Solution Interface. Nat. Energy 2023, 8, 859–869. [Google Scholar] [CrossRef]
- Park, S.; Liu, L.; Demirkır, C.; van der Heijden, O.; Lohse, D.; Krug, D.; Koper, M.T. Solutal Marangoni effect determines bubble dynamics during electrocatalytic hydrogen evolution. Nat. Chem. 2023, 15, 1532–1540. [Google Scholar] [CrossRef]
- Li, Z.H.; Lin, G.X.; Wang, L.Q.; Lee, H.; Du, J.; Tang, T.; Ding, G.H.; Ren, R.; Li, W.L.; Cao, X.; et al. Seed-assisted formation of NiFe anode catalysts for anion exchange membrane water electrolysis at industrial-scale current density. Nat. Catal. 2024, 7, 944–952. [Google Scholar] [CrossRef]
- Magnier, L.; Cossard, G.; Martin, V.; Pascal, C.; Roche, V.; Sibert, E.; Shchedrina, I.; Bousquet, R.; Parry, V.; Chatenet, M. Fe-Ni-based alloys as highly active and low-cost oxygen evolution reaction catalyst in alkaline media. Nat. Mater. 2024, 23, 252–261. [Google Scholar] [CrossRef]
- Chong, L.; Gao, G.; Wen, J.; Li, H.; Xu, H.; Green, Z.; Sugar, J.D.; Kropf, A.J.; Xu, W.; Lin, X.-M.; et al. La- and Mn-doped cobalt spinel oxygen evolution catalyst for proton exchange membrane electrolysis. Science 2023, 380, 609–616. [Google Scholar] [CrossRef]
- Ram, R.; Xia, L.; Benzidi, H.; Guha, A.; Golovanova, V.; Garzón Manjón, A.; Llorens Rauret, D.; Sanz Berman, P.; Dimitropoulos, M.; Mundet, B.; et al. Water-Hydroxide Trapping in Cobalt Tungstate for Proton Exchange Membrane Water Electrolysis. Science 2024, 384, 1373–1380. [Google Scholar] [CrossRef]
- Li, A.; Kong, S.; Adachi, K.; Ooka, H.; Fushimi, K.; Jiang, Q.; Ofuchi, H.; Hamamoto, S.; Oura, M.; Higashi, K.; et al. Atomically dispersed hexavalent iridium oxide from MnO2 reduction for oxygen evolution catalysis. Science 2024, 384, 666–670. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Chen, F.-Y.; Li, B.; Yu, S.-W.; Finfrock, Y.Z.; Meira, D.M.; Yan, Q.-Q.; Zhu, P.; Chen, M.-X.; Song, T.-W.; et al. Non–iridium–based electrocatalyst for durable acidic oxygen evolution reaction in proton exchange membrane water electrolysis. Nat. Mater. 2023, 22, 100–108. [Google Scholar] [CrossRef]
- Cai, Z.; Li, L.D.; Ding, P.J.; Pang, D.W.; Xu, M.Y.; Xu, Z.Y.; Kang, J.X.; Guo, T.Q.; Teobaldi, G.; Wang, Z.C.; et al. High-valence Cu induced by photoelectric reconstruction for dynamically stable oxygen evolution sites. J. Am. Chem. Soc. 2024, 146, 19295–19302. [Google Scholar] [CrossRef]
- Wei, S.; Ma, Y.; Raabe, D. One step from oxides to sustainable bulk alloys. Nature 2024, 633, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.F.; Yao, J.Q.; Wang, Y.S.; Gao, N.; Zhang, T.; Li, L.; Liu, Y.Y.; Chen, Z.J.; Peng, J.; Liu, X.W.; et al. Crystal facets-activity correlation for oxygen evolution reaction in compositional complex alloys. Adv. Sci. 2024, 11, 2404095. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Q.; Zou, P.C.; Zhang, Y.Y.; Pan, W.L.; Li, Y.; Liang, L.M.; Chen, C.; Liu, H.; Zheng, S.J. Activating Lattice Oxygen in High-Entropy LDH for Robust and Durable Water Oxidation. Nat. Commun. 2023, 14, 6019. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nie, K.; Qu, X.; Li, X.; Li, B.; Yuan, Y.; Chong, S.; Liu, P.; Li, Y.; Yin, Z.; et al. General Bottom-Up Colloidal Synthesis of Nano-Monolayer Transition-Metal Dichalcogenides with High 1T′-Phase Purity. J. Am. Chem. Soc. 2022, 144, 4863–4873. [Google Scholar] [CrossRef]
- Du, Y.; Li, B.; Xu, G.; Wang, L. Recent advances in interface engineering strategy for highly-efficient electrocatalytic water splitting. InfoMat. 2023, 5, e12377. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, T.; Stromme, M.; Xu, C. Electrochemical Doping and Structural Modulation of Conductive Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2024, 63, e202318387. [Google Scholar] [CrossRef]
- Jia, H.; Yao, N.; Liao, Z.; Wu, L.; Zhu, J.; Lao, Y.; Luo, W. Understanding the Role of Spin State in Cobalt Oxyhydroxides for Water Oxidation. Angew. Chem. Int. Ed. 2024, 63, e202408005. [Google Scholar] [CrossRef]
- An, Z.; Yang, P.; Duan, D.; Li, J.; Wan, T.; Kong, Y.; Caratzoulas, S.; Xiang, S.; Liu, J.; Huang, L.; et al. Highly active, ultra-low loading single-atom iron catalysts for catalytic transfer hydrogenation. Nat. Commun. 2023, 14, 6666. [Google Scholar] [CrossRef]
- Chen, L.; Yin, Z.H.; Cui, J.Y.; Li, C.Q.; Song, K.P.; Liu, H.; Wang, J.J. Unlocking Lattice Oxygen on Selenide-Derived NiCoOOH for Amine Electrooxidation and Efficient Hydrogen Production. J. Am. Chem. Soc. 2024, 146, 27090–27099. [Google Scholar] [CrossRef]
- Qin, Q.; Li, Z.; Zhao, X.; Zhao, H.; Zhai, L.; Kim, M.G.; Cho, J.; Jang, H.; Liu, S.; Liu, X. Atomically Dispersed Vanadium-Induced Ru–V Dual Active Sites Enable Exceptional Performance for Acidic Water Oxidation. Angew. Chem. Int. Ed. 2024, 63, e202413657. [Google Scholar]
- Wu, R.Z.; Meng, Q.L.; Yan, J.; Zhang, Z.R.; Chen, B.F.; Liu, H.Z.; Tai, J.; Zhang, G.K.; Zheng, L.R.; Zhang, J.; et al. Intermetallic synergy in platinum–cobalt electrocatalysts for selective C-O bond cleavage. Nat. Catal. 2024, 7, 702–718. [Google Scholar] [CrossRef]
- Fan, R.; Liu, C.; Li, Z.; Huang, H.; Feng, J.; Li, Z.; Zou, Z. Ultrastable Electrocatalytic Seawater Splitting at Ampere-Level Current Density. Nat. Sustain. 2024, 7, 158–167. [Google Scholar] [CrossRef]
- Sikma, R.E.; Butler, K.S.; Vogel, D.J.; Harvey, J.A.; Gallis, D.F.S. Quest for Multifunctionality: Current Progress in the Characterization of Heterometallic Metal-Organic Frameworks. J. Am. Chem. Soc. 2024, 146, 5715–5734. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, A.; Li, M.; Li, D.; O’Keeffe, M.; Yaghi, O.M. Structures of Metal-Organic Frameworks with Rod Secondary Building Units. Chem. Rev. 2016, 116, 12466–12535. [Google Scholar] [CrossRef]
- Wang, L.J.; Deng, H.; Furukawa, H.; Gándara, F.; Cordova, K.E.; Peri, D.; Yaghi, O.M. Synthesis and Characterization of Metal–Organic Framework-74 Containing 2, 4, 6, 8, and 10 Different Metals. Inorg. Chem. 2014, 53, 5881–5883. [Google Scholar] [CrossRef]
- Zhao, S.; Tan, C.; He, C.-T.; An, P.; Xie, F.; Jiang, S.; Zhu, Y.; Wu, K.-H.; Zhang, B.; Li, H.; et al. Structural transformation of highly active metal-organic framework electrocatalysts during the oxygen evolution reaction. Nat. Energy 2020, 5, 881–890. [Google Scholar] [CrossRef]
- Yao, B.H.; Chen, Y.T.; Yan, Y.Y.; Yang, Y.; Xing, H.H.; Xu, Y.C.; Jiao, D.X.; Xing, Z.C.; Wang, D.W.; Yang, X.R. Iron-Induced Localized Oxide Path Mechanism Enables Efficient and Stable Water Oxidation. Angew. Chem. Int. Ed. 2024, 63, e202416141. [Google Scholar]
- Xu, H.G.; Zhu, C.; Lin, H.Y.; Liu, J.K.; Wu, Y.X.; Fu, H.Q.; Zhang, X.Y.; Mao, F.X.; Yuan, H.Y.; Sun, C.H.; et al. Oxygen Plasma Triggered Co-O-Fe Motif in Prussian Blue Analogue for Efficient and Robust Alkaline Water Oxidation. Angew. Chem. Int. Ed. 2024, 63, e202415423. [Google Scholar]
- Wu, Y.L.; Sun, Z.P.; Yu, L.M.; Chen, Y.Y.; Li, Z.B.; Li, M.L.; Liu, D.; Yan, Z.; Cao, X.B. Synergistic effect of CoII, NiII and FeII/FeIII in trimetallic MOFs for enhancing electrocatalytic water oxidation. CrystEngComm 2024, 26, 6608–6617. [Google Scholar] [CrossRef]
- Furukawa, H.; Müller, U.; Yaghi, O.M. “Heterogeneity within Order” in Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 3417–3430. [Google Scholar] [CrossRef]
- Sun, D.; Sun, F.; Deng, X.; Li, Z. Mixed-Metal Strategy on Metal-Organic Frameworks (MOFs) for Functionalities Expansion: Co Substitution Induces Aerobic Oxidation of Cyclohexene over Inactive Ni-MOF-74. Inorg. Chem. 2015, 54, 8639–8643. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Blas, C.; de la Peña-O’Shea, V.A.; Puente-Orench, I.; de Paz, J.R.; Sáez-Puche, R.; Gutiérrez-Puebla, E.; Gándara, F.; Monge, A. Addressed realization of multication complex arrangements in metalorganic frameworks. Sci. Adv. 2017, 3, e1700773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Zhang, D.-X.; Chang, G.-G.; Ma, X.-C.; Wu, J.; Wang, Y.; Yu, H.-Z.; Tian, G.; Chen, J.; Yang, X.-Y. Bimetallic (Zn/Co) MOFs-Derived Highly Dispersed Metallic Co/HPC for Completely Hydrolytic Dehydrogenation of Ammonia–Borane. Ind. Eng. Chem. Res. 2019, 58, 7209–7216. [Google Scholar] [CrossRef]
- Kim, D.; Coskun, A. Template-Directed Approach Towards the Realization of Ordered Heterogeneity in Bimetallic Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2017, 56, 5071–5076. [Google Scholar] [CrossRef]
- Ayoub, G.; Karadeniz, B.; Howarth, A.J.; Farha, O.K.; Đilović, I.; Germann, L.S.; Dinnebier, R.E.; Užarević, K.; Friščić, T. Rational Synthesis of Mixed-Metal Microporous Metal-Organic Frameworks with Controlled Composition Using Mechanochemistry. Chem. Mater. 2019, 31, 5494–5501. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Rightmire, N.R.; Hanusa, T.P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352–2362. [Google Scholar] [CrossRef]
- Dietzel, P.D.C.; Morita, Y.; Blom, R.; Fjellvåg, H. An In Situ High-Temperature Single-Crystal Investigation of a Dehydrated Metal-Organic Framework Compound and Field-Induced Magnetization of One-Dimensional Metal-Oxygen Chains. Angew. Chem. Int. Ed. 2005, 44, 6354–6358. [Google Scholar] [CrossRef]
- Rosi, N.L.; Kim, J.; Eddaoudi, M.; Chen, B.; O’Keeffe, M.; Yaghi, O.M. Rod Packings and Metal-Organic Frameworks Constructed from Rod-Shaped Secondary Building Units. J. Am. Chem. Soc. 2005, 127, 1504–1518. [Google Scholar] [CrossRef]
- Yang, C.; Cai, W.-J.; Yu, B.-B.; Qiu, H.; Li, M.-L.; Zhu, L.-W.; Yan, Z.; Hou, L.; Wang, Y.-Y. Performance enhancement of oxygen evolution reaction through incorporating bimetallic electrocatalysts in two–dimensional metal−organic frameworks. Catal. Sci. Technol. 2020, 10, 3897–3903. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Yin, J.; Li, D.; Wu, C.; Zhang, C.; Fei, H. Highly Stable MOF-Type Lead Halide Luminescent Ferroelectrics. Angew. Chem. Int. Ed. 2024, 63, e202407102. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhou, D.; Wang, M.; Bak, S.M.; Wu, Y.; Wu, Z.; Tian, Y.; Xiong, X.; Li, Y.; Liu, W.; et al. Introducing Fe2+ into Nickel-Iron Layered Double Hydroxide: Local Structure Modulated Water Oxidation Activity. Angew. Chem. Int. Ed. 2018, 57, 9392–9396. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Rao, R.R.; Peng, J.; Huang, B.; Stephens, I.E.L.; Risch, M.; Xu, Z.J.; Shao-Horn, Y. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 2019, 31, 1806296. [Google Scholar] [CrossRef]
- Pan, S.; Li, H.; Wang, T.; Fu, Y.; Wang, S.; Xie, Z.; Wei, L.; Li, H.; Li, N. Er-Doping Enhances the Oxygen Evolution Performance of Cobalt Oxide in Acidic Medium. ACS Catal. 2024, 14, 13814–13824. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, G.; Guo, X.; Xiong, P.; Xu, Z.; Zhang, L.; Chen, C.; Xu, C.; Wu, T.-S.; Soo, Y.-L. Facet-Dependent Surface Restructuring on Nickel (Oxy)hydroxides: A Self-Activation Process for Enhanced Oxygen Evolution Reaction. J. Am. Chem. Soc. 2024, 146, 15219–15229. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, G.; Tan, H.; Mou, Y.; Zhang, J.; Guo, H.; Yang, S.; Lei, H.; Zheng, H.; Zhang, W.; et al. Constructing Co4(SO4)4 Clusters within Metal–Organic Frameworks for Efficient Oxygen Electrocatalysis. Adv. Mater. 2024, 36, 2408094. [Google Scholar] [CrossRef]
- Nguyen, A.I.; Van Allsburg, K.M.; Terban, M.W.; Bajdich, M.; Oktawiec, J.; Amtawong, J.; Ziegler, M.S.; Dombrowski, J.P.; Lakshmi, K.V.; Drisdell, W.S.; et al. Stabilization of reactive Co4O4 cubane oxygen-evolution catalysts within porous frameworks. Proc. Natl. Acad. Sci. USA 2019, 116, 11630–11639. [Google Scholar] [CrossRef]
- Cheng, W.; Lu, X.F.; Luan, D.; Lou, X.W. NiMn-Based bimetal–organic framework nanosheets supported on multi-channel carbon fibers for efficient oxygen electrocatalysis. Angew. Chem. Int. Ed. 2020, 59, 18234. [Google Scholar] [CrossRef]
- Wu, P.; Geng, S.; Wang, X.; Zhang, X.; Li, H.; Zhang, L.; Shen, Y.; Zha, B.; Zhang, S.; Huo, F.; et al. Exfoliation of Metal–Organic Frameworks to Give 2D MOF Nanosheets for the Electrocatalytic Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2024, 63, e202402969. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, Q.; Liang, Z.; Guo, H.; Wang, Y.; Lv, H.; Li, X.; Zhang, W.; Apfel, U.P.; Cao, R. Metal-corrole-based porous organic polymers for electrocatalytic oxygen reduction and evolution reactions. Angew. Chem. Int. Ed. 2022, 61, e202201104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qin, H.; Han, J.; Jin, X.; Xu, Y.; Yang, S.; Zhang, W.; Cao, R. A One-Pot Three-In-One Synthetic Strategy to Immobilize Cobalt Corroles on Carbon Nanotubes for Oxygen Electrocatalysis. Adv. Funct. Mater. 2024, 34, 2310820. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, X.P.; Guo, K.; Han, J.; Guo, H.; Lei, H.; Li, X.; Zhang, W.; Apfel, U.P.; Cao, R. Coordination tuning of metal porphyrins for improved oxygen evolution reaction. Angew. Chem. Int. Ed. 2023, 62, e202305938. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Guo, H.; Zhou, G.; Guo, K.; Wang, B.; Lei, H.; Zhang, W.; Zheng, H.; Apfel, U.P.; Cao, R. Metal–organic-framework-supported molecular electrocatalysis for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2021, 60, 8472. [Google Scholar] [CrossRef]
- Shen, J.Q.; Liao, P.Q.; Zhou, D.D.; He, C.T.; Wu, J.X.; Zhang, W.X.; Zhang, J.P.; Chen, X.M. Modular and stepwise synthesis of a hybrid metal–organic framework for efficient electrocatalytic oxygen evolution. J. Am. Chem. Soc. 2017, 139, 1778. [Google Scholar] [CrossRef]
- Aljabour, A.; Awada, H.; Song, L.; Sun, H.; Offenthaler, S.; Yari, F.; Bechmann, M.; Scharber, M.C.; Schöfberger, W. A Bifunctional Electrocatalyst for OER and ORR based on a Cobalt(II) Triazole Pyridine Bis-[Cobalt(III) Corrole] Complex. Angew. Chem. Int. Ed. 2023, 62, e202302208. [Google Scholar] [CrossRef]
- Liu, M.; Liu, S.; Cui, C.X.; Miao, Q.; He, Y.; Li, X.; Xu, Q.; Zeng, G. Construction of catalytic covalent organic frameworks with redox-active sites for the oxygen reduction and the oxygen evolution reaction. Angew. Chem. Int. Ed. 2022, 61, e202213522. [Google Scholar] [CrossRef]
- Zhuang, L.; Ge, L.; Liu, H.; Jiang, Z.; Jia, Y.; Li, Z.; Yang, D.; Hocking, R.K.; Li, M.; Zhang, L.; et al. A surfactant-free and scalable general strategy for synthesizing ultrathin two-dimensional metal–organic framework nanosheets for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2019, 58, 13565. [Google Scholar] [CrossRef]
- Lin, R.; Li, X.; Krajnc, A.; Li, Z.; Li, M.; Wang, W.; Zhuang, L.; Smart, S.; Zhu, Z.; Appadoo, D.; et al. Mechanochemically synthesised flexible electrodes based on bimetallic metal–organic framework glasses for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2021, 61, e202112880. [Google Scholar]
- Wang, X.L.; Dong, L.Z.; Qiao, M.; Tang, Y.J.; Liu, J.; Li, Y.; Li, S.L.; Su, J.X.; Lan, Y.Q. Exploring the performance improvement of the oxygen evolution reaction in a stable bimetal–organic framework system. Angew. Chem. Int. Ed. 2018, 57, 9660. [Google Scholar]
- Manna, P.; Debgupta, J.; Bose, S.; Das, S.K. A Mononuclear CoII Coordination Complex Locked in a Confined Space and Acting as an Electrochemical Water-Oxidation Catalyst: A “Ship-in-a-Bottle” Approach. Angew. Chem. Int. Ed. 2016, 55, 2425. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Sun, Z.; Chen, Y.; Liu, D.; Meng, Y.; Yan, Z. Assembly and Valence Modulation of Ordered Bimetallic MOFs for Highly Efficient Electrocatalytic Water Oxidation. Molecules 2024, 29, 5845. https://doi.org/10.3390/molecules29245845
Wu Y, Sun Z, Chen Y, Liu D, Meng Y, Yan Z. Assembly and Valence Modulation of Ordered Bimetallic MOFs for Highly Efficient Electrocatalytic Water Oxidation. Molecules. 2024; 29(24):5845. https://doi.org/10.3390/molecules29245845
Chicago/Turabian StyleWu, Yaling, Zhaopeng Sun, Yingying Chen, Dan Liu, Yan Meng, and Zheng Yan. 2024. "Assembly and Valence Modulation of Ordered Bimetallic MOFs for Highly Efficient Electrocatalytic Water Oxidation" Molecules 29, no. 24: 5845. https://doi.org/10.3390/molecules29245845
APA StyleWu, Y., Sun, Z., Chen, Y., Liu, D., Meng, Y., & Yan, Z. (2024). Assembly and Valence Modulation of Ordered Bimetallic MOFs for Highly Efficient Electrocatalytic Water Oxidation. Molecules, 29(24), 5845. https://doi.org/10.3390/molecules29245845





