Rearrangement of Arylsulfamates and Sulfates to Para-Sulfonyl Anilines and Phenols
Abstract
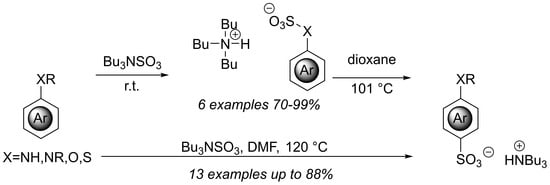
:1. Introduction

2. Results and Discussion
3. Control Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, D.-F.; Bradshaw, T.D.; Chua, M.-S.; Westwell, A.D.; Stevens, M.F.G. Antitumour Benzothiazoles. Part 15:1 The Synthesis and Physico-Chemical Properties of 2-(4-Aminophenyl)benzothiazole Sulfamate Salt Derivatives. Bioorg. Med. Chem. Lett. 2001, 11, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, K.K.D.; Evdokimov, A.G.; Xu, K.; Clark, C.M.; Maier, M.B.; Srivastava, A.; Colson, A.E.; Gerwe, G.S.; Stake, G.E.; Howard, B.W.; et al. Design and synthesis of potent, non-peptidic inhibitors of HPTPb. Bioorg. Med. Chem. Lett. 2006, 16, 4252–4256. [Google Scholar] [CrossRef]
- Gill, D.M.; Povinelli, A.P.R.; Zazeri, G.; Shamir, S.A.; Mahmoud, A.M.; Wilkinson, F.L.; Alexander, M.Y.; Cornelio, M.L.; Jones, A.M. The modulatory role of sulfated and non-sulfated small molecule heparan sulfate-glycomimetics in endothelial dysfunction: Absolute structural clarification, molecular docking and simulated dynamics, SAR analyses and ADME studies. RSC Med. Chem. 2021, 12, 779–790. [Google Scholar] [CrossRef]
- Sidgwick, G.P.; Weston, R.; Mahmoud, A.M.; Schiro, A.; Serracino-Inglott, F.; Tandel, S.M.; Skeoch, S.; Bruce, I.N.; Jones, A.M.; Alexander, M.Y.; et al. Novel Glycomimetics Protect against Glycated Low-Density Lipoprotein-Induced Vascular Calcification In Vitro via Attenuation of the RAGE/ERK/CREB Pathway. Cells 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Jones, A.M.; Sidgwick, G.; Arafat, A.M.; Wilkinson, F.L.; Alexander, M.Y. Small molecule glycomimetics inhibit vascular calcification via c-Met/Notch3/HES1 signalling. Cell. Phys. Biochem. 2019, 53, 323–336. [Google Scholar]
- Hawking, F. Suramin: With special reference to onchocerciasis. Adv. Pharmacol. Chemother. 1978, 15, 289–322. [Google Scholar]
- Mautner, H.G.; Merrill, R.E.; Currier, S.F.; Harvey, G. Interaction of aromatic dyes with the coenzyme A binding site of choline acetyltransferase. J. Med. Chem. 1981, 24, 1534–1537. [Google Scholar] [CrossRef]
- Kugimiya, A.; Tachibana, Y. Indolecarboxylic Acid Derivative Having PGD2 Receptor Antagonistic Activity. WO/2007/029629 A1, 27 March 2007. [Google Scholar]
- Benedetti, A.M.; Gill, D.M.; Tsang, C.W.; Jones, A.M. Chemical methods for N- and O-sulfation of small molecules, amino acids and peptides. ChemBioChem 2020, 21, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, J.A.; Benedetti, A.M.; Jones, A.M. A Novel Exchange Method to Access Sulfated Molecules. Sci. Rep. 2020, 10, 16559. [Google Scholar] [CrossRef]
- Blackburn, J.M.; Short, M.A.; Castanheiro, T.; Ayer, S.K.; Muellers, T.D.; Roizen, J.L. Synthesis of N-Substituted Sulfamate Esters from Sulfamic Acid Salts by Activation with Triphenylphosphine Ditriflate. Org. Lett. 2017, 19, 6012–6015. [Google Scholar] [CrossRef]
- Kanetani, F.; Okada, E.; Negoro, K. Insertion of Sulfur Trioxide into the N-Si Bond of Anilinotrimethylsilane. An Improved Method for the Preparation of Free Phenylamidosulfuric acid. Bull. Chem. Soc. Jpn. 1986, 59, 2517–2520. [Google Scholar] [CrossRef]
- Qingdao University of Science and Technology. Preparation of Amido Sulfonate Derivative Using Sulfur Trioxide. CN114605295 A, 10 June 2022.
- Mihai, M.T.; Williams, B.D.; Phipps, R.J. Para-Selective C-H Borylation of Common Arene Building Blocks Enabled by Ion-Pairing with a Bulky Countercation. J. Am. Chem. Soc. 2019, 141, 15477–15482. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.E.; Morrill, C.; Phipps, R.J. Regioselective Radical Arene Amination for the Concise Synthesis of ortho-Phenylenediamines. J. Am. Chem. Soc. 2021, 143, 9355–9360. [Google Scholar] [CrossRef] [PubMed]
- Matveev, L.G.; Chalykh, S.N.; Okhterova, I.A.; Nazarova, N.E.; Chalykh, E.A.; Gradov, V.A. Synthesis and Properties of Sulfate Salts of Para-Substituted Aromatic-Amines. J. Appl. Chem. USSR 1985, 58, 770–774. [Google Scholar]
- Li, H.-Z.; Xiao, L.-W.; Li, H.-Y.; Wang, K.-F.; Li, X. A Study on the Sulfonation of Aromatic Amines with Sulfuric Acid under Microwave Irradiation. J. Chem. Res. 2003, 2003, 493–494. [Google Scholar] [CrossRef]
- Yur’ev, Y.K.; Arbatskii, A.V. Nitrosation and sulfonation of 1-phenylpyrrolidine. Vestnik Moskovskogo Universiteta 1951, 6, 97–102. [Google Scholar]
- Kanetani, F.; Yamaguchi, H. Studies of Reactions of Amines with Sulfur Trioxide. VI. Thermal Reactions of Anilinium, Dimethylanilinium, and Trimethylanilinium Salts of Butylamidosulfuric acid. Bull. Chem. Soc. Jpn. 1981, 54, 3048–3058. [Google Scholar] [CrossRef]
- Kanetani, F. Preparation of Arylimidobis(sulfates). Bull. Chem. Soc. Jpn. 1986, 59, 952–954. [Google Scholar] [CrossRef]
- Koleva, G.; Galabov, B.; Kong, J.; Schaefer, H.F., III; von R. Schleyer, P. Electrophilic Aromatic Sulfonation with SO3: Concerted or Classic SEAr mechanism? J. Am. Chem. Soc. 2011, 133, 19094–19101. [Google Scholar] [CrossRef]
- Moors, S.L.C.; Deraet, X.; Assche, G.V.; Geerlings, P.; De Proft, F. Aromatic sulfonation with sulfur trioxide: Mechanism and kinetic model. Chem. Sci. 2017, 8, 680–688. [Google Scholar] [CrossRef]
- Morley, J.O.; Roberts, D.W. Molecular Modeling Studies on Aromatic Sulfonation. 1. Intermediates Formed in the Sulfonation of Toluene. J. Org. Chem. 1997, 62, 7358–7363. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.O.; Roberts, D.W.; Watson, S.P. Experimental and molecular modelling studies on aromatic sulfonation. J. Chem. Soc. Perkin Trans. II 2002, 2, 538–544. [Google Scholar] [CrossRef]
- Galabov, B.; Nalbantova, D.; von R. Schleyer, P.; Schaefer, H.F., III. Electrophilic Aromatic Substitution: New Insights into an Old Class of Reactions. Acc. Chem. Res. 2016, 49, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Bochkareva, T.P.; Passat, B.V.; Popov, K.R.; Platonova, N.V.; Koval’cuk, T.I. Sulfonation of substituted azoles with sulfur trioxide in dichloroethane. Khimiya Geterotsiklicheskikh Soedin. 1987, 23, 1084–1089. [Google Scholar] [CrossRef]
- Lally, J.M.; Spillane, W.J. The Photochemistry of Phenylsulphamic Acid: Photorearrangement and Photodegradation. J. Chem. Soc. Chem. Commun. 1987, 1, 8–9. [Google Scholar] [CrossRef]
- Benson, G.A.; Spillane, W.J. Sulfamic Acids and Its N-Substituted Derivatives. Chem. Rev. 1980, 80, 151–186. [Google Scholar] [CrossRef]
- Maarsen, P.K.; Cerfontain, H. Aromatic Sulphonation. Part 56. The Rearrangment of Phenylsulphamic Acid to Aniliniumsulphonic Acids in Concentrated Sulphuric Acid: Evidence for an Intermolecular Reaction Pathway. J. Chem. Soc. Perkin Trans. II 1977, 2, 921–928. [Google Scholar] [CrossRef]
- Newcomer, R.; McKee, J.; Zanger, M. Triflic acid-catalyzed rearrangement of unalkylated benzene sulfonamides. Synth. Commun. 2016, 46, 949–955. [Google Scholar] [CrossRef]
- Kanetani, F.; Yamaguchi, H. Studies of Reactions of Amines with Sulfur Trioxide. V. Transsulfonation of Amine Salts of Some N-Substituted Amidosulfuric Acids. Bull. Chem. Soc. Jpn. 1978, 51, 3039–3046. [Google Scholar] [CrossRef]
- Hopkins, A.; Day, R.A.; Williams, A. Sulfate Group Transfer between Nitrogen and Oxygen: Evidence Consistent with an Open “Exploded” Transition State. J. Am. Chem. Soc. 1983, 105, 6062–6070. [Google Scholar] [CrossRef]
- Gilbert, E.E. The Reactions of Sulfur Trioxide, and of its Adducts, with organic compounds. J. Am. Chem. Soc. 1962, 62, 549–589. [Google Scholar] [CrossRef]
- Tyrer, D. Sulfonation of Hydrocarbons. U.S. Patent 1210725, 2 January 1917. [Google Scholar]
- Roeges, N. A simple preparation of sulfanilic acid. J. Chem. Educ. 1968, 45, 274. [Google Scholar] [CrossRef]
- Bamberger, E.; Hindermann, E. Umlagerung der Phenylsulfaminsäure. Chem. Ber. 1897, 30, 654. [Google Scholar] [CrossRef]
- Illuminati, G. A Reinvestigation of the Role of Phenylsulfamic Acid in the Formation of Aminobenzenesulfonic Acids. J. Am. Chem. Soc. 1956, 78, 2603–2606. [Google Scholar] [CrossRef]
- Spillane, W.J.; Scott, F.L. Radiosulphur studies on the rearrangement of phenylsulphamic acid to sulphanilic acid. Tetrahedron Lett. 1967, 8, 1251–1253. [Google Scholar] [CrossRef]
- Spillane, W.J.; Scott, F.L. The Rearrangement of Phenylsulphamic Acid to Sulphanilic Acid in the Presence of [35S] Sulphuric Acid. J. Chem. Soc. B 1968, 779–781. [Google Scholar] [CrossRef]
- Jones, A.M. Tributylsulfoammonium Betaine. The Encyclopaedia of Reagents for Organic Synthesis (e-EROS). 2021. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/047084289X.RN02393 (accessed on 4 March 2024).
- Gill, D.M.; Male, L.; Jones, A.M. Sulfation made simple: A strategy for synthesising sulfated molecules. Chem. Commun. 2019, 55, 4319–4322. [Google Scholar] [CrossRef] [PubMed]
- Montero Bastidas, J.R.; Oleskey, T.J.; Miller, S.L.; Smith, M.R., III; Maleczka, R.E., Jr. Para-Selective, Iridium-Catalyzed C−H Borylations of Sulfated Phenols, Benzyl Alcohols, and Anilines Directed by Ion-Pair Electrostatic Interactions. J. Am. Chem. Soc. 2019, 141, 15483–15487. [Google Scholar] [CrossRef]
- Prinsen, A.J.; Koeberg-Telder, A.; Cerfontain, H. Sulphonation of polymetylbenzenesulphonic acids. Evidence for a buttressing effect. Tetrahedron 1970, 26, 1953–1960. [Google Scholar] [CrossRef]
- Alexander, E.R. Mechanism of the Sulfonation of Aromatic Amines. II. Sulfonation at Elevated Temperatures with Sulfuric Acid. J. Am. Chem. Soc. 1947, 69, 1599–1602. [Google Scholar] [CrossRef]
- Pereira, M.R.R.C.; Ribeiro, A.F.G.; Silva, A.M.S.; Silva, V.L.M. Ohmic heating-assisted regioselective sulfonation of aniline: Synthesis of sulfanilic acid. New J. Chem. 2022, 46, 20481–20489. [Google Scholar] [CrossRef]
- Morrill, C.; Gillespie, J.E.; Phipps, R.J. An Aminative Rearrangement of O-(Arenesulfonyl)hydroxylamines: Facile Access to ortho-Sulfonyl Anilines. Angew. Chem. Int. Ed. 2022, 61, e202204025, Angew. Chem. 2022, 134, e202204025. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jones, A.M. A Sulfonative Rearrangement of N-Aryl Sulfamates to para-Sulfonyl Anilines. ChemRxiv 2022. [Google Scholar] [CrossRef]









| Entry | Eq. | T (°C) | Atmosphere | Solvent | p-4a at t = 2 h (%) a | p-4a at t = 24 h (%) a | Selectivity p:o a |
|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 101 | Ar | 1,4-Dioxane | 6 | 7 | - |
| 2 | 1.0 | 101 | Ar | 1,4-Dioxane | 1 | 6 | - |
| 3 | 1.5 | 101 | Ar | 1,4-Dioxane | 7 | 10 | - |
| 4 | 2 | 101 | Ar | 1,4-Dioxane | 4 | 12 | - |
| 5 | 4 | 101 | Ar | 1,4-Dioxane | 4 | 9 | - |
| 6 | 2 | 101 | air | 1,4-Dioxane | 3 | 11 | - |
| 7 b | 2 | 80 | Ar | 1,4-Dioxane | - | - | - |
| 8 | 2 | 100 | Ar | Formic Acid | - | - | - |
| 9 | 2 | 100 | Ar | Butan-2-ol | - | - | - |
| 10 | 2 | 80 | Ar | DMF | - | - | - |
| 11 | 2 | 100 | Ar | DMF | 4 | 13 | - |
| 12 | 2 | 120 | Ar | DMF | 32 | 58 | >10:1 |
| 13 | 2 | 140 | Ar | DMF | 30 | 49 | 5:1 |
| 14 | 2 | 120 | Ar | DMSO | 25 | 48 | 8:1 |
| 15 | 2 | 140 | Ar | DMSO | 15 | 35 | 2:1 |
| 16 | 2 | 160 | Ar | DMSO | - | - | - |
| 17 | 2 | 180 | Ar | DMSO | - | - | - |
| 18 | 2 | 120 | Ar | 1,2-dichlorobenzene | 27 | 52 | >10:1 |
| 19 | 2 | 140 | Ar | 1,2-dichlorobenzene | 20 | 44 | 4:1 |
| 20 c | 2 | 160 | Ar | 1,2-dichlorobenzene | - | - | - |
| 21 c | 2 | 180 | Ar | 1,2-dichlorobenzene | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Jones, A.M. Rearrangement of Arylsulfamates and Sulfates to Para-Sulfonyl Anilines and Phenols. Molecules 2024, 29, 1445. https://doi.org/10.3390/molecules29071445
Zhou Y, Jones AM. Rearrangement of Arylsulfamates and Sulfates to Para-Sulfonyl Anilines and Phenols. Molecules. 2024; 29(7):1445. https://doi.org/10.3390/molecules29071445
Chicago/Turabian StyleZhou, Yifei, and Alan M. Jones. 2024. "Rearrangement of Arylsulfamates and Sulfates to Para-Sulfonyl Anilines and Phenols" Molecules 29, no. 7: 1445. https://doi.org/10.3390/molecules29071445
APA StyleZhou, Y., & Jones, A. M. (2024). Rearrangement of Arylsulfamates and Sulfates to Para-Sulfonyl Anilines and Phenols. Molecules, 29(7), 1445. https://doi.org/10.3390/molecules29071445