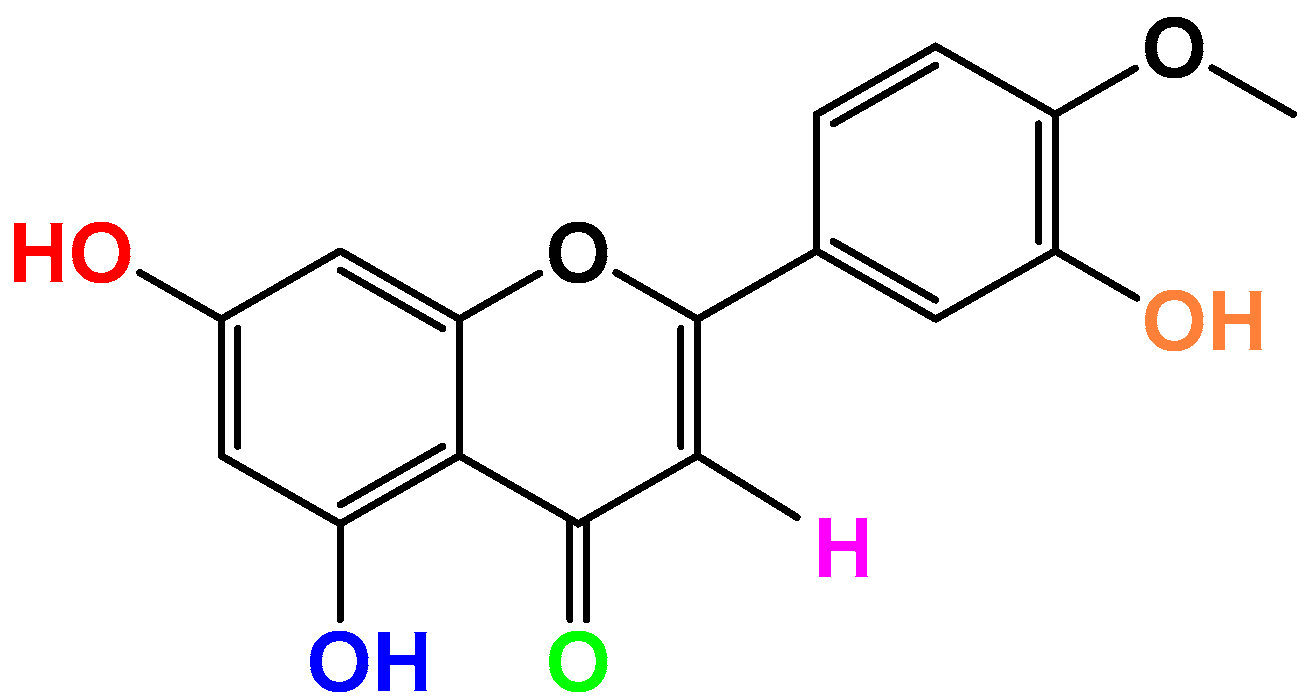
Anti-Inflammatory Properties of the Citrus Flavonoid Diosmetin: An Updated Review of Experimental Models
Abstract
:1. Introduction
2. Anti-Inflammatory Effects
3. Cellular Models
3.1. LPS-Induced Inflammatory Models
3.2. Other Cellular Models of Inflammation
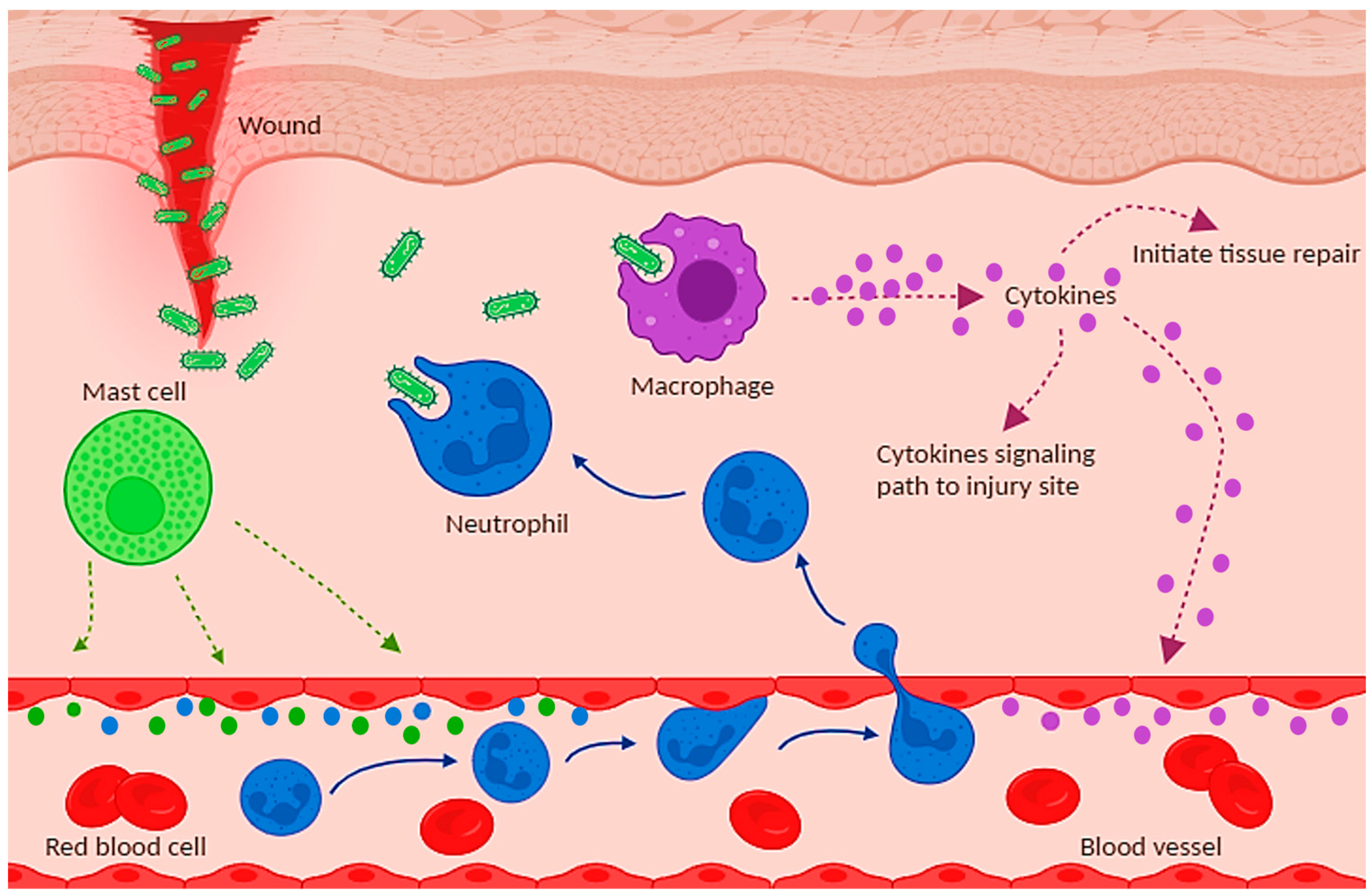
4. Animal Models of Inflammation
4.1. Skin
4.2. Brain
4.3. Lung
4.4. Liver
4.5. Pancreas
4.6. Kidney
4.7. Intestine
4.8. Reproductive System
5. Discussion
6. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanches, V.L.; de Souza Mesquita, L.M.; Viganó, J.; Contieri, L.S.; Pizani, R.; Chaves, J.; da Silva, L.C.; de Souza, M.C.; Breitkreitz, M.C.; Rostagno, M.A. Insights on the Extraction and Analysis of Phenolic Compounds from Citrus Fruits: Green Perspectives and Current Status. Crit. Rev. Anal. Chem. 2022, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Ranjit, A.; Sharma, K.; Prasad, P.; Shang, X.; Gowda, K.G.M.; Keum, Y.-S. Bioactive Compounds of Citrus Fruits: A Review of Composition and Health Benefits of Carotenoids, Flavonoids, Limonoids, and Terpenes. Antioxidants 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Roowi, S.; Crozier, A. Flavonoids in Tropical Citrus Species. J. Agric. Food. Chem. 2011, 59, 12217–12225. [Google Scholar] [CrossRef] [PubMed]
- Caristi, C.; Bellocco, E.; Gargiulli, C.; Toscano, G.; Leuzzi, U. Flavone-di--glycosides in juices from Southern Italy. Food Chem. 2006, 95, 431–437. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, X.; Gao, J.; Wu, Y.; Li, Y. Inhibition of TGF-β Signaling in Gliomas by the Flavonoid Diosmetin Isolated from Dracocephalum peregrinum L. Molecules 2020, 25, 192. [Google Scholar] [CrossRef] [PubMed]
- Wójciak, M.; Feldo, M.; Borowski, G.; Kubrak, T.; Płachno, B.J.; Sowa, I. Antioxidant Potential of Diosmin and Diosmetin against Oxidative Stress in Endothelial Cells. Molecules 2022, 27, 8232. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-h.; Park, J.-k.; Choi, J.; Jang, H.; Seol, J.-w. Anti-inflammatory effects of natural flavonoid diosmetin in IL-4 and LPS-induced macrophage activation and atopic dermatitis model. Int. Immunopharmacol. 2020, 89, 107046–107053. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Pan, D.; Liu, H.; Liu, M.; Shi, X.; Chu, X.; Lu, J.; Zhu, M.; Xia, B.; Wu, J. Diosmetin Protects Against Obesity and Metabolic Dysfunctions Through Activation of Adipose Estrogen Receptors in Mice. Mol. Nutr. Food Res. 2021, 65, 2100070–2100083. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Teng, H.; Xie, Z.; Cao, H.; Cheang, W.S.; Skalicka-Woniak, K.; Georgiev, M.I.; Xiao, J. Modifications of dietary flavonoids towards improved bioactivity: An update on structure–activity relationship. Crit. Rev. Food Sci. Nutr. 2017, 58, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Ritter, B.; Greten, F.R. Modulating inflammation for cancer therapy. J. Exp. Med. 2019, 216, 1234–1243. [Google Scholar] [CrossRef]
- Silva, D.F.; Empadinhas, N.; Cardoso, S.M.; Esteves, A.R. Neurodegenerative Microbially-Shaped Diseases: Oxidative Stress Meets Neuroinflammation. Antioxidants 2022, 11, 2141. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Weintraub, W.S. Safety of non-steroidal anti-inflammatory drugs. Eur. Heart J. 2017, 38, 3293–3295. [Google Scholar] [CrossRef]
- Wu, X.; Schauss, A.G. Mitigation of Inflammation with Foods. J. Agric. Food. Chem. 2012, 60, 6703–6717. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Natural products and drug discovery. Natl. Sci. Rev. 2022, 9, 206–226. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018, 9, 2160–2166. [Google Scholar] [CrossRef]
- Maddipati, K.R. Non-inflammatory Physiology of “Inflammatory” Mediators—Unalamation, a New Paradigm. Front. Immunol. 2020, 11, 580117–580126. [Google Scholar] [CrossRef] [PubMed]
- Freise, N.; Burghard, A.; Ortkras, T.; Daber, N.; Chasan, A.I.; Jauch, S.-L.; Fehler, O.; Hillebrand, J.; Schakaki, M.; Rojas, J.; et al. Signaling mechanisms inducing hypo-responsiveness of phagocytes during systemic inflammation. Blood 2019, 134, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Hawiger, J.; Zienkiewicz, J. Decoding inflammation, its causes, genomic responses, and emerging countermeasures. Scand. J. Immunol. 2019, 90, 12812–12844. [Google Scholar] [CrossRef]
- Medzhitov, R. The spectrum of inflammatory responses. Science 2021, 374, 1070–1075. [Google Scholar] [CrossRef]
- Margetic, S. Inflammation and hemostasis. Biochem. Med. 2018, 22, 49–62. [Google Scholar] [CrossRef]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M.; et al. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Gaber, T.; Strehl, C.; Buttgereit, F. Metabolic regulation of inflammation. Nat. Rev. Rheumatol. 2017, 13, 267–279. [Google Scholar] [CrossRef]
- Kang, S.R.; Park, K.I.; Park, H.S.; Lee, D.H.; Kim, J.A.; Nagappan, A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Park, M.K.; et al. Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chem. 2011, 129, 1721–1728. [Google Scholar] [CrossRef]
- Lee, S.-B.; Lee, W.S.; Shin, J.-S.; Jang, D.S.; Lee, K.T. Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 49, 21–29. [Google Scholar] [CrossRef]
- Berköz, M. Diosmin suppresses the proinflammatory mediators in lipopolysaccharide-induced RAW264.7 macrophages via NF-κB and MAPKs signal pathways. Gen. Physiol. Biophys. 2019, 38, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Feldo, M.; Wójciak, M.; Ziemlewska, A.; Dresler, S.; Sowa, I. Modulatory Effect of Diosmin and Diosmetin on Metalloproteinase Activity and Inflammatory Mediators in Human Skin Fibroblasts Treated with Lipopolysaccharide. Molecules 2022, 27, 4264. [Google Scholar] [CrossRef]
- Comalada, M.; Ballester, I.; Bailón, E.; Sierra, S.; Xaus, J.; Gálvez, J.; de Medina, F.S.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure–activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, P.; Wu, D. Diosmetin alleviates periodontitis by inhibiting oxidative stress and pyroptosis through Nrf2/NF κB/NLRP3 axis. Trop. J. Pharm. Res. 2023, 21, 2519–2524. [Google Scholar] [CrossRef]
- Chandler, D.; Woldu, A.; Rahmadi, A.; Shanmugam, K.; Steiner, N.; Wright, E.; Benavente-García, O.; Schulz, O.; Castillo, J.; Münch, G. Effects of plant-derived polyphenols on TNF-α and nitric oxide production induced by advanced glycation endproducts. Mol. Nutr. Food Res. 2010, 54, 141–150. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Liu, M.; Zhou, B.; Yang, G. Diosmetin exhibits anti-proliferative and anti-inflammatory effects on TNF-α-stimulated human rheumatoid arthritis fibroblast-like synoviocytes through regulating the Akt and NF-κB signaling pathways. Phytother. Res. 2019, 34, 1310–1319. [Google Scholar] [CrossRef]
- López-Posadas, R.; Ballester, I.; Abadía-Molina, A.C.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effect of flavonoids on rat splenocytes, a structure–activity relationship study. Biochem. Pharmacol. 2008, 76, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Hasegawa, N. Therapeutic efficacy of pycnogenol in experimental inflammatory bowel diseases. Phytother. Res. 2004, 18, 1027–1028. [Google Scholar] [CrossRef] [PubMed]
- Minin, A.; Tiuchai, M.; Rodionov, S.; Blatov, I.; Zubarev, I. Magnetocontrolled protein membranes for cell cultures co-cultivation. bioRxiv 2020. [Google Scholar] [CrossRef]
- Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory effect of diosmetin on inflammation and lipolysis in coculture of adipocytes and macrophages. J. Food Biochem. 2020, 44, e13261. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hong, S.; Jung, K.; Choi, S.; Kang, K.S. Suppressive Effects of Flavonoids on Macrophage-Associated Adipocyte Inflammation in a Differentiated Murine Preadipocyte 3T3-L1 Cells Co-Cultured with a Murine Macrophage RAW264.7 Cells. Plants 2022, 11, 3552. [Google Scholar] [CrossRef]
- Kulkarni, O.P.; Lichtnekert, J.; Anders, H.-J.; Mulay, S.R. The Immune System in Tissue Environments Regaining Homeostasis after Injury: Is “Inflammation” Always Inflammation? Mediat. Inflamm. 2016, 2016, 2856213. [Google Scholar] [CrossRef]
- Park, S.-a.; Bong, S.-K.; Lee, J.W.; Park, N.-J.; Choi, Y.; Kim, S.M.; Yang, M.H.; Kim, Y.K.; Kim, S.-N. Diosmetin and Its Glycoside, Diosmin, Improve Atopic Dermatitis- Like Lesions in 2,4-Dinitrochlorobenzene-Induced Murine Models. Biomol. Ther. 2020, 28, 542–548. [Google Scholar] [CrossRef]
- Park, N.-J.; Jo, B.-G.; Bong, S.-K.; Park, S.-a.; Lee, S.; Kim, Y.K.; Yang, M.H.; Kim, S.-N. Lobelia chinensis Extract and Its Active Compound, Diosmetin, Improve Atopic Dermatitis by Reinforcing Skin Barrier Function through SPINK5/LEKTI Regulation. Int. J. Mol. Sci. 2022, 23, 8687. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhan, M.; Chen, Z.; Li, L.; Lu, J.; Yang, M.; Gao, X. Diosmetin ameliorates imiquimod-induced psoriasis by regulating apoptosis and inflammation via toll-like receptor 4/nuclear factor kappa B pathway. Dermatol. Sin. 2022, 40, 207–213. [Google Scholar] [CrossRef]
- Camponogara, C.; Brum, E.S.; Pegoraro, N.S.; Brusco, I.; Brucker, N.; Oliveira, S.M. Diosmetin, a novel transient receptor potential vanilloid 1 antagonist, alleviates the UVB radiation-induced skin inflammation in mice. Inflammopharmacology 2021, 29, 879–895. [Google Scholar] [CrossRef]
- Yu, G.; Wan, R.; Yin, G.; Xiong, J.; Hu, Y.; Xing, M.; Cang, X.; Fan, Y.; Xiao, W.; Qiu, L. Diosmetin ameliorates the severity of cerulein-induced acute pancreatitis in mice by inhibiting the activation of the nuclear factor-κB. Int. J. Clin. Exp. Pathol. 2014, 7, 2133–2142. [Google Scholar]
- Yang, Y.; Gong, X.B.; Huang, L.G.; Wang, Z.X.; Zhang, B.S. Diosmetin exerts anti-oxidative, anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget 2017, 8, 30723–30733. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Yang, C.; Zhu, Y.; Chen, Q.; Zhang, B. Diosmetin Ameliorates Nonalcoholic Steatohepatitis through Modulating Lipogenesis and Inflammatory Response in a STAT1/CXCL10-Dependent Manner. J. Agric. Food. Chem. 2021, 69, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Azmat, R.; Ijaz, M.U.; Ehsan, N.; Afsar, T.; Almajwal, A.; Amor, H.; Alruwaili, N.W.; Razak, S. Diosmetin alleviates nonylphenol-induced liver damage by improving biochemical, inflammatory, apoptotic and histological profile in rats. J. King Saud Univ.—Sci. 2023, 35, 102392–102396. [Google Scholar] [CrossRef]
- Liu, Q.; Ci, X.; Wen, Z.; Peng, L. Diosmetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury through Activating the Nrf2 Pathway and Inhibiting the NLRP3 Inflammasome. Biomol. Ther. 2018, 26, 157–166. [Google Scholar] [CrossRef]
- Xia, J.; Li, J.; Deng, M.; Yin, F.; Liu, J.; Wang, J. Diosmetin alleviates acute lung injury caused by lipopolysaccharide by targeting barrier function. Inflammopharmacology 2023, 31, 2037–2047. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, L.; Yang, S.; Liang, Y.; Zhang, Y.; Pan, X.; Li, J. Diosmetin alleviates benzo[a]pyrene-exacerbated H1N1 influenza virus-induced acute lung injury and dysregulation of inflammation through modulation of the PPAR-γ-NF-κB/P38 MAPK signaling axis. Food Funct. 2023, 14, 3357–3378. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.; Zhou, Z.; Liu, K.; Liu, C. Diosmetin Attenuates Akt Signaling Pathway by Modulating Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB)/Inducible Nitric Oxide Synthase (iNOS) in Streptozotocin (STZ)-Induced Diabetic Nephropathy Mice. Med. Sci. Monit. 2018, 24, 7007–7014. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y. Diosmetin attenuate experimental ulcerative colitis in rats via suppression of NF-κB, TNF-α and IL-6 signalling pathways correlated with down-regulation of apoptotic events. Eur. J. Inflamm. 2021, 19, 20587392211067292. [Google Scholar] [CrossRef]
- Liu, J.; Fu, L.; Yin, F.; Yin, L.; Song, X.; Guo, H.; Liu, J. Diosmetin Maintains Barrier Integrity by Reducing the Expression of ABCG2 in Colonic Epithelial Cells. J. Agric. Food. Chem. 2023, 71, 8931–8940. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-l.; Wei, Y.-y.; Li, X.-h.; Zhang, S.-s.; Zhang, R.-t.; Li, J.-h.; Ma, B.-w.; Shao, S.-b.; Lv, Z.-w.; Ruan, H.; et al. Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-Sirt1/Sirt1 axis. Acta Pharmacol. Sin. 2021, 43, 919–932. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, L.; Yang, B. Diosmetin alleviates S. aureus-induced mastitis by inhibiting SIRT1/GPX4 mediated ferroptosis. Life Sci. 2023, 331, 122060–122072. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Lu, D. Diosmetin Suppresses Neuronal Apoptosis and Inflammation by Modulating the Phosphoinositide 3-Kinase (PI3K)/AKT/Nuclear Factor-κB (NF-κB) Signaling Pathway in a Rat Model of Pneumococcal Meningitis. Med. Sci. Monit. 2019, 25, 2238–2245. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wang, J.; Bi, F.; Bai, Z. Diosmetin alleviates cerebral ischemia-reperfusion injury through Keap1-mediated Nrf2/ARE signaling pathway activation and NLRP3 inflammasome inhibition. Environ. Toxicol. 2022, 37, 1529–1542. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab Treatment in Adults with Moderate-to-Severe Atopic Dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef]
- Park, N.-J.; Bong, S.-K.; Lee, S.; Jung, Y.; Jegal, H.; Kim, J.; Kim, S.-K.; Kim, Y.K.; Kim, S.-N. Compound K improves skin barrier function by increasing SPINK5 expression. J. Ginseng Res. 2020, 44, 799–807. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef]
- Huang, T.-H.; Lin, C.-F.; Alalaiwe, A.; Yang, S.-C.; Fang, J.-Y. Apoptotic or Antiproliferative Activity of Natural Products against Keratinocytes for the Treatment of Psoriasis. Int. J. Mol. Sci. 2019, 20, 2558. [Google Scholar] [CrossRef]
- Zhou, M.; Shi, J.; Lan, S.; Gong, X. FOXM1 regulates the proliferation, apoptosis and inflammatory response of keratinocytes through the NF-κB signaling pathway. Hum. Exp. Toxicol. 2021, 40, 1130–1140. [Google Scholar] [CrossRef]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. Semin. Immunopathol. 2018, 40, 249–259. [Google Scholar] [CrossRef]
- La Russa, F.; Lopes, D.M.; Hobbs, C.; Argunhan, F.; Brain, S.; Bevan, S.; Bennett, D.L.H.; McMahon, S.B. Disruption of the Sensory System Affects Sterile Cutaneous Inflammation In Vivo. J. Invest. Dermatol. 2019, 139, 1936–1945. [Google Scholar] [CrossRef]
- Camponogara, C.; Casoti, R.; Brusco, I.; Piana, M.; Boligon, A.A.; Cabrini, D.A.; Trevisan, G.; Ferreira, J.; Silva, C.R.; Oliveira, S.M. Tabernaemontana catharinensis leaves exhibit topical anti-inflammatory activity without causing toxicity. J. Ethnopharmacol. 2019, 231, 205–216. [Google Scholar] [CrossRef]
- Huang, K.-F.; Ma, K.-H.; Jhap, T.-Y.; Liu, P.-S.; Chueh, S.-H. Ultraviolet B irradiation induced Nrf2 degradation occurs via activation of TRPV1 channels in human dermal fibroblasts. Free Radic. Biol. Med. 2019, 141, 220–232. [Google Scholar] [CrossRef]
- Saito, P.; Melo, C.P.B.; Martinez, R.M.; Fattori, V.; Cezar, T.L.C.; Pinto, I.C.; Bussmann, A.J.C.; Vignoli, J.A.; Georgetti, S.R.; Baracat, M.M.; et al. The Lipid Mediator Resolvin D1 Reduces the Skin Inflammation and Oxidative Stress Induced by UV Irradiation in Hairless Mice. Front. Pharmacol. 2018, 9, 1242–1256. [Google Scholar] [CrossRef]
- Cho, B.O.; Che, D.N.; Shin, J.Y.; Kang, H.J.; Kim, J.H.; Kim, H.Y.; Cho, W.G.; Jang, S.I. Ameliorative effects of Diospyros lotus leaf extract against UVB-induced skin damage in BALB/c mice. Biomed. Pharmacother. 2017, 95, 264–274. [Google Scholar] [CrossRef]
- Adamante, G.; de Almeida, A.S.; Rigo, F.K.; da Silva Silveira, E.; Coelho, Y.O.; De Prá, S.D.-T.; Milioli, A.M.; Camponogara, C.; Casoti, R.; Bellinaso, F.; et al. Diosmetin as a novel transient receptor potential vanilloid 1 antagonist with antinociceptive activity in mice. Life Sci. 2019, 216, 215–226. [Google Scholar] [CrossRef]
- Lünemann, J.D.; Malhotra, S.; Shinohara, M.L.; Montalban, X.; Comabella, M. Targeting Inflammasomes to Treat Neurological Diseases. Ann. Neurol. 2021, 90, 177–188. [Google Scholar] [CrossRef]
- Farmen, K.; Tofiño-Vian, M.; Iovino, F. Neuronal Damage and Neuroinflammation, a Bridge Between Bacterial Meningitis and Neurodegenerative Diseases. Front. Cell. Neurosci. 2021, 15, 680858. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, H.; Xiong, Y.; Gui, X.; Zhang, Y.; Deng, L.; Gao, S.; Luo, M.; Hou, W.; Guo, D. Molecular diagnosis of central nervous system opportunistic infections and mortality in HIV-infected adults in Central China. AIDS Res. Ther. 2017, 14, 24. [Google Scholar] [CrossRef]
- Mehta, A. Neurologic complications and neurodevelopmental outcome with extracorporeal life support. World J. Crit. Care Med. 2013, 2, 40–47. [Google Scholar] [CrossRef]
- Mook-Kanamori, B.B.; Geldhoff, M.; van der Poll, T.; van de Beek, D. Pathogenesis and Pathophysiology of Pneumococcal Meningitis. Clin. Microbiol. Rev. 2011, 24, 557–591. [Google Scholar] [CrossRef]
- Walford, H.H.; Doherty, T.A. STAT6 and lung inflammation. Jak-Stat 2014, 2, 25301–25312. [Google Scholar] [CrossRef]
- Baetz, D.; Shaw, J.; Kirshenbaum, L.A. Nuclear Factor-κB Decoys Suppress Endotoxin-Induced Lung Injury. Mol. Pharmacol. 2005, 67, 977–979. [Google Scholar] [CrossRef]
- Zhao, Y.; Ridge, K.; Zhao, J. Acute Lung Injury, Repair, and Remodeling: Pulmonary Endothelial and Epithelial Biology. Mediat. Inflamm. 2017, 2017, 9081521. [Google Scholar] [CrossRef]
- Carvalho, M.V.d.; Gonçalves-de-Albuquerque, C.F.; Silva, A.R. PPAR Gamma: From Definition to Molecular Targets and Therapy of Lung Diseases. Int. J. Mol. Sci. 2021, 22, 805. [Google Scholar] [CrossRef]
- Lai, M.C.; Liu, W.Y.; Liou, S.-S.; Liu, I.M. Diosmetin Targeted at Peroxisome Proliferator-Activated Receptor Gamma Alleviates Advanced Glycation End Products Induced Neuronal Injury. Nutrients 2022, 14, 2248. [Google Scholar] [CrossRef]
- Brenner, C.; Galluzzi, L.; Kepp, O.; Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 2013, 59, 583–594. [Google Scholar] [CrossRef]
- Beg, A.A.; Sha, W.C.; Bronson, R.T.; Ghosh, S.; Baltimore, D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 1995, 376, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Sun, R.; Wei, H.; Tian, Z. Toll-like receptor 3 ligand attenuates LPS-induced liver injury by down-regulation of toll-like receptor 4 expression on macrophages. Proc. Natl. Acad. Sci. USA 2005, 102, 17077–17082. [Google Scholar] [CrossRef]
- Tomita, K.; Freeman, B.L.; Bronk, S.F.; LeBrasseur, N.K.; White, T.A.; Hirsova, P.; Ibrahim, S.H. CXCL10-Mediates Macrophage, but not Other Innate Immune Cells-Associated Inflammation in Murine Nonalcoholic Steatohepatitis. Sci. Rep. 2016, 6, 28786–28798. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef]
- Hallsworth, K.; Adams, L.A. Lifestyle modification in NAFLD/NASH: Facts and figures. JHEP Rep. 2019, 1, 468–479. [Google Scholar] [CrossRef]
- Sharma, M.; Premkumar, M.; Kulkarni, A.V.; Kumar, P.; Reddy, D.N.; Rao, N.P. Drugs for Non-alcoholic Steatohepatitis (NASH): Quest for the Holy Grail. J. Clin. Transl. Hepatol. 2020, 9, 40–50. [Google Scholar] [CrossRef]
- Silvestro, L.; Tarcomnicu, I.; Dulea, C.; Attili, N.R.B.N.; Ciuca, V.; Peru, D.; Rizea Savu, S. Confirmation of diosmetin 3-O-glucuronide as major metabolite of diosmin in humans, using micro-liquid-chromatography–mass spectrometry and ion mobility mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 8295–8310. [Google Scholar] [CrossRef]
- Grün, F.; Blumberg, B. Endocrine disrupters as obesogens. Mol. Cell. Endocrinol. 2009, 304, 19–29. [Google Scholar] [CrossRef]
- Ke, Q.; Yang, J.; Liu, H.; Huang, Z.; Bu, L.; Jin, D.; Liu, C. Dose- and time-effects responses of Nonylphenol on oxidative stress in rat through the Keap1-Nrf2 signaling pathway. Ecotoxicol. Environ. Saf. 2021, 216, 112185–112192. [Google Scholar] [CrossRef]
- Manohar, M.; Verma, A.K.; Venkateshaiah, S.U.; Sanders, N.L.; Mishra, A. Pathogenic mechanisms of pancreatitis. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 10–25. [Google Scholar] [CrossRef]
- Petrov, M.S.; Shanbhag, S.; Chakraborty, M.; Phillips, A.R.J.; Windsor, J.A. Organ Failure and Infection of Pancreatic Necrosis as Determinants of Mortality in Patients with Acute Pancreatitis. Gastroenterology 2010, 139, 813–820. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Y.; Daniluk, J.; Gaiser, S.; Chu, J.; Wang, H.; Li, Z.S.; Logsdon, C.D.; Ji, B. Activation of Nuclear Factor-κB in Acinar Cells Increases the Severity of Pancreatitis in Mice. Gastroenterology 2013, 144, 202–210. [Google Scholar] [CrossRef]
- Treiber, M.; Neuhöfer, P.; Anetsberger, E.; Einwächter, H.; Lesina, M.; Rickmann, M.; Liang, S.; Kehl, T.; Nakhai, H.; Schmid, R.M.; et al. Myeloid, but Not Pancreatic, RelA/p65 Is Required for Fibrosis in a Mouse Model of Chronic Pancreatitis. Gastroenterology 2011, 141, 1473–1485. [Google Scholar] [CrossRef]
- Werawatganon, D.; Vivatvakin, S.; Somanawat, K.; Tumwasorn, S.; Klaikeaw, N.; Siriviriyakul, P.; Chayanupatkul, M. Effects of probiotics on pancreatic inflammation and intestinal integrity in mice with acute pancreatitis. BMC Complement. Med. Ther. 2023, 23, 166–176. [Google Scholar] [CrossRef]
- Ernandez, T.; Mayadas, T.N. The Changing Landscape of Renal Inflammation. Trends Mol. Med. 2016, 22, 151–163. [Google Scholar] [CrossRef]
- Lim, A. Diabetic nephropathy—Complications and treatment. Int. J. Nephrol. Renov. Dis. 2014, 2014, 361–381. [Google Scholar] [CrossRef]
- Duran-Salgado, M.B. Diabetic nephropathy and inflammation. World J. Diabetes 2014, 5, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Martín-Núñez, E.; Muros-de-Fuentes, M.; Mora-Fernández, C.; Navarro-González, J.F. Inflammatory Cytokines in Diabetic Nephropathy. J. Diabetes Res. 2015, 2015, 948417. [Google Scholar] [CrossRef]
- Kirchmair, R.; Pan, Y.; Zhang, X.; Wang, Y.; Cai, L.; Ren, L.; Tang, L.; Wang, J.; Zhao, Y.; Wang, Y.; et al. Targeting JNK by a New Curcumin Analog to Inhibit NF-kB-Mediated Expression of Cell Adhesion Molecules Attenuates Renal Macrophage Infiltration and Injury in Diabetic Mice. PLoS ONE 2013, 8, 79084–79093. [Google Scholar] [CrossRef]
- Hsieh, M.-Y.; Chang, M.Y.; Chen, Y.-J.; Li, Y.K.; Chuang, T.-H.; Yu, G.-Y.; Cheung, C.H.A.; Chen, H.-C.; Maa, M.-C.; Leu, T.-H. The Inducible Nitric-oxide Synthase (iNOS)/Src Axis Mediates Toll-like Receptor 3 Tyrosine 759 Phosphorylation and Enhances Its Signal Transduction, Leading to Interferon-β Synthesis in Macrophages. J. Biol. Chem. 2014, 289, 9208–9220. [Google Scholar] [CrossRef]
- van Gennep, S.; de Boer, N.K.H.; Gielen, M.E.; Rietdijk, S.T.; Gecse, K.B.; Ponsioen, C.Y.; Duijvestein, M.; D’Haens, G.R.; Löwenberg, M.; de Boer, A.G.E.M. Impaired Quality of Working Life in Inflammatory Bowel Disease Patients. Dig. Dis. Sci. 2020, 66, 2916–2924. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef]
- Khajah, M.A.; Orabi, K.Y.; Hawai, S.; Sary, H.G.; El-Hashim, A.Z. Onion bulb extract reduces colitis severity in mice via modulation of colonic inflammatory pathways and the apoptotic machinery. J. Ethnopharmacol. 2019, 241, 112008–112017. [Google Scholar] [CrossRef]
- Sabino, J.; Verstockt, B.; Vermeire, S.; Ferrante, M. New biologics and small molecules in inflammatory bowel disease: An update. Ther. Adv. Gastroenterol. 2019, 12, 1756284819853208. [Google Scholar] [CrossRef]
- Kaser, A.; Blumberg, R.S. The road to Crohn’s disease. Science 2017, 357, 976–977. [Google Scholar] [CrossRef]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef]
- Wellman, A.S.; Metukuri, M.R.; Kazgan, N.; Xu, X.; Xu, Q.; Ren, N.S.X.; Czopik, A.; Shanahan, M.T.; Kang, A.; Chen, W.; et al. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology 2017, 153, 772–786. [Google Scholar] [CrossRef]
- Ye, Y.-L.; Yin, J.; Hu, T.; Zhang, L.-P.; Wu, L.-Y.; Pang, Z. Increased circulating circular RNA_103516 is a novel biomarker for inflammatory bowel disease in adult patients. World J. Gastroenterol. 2019, 25, 6273–6288. [Google Scholar] [CrossRef]
- Kong, P.; Yu, Y.; Wang, L.; Dou, Y.-Q.; Zhang, X.-H.; Cui, Y.; Wang, H.-Y.; Yong, Y.-T.; Liu, Y.-B.; Hu, H.-J.; et al. circ-Sirt1 controls NF-κB activation via sequence-specific interaction and enhancement of SIRT1 expression by binding to miR-132/212 in vascular smooth muscle cells. Nucleic Acids Res. 2019, 47, 3580–3593. [Google Scholar] [CrossRef]
- Hu, X.; He, Z.; Zhao, C.; He, Y.; Qiu, M.; Xiang, K.; Zhang, N.; Fu, Y. Gut/rumen-mammary gland axis in mastitis: Gut/rumen microbiota–mediated “gastroenterogenic mastitis”. J. Adv. Res. 2023, 55, 159–171. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef]
- Hu, X.; Guo, J.; Zhao, C.; Jiang, P.; Maimai, T.; Yanyi, L.; Cao, Y.; Fu, Y.; Zhang, N. The gut microbiota contributes to the development of Staphylococcus aureus-induced mastitis in mice. ISME J. 2020, 14, 1897–1910. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting Myeloperoxidase (MPO) Mediated Oxidative Stress and Inflammation for Reducing Brain Ischemia Injury: Potential Application of Natural Compounds. Front. Physiol. 2020, 11, 433–448. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Zhang, L. Negative regulation of inflammation by SIRT1. Pharmacol. Res. 2013, 67, 60–67. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168–831183. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, S.; Zhao, A.; Mi, Y.; Zhang, C. Protective effect of rutin on ferroptosis-induced oxidative stress in aging laying hens through Nrf2/HO-1 signaling. Cell Biol. Int. 2022, 47, 598–611. [Google Scholar] [CrossRef]
- Meng, M.; Huo, R.; Wang, Y.; Ma, N.; Shi, X.; Shen, X.; Chang, G. Lentinan inhibits oxidative stress and alleviates LPS-induced inflammation and apoptosis of BMECs by activating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 2022, 222, 2375–2391. [Google Scholar] [CrossRef]
- Hoang, T.K.-D.; Huynh, T.K.-C.; Nguyen, T.-D. Synthesis, characterization, anti-inflammatory and anti-proliferative activity against MCF-7 cells of O-alkyl and O-acyl flavonoid derivatives. Bioorg. Chem. 2015, 63, 45–52. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642–118662. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-X.; Zhang, C.-t.; Yu, X.-N.; Guo, J.-b.; Ma, H.; Liu, K.; Luo, P.; Ren, J. Preparation and pharmacokinetic study of diosmetin long-circulating liposomes modified with lactoferrin. J. Funct. Foods 2022, 91, 105027–105036. [Google Scholar] [CrossRef]
- Gu, Z.; Xue, Y.; Li, S.; Adu-Frimpong, M.; Xu, Y.; Yu, J.; Xu, X.; Zhu, Y. Design, Characterization, and Evaluation of Diosmetin-Loaded Solid Self-microemulsifying Drug Delivery System Prepared by Electrospray for Improved Bioavailability. AAPS PharmSciTech 2022, 23, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Brad, K.; Chen, C. Physicochemical Properties of Diosmetin and Lecithin Complex. Trop. J. Pharm. Res 2013, 12, 453–456. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Chik, Z.; Alshawsh, M.A. Diosmetin Exerts Synergistic Effects in Combination with 5-Fluorouracil in Colorectal Cancer Cells. Biomedicines 2022, 10, 531. [Google Scholar] [CrossRef]
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants 2020, 9, 288. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Guo, S.; Wang, Z.; Jiang, L.; Wang, F.; Zhang, J.; Liu, B. Profiling and comparison of the metabolites of diosmetin and diosmin in rat urine, plasma and feces using UHPLC-LTQ-Orbitrap MSn. J. Chromatogr. B 2019, 1124, 58–71. [Google Scholar] [CrossRef]

| Cell Studies | |||
|---|---|---|---|
| Study Model | Dose(s) | Major Findings | Ref(s). |
| LPS-induced human skin fibroblast cells | 150, 300 μM |  IL-6, IL-1β, COX-2, and PGE2. IL-6, IL-1β, COX-2, and PGE2. | [29] |
| LPS-induced BMDM cells | 25, 50, 100 μM |  TNF-α, NO, iNOS, and IκB-α phosphorylation. TNF-α, NO, iNOS, and IκB-α phosphorylation. | [30] |
| LPS-induced HPDL cells | 10, 20, 40 μM |  IL-6, IL-1β, TNF-α, and NF-κB/NLRP3 signaling. IL-6, IL-1β, TNF-α, and NF-κB/NLRP3 signaling. Nrf2 activity. Nrf2 activity. | [31] |
| AGEs-induced N-11 murine microglial cells | 100 μM |  NO and TNF-α. NO and TNF-α. | [32] |
| TNF-α-induced MH7A cells | 5, 10, 20 μM |  IL-6, IL-8, and IL-1β. IL-6, IL-8, and IL-1β. | [33] |
| Quiescent and concanavalin A-induced rat splenocyte cells | 50 μM |  IL-2, TNF-α, IFN-g, COX-2, and iNOS. IL-2, TNF-α, IFN-g, COX-2, and iNOS. | [34] |
| Co-culture 3T3-L1 cells and RAW 264.7 cells | 10, 25, 50, 100 μM |  NO, TNF-α, monocyte chemoattractant protein, and iNOS. NO, TNF-α, monocyte chemoattractant protein, and iNOS. Mitogen-activated protein kinase phosphorylation, and p65 and p50 translocation. Mitogen-activated protein kinase phosphorylation, and p65 and p50 translocation. | [37] |
 indicates inhibition/reduction,
indicates inhibition/reduction,  indicates activate/increase.
indicates activate/increase.| Animal Studies | ||||
|---|---|---|---|---|
| Study Model | Condition(s) | Studied Sample | Major Findings | Ref(s). |
| DNCB-induced AD in hairless mice | 5 mg kg−1 d−1 (2 weeks) | Skin |  TNF-α, IL-4, IL-1β, iNOS, and MAP kinase phosphorylation (ERK 1/2, p38, and JNK). TNF-α, IL-4, IL-1β, iNOS, and MAP kinase phosphorylation (ERK 1/2, p38, and JNK). JAK/STAT signaling pathway. JAK/STAT signaling pathway. | [7,40] |
| 200 μL 0.5% |  SPINK5 promoter transcriptional activation. SPINK5 promoter transcriptional activation. | [41] | ||
| IMQ-induced psoriasis in mice | 5 mg kg−1 d−1 (1 week) |  IL-6, IL-8, p65, and IκB-α phosphorylation. IL-6, IL-8, p65, and IκB-α phosphorylation. | [42] | |
| UVB-induced inflammation in mice | 0.01–1% of semisolid formulations |  IL-1β and MIP-2. IL-1β and MIP-2. | [43] | |
| Cerulean-induced AP in mice | 100 mg kg−1 | Pancreas |  TNF-α, IL-6, IL-1β, iNOS, MPO, TAP, and NF-κB signaling. TNF-α, IL-6, IL-1β, iNOS, MPO, TAP, and NF-κB signaling. | [44] |
| LPS/D-GalN-induced AHF in murine | 50 mg kg−1 d−1 (6 days) | Liver |  TNF-α, IL-6 and IL-1β. TNF-α, IL-6 and IL-1β. IKK, IκBα, p65 phosphorylation (NF-κB signaling pathway), and JNK and p38 (MAPK signaling pathway). IKK, IκBα, p65 phosphorylation (NF-κB signaling pathway), and JNK and p38 (MAPK signaling pathway). | [45] |
| HFD-induced NASH in mice | 60 mg kg−1 d−1 (4 weeks) |  TNF-α, IL-6. TNF-α, IL-6. STAT1/CXCL10 signaling via NF-κB. STAT1/CXCL10 signaling via NF-κB. | [46] | |
| NP-induced liver damage in rats | 100 mg kg−1 d−1 (30 days) |  NF-κB, TNF-α, IL-6, IL-1β, COX-2, and anti-apoptotic protein (Bcl-2). NF-κB, TNF-α, IL-6, IL-1β, COX-2, and anti-apoptotic protein (Bcl-2). Pro-apoptotic proteins (Bax, caspase-3, and caspase-9). Pro-apoptotic proteins (Bax, caspase-3, and caspase-9). | [47] | |
| LPS-induced ALI in mice | 5, 25 mg kg−1 | Lung |  TNF-α, IL-6, IL-1β, and NLRP3 inflammasome. TNF-α, IL-6, IL-1β, and NLRP3 inflammasome. Nrf2/HO-1 pathway Nrf2/HO-1 pathway | [48] |
| 5, 10, 20 mg kg−1 |  TNF-α, IL-6, and NO. TNF-α, IL-6, and NO. Barrier-related protein expression. Barrier-related protein expression. | [49] | ||
| H1N1 virus and B[a]P-mediated lung injury in mice | 50, 100 mg kg−1 week−1 (27 weeks); 100 mg kg−1 d−1 (7 days) |  IL-6, IL-8, IP-10, MCP-1, RANTES, TNF-α, COX-2, and PGE2. IL-6, IL-8, IP-10, MCP-1, RANTES, TNF-α, COX-2, and PGE2. NF-κB and P38 MAPK signaling. NF-κB and P38 MAPK signaling. | [50] | |
| STZ-induced DN in mice | 25, 50, 100 mg kg−1 d−1 (8 weeks) | Kidney |  TNF-α, IL-6, NO, Akt, NF-κB, and iNOS. TNF-α, IL-6, NO, Akt, NF-κB, and iNOS. | [51] |
| TNBS-induced UC in rats | 50, 100, 200 mg kg−1 d−1 (28 days) | Intestine |  TNF-α, IL-6, and NF-κB. TNF-α, IL-6, and NF-κB. | [52] |
| TNBS-induced CD in mice | 5, 10, 20 mg kg−1 (once every other day for 2 weeks) |  IL-1β, IL-6, and TNF-α. IL-1β, IL-6, and TNF-α. ZO-1, occludin, and claudin-1 expression. ZO-1, occludin, and claudin-1 expression. | [53] | |
| DSS-induced colitis in a mouse | 25, 50 mg kg−1 d−1 (8 days) |  IL-1β, IL-6, TNF-α, COX-2, and acetylated NF-κB via circ-Sirt1/Sirt1. IL-1β, IL-6, TNF-α, COX-2, and acetylated NF-κB via circ-Sirt1/Sirt1. Nrf2 and HO-1. Nrf2 and HO-1. | [54] | |
| S. aureus-induced mastitis in a mouse | 12.5, 25, 50 mg kg−1 | Mammary gland |  MPO, TNF-α, IL-1β, IκB, and NF-κB p65 phosphorylation. MPO, TNF-α, IL-1β, IκB, and NF-κB p65 phosphorylation. | [55] |
| Streptococcus pneumonia-induced bacterial meningitis in rats | 100, 200 mg kg−1 d−1 (4 days) | Brain |  TNF-α, IL-1b, IL-6, Akt, PI3K, MyD88, and NF-κB proteins. TNF-α, IL-1b, IL-6, Akt, PI3K, MyD88, and NF-κB proteins. | [56] |
| Cerebral ischemia-reperfusion neurological injury in rats | 100 mg kg−1 d−1 (3 days) |  IL-1β, IL-18, and NLRP3. IL-1β, IL-18, and NLRP3. Keap1-mediated Nrf2/ARE signaling. Keap1-mediated Nrf2/ARE signaling. | [57] | |
 indicates inhibition/reduction,
indicates inhibition/reduction,  indicates activate/increase.
indicates activate/increase.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Xiang, W.; Cui, J.; Jiao, B.; Su, X. Anti-Inflammatory Properties of the Citrus Flavonoid Diosmetin: An Updated Review of Experimental Models. Molecules 2024, 29, 1521. https://doi.org/10.3390/molecules29071521
Fang Y, Xiang W, Cui J, Jiao B, Su X. Anti-Inflammatory Properties of the Citrus Flavonoid Diosmetin: An Updated Review of Experimental Models. Molecules. 2024; 29(7):1521. https://doi.org/10.3390/molecules29071521
Chicago/Turabian StyleFang, Yangyang, Wei Xiang, Jinwei Cui, Bining Jiao, and Xuesu Su. 2024. "Anti-Inflammatory Properties of the Citrus Flavonoid Diosmetin: An Updated Review of Experimental Models" Molecules 29, no. 7: 1521. https://doi.org/10.3390/molecules29071521
APA StyleFang, Y., Xiang, W., Cui, J., Jiao, B., & Su, X. (2024). Anti-Inflammatory Properties of the Citrus Flavonoid Diosmetin: An Updated Review of Experimental Models. Molecules, 29(7), 1521. https://doi.org/10.3390/molecules29071521






