Three-Dimensional Zeolitic Imidazolate Framework-8 as Sorbent Integrated with Active Capillary Plasma Mass Spectrometry for Rapid Assessment of Low-Level Wine and Grape Quality-Related Volatiles
Abstract
:1. Introduction
2. Results and Discussion
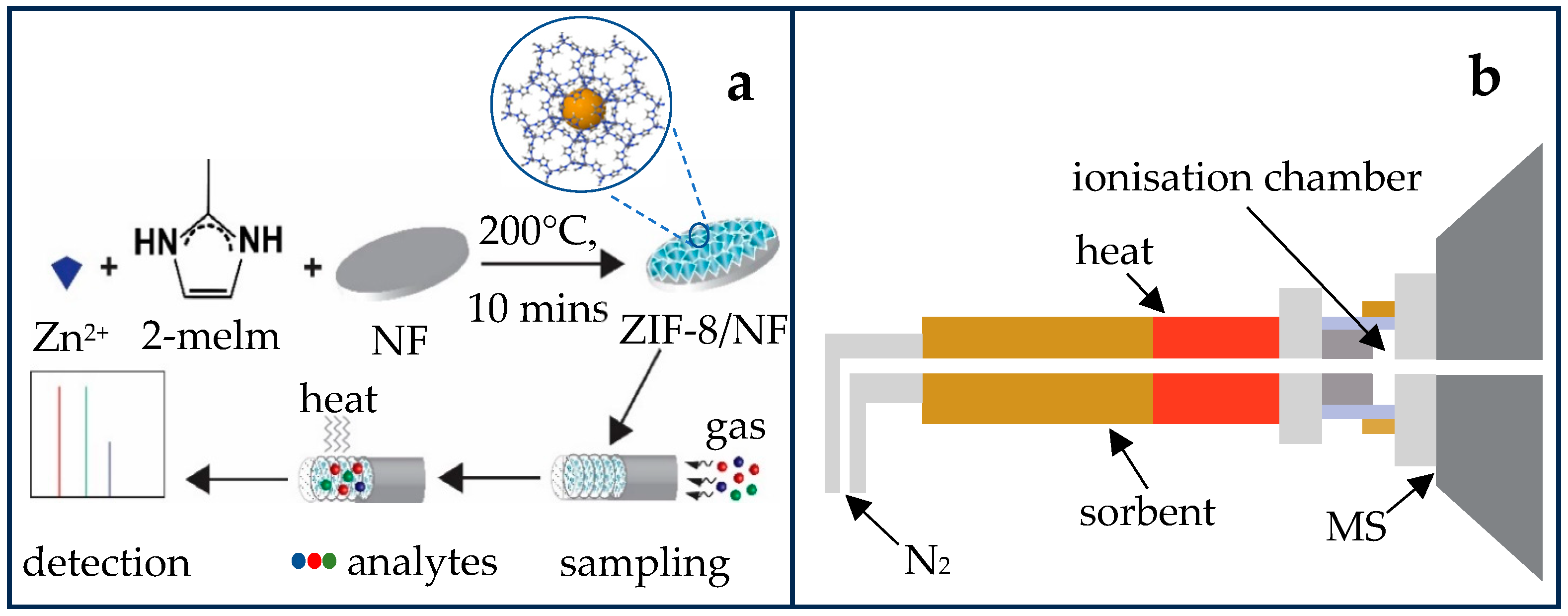
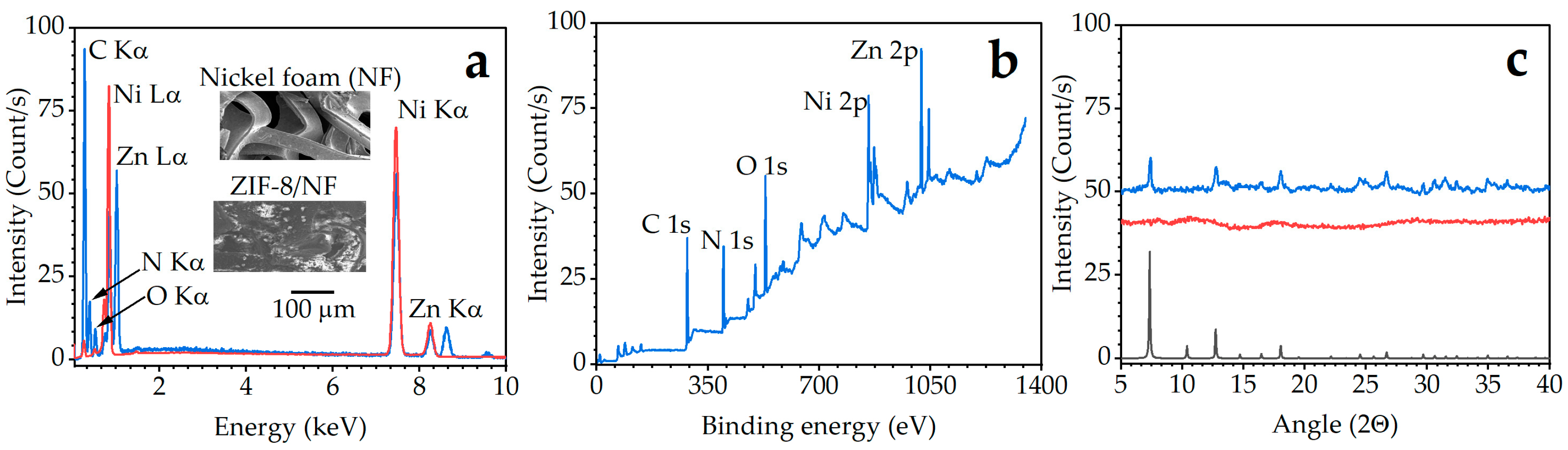
2.1. Fabrication and Surface Characterisation of ZIF-8 on NF
2.2. Loading and Temperature Dependent Study of ZIF-8/NF
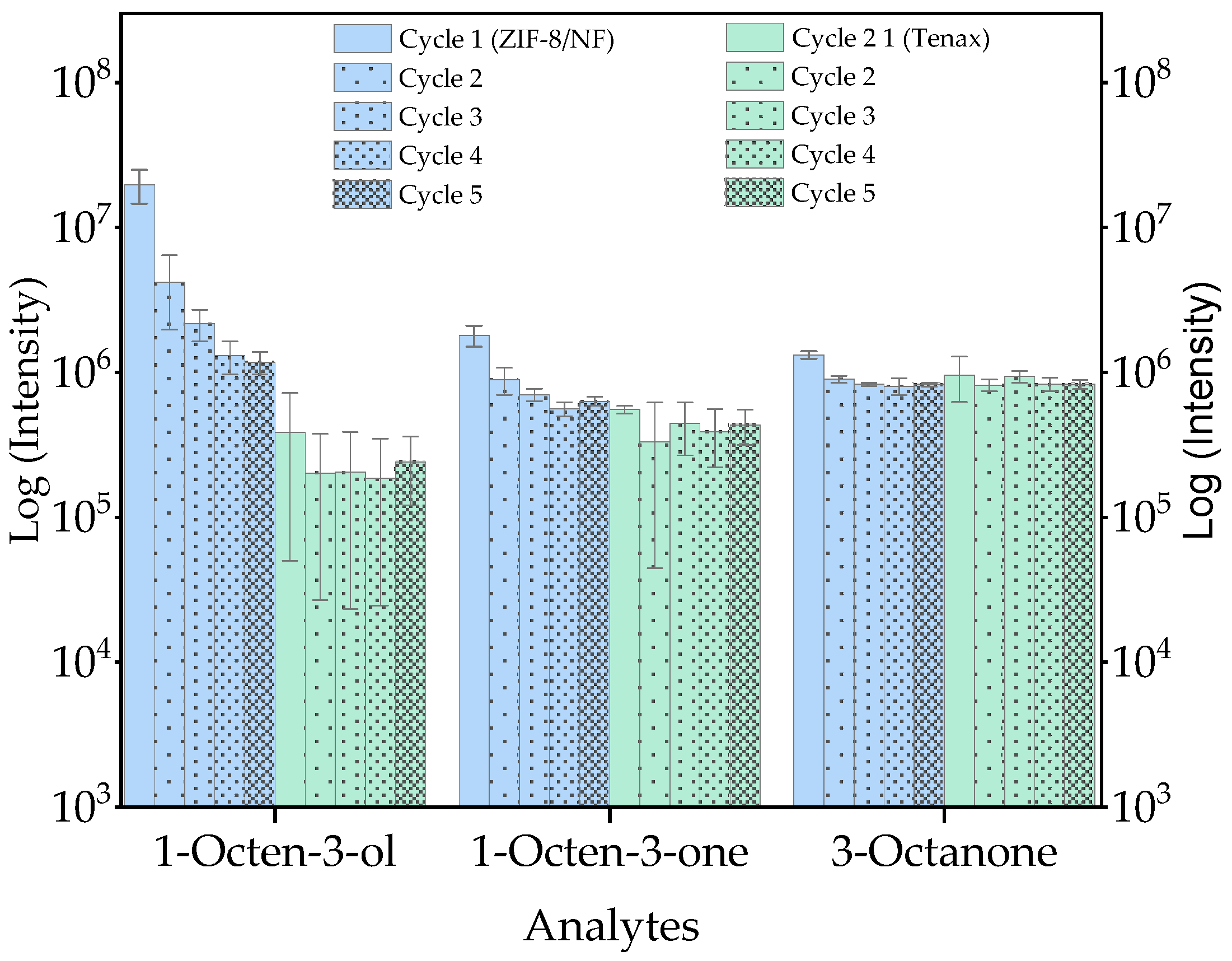
2.3. Optimisation of ZIF-8/NF as Sorbent Material via Thermal Desorption Coupled to Gas Chromatography–Mass Spectrometry (TD-GC/MS)
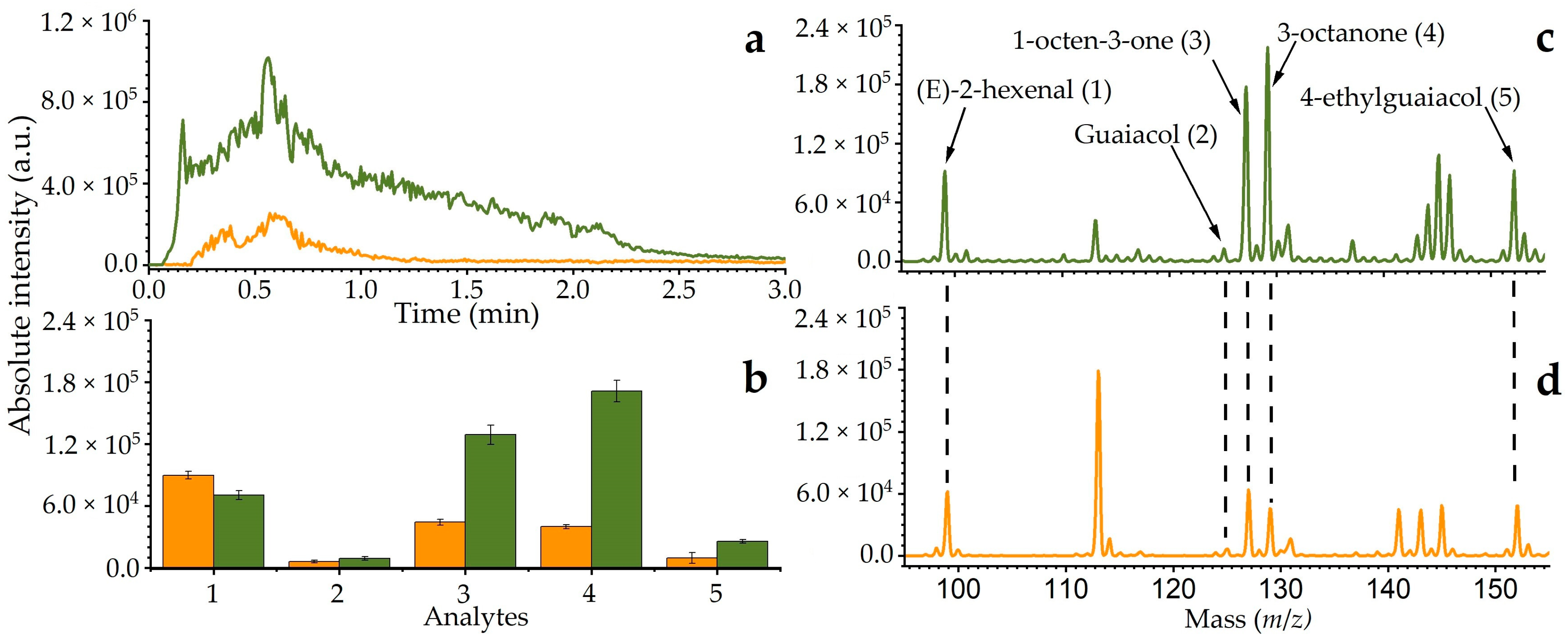
2.4. Direct Thermal Desorption Active Capillary Plasma Mass Spectrometry (TD-LTP-MS)
3. Materials and Methods
3.1. Synthesis of ZIF-8 on NF
3.2. Surface Characterisation of ZIF-8/NF
3.3. Sorbent Tubes Preparation and Sampling
3.4. Thermal Desorption Gas Chromatography–Mass Spectrometry (TD-GC/MS)
3.5. Direct Thermal Desorption Active Capillary Plasma Mass Spectrometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Lee, J. Current Research Related to Wine Sensory Perception Since 2010. Beverages 2020, 6, 47. [Google Scholar] [CrossRef]
- Shapin, S. A taste of science: Making the subjective objective in the California wine world. Soc. Stud. Sci. 2016, 46, 436–460. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.P.; Busto, O.; Guasch, J. Application of a headspace mass spectrometry system to the differentiation and classification of wines according to their origin, variety and ageing. J. Chromatogr. A 2004, 1057, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Weingart, G.; Schwartz, H.; Eder, R.; Sontag, G. Determination of geosmin and 2,4,6-trichloroanisole in white and red Austrian wines by headspace SPME-GC/MS and comparison with sensory analysis. Eur. Food Res. Technol. 2010, 231, 771–779. [Google Scholar] [CrossRef]
- Darriet, P.; Pons, M.; Lamy, S.; Dubourdieu, D. Identification and Quantification of Geosmin, an Earthy Odorant Contaminating Wines. J. Agric. Food. Chem. 2000, 48, 4835–4838. [Google Scholar] [CrossRef] [PubMed]
- Steel, C.C.; Schwarz, L.J.; Qiu, Y.; Schueuermann, C.; Blackman, J.W.; Clark, A.C.; Schmidtke, L.M. Thresholds for Botrytis bunch rot contamination of Chardonnay grapes based on the measurement of the fungal sterol, ergosterol. Aust. J. Grape Wine Res. 2020, 26, 79–89. [Google Scholar] [CrossRef]
- Jiang, L.; Qiu, Y.; Dumlao, M.C.; Donald, W.A.; Steel, C.C.; Schmidtke, L.M. Detection and prediction of Botrytis cinerea infection levels in wine grapes using volatile analysis. Food Chem. 2023, 421, 136120. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Sarais, G.; Cabras, M.; Angioni, A. Determination of 4-Ethylphenol and 4-Ethylguaiacol in Wines by LC-MS-MS and HPLC-DAD-Fluorescence. J. Agric. Food. Chem. 2007, 55, 7288–7293. [Google Scholar] [CrossRef] [PubMed]
- Krstic, M.P.; Johnson, D.L.; Herderich, M.J. Review of smoke taint in wine: Smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Aust. J. Grape Wine Res. 2015, 21, 537–553. [Google Scholar] [CrossRef]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of Several Volatile Phenols and Their Glycoconjugates to Smoke-Related Sensory Properties of Red Wine. J. Agric. Food. Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.-n.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Pollnitz, A.P.; Pardon, K.H.; Sefton, M.A. Quantitative analysis of 4-ethylphenol and 4-ethylguaiacol in red wine. J. Chromatogr. A 2000, 874, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Spillman, P.J.; Sefton, M.A.; Gawel, R. The effect of oak wood source, location of seasoning and coopering on the composition of volatile compounds in oak-matured wines. Aust. J. Grape Wine Res. 2004, 10, 216–226. [Google Scholar] [CrossRef]
- Kennison, K.R.; Wilkinson, K.L.; Williams, H.G.; Smith, J.H.; Gibberd, M.R. Smoke-derived Taint in Wine: Effect of Postharvest Smoke Exposure of Grapes on the Chemical Composition and Sensory Characteristics of Wine. J. Agric. Food. Chem. 2007, 55, 10897–10901. [Google Scholar] [CrossRef] [PubMed]
- Summerson, V.; Gonzalez Viejo, C.; Pang, A.; Torrico, D.D.; Fuentes, S. Review of the Effects of Grapevine Smoke Exposure and Technologies to Assess Smoke Contamination and Taint in Grapes and Wine. Beverages 2021, 7, 7. [Google Scholar] [CrossRef]
- Scholefield, P. Assessment of Economic Cost of Endemic Pests and Diseases on the Australian Grape and Wine Industry; Wine Australia: Adelaide, SA, Australia, 2010. [Google Scholar]
- Rice, S.; Maurer, D.L.; Fennell, A.; Dharmadhikari, M.; Koziel, J.A. Evaluation of Volatile Metabolites Emitted In-Vivo from Cold-Hardy Grapes during Ripening Using SPME and GC-MS: A Proof-of-Concept. Molecules 2019, 24, 536. [Google Scholar] [CrossRef] [PubMed]
- Cappellin, L.; Lopez-Hilfiker, F.D.; Pospisilova, V.; Ciotti, L.; Pastore, P.; Gonin, M.; Hutterli, M.A. Thermal Desorption–Vocus Enables Online Nondestructive Quantification of 2,4,6-Trichloroanisole in Cork Stoppers below the Perception Threshold. Anal. Chem. 2020, 92, 9823–9829. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Serpa, A.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Metal–Organic Frameworks as Key Materials for Solid-Phase Microextraction Devices—A Review. Separations 2019, 6, 47. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Gutiérrez-Serpa, A.; Cruz, A.J.; Ameloot, R.; Ayala, J.H.; Afonso, A.M.; Pasán, J.; Rodríguez-Hermida, S.; Pino, V. Solid-phase microextraction coatings based on the metal-organic framework ZIF-8: Ensuring stable and reusable fibers. Talanta 2020, 215, 120910. [Google Scholar] [CrossRef] [PubMed]
- Suwannakot, P.; Lisi, F.; Ahmed, E.; Liang, K.; Babarao, R.; Gooding, J.J.; Donald, W.A. Metal–Organic Framework-Enhanced Solid-Phase Microextraction Mass Spectrometry for the Direct and Rapid Detection of Perfluorooctanoic Acid in Environmental Water Samples. Anal. Chem. 2020, 92, 6900–6908. [Google Scholar] [CrossRef]
- Ahmad, R.; Wong-Foy, A.G.; Matzger, A.J. Microporous Coordination Polymers As Selective Sorbents for Liquid Chromatography. Langmuir 2009, 25, 11977–11979. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, L.; Maes, M.; Giebeler, L.; Jacobs, P.A.; Martens, J.A.; Denayer, J.F.M.; Kirschhock, C.E.A.; De Vos, D.E. Selective Adsorption and Separation of ortho-Substituted Alkylaromatics with the Microporous Aluminum Terephthalate MIL-53. J. Am. Chem. Soc. 2008, 130, 14170–14178. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.-Y.; Gu, Z.-Y.; Jiang, D.-Q.; Li, Y.; Wang, H.-F.; Yan, X.-P. In Situ Hydrothermal Growth of Metal−Organic Framework 199 Films on Stainless Steel Fibers for Solid-Phase Microextraction of Gaseous Benzene Homologues. Anal. Chem. 2009, 81, 9771–9777. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Brown, R.J.C.; Kim, Y.-H.; Boukhvalov, D. Metal-organic framework and Tenax-TA as optimal sorbent mixture for concurrent GC-MS analysis of C1 to C5 carbonyl compounds. Sci. Rep. 2018, 8, 5033. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Wang, G.; Yan, X.P. MOF-5 metal-organic framework as sorbent for in-field sampling and preconcentration in combination with thermal desorption GC/MS for determination of atmospheric formaldehyde. Anal. Chem. 2010, 82, 1365–1370. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal–Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef]
- Liu, X.L.; Li, Y.S.; Zhu, G.Q.; Ban, Y.J.; Xu, L.Y.; Yang, W.S. An organophilic pervaporation membrane derived from metal-organic framework nanoparticles for efficient recovery of bio-alcohols. Angew. Chem. Int. Ed. Engl. 2011, 50, 10636–10639. [Google Scholar] [CrossRef]
- Avci, C.; Imaz, I.; Carné-Sánchez, A.; Pariente, J.A.; Tasios, N.; Pérez-Carvajal, J.; Alonso, M.I.; Blanco, A.; Dijkstra, M.; López, C.; et al. Self-assembly of polyhedral metal–organic framework particles into three-dimensional ordered superstructures. Nat. Chem. 2018, 10, 78–84. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, W.; Liu, D.; Zhong, C. Understanding the Effect of Trace Amount of Water on CO2 Capture in Natural Gas Upgrading in Metal–Organic Frameworks: A Molecular Simulation Study. Ind. Eng. Chem. Res. 2012, 51, 10031–10038. [Google Scholar] [CrossRef]
- Luebbers, M.T.; Wu, T.; Shen, L.; Masel, R.I. Effects of Molecular Sieving and Electrostatic Enhancement in the Adsorption of Organic Compounds on the Zeolitic Imidazolate Framework ZIF-8. Langmuir 2010, 26, 15625–15633. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.-Y.; Yu, L.-Q.; Wang, S.-W.; Meng, Y.; Lv, Y.-K. Hybrid ZIF-8-90 for Selective Solid-Phase Microextraction of Exhaled Breath from Gastric Cancer Patients. ACS Appl. Bio Mater. 2021, 4, 3608–3613. [Google Scholar] [CrossRef] [PubMed]
- Rankin-Turner, S.; Heaney, L.M. Applications of ambient ionization mass spectrometry in 2020: An annual review. Anal. Sci. Adv. 2021, 2, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Shelley, J.T.; Badal, S.P.; Engelhard, C.; Hayen, H. Ambient desorption/ionization mass spectrometry: Evolution from rapid qualitative screening to accurate quantification tool. Anal. Bioanal. Chem. 2018, 410, 4061–4076. [Google Scholar] [CrossRef] [PubMed]
- Nudnova, M.M.; Zhu, L.; Zenobi, R. Active capillary plasma source for ambient mass spectrometry. Rapid Commun. Mass. Spectrom. 2012, 26, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Stephens, E.R.; Dumlao, M.; Xiao, D.; Zhang, D.; Donald, W.A. Benzylammonium Thermometer Ions: Internal Energies of Ions Formed by Low Temperature Plasma and Atmospheric Pressure Chemical Ionization. J. Am. Soc. Mass. Spectrom. 2015, 26, 2081–2084. [Google Scholar] [CrossRef] [PubMed]
- Dumlao, M.; Khairallah, G.N.; Donald, W.A. Internal Energy Deposition in Dielectric Barrier Discharge Ionization is Significantly Lower than in Direct Analysis in Real-Time Mass Spectrometry. Aust. J. Chem. 2017, 70, 1219–1226. [Google Scholar] [CrossRef]
- Feider, C.L.; Krieger, A.; DeHoog, R.J.; Eberlin, L.S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem. 2019, 91, 4266–4290. [Google Scholar] [CrossRef]
- Chen, J.; Tang, F.; Guo, C.A.; Zhang, S.; Zhang, X. Plasma-based ambient mass spectrometry: A step forward to practical applications. Anal. Methods 2017, 9, 4908–4923. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Pei, X.; Zhou, J.; Feng, X.; Zhang, S.; Cheng, Y.; Li, H.; Han, R.; Wang, B. A Solvent-Free Hot-Pressing Method for Preparing Metal–Organic-Framework Coatings. Angew. Chem. Int. Ed. 2016, 55, 3419–3423. [Google Scholar] [CrossRef] [PubMed]
- Verougstraete, B.; Schuddinck, D.; Lefevere, J.; Baron, G.V.; Denayer, J.F.M. A 3D-Printed Zeolitic Imidazolate Framework-8 Monolith For Flue- and Biogas Separations by Adsorption: Influence of Flow Distribution and Process Parameters. Front. Chem. Eng. 2020, 2, 589686. [Google Scholar] [CrossRef]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling Zeolitic Imidazolate Framework Nano- and Microcrystal Formation: Insight into Crystal Growth by Time-Resolved In Situ Static Light Scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Tian, F.; Cerro, A.M.; Mosier, A.M.; Wayment-Steele, H.K.; Shine, R.S.; Park, A.; Webster, E.R.; Johnson, L.E.; Johal, M.S.; Benz, L. Surface and Stability Characterization of a Nanoporous ZIF-8 Thin Film. J. Phys. Chem. C 2014, 118, 14449–14456. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, Y.; Ding, B.; Li, Z.; Xie, F.; Zhang, F.; Wang, H.; Liu, J.; Wang, X. Structure induced selective adsorption performance of ZIF-8 nanocrystals in water. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 661–667. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Li, M.; Hou, L.A. Influence of the 2-methylimidazole/zinc nitrate hexahydrate molar ratio on the synthesis of zeolitic imidazolate framework-8 crystals at room temperature. Sci. Rep. 2018, 8, 9597. [Google Scholar] [CrossRef]
- James, J.B.; Lin, Y.S. Kinetics of ZIF-8 Thermal Decomposition in Inert, Oxidizing, and Reducing Environments. J. Phys. Chem. C 2016, 120, 14015–14026. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186. [Google Scholar] [CrossRef]
- Dryahina, K.; Polášek, M.; Jašík, J.; Sovová, K.; Španěl, P. Ion Chemistry in Dielectric Barrier Discharge Ionization: Recent Advances in Direct Gas Phase Analyses. Mass. Spectrom. Rev. 2024, 1–25. [Google Scholar] [CrossRef]
- Kamarulzaman, N.H.; Le-Minh, N.; Fisher, R.M.; Stuetz, R.M. Quantification of VOCs and the development of odour wheels for rubber processing. Sci. Total Environ. 2019, 657, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Dumlao, M.C.; Jeffress, L.E.; Gooding, J.J.; Donald, W.A. Solid-phase microextraction low temperature plasma mass spectrometry for the direct and rapid analysis of chemical warfare simulants in complex mixtures. Analyst 2016, 141, 3714–3721. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Xiao, D.; Dumlao, M.C.; Steel, C.C.; Schmidtke, L.M.; Fletcher, J.; Donald, W.A. Nanosecond Pulsed Dielectric Barrier Discharge Ionization Mass Spectrometry. Anal. Chem. 2020, 92, 4468–4474. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumlao, M.C.; Jiang, L.; Bhattacharyya, S.K.; Donald, W.A.; Steel, C.C.; Schmidtke, L.M. Three-Dimensional Zeolitic Imidazolate Framework-8 as Sorbent Integrated with Active Capillary Plasma Mass Spectrometry for Rapid Assessment of Low-Level Wine and Grape Quality-Related Volatiles. Molecules 2024, 29, 6053. https://doi.org/10.3390/molecules29246053
Dumlao MC, Jiang L, Bhattacharyya SK, Donald WA, Steel CC, Schmidtke LM. Three-Dimensional Zeolitic Imidazolate Framework-8 as Sorbent Integrated with Active Capillary Plasma Mass Spectrometry for Rapid Assessment of Low-Level Wine and Grape Quality-Related Volatiles. Molecules. 2024; 29(24):6053. https://doi.org/10.3390/molecules29246053
Chicago/Turabian StyleDumlao, Morphy C., Liang Jiang, Saroj Kumar Bhattacharyya, William A. Donald, Christopher C. Steel, and Leigh M. Schmidtke. 2024. "Three-Dimensional Zeolitic Imidazolate Framework-8 as Sorbent Integrated with Active Capillary Plasma Mass Spectrometry for Rapid Assessment of Low-Level Wine and Grape Quality-Related Volatiles" Molecules 29, no. 24: 6053. https://doi.org/10.3390/molecules29246053
APA StyleDumlao, M. C., Jiang, L., Bhattacharyya, S. K., Donald, W. A., Steel, C. C., & Schmidtke, L. M. (2024). Three-Dimensional Zeolitic Imidazolate Framework-8 as Sorbent Integrated with Active Capillary Plasma Mass Spectrometry for Rapid Assessment of Low-Level Wine and Grape Quality-Related Volatiles. Molecules, 29(24), 6053. https://doi.org/10.3390/molecules29246053





