Organophosphate Triesters and Their Transformation Products in Sediments of Mangrove Wetlands in the Beibu Gulf, South China Sea
Abstract
1. Introduction
2. Results and Discussion
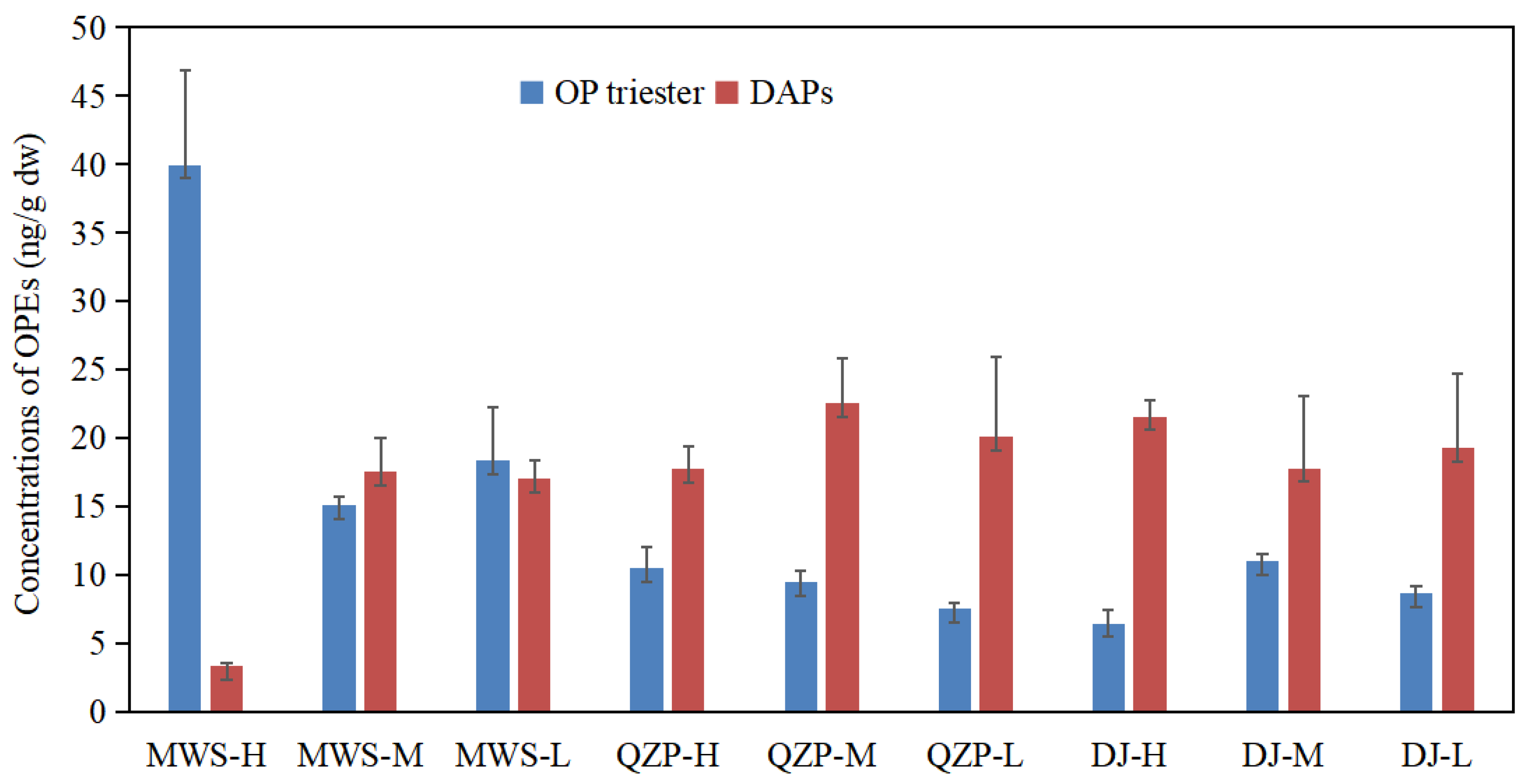
2.1. Occurrence of OPEs in Mangrove Sediment
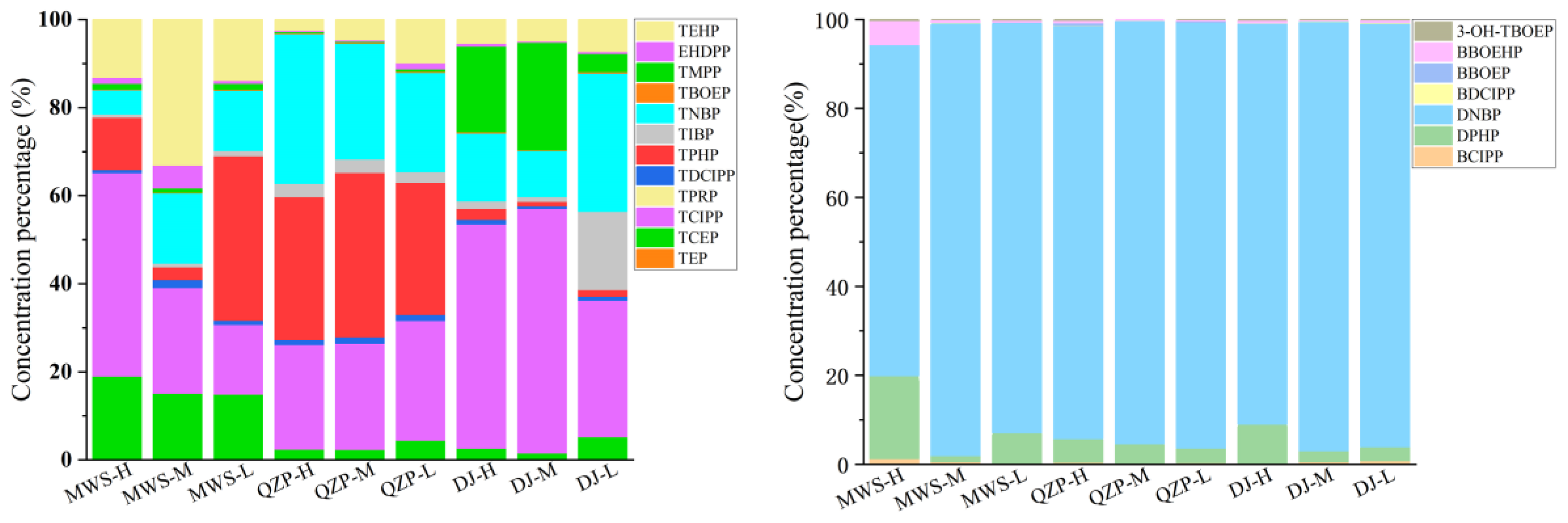
2.2. Composition Profiles of OPEs in Mangrove Sediments
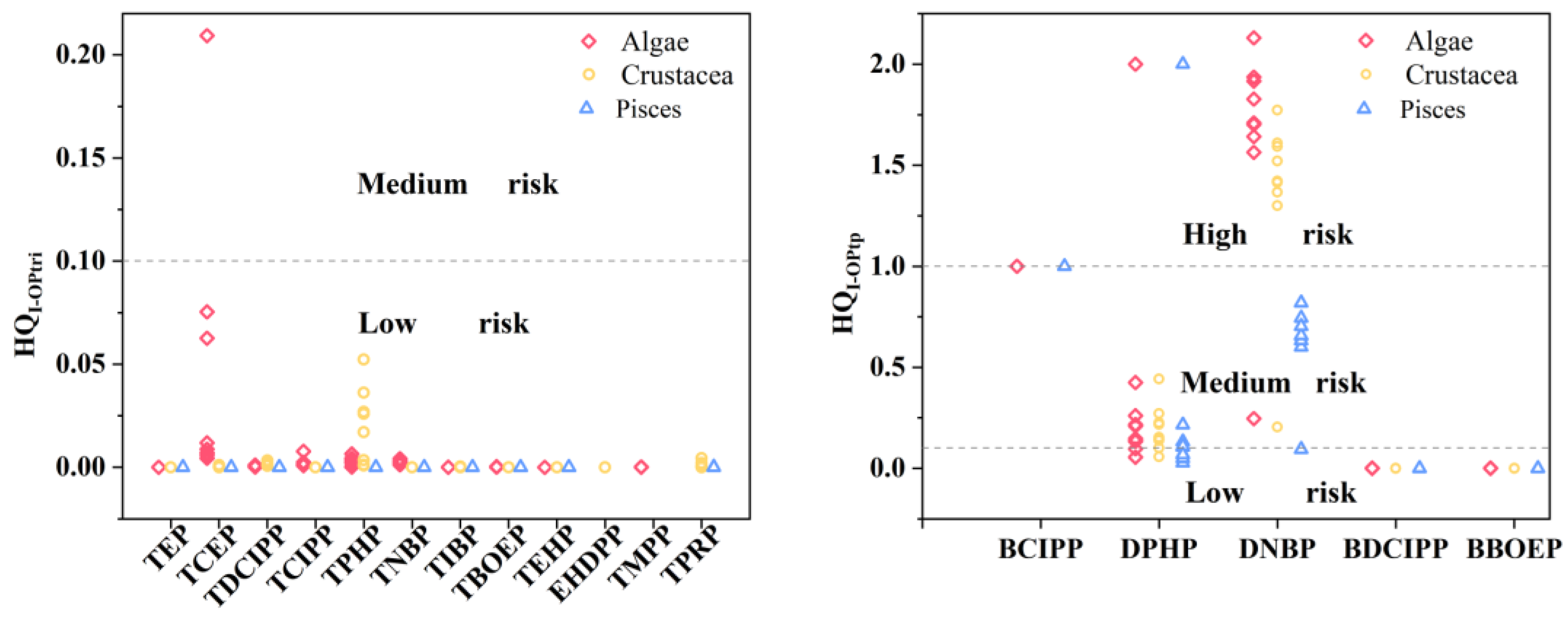
2.3. Ecological Risk Assessment
3. Materials and Methods
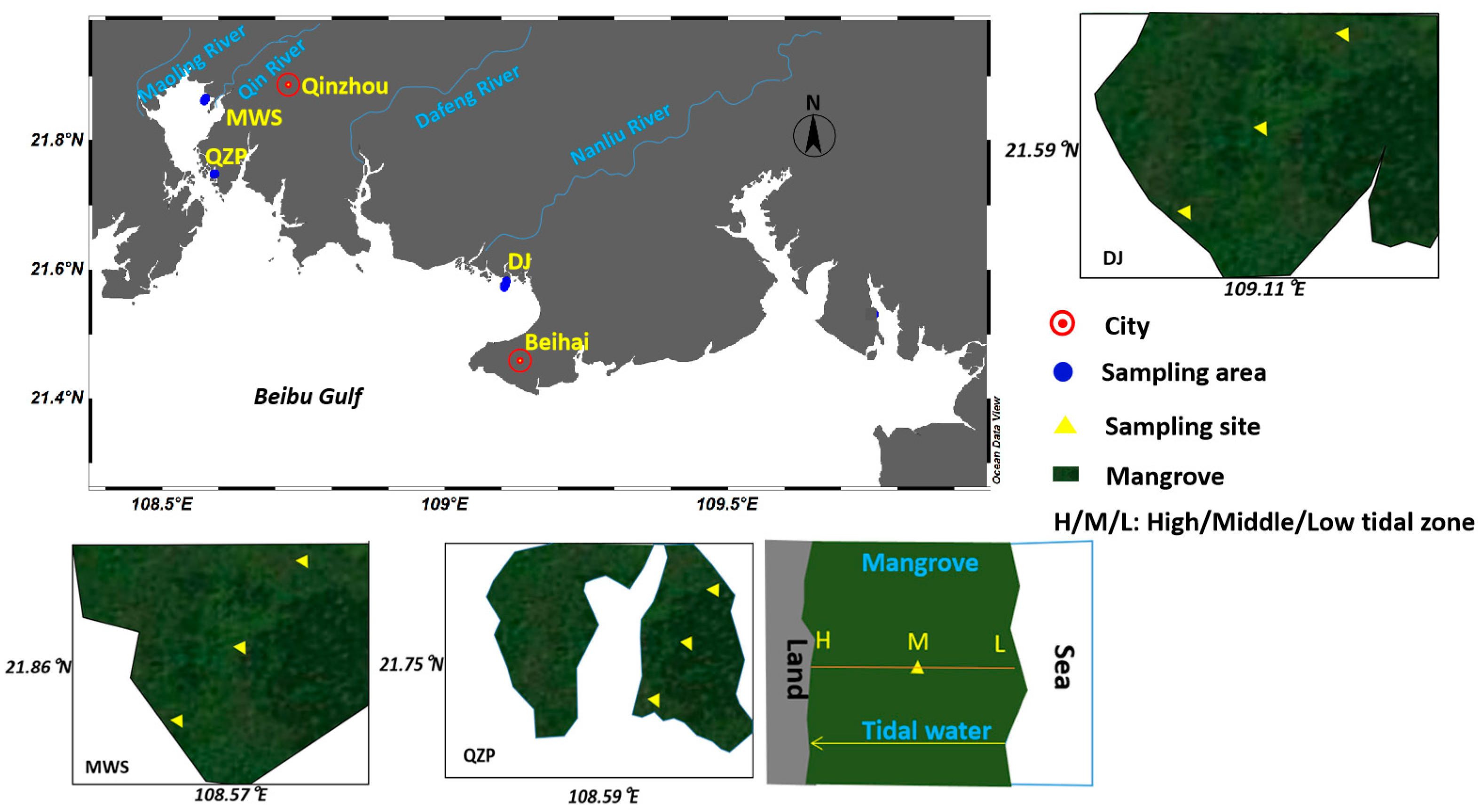
3.1. Studied Areas and Sample Collection
3.2. Chemical and Reagents
3.3. Sample Pretreatment and Instrumental Analysis
3.4. Ecological Risk Assessment of OPEs
3.5. Quality Assurance and Quality Control
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Lu, L.; Zhu, W.; Yang, B.; Lu, D.; Dan, S.F.; Zhang, S. Organophosphorus flame retardants (OPFRs) in the seawater and sediments of the Qinzhou Bay, Northern Beibu Gulf: Occurrence, distribution, and ecological risks. Mar. Pollut. Bull. 2021, 168, 112368. [Google Scholar] [CrossRef]
- Wei, G.L.; Li, D.Q.; Zhuo, M.N.; Liao, Y.S.; Xie, Z.Y.; Guo, T.L.; Li, J.J.; Zhang, S.Y.; Liang, Z.Q. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, L.; Wang, H.; Lu, D.; Luo, X. Development and validation of a liquid chromatography-tandem mass spectrometry method for comprehensive detection of organophosphate esters and their degradation products in sediment. J. Chromatogr. A 2002, 1665, 462826. [Google Scholar] [CrossRef]
- Pantelaki, I.; Voutsa, D. Organophosphate flame retardants (OPFRs): A review on analytical methods and occurrence in wastewater and aquatic environment. Sci. Total Environ. 2019, 649, 247–263. [Google Scholar] [CrossRef]
- Bika, S.H.; Adeniji, A.O.; Okoh, A.I.; Okoh, O.O. Spatiotemporal Distribution and Analysis of Organophosphate Flame Retardants in the Environmental Systems: A Review. Molecules 2022, 27, 573. [Google Scholar] [CrossRef]
- Chen, Z.; An, C.; Elektorowicz, M.; Tian, X. Sources, behaviors, transformations, and environmental risks of organophosphate esters in the coastal environment: A review. Mar. Pollut. Bull. 2022, 180, 113779. [Google Scholar] [CrossRef]
- Xing, R.; Zhang, P.; Zheng, N.; Ji, H.; Shi, R.; Ge, L.; Ma, H. Organophosphate esters in the seawater of the Bohai Sea: Environmental occurrence, sources and ecological risks. Mar. Pollut. Bull. 2023, 191, 114883. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, P.; Wang, X.; Castro-Jiménez, J.; Kallenborn, R.; Liao, C.; Mi, W.; Lohmann, R.; Vila-Costa, M.; Dachs, J. Organophosphate ester pollution in the oceans. Nat. Rev. Earth Environ. 2022, 3, 309–322. [Google Scholar] [CrossRef]
- Wang, Q.; Lam, J.C.W.; Han, J.; Wang, X.; Guo, Y.; Lam, P.K.S.; Zhou, B. Developmental exposure to the organophosphorus flame retardant tris(1,3-dichloro-2-propyl) phosphate: Estrogenic activity, endocrine disruption and reproductive effects on zebrafish. Aquat. Toxicol. 2015, 160, 163–171. [Google Scholar] [CrossRef]
- Kim, J.W.; Isobe, T.; Chang, K.H.; Amano, A.; Maneja, R.H.; Zamora, P.B.; Siringan, F.P.; Tanabe, S. Levels and distribution of organophosphorus flame retardants and plasticizers in fishes from Manila Bay, the Philippines. Environ. Pollut. 2011, 159, 3653–3659. [Google Scholar] [CrossRef]
- Lu, S.Y.; Li, Y.X.; Zhang, T.; Cai, D.; Ruan, J.J.; Huang, M.Z.; Wang, L.; Zhang, J.Q.; Qiu, R.L. Effect of E-waste Recycling on Urinary Metabolites of Organophosphate Flame Retardants and Plasticizers and Their Association with Oxidative Stress. Environ. Sci. Technol. 2017, 51, 2427–2437. [Google Scholar] [CrossRef]
- Liu, Y.; Song, N.; Guo, R.; Xu, H.; Zhang, Q.; Han, Z.; Feng, M.; Li, D.; Zhang, S.; Chen, J. Occurrence and partitioning behavior of organophosphate esters in surface water and sediment of a shallow Chinese freshwater lake (Taihu Lake): Implication for eco-toxicity risk. Chemosphere 2018, 202, 255–263. [Google Scholar] [CrossRef]
- Liao, C.Y.; Kim, U.J.; Kannan, K. Occurrence and distribution of organophosphate esters in sediment from northern Chinese coastal waters. Sci. Total Environ. 2020, 704, 135328. [Google Scholar] [CrossRef]
- Tang, B.; Poma, G.; Bastiaensen, M.; Yin, S.S.; Luo, X.J.; Mai, B.X.; Covaci, A. Bioconcentration and biotransformation of organophosphorus flame retardants (PFRs) in common carp (Cyprinus carpio). Environ. Int. 2019, 126, 512–522. [Google Scholar] [CrossRef]
- Su, G.; Letcher, R.J.; Crump, D.; Gooden, D.M.; Stapleton, H.M. In Vitro Metabolism of the Flame Retardant Triphenyl Phosphate in Chicken Embryonic Hepatocytes and the Importance of the Hydroxylation Pathway. Environ. Sci. Technol. Lett. 2015, 2, 100–104. [Google Scholar] [CrossRef]
- Van den Eede, N.; Maho, W.; Erratico, C.; Neels, H.; Covaci, A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol. Lett. 2013, 223, 9–15. [Google Scholar] [CrossRef]
- Hou, R.; Luo, X.; Liu, C.; Zhou, L.; Wen, J.; Yuan, Y. Enhanced degradation of triphenyl phosphate (TPHP) in bioelectrochemical systems: Kinetics, pathway and degradation mechanisms. Environ. Pollut. 2019, 254, 113040. [Google Scholar] [CrossRef]
- Su, G.; Letcher, R.J.; Yu, H. Organophosphate Flame Retardants and Plasticizers in Aqueous Solution: pH-Dependent Hydrolysis, Kinetics, and Pathways. Environ. Sci. Technol. 2016, 50, 8103–8111. [Google Scholar] [CrossRef]
- Ou, H.S.; Liu, J.; Ye, J.S.; Wang, L.L.; Gao, N.Y.; Ke, J. Degradation of tris(2-chloroethyl) phosphate by ultraviolet-persulfate: Kinetics, pathway and intermediate impact on proteome of Escherichia coli. Chem. Eng. J. 2017, 308, 386–395. [Google Scholar] [CrossRef]
- Fu, L.; Du, B.; Wang, F. Organophosphate Triesters and Diester Degradation Products in Municipal Sludge from Wastewater Treatment Plants in China: Spatial Patterns and Ecological Implications. Environ. Sci. Technol. 2017, 51, 13614–13623. [Google Scholar] [CrossRef]
- Cooper, E.M.; Covaci, A.; van Nuijs, A.L.N.; Webster, T.F.; Stapleton, H.M. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 2123–2132. [Google Scholar] [CrossRef]
- Hou, R.; Liu, C.; Gao, X.; Xu, Y.; Zha, J.; Wang, Z. Accumulation and distribution of organophosphate flame retardants (PFRs) and their di-alkyl phosphates (DAPs) metabolites in different freshwater fish from locations around Beijing, China. Environ. Pollut. 2017, 229, 548–556. [Google Scholar] [CrossRef]
- Hou, R.; Huang, C.; Rao, K.; Xu, Y.; Wang, Z. Characterized in Vitro Metabolism Kinetics of Alkyl Organophosphate Esters in Fish Liver and Intestinal Microsomes. Environ. Sci. Technol. 2018, 52, 3202–3210. [Google Scholar] [CrossRef]
- Chokwe, T.B.; Abafe, O.A.; Mbelu, S.P.; Okonkwo, J.O.; Sibali, L.L. A review of sources, fate, levels, toxicity, exposure and transformations of organophosphorus flame-retardants and plasticizers in the environment. Emerg. Contam. 2020, 6, 345–366. [Google Scholar] [CrossRef]
- Hoffman, K.; Daniels, J.L.; Stapleton, H.M. Urinary metabolites of organophosphate flame retardants and their variability in pregnant women. Environ. Int. 2014, 63, 169–172. [Google Scholar] [CrossRef]
- Hu, Y.X.; Sun, Y.X.; Li, X.; Xu, W.H.; Zhang, Y.; Luo, X.J.; Dai, S.H.; Xu, X.R.; Mai, B.X. Organophosphorus flame retardants in mangrove sediments from the Pearl River Estuary, South China. Chemosphere 2017, 181, 433–439. [Google Scholar] [CrossRef]
- Alegria, H.; Martinez-Colon, M.; Birgul, A.; Brooks, G.; Hanson, L.; Kurt-Karakus, P. Historical sediment record and levels of PCBs in sediments and mangroves of Jobos Bay, Puerto Rico. Sci. Total Environ. 2016, 573, 1003–1009. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhou, K.; Sun, X.L.; Zhao, L.R.; Zhang, Y.B. Occurrence and distribution of the environmental pollutant antibiotics in Gaoqiao mangrove area, China. Chemosphere 2016, 147, 25–35. [Google Scholar] [CrossRef]
- Zhang, Z.; Pei, N.; Sun, Y.; Li, J.; Li, X.; Yu, S.; Xu, X.; Hu, Y.; Mai, B. Halogenated organic pollutants in sediments and organisms from mangrove wetlands of the Jiulong River Estuary, South China. Environ. Rev. 2019, 171, 145–152. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B. Rare Earth Elements and Bioavailability in Northern and Southern Central Red Sea Mangroves, Saudi Arabia. Molecules 2022, 27, 4335. [Google Scholar] [CrossRef]
- Bayen, S.; Wurl, O.; Karuppiah, S.; Sivasothi, N.; Lee, H.K.; Obbard, J.P. Persistent organic pollutants in mangrove food webs in Singapore. Chemosphere 2005, 61, 303–313. [Google Scholar] [CrossRef]
- Bodin, N.; N’Gom Ka, R.; Le Loc’h, F.; Raffray, J.; Budzinski, H.; Peluhet, L.; Tito de Morais, L. Are exploited mangrove molluscs exposed to Persistent Organic Pollutant contamination in Senegal, West Africa? Chemosphere 2011, 84, 318–327. [Google Scholar] [CrossRef]
- Youssef, T. Physiological responses of Avicennia marina seedlings to the phytotoxic effects of the water-soluble fraction of light Arabian crude oil. Environmentalist 2002, 22, 149–159. [Google Scholar] [CrossRef]
- Kruitwagen, G.; Pratap, H.B.; Covaci, A.; Wendelaar Bonga, S.E. Status of pollution in mangrove ecosystems along the coast of Tanzania. Mar. Pollut. Bull. 2008, 56, 1022–1031. [Google Scholar] [CrossRef]
- Chen, J.; Wang, C.; Pan, Y.; Farzana, S.S.; Tam, N.F.Y. Biochar accelerates microbial reductive debromination of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in anaerobic mangrove sediments. J. Hazard. 2018, 341, 177–186. [Google Scholar] [CrossRef]
- Yang, C.W.; Lee, C.C.; Ku, H.; Chang, B.V. Bacterial communities associated with anaerobic debromination of decabromodiphenyl ether from mangrove sediment. Environ. Sci. Pollut. Res. 2017, 24, 5391–5403. [Google Scholar] [CrossRef]
- Zapata-Pérez, O.; Ceja-Moreno, V.; Olmos, M.R.; Pérez, M.T.; Río-García, M.D.; Yarto, M.; Mendoza-Cantú, A.; Ize-Lema, A.I.; Gavilán-García, A.; Felipe, S.T.; et al. Ecotoxicological effects of POPs on ariidae Ariopsis felis (Linnaeus, 1766) from three coastal ecosystems in the Southern Gulf of Mexico and Yucatan Peninsula. J. Environ. Sci. Health A 2007, 42, 1513–1520. [Google Scholar] [CrossRef]
- Xie, J.; Pei, N.; Sun, Y.; Chen, Z.; Cheng, Y.; Chen, L.; Xie, C.; Dai, S.; Zhu, C.; Luo, X.; et al. Bioaccumulation and translocation of organophosphate esters in a Mangrove Nature Reserve from the Pearl River Estuary, South China. J. Hazard. Mater. 2022, 427, 127909. [Google Scholar] [CrossRef]
- Ma, F.; Wang, Y.; Li, Y.; Ye, C.; Xu, Z.; Zhang, F. The application of geostatistics in grain size trend analysis: A case study of eastern Beibu Gulf. J. Geogr. Sci. 2010, 20, 77–90. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Z.; Zhang, Y.; Ren, C.; Song, K. Landsat-Based Estimation of Mangrove Forest Loss and Restoration in Guangxi Province, China, Influenced by Human and Natural Factors. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 311–323. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Jian, Q.; Zhang, P.; Lu, Y.; Liu, H. Tidal variation shaped microplastic enrichment patterns in mangrove blue carbon ecosystem of northern Beibu Gulf, China. Front. Mar. Sci. 2022, 9, 927884. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Ke, L.; Wang, X.H.; Wong, Y.S. Contamination of polycyclic aromatic hydrocarbons in surface sediments of mangrove swamps. Environ. Pollut. 2001, 114, 255–263. [Google Scholar] [CrossRef]
- Tan, X.; Luo, X.; Zheng, X.; Li, Z.; Sun, R.; Mai, B. Distribution of organophosphorus flame retardants in sediments from the Pearl River Delta in South China. Sci. Total Environ. 2016, 544, 77–84. [Google Scholar] [CrossRef]
- Hu, Q.; Xu, L.; Liu, Y.; Zeng, X.; Yu, Z. Co-occurrence and distribution of organophosphate tri- and di-esters in indoor dust from different indoor environments in Guangzhou and their potential human health risk. Environ. Pollut. 2020, 262, 114311. [Google Scholar] [CrossRef]
- Tan, H.; Yang, L.; Yu, Y.; Guan, Q.; Liu, X.; Li, L.; Chen, D. Co-Existence of Organophosphate Di- and Tri-Esters in House Dust from South China and Midwestern United States: Implications for Human Exposure. Environ. Sci. Technol. 2019, 53, 4784–4793. [Google Scholar] [CrossRef]
- Van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Mo, L.; Zheng, J.; Wang, T.; Shi, Y.G.; Chen, B.J.; Liu, B.; Ma, Y.H.; Li, M.; Zhuo, L.; Chen, S.J. Legacy and emerging contaminants in coastal surface sediments around Hainan Island in South China. Chemosphere 2019, 215, 133–141. [Google Scholar] [CrossRef]
- Chen, M.; Gan, Z.; Qu, B.; Chen, S.; Dai, Y.; Bao, X. Temporal and seasonal variation and ecological risk evaluation of flame retardants in seawater and sediments from Bohai Bay near Tianjin, China during 2014 to 2017. Mar. Pollut. Bull. 2019, 146, 874–883. [Google Scholar] [CrossRef]
- Aznar-Alemany, Ò.; Aminot, Y.; Vilà-Cano, J.; Köck-Schulmeyer, M.; Readman, J.W.; Marques, A.; Godinho, L.; Botteon, E.; Ferrari, F.; Boti, V.; et al. Halogenated and organophosphorus flame retardants in European aquaculture samples. Sci. Total Environ. 2018, 612, 492–500. [Google Scholar] [CrossRef]
- Stachel, B.; Jantzen, E.; Knoth, W.; Krüger, F.; Lepom, P.; Oetken, M.; Reincke, H.; Sawal, G.; Schwartz, R.; Uhlig, S. The Elbe flood in August 2002—Organic contaminants in sediment samples taken after the flood event. J. Environ. Sci. 2005, 40, 265–287. [Google Scholar] [CrossRef]
- Liu, J.; He, L.; Zeng, X.; Yu, Z.; Ran, Y.; Sheng, G.; Fu, J. Occurrence and Distribution of Organophosphorus Flame Retardants/Plasticizer in Surface Sediments from the Pearl River and Dongjiang River. Asian J. Ecotoxicol. 2016, 11, 436–443. [Google Scholar]
- Hou, R.; Xu, Y.; Wang, Z. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 2016, 153, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Funk, S.P.; Duffin, L.; He, Y.; McMullen, C.; Sun, C.; Utting, N.; Martin, J.W.; Goss, G.G.; Alessi, D.S. Assessment of impacts of diphenyl phosphate on groundwater and near-surface environments: Sorption and toxicity. J. Contam. Hydrol. 2019, 221, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Crump, D.; Letcher, R.J.; Kennedy, S.W. Rapid in Vitro Metabolism of the Flame Retardant Triphenyl Phosphate and Effects on Cytotoxicity and mRNA Expression in Chicken Embryonic Hepatocytes. Environ. Sci. Technol. 2014, 48, 13511–13519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, J.; Liu, P.; Qi, A.; Jin, H.; Jia, R.; Zheng, Z.; Yan, C.; Cai, M. Contamination characteristics, spatial distribution and ecological-health risk assessment of legacy and current-use pesticides: A case study in the Beibu Gulf. Front. Mar. Sci. 2023, 10, 1167712. [Google Scholar] [CrossRef]
- Zhang, L.; Ni, L.; Wang, H.; Zhang, Z.; Wu, Y.; Jia, R.; He, J.; Zhu, Z.; Jin, H.; Ren, X.; et al. Higher ecological risks and lower bioremediation potentials identified for emerging OPEs than legacy PCBs in the Beibu Gulf, China. Environ. Rev. 2023, 231, 116244. [Google Scholar] [CrossRef]
- European Commission, Technical Guidance Document on Risk Assessment. Part II. Available online: http://refhub.elsevier.com/S0160-4120(13)00129-3/rf0165 (accessed on 29 July 2019).
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World Health Organization Reevaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-Like Compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef]
- Verbruggen, E.M.J.; Rila, J.P.; Traas, T.P.; Posthuma-Doodeman, C.J.A.M.; Posthumus, R. Environmental Risk Limits for Several Phosphate Esters, with Possible Application as Flame Retardant. RIVM Report. 2005. Available online: https://rivm.openrepository.com/handle/10029/255802 (accessed on 21 December 2023).
- Liu, Y.; Gong, S.; Ye, L.; Li, J.; Liu, C.; Chen, D.; Fang, M.; Letcher, R.J.; Su, G. Organophosphate (OP) diesters and a review of sources, chemical properties, environmental occurrence, adverse effects, and future directions. Environ. Int. 2021, 155, 106691. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Yan, X.; Wang, Y.; Liao, C.; Jiang, G. A review of organophosphate flame retardants and plasticizers in the environment: Analysis, occurrence and risk assessment. Sci. Total Environ. 2020, 731, 139071. [Google Scholar] [CrossRef]
| Stations | MWS | QZP | DJ | Mean | DF/% |
|---|---|---|---|---|---|
| Organophosphate triesters | |||||
| TEP | n.d. | n.d. | n.d. | n.d. | 0 |
| TCEP | 2.27–7.58 | <LOQ | <LOQ | 1.57 | 100 |
| TCIPP | 2.90–18.45 | 2.04–2.48 | 2.69–6.10 | 4.87 | 100 |
| TPRP | n.d. | n.d. | n.d. | n.d. | 0 |
| TDCIPP | 0.18–0.28 | 0.10–0.14 | 0.06–0.08 | 0.14 | 100 |
| TPHP | 0.44–6.86 | 2.25–3.53 | 0.11–0.16 | 2.40 | 100 |
| TIBP | <LOQ | <LOQ | <LOQ | <LOQ | 100 |
| TNBP | 2.21–2.51 | 1.69–3.55 | 0.99–2.72 | 2.19 | 100 |
| TBOEP | 0.03–0.06 | 0.02 | 0.02–0.03 | 0.03 | 100 |
| TMPP | 0.14–0.52 | 0.02–0.04 | 0.36–2.68 | 0.59 | 100 |
| EHDPP | <LOQ–0.79 | <LOQ | <LOQ | 0.19 | 100 |
| TEHP | 2.54–5.27 | <LOQ–0.74 | 0.35–0.63 | 1.75 | 100 |
| Sum | 15.09–39.96 | 7.48–10.46 | 6.43–10.98 | 14.1 | / |
| Transformation products | |||||
| BCIPP | <LOQ | <LOQ | <LOQ | <LOQ | 100 |
| DPHP | <LOQ–1.17 | 0.66–0.93 | 0.43–1.90 | 0.84 | 100 |
| DNBP | 2.47–15.71 | 16.5–21.4 | 17.17–19.45 | 16.38 | 100 |
| BDCIPP | n.d. | n.d. | n.d.–<LOQ | <LOQ | 11 |
| BBOEP | n.d. | n.d.–<LOQ | n.d. | <LOQ | 22 |
| BBOEHP | <LOQ | <LOQ | <LOQ | <LOQ | 100 |
| 3-OH-TBOEP | n.d.– < LOQ | n.d.– < LOQ | n.d.– < LOQ | <LOQ | 33 |
| Sum | 3.33–17.57 | 17.76–22.50 | 17.78–21.57 | 17.44 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Xing, Y.; Zhang, P.; Luo, X.; Niu, Z. Organophosphate Triesters and Their Transformation Products in Sediments of Mangrove Wetlands in the Beibu Gulf, South China Sea. Molecules 2024, 29, 736. https://doi.org/10.3390/molecules29030736
Zhang L, Xing Y, Zhang P, Luo X, Niu Z. Organophosphate Triesters and Their Transformation Products in Sediments of Mangrove Wetlands in the Beibu Gulf, South China Sea. Molecules. 2024; 29(3):736. https://doi.org/10.3390/molecules29030736
Chicago/Turabian StyleZhang, Li, Yongze Xing, Peng Zhang, Xin Luo, and Zengyuan Niu. 2024. "Organophosphate Triesters and Their Transformation Products in Sediments of Mangrove Wetlands in the Beibu Gulf, South China Sea" Molecules 29, no. 3: 736. https://doi.org/10.3390/molecules29030736
APA StyleZhang, L., Xing, Y., Zhang, P., Luo, X., & Niu, Z. (2024). Organophosphate Triesters and Their Transformation Products in Sediments of Mangrove Wetlands in the Beibu Gulf, South China Sea. Molecules, 29(3), 736. https://doi.org/10.3390/molecules29030736