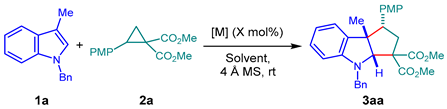
Abstract
This article describes the development of a nickel-catalyzed regio- and diastereoselective formal [3+2] cycloaddition between N-substituted indoles and donor–acceptor cyclopropanes to synthesize cyclopenta[b]indoles. Optimized reaction conditions provide the desired nitrogen-containing cycloadducts in up to 93% yield and dr 8.6:1 with complete regioselectivity. The substrate scope showed high tolerance to various substituted indoles and cyclopropanes, resulting in the synthesis of six new cyclopenta[b]indoles and the isolation of five derivatives previously reported in the literature. In addition, a mechanistic proposal for the reaction was studied through online reaction monitoring by ESI-MS, allowing for the identification of the reactive intermediates in the Ni(II) catalyzed process. X-ray crystallography confirmed the structure and relative endo stereochemistry of the products. This method enables the fast and efficient construction of fused indolines from readily accessible starting materials.
1. Introduction
Fused nitrogen heterocycles, such as indoles and indolines, are ubiquitous in natural products, pharmaceuticals, and bioactive compounds [1,2,3,4]. Notably, derivatives like cyclopenta[b]indoles, a type of C2,C3-fused indoline, are prevalent scaffolds in a plethora of biologically active alkaloids [1,2,3,4,5,6]. For example, terpendole E is an anticarcinogen inhibitor of cellular mitosis [7,8], while vindolinine, an antidiabetic compound isolated from Catharanthus roseus [9,10], and borreverine are strongly active against Gram-positive bacteria (Figure 1) [11,12].

Figure 1.
Cyclopenta[b]indoles in natural products.
Due to the interesting biological properties and structural complexity of these indolic compounds, their synthesis stands out as a prominent area of interest in the field of synthetic chemistry. The reported methods include Nazarov cyclization [13,14], addition-cyclization strategies [15,16], Dieckmann condensation [17], variations in Fischer indole synthesis [18,19,20], organocatalysis [21,22], tin-promoted cycloadditions [23], aluminum-promoted intramolecular imino–ene reactions [24], palladium-catalyzed cross-couplings [25,26,27], and cycloadditions catalyzed by Rh [28,29], Ir [30,31], or Au complexes [24,32,33,34]. However, many of these strategies lack atom economy, rely on the use of precious metal catalysts, or involve relatively elaborate substrates.
In this context, intermolecular [3+2] cycloadditions have emerged as powerful for the construction of five-membered rings in a single synthetic step. Significant contributions to this field have been made through catalytic reactions of vinyldiazo compounds [29,35], azomethine ylides [36], and trimethylenemethane (TMM) [37] and methodologies employing donor–acceptor cyclopropanes [20]. This latter strategy is particularly appealing because it would provide a straightforward and atom-economical strategy for gaining access to cyclopenta[b]indole cores [38,39,40,41,42,43,44].
In this regard, the Kerr group pioneered the synthesis of this skeleton through a [3+2] cycloaddition of donor–acceptor cyclopropanes and 3-methylindoles as a two-carbon synthon catalyzed by Yb(OTf)3 (Scheme 1A) [45,46,47]. Subsequently, in 2013, Xie and Tang reported the use of Cu(II) catalysts (Scheme 1A), in combination with BOX-type chiral ligands, for an enantioselective version of this cyclization [48]. Recently, during the development of this project, Doyle’s group reported the use of 3-alkylindoles as an interesting strategy for the construction of carbocyclic systems fused to indoles via a Ni(II) catalyzed stereoretentive [3+2] cycloaddition, followed by a one-pot Sc(III)-catalyzed decarboxylative ring expansion to afford the corresponding cyclopenta[b]indoles and dihydro-3H-carbazoles (Scheme 1B) [49]. In the initial stage catalyzed by Ni(II), the authors describe cyclopenta[b]indole formation via [3+2] cycloaddition with good retention of the chirality of the starting product, but with a low diastereoselectivity, the inconvenience of which is elegantly overcome in a subsequent rearrangement/decarboxylation stage catalyzed by Sc(OTf)3 [49]. On the other hand, other research groups have explored the use of 3-nitroindoles as more reactive species in [3+2] cycloadditions, using vinylcyclopropanes (VCPs) in Pd(0)-catalyzed processes to obtain cyclopenta[b]indole systems (Scheme 1C) [50,51,52,53]. Following a similar strategy, but employing phenylaziridines or oxiranyl-dicarboxylates instead of VCPs, other authors have described the efficient synthesis of pyrrolo[2,3-b]indole and furo[3,4-b]indole scaffolds [54,55,56]. These cyclopropanes can be activated by a variety of Lewis acids, including those based on non-precious metals that are highly desirable for their abundance and cost-effectiveness [57,58,59,60].
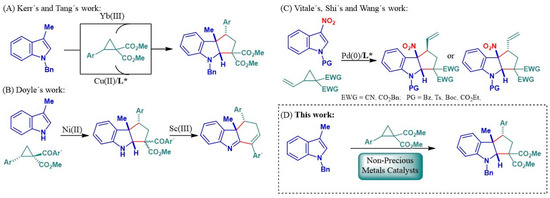
Scheme 1.
[3+2] cycloadditions of 3-methylindole and 3-nitroindole with donor–acceptor cyclopropanes. L* = Chiral ligand.
In this context, and despite the precedents for the use of non-precious metals to access cyclopenta[b]indole skeletons from skatole derivatives, this field is still underdeveloped. Therefore, starting from the general concept of opening metal-catalyzed cyclopropane derivatives, the main objective of this work is the application of non-precious-metal-based catalysts in the diastereoselective synthesis of cyclopenta[b]indole-type skeletons in a single synthetic stage by opening donor–acceptor cyclopropanes and guiding their subsequent formal [3+2] cycloaddition with C3-substituted indoles (Scheme 1D).
2. Results and Discussion
We started our study by employing different Lewis acids based on non-precious metals, using N-benzylskatole (1a) and cyclopropane 2a as model substrates. As an initial control experiment, we attempted to reproduce the reported reaction conditions when employing Cu(OTf)2 as a catalyst [48], affording the desired cycloadduct 3aa at a 79% yield and a diastereoismeric ratio (dr) of 5:1, consistent with the literature reports (Table 1, entry 1) [48]. Next, the reactivity of different metal salts was evaluated. The use of Co(ClO4)2·6H2O led to 42% 3aa with a modest increase in diastereoselectivity (dr = 6:1, Table 1, entry 2). On the other hand, Zn(ClO4)2·6H2O showed a similar enhancement of diastereoselectivity (dr = 6.4:1) but with a significant decrease in yield (28%, Table 1, entry 3). Interestingly, the use of Ni(ClO4)2·6H2O as the catalyst led to 3aa in an excellent 95% yield and with a 6:1 ratio of isomers (Table 1, entry 4). This diastereomeric ratio is considerably higher than the 1:1 ratio described by the Doyle group under similar reaction conditions [49]. Furthermore, the use of Ni(II) salts, such as Ni(ClO4)2·6H2O, in cycloaddition reactions of donor–acceptor cyclopropanes has been previously described in the literature [56,61,62,63], which supports the observed results. Other metal salts of Mn2+, Fe2+, Fe3+, and Al3+ were also evaluated (see Table S1 in the Supplementary Materials), but they were inefficient in obtaining cyclopenta[b]indole 3aa. Based on these results, we continued our study by evaluating different Ni2+ salts. When using a catalytic load of 10 mol%, Ni(ClO4)2·6H2O showed a significantly slower conversion, although it led to a 48% yield (Table 1, entry 5). On the other hand, when Ni(OAc)2·4H2O was employed, a negligible conversion and reaction yield were observed after 24 h (Table 1, entry 6). Other salts such as NiSO4·6H2O, Ni(NO3)2·6H2O, or NiCl2 were evaluated, providing the desired product in modest yields and incomplete conversions (Table 1, entries 7 to 9). Notably, the use of Ni(OTf)2 (Table 1, entry 10) resulted in a significant increase in the yield of the product, reaching 78%, with a diastereomeric ratio of 5:1. At this point, we evaluated the influence of different solvents on the reaction. The use of DCM led to a decrease in yield (47%) with a dr = 7.7:1 (Table 1, entry 11), while with CHCl3, it provided only a 25% yield with an important decrease in diastereoselectivity (Table 1, entry 12). Given the reports of Tang and Doyle [48,49], we also evaluated the use of toluene as a solvent, observing a poor 29% yield and no diastereoselectivity (Table 1, entry 13). Acetonitrile and ethyl acetate were also evaluated, although the starting material was fully recovered in both cases (Table 1, entries 14 and 15). To consult the other solvents evaluated, see Table S2 in the Supplementary Materials.

Table 1.
Screening of the metal salts 1.
Having identified Ni(OTf)2 as the most suitable promoter of the [3+2] cycloaddition, we initially evaluated different substituted indoles. Unfortunately, we observed the formation of the desired products with low yields and diastereoselectivities (Table S3 in the Supplementary Materials). For this reason, we decided to assess the influence of different ligands commonly employed in this type of reaction, including bidentate nitrogenous ligands, bisphosphines, and N,N’-dioxides (Table 2) [56,64,65]. We started by evaluating nitrogen- and phosphorous-based bidentate ligands such as Bipy, Phen, dppe, dFppe, L2-PrPr2, and L3-PrPr2 (Table 2, entries 1 to 6), but unfortunately, none of these catalysts improved the results observed with Ni(OTf)2 (see Table 1, entry 10). On this basis, we decided to reevaluate Ni(ClO4)2·6H2O, considering that it proved to be a competent catalyst (Table 1, entry 4) and taking advantage of the labile nature of the perchlorate ion to facilitate the formation of cationic metal–ligand complexes [66]. With the use of bidentate ligands such as Phen or dppe, the formation of compound 3aa was observed with low yields (Table 2, entries 7 and 8). In contrast, bisphosphines such as dfppe and dppBz afforded the product in modest 43% and 53% yields, respectively (Table 2, entries 9 and 10), while with dppf, no reactivity was observed (Table 2, entry 11). Gratifyingly, the evaluation of rac-BINAP [67,68] resulted in a significant acceleration of the reaction, providing the desired 3aa product in a 73% yield with a diastereoisomeric ratio of 6:1 after 5 h at room temperature (Table 2, entry 12). For more details on ligand optimization, see Table S4 in the Supplementary Materials. The effect of temperature was also evaluated, with us observing that at both 50 and 80 °C, there was a decrease in the isolated yield of 3aa, possibly due to the degradation of the starting materials, along with a decrease in the diastereoisomeric ratio (Table 2, entries 13 and 14).

Table 2.
Screening of the ligand influence 1.
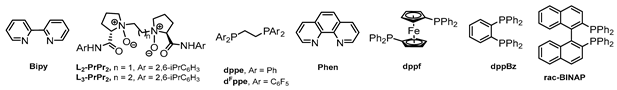 The results listed in Table 2 suggest that the rigidity and bite angle of bidentate phosphines might be crucial factors in the system’s reactivity. Ligands such as dppe (flexible ligand) and dppBz (rigid ligand) [69], with approximately 90° angles, exhibited a reduced yield and a significant increase in reaction time compared to the BINAP version, which features a greater bite angle (approximately 93°) than the previously mentioned ligands [70]. Ligands with larger angles, such as dppf (99°) [71], also fail to provide ideal conditions for this [3+2] cycloaddition. We primarily evaluated a possible enantioselective version of the cycloaddition using chiral bisphosphines such as (R)-BINAP, (R)-DM-BINAP, and (S)-DTBM-DEGPHOS. Among these, only the BINAP derivatives effectively catalyzed the reaction, yielding up to 81%, but not exceeding 40% ee in any case (see details in Table S5 in the Supplementary Materials). Further studies to develop a highly enantioselective version are under development.
The results listed in Table 2 suggest that the rigidity and bite angle of bidentate phosphines might be crucial factors in the system’s reactivity. Ligands such as dppe (flexible ligand) and dppBz (rigid ligand) [69], with approximately 90° angles, exhibited a reduced yield and a significant increase in reaction time compared to the BINAP version, which features a greater bite angle (approximately 93°) than the previously mentioned ligands [70]. Ligands with larger angles, such as dppf (99°) [71], also fail to provide ideal conditions for this [3+2] cycloaddition. We primarily evaluated a possible enantioselective version of the cycloaddition using chiral bisphosphines such as (R)-BINAP, (R)-DM-BINAP, and (S)-DTBM-DEGPHOS. Among these, only the BINAP derivatives effectively catalyzed the reaction, yielding up to 81%, but not exceeding 40% ee in any case (see details in Table S5 in the Supplementary Materials). Further studies to develop a highly enantioselective version are under development.Then, with optimized conditions in hand, we investigated the scope of the reaction by varying the substitution patterns of both substrates (Scheme 2). Regarding cyclopropanes, diverse functional groups on the aryl ring, such as OMe, H, Cl, Br, F, and NO2, were well-tolerated, affording the corresponding products with yields ranging from 51% to 90%, although with lower diastereomer selectivity (3aa–3ag). In particular, electron–donor groups led to shorter reaction times (2 to 3 h), while strongly attracting substituents like the nitro group significantly extended the reaction time (5 days). However, the use of a cyclopropane bearing a 2,4-dichloro-substituted arene did not lead to the corresponding cycloadduct 3af, with us instead recovering the starting material. This result may be explained by steric hindrance having occurred between cyclopropane and the catalyst, rather than there being an electronic effect. Importantly, we also evaluated a vinylcyclopropane (VCP), a less stabilized 1,3-dipole system, widely used with palladium catalysis. After 5 days of reaction, only traces of the product 3ah were observed by mass spectrometry. Inspired by these results, we decided to evaluate a styrylcyclopropane derivative, a more resonance-stabilized VCP. Gratifyingly, this substrate provided cyclopenta[b]indole 3ai at a 93% yield with a dr of 1.7:1. Regarding the effect of the indolic ring, substitutions such as OMe, Cl, or Br in positions 5 and 6 were well-tolerated, affording the desired products with good yields and diastereoisomeric ratios of between 8.6:1 to 4:1, albeit with longer reaction times (3ca–3fa). In contrast, introducing a nitro group in C5, disubstituted C2,C3, or an electron-withdrawing group in the C3 position of the indole did not lead to the desired tricyclic compounds (3ga–3ja). Similarly, it was observed that the N-protection of the indole ring with attractor groups such as Ts, Boc, or Bz did not lead to the formation of the desired skeleton (3ka–3ma). These results are consistent with a decreased nucleophilicity in the indole ring due to the electronic effects of the abovementioned substituents. On the other hand, incorporating a methyl group at the C7 position of the ring did not provide the desired product (3ba), resulting in a complex mixture of products in all cases. This result, along with the case of double substitution in the phenyl ring of cyclopropane (3af), indicated that this cycloaddition is also sensitive to steric factors in both reaction components. Finally, we evaluated sktole (deprotected N) in the reaction, but the formation of 3na was not observed and the starting material was recovered. This observation indicated that this Ni(II)-based catalytic system differs from the reaction described by Doyle’s group [49]. The structure of all reaction products was completely characterized by spectroscopic and spectrometric techniques. Additionally, the structure and relative endo stereochemistry could be confirmed by X-ray analysis of the 3ac crystal (CCDC: 2302398) [72].
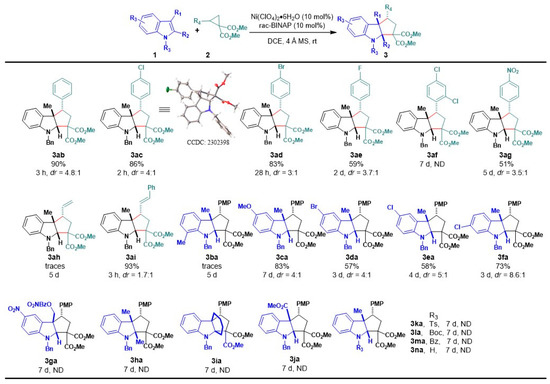
Scheme 2.
Scope of the reaction 1, 2, 3. 1 Reaction conditions: Indole 1 (1.0 eq.) and cyclopropane 2 (1.2 eq.), was treated with Ni(ClO4)2·6H2O (10 mol%) and rac-BINAP (10 mol%) in DCE (0.1 M) at rt and time (h). 2 Isolated yield. 3 Calculated from the 1H NMR. h = hours, d = days, ND = not detected.
Based on the experimental results and previous reports of [3+2] cycloadditions between donor–acceptor cyclopropanes and indoles [48,49,55], a plausible stepwise mechanism is proposed (Scheme 3). Based on the crystal structure of the cationic BINAP/Ni2+ complex (A) [73,74] and the labile nature of the perchlorate ion for the formation of metal–ligand complexes [66], we propose that an exchange of the labile ligands of the complex occurs when it interacts with the 1,1-dicarbonyl system of the donor–acceptor cyclopropane 2. This exchange leads to the formation of intermediate B, which, in turn, stabilizes the formation of the polarized species C. In view of the steric demand, the nucleophilic attack of the C3 position of indole 1 on the sp3 stabilized carbon of cyclopropane, which bears a cationic character (δ+), leads to the formation of the intermediate D [75,76]. Subsequently, an intramolecular cyclization occurs through the attack of the malonic anion on the iminium ion generating the indole. Finally, the decoordination of the obtained cyclopenta[b]indole 3aa regenerates the catalytic species A.
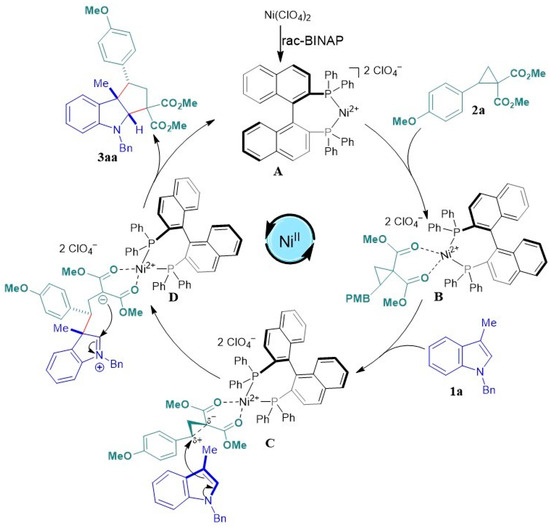
Scheme 3.
Proposed mechanism for formal [3+2] cycloaddition.
To gain further insight into the proposed mechanism, we performed online reaction monitoring by ESI-MS, a technique of great relevance in the advancement of organic catalysis [77,78,79], since molecules and transient intermediates [80] of high polarity and moiety complexity can be easily studied by mass spectrometry. ESI-MS has proven to be a remarkable “ion-fishing” technique, as it gently transfers preformed ions in solution directly to the gas phase. Thus, Figure 2 shows the screening of the reaction of 1a and 2a catalyzed with Ni(ClO4)2⋅6H2O (10 mol%) and rac-BINAP (10 mol%) in DCE by online reaction monitoring by ESI-MS. The goal of this study was to intercept the cationic species resulting from the Ni-catalyzed reaction using ESI-MS in the positive-ion mode (Scheme 3) [81,82,83]. The main advantage of using ESI is its capacity to transfer ions to the gas phase without inducing unwanted side reactions, and the composition of ESI-transferred ions often reflects that in solution [77,78,79]. The ESI-MS spectra collected for such a reaction are particularly clean and mechanistically enlightening. Shortly after four to five minutes of reaction of Ni(ClO4)2⋅6H2O and rac-BINAP, two cationic species directly related to the proposed catalytic cycle (Scheme 3) were detected as major ions (Figure 2a): [A + ClO4−]+ of m/z 779, and [A + Cl−]+ of m/z 715. After 10 min of reaction, B was observed as m/z 1059 (Figure 2b). The isotopic patterns of Ni species were in accordance with the calculated compounds. Then, following the proposed mechanism depicted in Scheme 3, the search began for the species C and D of m/z 1165 that have the same m/z. The formation of intermediates C and D of m/z 1165 was observed after 19 min of reaction time, as shown in Figure 2d. The overall appearance of the spectrum and relative intensities of the ions changed little from 19 to 120 min of reaction in solution, as revealed by continuous ESI-MS monitoring. Intermediates C and D in Figure 2e are isobars, but they have a different bond behavior, as consistent with Scheme 2. Furthermore, the ESI-MS/MS spectrum of the ion of m/z 1165 did not change with time, showing that these intermediates participate in the catalytic process. However, spectra of samples taken after 20 min to 2 h of reaction (Figure 2e) showed that the ion of m/z 1165 dissociates in the gas phase mainly into two pathways. A fragment ion of m/z 265 (2a) was formed from intermediate C, whereas a dissociated ion of m/z 485 could be distinguished from intermediate D, which afforded the observed final product 3aa, as depicted in Figure 2e,f. Furthermore, this study demonstrates the important role of BINAP in the catalytic cycle, since its participation in forming all the key intermediates of the proposed mechanism was detected by ESI-MS.
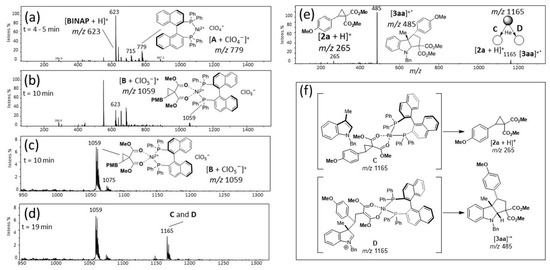
Figure 2.
Online ESI-MS monitoring of the reaction of indole 1a (1.0 eq.) and cyclopropane 2a (1.2 eq.) treated with Ni(ClO4)2·6H2O (10 mol%) and rac-BINAP (10 mol%) in DCE at rt in different reactional times: (a) 4–5 min; (b) 10 min; (c) 10 min, m/z 950 to 1300; (d) 19 min. (e) ESI-MS/MS of isobaric intermediates C and D: C gives 2a; D give final product 3aa. (f) ESI-MS/MS experiments for the characterization of intermediates C (that affords 2a) and D (that produces the final product 3aa), which have the same m/z.
In several studies, it is proposed that cycloadditions with catalyst-activated donor–acceptor cyclopropanes can occur via the SN2 or SN1 pathway [49,84,85,86,87]. However, according to the ESI-MS experiments and synthetic results in this work, the SN1 pathway could not be discarded. Therefore, C and D are undoubtedly present in the reaction mixture in the course of the reaction. This observation, made according to ESI-MS/MS experiments, suggests that intermediate D is the only species that produces the final product 3aa.
3. Experimental Section
3.1. General Section
All the reactions were conducted in dry solvents under a N2 atmosphere unless otherwise stated. All reagents used were obtained from commercial suppliers and used without further purification. The abbreviation “rt” refers to reactions carried out at approximately 25 °C. Reaction mixtures were stirred using Teflon-coated magnetic stirring bars. Reaction temperatures were maintained using Thermowatch-controlled silicone oil baths. The reactions were monitored by thin-layer chromatography (TLC), which was performed on silica gel Merck 60 F254, and the components were visualized by observation under UV light (254 and 365 nm) and/or by treating the plates with p-anisaldehyde, oleum, phosphomolybdic acid, or cerium nitrate solutions, followed by heating. Flash chromatography was carried out on silica gel (63–200 µm) unless otherwise stated. Drying was performed with anhydrous Na2SO4. Concentration refers to the removal of volatile solvents via distillation using a Büchi rotary evaporator R-300 followed by residual solvent removal under a high vacuum. Melting points were determined using a Stuart SMP3 apparatus. Infrared spectra were measured using a Perkin-Elmer FT-IR Spectrometer Spectrum Two (Llantrisant, UK) with KBr pellets. NMR spectra were recorded in CDCl3, at 300, 400, 500, or 700 MHz (Bruker Advance III, Oxford, UK). Chemical shifts were reported in parts per million (δ) using the residual solvent signals (CDCl3: δH 7.26, δC 77.16) as the internal standards for the 1H and 13C NMR spectra and coupling constants (J) in Hz. Carbon types and structure assignments were determined from DEPT-NMR and two-dimensional experiments (HSQC and HMBC, COSY and NOESY). Mass spectra (ESI-MS) were acquired using an Agilent 1200 ESI/APCI Q-TOF in tandem with an Agilent Mass Q-TOF 6520 (Santa Clara, CA, USA). For the crystal structure determination, data were collected by applying the omega and phi scan method on a Bruker D8 VENTURE PHOTON III-14 diffractometer (Karlsurehe, Germany) using an Incoatec multilayer mirror monochromated with Mo-Kα radiation (λ = 0.71073Å) from a microfocus sealed tube source at 100 K, with a detector resolution of 7.3910 pixels mm−1. For details of the synthesis and characterization of indoles 1a–m and donor–acceptor cyclopropanes 2a–i, see the Supplementary Materials.
3.2. General Procedure for [3+2] Cycloaddition (Exemplified for the Synthesis of Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-1-(4-methoxyphenyl)-8b-methyl-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3aa))
A solution of N-benzylskatole 1a (20 mg, 90.37 μmol, 1.0 eq), cyclopropane 2a (28.7 mg, 108.45 μmol, 1.2 eq), Ni(ClO4)2·6H2O (3.3 mg, 9.04 μmol, 10 mol%), and rac-BINAP (5.63 mg, 9.04 μmol, 10 mol%) and 400 mg of 4 Å molecular sieves in dry DCE (903 μL, 0.1 M) were stirred under a nitrogen atmosphere at room temperature for 5 h. After completing the reaction, as indicated by TLC, the mixture was filtered through celite® and washed AcOEt (50 mL). The filtrate was concentrated and purified by flash chromatography (SiO2, 63-200 μm, 10% AcOEt/hexane) to afford cyclopenta[b]indole 3aa (32 mg, 73% yield) as a colorless oil (dr = 6:1). IR (KBr, cm−1) 𝜈: 2994, 2950, 2834, 1731, 1599, 1514, 1275. 1H NMR: (500 MHz, CDCl3) δ 7.24–7.17 (m, 2.43H), 7.17–7.10 (m, 2.76H), 7.05 (t, J = 9.5 Hz, 0.57H), 6.95 (t, J = 7.7 Hz, 0.20H), 6.90–6.78 (m, 2H), 6.73 (d, J = 8.2 Hz, 1.84H), 6.63 (d, J = 7.2 Hz, 0.14H), 6.59 (t, J = 7.3 Hz, 0.14H), 6.36 (d, J = 8.0 Hz, 0.14H), 6.32 (d, J = 7.9 Hz, 0.86H), 6.28 (t, J = 7.4 Hz, 0.86H), 5.47 (d, J = 7.5 Hz, 0.86H), 4.58 (d, J = 16.5 Hz, 0.18H), 4.55–4.49 (m, 1.78H), 4.25 (d, J = 16.3 Hz, 0.16H), 4.08 (d, J = 16.1 Hz, 0.89H), 3.75 (s, 0.5H), 3.73 (s, 2.79H), 3.71 (s, 0.36H), 3.69 (s, 2.52H), 3.39 (s, 2.56H), 3.34 (s, 0.44H), 2.90 (dd, J = 14.8, 4.6 Hz, 0.82H), 2.68 (dd, J = 14.8, 12.7 Hz, 0.90H), 2.60 (dd, J = 13.1, 5.7 Hz, 0.16H), 2.30 (t, J = 13.3 Hz, 0.21H), 2.26–2.21 (m, 0.94H), 1.26 (s, 2.82H), 1.18 (s, 1.53H). 13C NMR: (126 MHz, CDCl3) δ 172.84, 171.97, 169.21, 158.79, 153.45, 150.88, 138.77, 138.62, 137.21, 133.00, 130.82, 130.44, 130.08, 129.75, 128.45, 128.37, 127.86, 127.85, 127.76, 127.59, 127.05, 126.89, 125.95, 118.39, 118.21, 114.14, 113.99, 113.73, 113.50, 113.25, 109.67, 109.54, 82.33, 80.19, 65.30, 64.12, 57.57, 56.66, 55.39, 55.37, 55.13, 53.74, 53.41, 52.90, 52.55, 52.44, 39.36, 36.87, 29.83, 28.15. HRMS (ESI): Calculated for C30H32NO5 [M + H]+ = 486.2275; found: 486.2286. NMR data of the major isomer deduced from the 6:1 mixture: 1H NMR: (500 MHz, CDCl3) δ 7.24–7.09 (m, 6H), 6.89–6.78 (m, 2H), 6.73 (d, J = 8.3 Hz, 2H), 6.34–6.25 (m, 2H), 5.47 (d, J = 7.5 Hz, 1H), 4.52 (d, 2H), 4.08 (d, J = 16.1 Hz, 1H), 3.73 (s, 3H), 3.69 (s, 3H), 3.39 (s, 3H), 2.90 (dd, J = 14.8, 4.6 Hz, 1H), 2.73–2.63 (m, 1H), 2.23 (dd, J = 13.0, 4.4 Hz, 1H), 1.26 (s, 3H). 13C NMR: (126 MHz, CDCl3) δ 171.97, 169.21, 158.79, 153.45, 138.77, 133.00, 130.44, 130.08, 128.45, 128.37, 127.86, 127.83, 127.76, 127.59, 127.05, 125.95, 118.21, 113.73, 113.50, 113.25, 109.67, 80.19, 65.30, 57.57, 56.66, 55.39, 55.37, 52.90, 52.55, 52.44, 36.87, 29.83, 28.15. The spectroscopic data agreed with the literature [48].
3.2.1. Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-8b-methyl-1-phenyl-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3ab)
Colorless oil (37 mg, 90%, dr = 4.8:1) from N-benzylskatole 1a (20 mg, 90.37 μmol) and cyclopropane 2b (25.4 mg, 108.45 μmol). IR (KBr, cm−1) 𝜈: 3026, 2949, 2849, 1731, 1599, 1484, 1452, 1264, 698. 1H NMR: (700 MHz, CDCl3) δ 7.34 (t, J = 7.4 Hz, 0.74H), 7.32–7.25 (m, 3.26H), 7.24–7.21 (m, 1.38H), 7.21–7.18 (m, 1.69H), 7.08–7.01 (m, 1.67H), 7.02 (s, 0.21H), 6.91 (td, J = 7.6, 1.3 Hz, 0.95H), 6.72 (dd, J = 7.4, 1.4 Hz, 0.30H), 6.68 (d, J = 7.3 Hz, 0.32H), 6.45 (d, J = 8.0 Hz, 0.33H), 6.40 (d, J = 7.9 Hz, 0.81H), 6.35–6.30 (m, 0.85H), 5.49–5.44 (m, 0.74H), 4.71 (s, 0.26H), 4.66 (d, J = 16.3 Hz, 0.43H), 4.63 (d, J = 1.5 Hz, 0.71H), 4.60 (d, J = 16.1 Hz, 0.79H), 4.34 (d, J = 16.3 Hz, 0.35H), 4.16 (d, J = 16.0 Hz, 0.80H), 3.81 (s, 0.81H), 3.78 (s, 2.22H), 3.47 (s, 2.22H), 3.42 (s, 0.72H), 3.02 (dd, J = 14.8, 4.7 Hz, 0.79H), 2.82 (dd, J = 14.9, 12.8 Hz, 0.88H), 2.71 (dd, J = 13.1, 5.8 Hz, 0.37H), 2.44 (t, J = 13.4 Hz, 0.42H), 2.37–2.32 (m, 1H), 1.37 (s, 2.15H), 0.94 (s, 0.87H). 13C NMR: (176 MHz, CDCl3) δ 172.70, 171.84, 170.28, 170.22, 169.04, 167.07, 153.34, 150.77, 138.73, 138.65, 138.49, 138.27, 136.95, 134.61, 132.77, 129.10, 129.07, 128.64, 128.61, 128.46, 128.43, 128.35, 128.28, 128.27, 128.25, 128.20, 127.99, 127.80, 127.76, 127.64, 127.58, 127.46, 127.43, 127.34, 127.01, 126.94, 126.91, 126.78, 125.79, 125.68, 124.85, 122.79, 118.30, 118.07, 109.60, 109.45, 82.28, 80.18, 65.20, 64.06, 57.55, 56.60, 55.08, 53.97, 53.61, 53.14, 52.83, 52.81, 52.58, 52.46, 52.36, 52.23, 38.99, 36.44, 32.59, 29.72, 28.06, 22.92, 19.14. HRMS (ESI): Calculated for C29H30NO4 [M + H]+ = 456.2169; found: 456.2181. NMR data of the major isomer deduced from the 4.8:1 mixture: 1H NMR: (700 MHz, CDCl3) δ 7.32–7.25 (m, 4H), 7.24–7.21 (m, 2H), 7.21–7.18 (m, 2H), 7.08–7.01 (m, 2H), 6.91 (td, J = 7.6, 1.3 Hz, 1H), 6.40 (d, J = 7.9 Hz, 1H), 6.35–6.30 (m, 1H), 5.49–5.44 (m, 1H), 4.63 (d, J = 1.5 Hz, 1H), 4.60 (d, J = 16.1 Hz, 1H), 4.16 (d, J = 16.0 Hz, 1H), 3.78 (s, 3H), 3.47 (s, 3H), 3.02 (dd, J = 14.8, 4.7 Hz, 1H), 2.82 (dd, J = 14.9, 12.8 Hz, 1H), 2.37–2.32 (m, 1H), 1.37 (s, 3H). 13C NMR: (176 MHz, CDCl3) δ 171.84, 169.04, 153.34, 138.65, 138.27, 132.77, 129.10, 129.07, 128.46, 128.35, 128.27, 128.20, 127.99, 127.80, 127.76, 127.64, 127.46, 127.01, 126.94, 126.78, 125.68, 122.79, 118.30, 118.07, 109.60, 109.45, 80.18, 65.20, 57.55, 56.60, 53.14, 52.83, 52.81, 52.36, 36.44, 28.06. The spectroscopic data agreed with the literature [48].
3.2.2. Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-1-(4-chlorophenyl)-8b-methyl-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3ac)
White solid (38.1 mg, 86%, dr = 4:1) from N-benzylskatole 1a (20 mg, 90.37 μmol) and cyclopropane 2c (29.2 mg, 108.45 μmol). mp: 117–120 °C. IR (KBr, cm−1) 𝜈: 3026, 2949, 2849, 1731, 1599, 1484, 1452, 1264, 698. 1H NMR: (700 MHz, CDCl3) δ 7.34–7.25 (m, 3H), 7.23 (d, J = 8.2 Hz, 2H), 7.18 (d, J = 7.7 Hz, 1.39H), 7.04 (d, J = 7.7 Hz, 0.41H), 7.00–6.91 (m, 1.42H), 6.45 (d, J = 8.0 Hz, 0.24H), 6.41 (d, J = 8.0 Hz, 0.70H), 6.38 (t, J = 7.4 Hz, 0.69H), 5.53 (d, J = 7.5 Hz, 0.76H), 4.71 (s, 0.19H), 4.65 (d, J = 16.3 Hz, 0.30H), 4.62 (s, 0.68H), 4.59 (d, J = 16.1 Hz, 0.71H), 4.32 (d, J = 16.3 Hz, 0.22H), 4.15 (d, J = 16.1 Hz, 0.76H), 3.81 (s, 0.57H), 3.78 (s, 2.08H), 3.47 (s, 2.11H), 3.42 (s, 0.58H), 2.98 (dd, J = 14.8, 4.7 Hz, 0.75H), 2.76 (dd, J = 14.7, 12.8 Hz, 0.82H), 2.69 (dd, J = 13.1, 5.7 Hz, 0.22H), 2.39 (d, J = 13.5 Hz, 0.27H), 2.31 (dd, J = 12.8, 4.7 Hz, 0.75H), 1.34 (s, 2.18H), 0.93 (s, 0.58H). 13C NMR: (176 MHz, CDCl3) δ 172.65, 171.84, 170.25, 169.03, 153.50, 150.95, 138.65, 138.51, 137.31, 136.97, 136.68, 132.94, 132.90, 132.51, 130.52, 130.45, 129.04, 128.79, 128.56, 128.50, 128.41, 128.28, 128.12, 128.07, 128.02, 127.93, 127.74, 127.58, 127.16, 127.12, 126.95, 125.87, 125.73, 124.98, 122.73, 122.19, 119.44, 118.63, 118.54, 118.37, 109.91, 109.81, 109.60, 82.38, 80.20, 65.27, 64.09, 57.63, 56.75, 55.18, 53.90, 53.56, 53.00, 52.82, 52.79, 52.69, 52.65, 52.53, 49.71, 47.05, 39.13, 36.74, 32.08, 29.85, 29.81, 28.07, 22.84, 22.72. HRMS (ESI): Calculated for C29H29ClNO4 [M + H]+ = 490.1780; found: 490.1796. NMR data of the major isomer deduced from the 4:1 mixture: 1H NMR: (700 MHz, CDCl3) δ 7.34–7.25 (m, 4H), 7.23 (d, J = 8.2 Hz, 3H), 7.18 (d, J = 7.7 Hz, 2H), 7.00–6.91 (m, 1H), 6.41 (d, J = 8.0 Hz, 1H), 6.38 (t, J = 7.4 Hz, 1H), 5.53 (d, J = 7.5 Hz, 1H), 4.62 (s, 1H), 4.59 (d, J = 16.1 Hz, 1H), 4.15 (d, J = 16.1 Hz, 1H), 3.78 (s, 3H), 3.47 (s, 3H), 2.98 (dd, J = 14.8, 4.7 Hz, 1H), 2.76 (dd, J = 14.7, 12.8 Hz, 1H), 2.31 (dd, J = 12.8, 4.7 Hz, 1H), 1.34 (s, 3H). 13C NMR: (176 MHz, CDCl3) δ 171.72, 168.90, 153.37, 138.52, 136.85, 132.81, 132.38, 130.39, 130.32, 128.67, 128.37, 128.28, 128.15, 127.99, 127.89, 127.62, 127.46, 126.99, 125.60, 118.24, 109.79, 80.07, 65.14, 57.50, 56.62, 52.87, 52.69, 52.41, 36.61, 27.94. Further confirmation of the structure of 3ac was obtained by X-ray crystallography (CCDC: 2302398) of single crystals obtained from a hexane/EtOAc (9:1) mixture [72].


3.2.3. Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-1-(4-bromophenyl)-8b-methyl-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3ad)
Yellow solid (40.1 mg, 83%, dr = 3:1) from N-benzylskatole 1a (20 mg, 90.37 μmol) and cyclopropane 2d (34 mg, 108.45 μmol). IR (KBr, cm−1) 𝜈: 2945, 2913, 2863, 1731, 1594, 1487, 1271, 756. 1H NMR: (700 MHz, CDCl3) δ 7.48–7.45 (m, 0.32H), 7.38 (d, J = 8.2 Hz, 1.59H), 7.31–7.25 (m, 1.76H), 7.24–7.21 (m, 0.85H), 7.18 (d, J = 7.7 Hz, 1.45H), 7.09 (dd, J = 8.4, 1.6 Hz, 0.30H), 7.08–7.06 (m, 0.17H), 7.04 (dd, J = 8.3, 5.2 Hz, 0.23H), 6.99 (d, J = 8.0 Hz, 0.11H), 6.94 (t, J = 7.8 Hz, 0.68H), 6.90 (d, J = 7.9 Hz, 1.09H), 6.80 (s, 0.16H), 6.68–6.67 (m, 0.28H), 6.45 (d, J = 8.1 Hz, 0.17H), 6.41 (d, J = 8.0 Hz, 0.58H), 6.38 (t, J = 7.4 Hz, 0.60H), 5.54 (d, J = 7.5 Hz, 0.54H), 4.71 (d, J = 1.5 Hz, 0.16H), 4.65 (d, J = 16.2 Hz, 0.18H), 4.62 (s, 0.58H), 4.59 (d, J = 16.0 Hz, 0.64H), 4.32 (d, J = 16.3 Hz, 0.17H), 4.15 (d, J = 16.0 Hz, 0.62H), 3.81 (s, 0.51H), 3.78 (s, 2.05H), 3.47 (d, J = 1.7 Hz, 1.84H), 3.42 (s, 0.64H), 2.97 (dd, J = 14.7, 4.6 Hz, 0.68H), 2.75 (t, J = 12.9 Hz, 0.70H), 2.69 (dd, J = 13.1, 5.5 Hz, 0.17H), 2.39 (d, J = 13.5 Hz, 0.15H), 2.31 (dd, J = 12.8, 4.6 Hz, 0.64H), 1.34 (s, 1.87H), 0.93 (s, 0.45H). 13C NMR: (176 MHz, CDCl3) δ 172.63, 171.83, 170.23, 169.01, 153.49, 150.95, 138.63, 138.50, 137.83, 137.49, 136.65, 132.47, 131.74, 131.47, 131.23, 130.96, 130.91, 130.84, 130.33, 129.41, 128.80, 128.55, 128.50, 128.47, 128.41, 128.13, 128.08, 127.93, 127.74, 127.58, 127.50, 127.12, 126.95, 125.87, 125.73, 122.73, 121.06, 121.02, 118.55, 118.39, 109.92, 109.82, 82.38, 80.18, 65.27, 64.08, 57.59, 56.74, 55.14, 53.90, 53.62, 53.04, 53.00, 52.89, 52.69, 52.66, 52.56, 52.53, 49.70, 39.05, 36.69, 31.95, 29.85, 28.06, 22.71 HRMS (ESI): Calculated for C29H29BrNO4 [M + H]+ = 534.1274; found: 534.1257. NMR data of the major isomer deduced from the 3:1 mixture: 1H NMR: (700 MHz, CDCl3) δ 7.38 (d, J = 8.2 Hz, 2H), 7.31–7.25 (m, 3H), 7.24–7.21 (m, 1H), 7.18 (d, J = 7.7 Hz, 2H), 6.94 (t, J = 7.8 Hz, 1H), 6.90 (d, J = 7.9 Hz, 1H), 6.41 (d, J = 8.0 Hz, 1H), 6.38 (t, J = 7.4 Hz, 1H), 5.54 (d, J = 7.5 Hz, 1H), 4.62 (s, 1H), 4.59 (d, J = 16.0 Hz, 1H), 4.15 (d, J = 16.0 Hz, 1H), 3.78 (d, J = 1.6 Hz, 3H), 3.47 (s, 3H), 2.97 (dd, J = 14.7, 4.6 Hz, 1H), 2.75 (t, J = 12.9 Hz, 1H), 2.31 (dd, J = 12.8, 4.6 Hz, 1H), 1.34 (s, 3H). 13C NMR: (176 MHz, CDCl3) δ 171.83, 169.01, 153.49, 138.63, 137.49, 132.47, 131.23, 130.96, 130.91, 130.84, 128.50, 128.41, 128.13, 127.74, 127.58, 127.12, 125.73, 121.06, 118.39, 109.92, 80.18, 65.27, 57.59, 56.74, 53.00, 52.89, 52.53, 36.69, 28.06.
3.2.4. Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-1-(4-fluorophenyl)-8b-methyl-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3ae)
White solid (25.3 mg, 59%, dr = 3.7:1) from N-benzylskatole 1a (20 mg, 90.37 μmol) and cyclopropane 2e (27.4mg, 108.45 μmol). IR (KBr, cm−1) 𝜈: 2995, 2995, 2920, 2848, 1731, 1600, 1513, 1273, 1222. 1H NMR: (700 MHz, CDCl3) δ 7.31–7.27 (m, 1.70H), 7.22 (t, J = 7.3 Hz, 0.91H), 7.20–7.15 (m, 3.11H), 7.11 (d, J = 7.7 Hz, 0.43H), 7.03 (t, J = 8.4 Hz, 0.68H), 6.96 (dd, J = 9.8, 7.5 Hz, 3.59H), 6.94–6.89 (m, 1.25H), 6.69–6.65 (m, 0.34H), 6.45 (d, J = 8.0 Hz, 0.31H), 6.40 (d, J = 8.0 Hz, 0.68H), 6.36 (td, J = 7.2, 2.9 Hz, 0.70H), 5.49 (d, J = 7.0 Hz, 0.64H), 4.71 (s, 0.23H), 4.65 (d, J = 16.3 Hz, 0.38H), 4.62 (s, 0.56H), 4.59 (d, J = 16.0 Hz, 0.68H), 4.32 (d, J = 16.3 Hz, 0.36H), 4.15 (d, J = 16.1 Hz, 0.74H), 3.81 (s, 0.49H), 3.79 (s, 2.26H), 3.42 (s, 0.49H), 3.39 (s, 2.26H), 2.99 (dd, J = 14.8, 4.6 Hz, 0.73H), 2.75 (dd, J = 14.8, 12.8 Hz, 0.78H), 2.69 (dd, J = 13.1, 5.6 Hz, 0.35H), 2.37 (t, J = 13.5 Hz, 0.39H), 2.35–2.29 (m, 0.79H), 1.34 (s, 1.58H), 0.92 (s, 0.53H). 13C NMR: (176 MHz, CDCl3) δ 171.88, 170.26, 169.08, 167.11, 162.29 (d, J = 246.13 Hz), 162.18 (d, J = 245.07 Hz)153.53, 138.69, 134.15, 132.65, 130.30 (d, J = 8.17 Hz), 128.50, 128.41, 128.06, 128.02, 127.75, 127.58, 127.11, 126.94, 125.74, 118.51, 118.31, 115.27 (d, J = 21.59 Hz), 114.70 (d, J = 21.00 Hz), 109.87, 109.77, 82.37, 80.21, 65.26, 64.08, 57.61, 56.76, 53.88, 53.42, 53.00, 52.99, 52.66, 52.64, 52.52, 52.46, 37.23, 36.87, 31.91, 29.86, 28.07, 19.39. HRMS (ESI): Calculated for C29H29FNO4 [M + H]+ = 474.2075l; found: 474.2052. NMR data of the major isomer deduced from the 3.7:1 mixture: 1H NMR: (700 MHz, CDCl3) δ 7.30–7.27 (m, 2H), 7.20–7.14 (m, 4H), 6.96 (t, J = 8.6 Hz, 4H), 6.40 (d, J = 8.0 Hz, 1H), 6.36 (t, J = 7.2 Hz, 1H), 5.51–5.48 (m, 1H), 4.62 (s, 1H), 4.59 (d, J = 16.0 Hz, 1H), 4.15 (d, J = 16.1 Hz, 1H), 3.79 (s, 3H), 3.39 (s, 3H), 2.99 (dd, J = 14.8, 4.6 Hz, 1H), 2.75 (dd, J = 14.8, 12.8 Hz, 1H), 2.35–2.28 (m, 1H), 1.34 (s, 3H). 13C NMR: (176 MHz, CDCl3) δ 171.88, 170.26, 169.08, 167.11, 162.29 (d, J = 246.1 Hz), 153.53, 138.69, 132.65, 130.47, 130.45, 130.30 (d, J = 8.2 Hz), 128.50, 128.06, 127.75, 127.58, 127.11, 125.74, 118.31, 115.27 (d, J = 21.6 Hz), 114.76, 114.64, 109.87, 80.21, 65.26, 57.61, 56.76, 53.00, 52.99, 52.66, 52.52, 52.46, 37.23, 36.87, 31.91, 28.07, 19.39.
3.2.5. Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-1-(4-nitrophenyl)-8b-methyl-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3ag)
Yellow solid (23.1 mg, 51%, dr = 3.5:1) from N-benzylskatole 1a (20 mg, 90.37 μmol) and cyclopropane 2g (30.3 mg, 108.45 μmol). mp: 134–140 °C. IR (KBr, cm−1) 𝜈: 3077, 3022, 2948, 1728, 1599, 1515, 1346. 1H NMR: (700 MHz, CDCl3) δ 8.15 (d, J = 8.5 Hz, 1.61H), 8.12 (d, J = 8.3 Hz, 1.15H), 7.36 (d, J = 8.3 Hz, 1.77H), 7.29 (t, J = 7.5 Hz, 1.81H), 7.23 (t, J = 7.4 Hz, 1.22H), 7.18 (t, J = 5.9 Hz, 2.85H), 6.97–6.92 (m, 0.79H), 6.70 (t, J = 7.4 Hz, 0.22H), 6.63 (d, J = 7.4 Hz, 0.18H), 6.48 (d, J = 8.0 Hz, 0.20H), 6.44 (d, J = 8.0 Hz, 0.73H), 6.34 (t, J = 7.4 Hz, 0.71H), 5.44 (d, J = 7.5 Hz, 0.59H), 4.73 (s, 0.17H), 4.66 (d, J = 16.6 Hz, 0.30H), 4.64 (s, 0.62H), 4.60 (d, J = 16.0 Hz, 0.76H), 4.32 (d, J = 16.3 Hz, 0.23H), 4.15 (d, J = 16.0 Hz, 0.78H), 3.83 (s, 0.71H), 3.81 (s, 2.34H), 3.53 (s, 0.34H), 3.42 (s, 2.69H), 3.12 (dd, J = 14.7, 4.8 Hz, 0.71H), 2.83 (dd, J = 14.7, 12.8 Hz, 0.85H), 2.74 (dd, J = 13.0, 5.5 Hz, 0.26H), 2.45 (t, J = 13.4 Hz, 0.28H), 2.39–2.34 (m, 0.78H), 1.38 (s, 2.16H), 0.94 (s, 0.49H). 13C NMR: (176 MHz, CDCl3) δ 171.63, 170.09, 169.68, 168.78, 166.65, 153.55, 147.39, 147.26, 147.23, 146.51, 142.60, 138.44, 131.89, 130.06, 129.92, 129.49, 128.78, 128.55, 128.53, 128.48, 128.45, 128.41, 127.73, 127.57, 127.31, 127.22, 127.04, 125.87, 125.28, 124.97, 123.80, 123.56, 123.33, 123.04, 122.59, 119.69, 118.78, 118.73, 118.51, 110.28, 110.16, 82.37, 80.21, 65.28, 64.14, 58.07, 56.82, 55.60, 54.04, 54.00, 53.44, 53.25, 53.11, 52.91, 52.80, 52.78, 52.74, 52.70, 52.64, 49.57, 47.11, 38.97, 37.86, 36.70, 31.71, 29.84, 29.81, 28.03, 22.55, 19.49, 9.79, 1.16. HRMS (ESI): Calculated for C29H29N2O6 [M + H]+ = 501.2020; found: 501.1997. NMR data of the major isomer deduced from the 3.5:1 mixture: 1H NMR: (700 MHz, CDCl3) δ 8.15 (d, J = 8.5 Hz, 2H), 7.36 (d, J = 8.3 Hz, 2H), 7.29 (t, J = 7.5 Hz, 2H), 7.18 (t, J = 5.9 Hz, 3H), 6.97–6.92 (m, 1H), 6.44 (d, J = 8.0 Hz, 1H), 6.34 (t, J = 7.4 Hz, 1H), 5.44 (d, J = 7.5 Hz, 1H), 4.64 (s, 1H), 4.60 (d, J = 16.0 Hz, 1H), 4.15 (d, J = 16.0 Hz, 1H), 3.80 (s, 3H), 3.49 (s, 3H), 3.12 (dd, J = 14.7, 4.8 Hz, 1H), 2.83 (dd, J = 14.7, 12.8 Hz, 1H), 2.39–2.34 (m, 1H), 1.38 (s, 3H). 13C NMR: (176 MHz, CDCl3) δ 171.63, 169.68, 168.78, 166.65, 153.55, 146.51, 142.60, 138.44, 131.89, 130.06, 129.49, 128.55, 128.48, 128.45, 127.57, 127.22, 125.28, 123.56, 123.04, 118.51, 110.28, 80.21, 65.28, 58.07, 56.82, 53.44, 53.25, 53.11, 52.74, 52.64, 37.86, 36.70, 31.71, 29.84, 28.03, 19.49, 1.16.
3.2.6. Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-8b-methyl-1-((E)-styryl)-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3ai)
White solid (40.4 mg, 93%, dr = 1.7:1) from N-benzylskatole 1a (20 mg, 90.37 μmol) and cyclopropane 2i (28.2 mg, 108.45 μmol). IR (KBr, cm−1) 𝜈: 3023, 2949, 2916, 2847, 1731, 1598, 1483, 1259. 1H NMR: (700 MHz, CDCl3) δ 7.41–7.39 (m, 0.65H), 7.36 (d, J = 7.1 Hz, 1.48H), 7.34–7.32 (m, 1.76H), 7.29–7.27 (m, 2.58H), 7.25–7.20 (m, 2.25H), 7.19 (d, J = 7.6 Hz, 1.59H), 7.17–7.14 (m, 0.45H), 7.11 (t, J = 7.6 Hz, 0.69H), 7.08–7.05 (m, 0.30H), 7.05–7.01 (m, 0.89H), 6.99 (dd, J = 7.3, 1.3 Hz, 0.32H), 6.87–6.86 (m, 0.65H), 6.69 (q, J = 7.2 Hz, 0.92H), 6.50–6.43 (m, 1.84H), 6.25 (dd, J = 15.8, 8.6 Hz, 0.34H), 6.02 (dd, J = 15.8, 8.8 Hz, 0.65H), 4.63 (s, 0.40H), 4.59 (s, 0.60H), 4.58 (d, J = 6.7 Hz, 0.59H), 4.32 (d, J = 16.2 Hz, 0.31H), 4.17 (d, J = 16.1 Hz, 0.65H), 3.79 (s, 3H), 3.46 (s, 0.78H), 3.45 (s, 1.69H), 2.64 (dd, J = 13.23, 6.08 Hz, 0.31H), 2.51 (ddd, J = 13.8, 8.8, 4.9 Hz, 0.67H), 2.42 (t, J = 13.3 Hz, 0.76H), 2.27 (ddd, J = 12.7, 4.9, 1.5 Hz, 0.68H), 2.04 (t, J = 12.6 Hz, 0.36H), 1.33 (s, 1.96H), 1.26 (s, 0.81H). 13C NMR: (176 MHz, CDCl3) δ 172.73, 171.89, 170.24, 169.06, 153.43, 150.58, 138.70, 138.48, 137.54, 137.52, 137.38, 133.20, 132.21, 131.31, 129.87, 128.79, 128.74, 128.49, 128.41, 128.24, 128.20, 128.18, 128.15, 127.89, 127.53, 127.46, 127.06, 127.00, 126.37, 126.35, 125.55, 122.13, 121.74, 118.67, 118.60, 110.16, 109.35, 81.71, 80.16, 77.34, 77.16, 76.98, 72.26, 65.81, 65.36, 57.61, 56.59, 55.91, 53.25, 52.94, 52.92, 52.81, 52.53, 52.45, 52.03, 40.02, 38.35, 29.85, 27.74, 22.88. HRMS (ESI): Calculated for C30H32NO4 [M + H]+ = 482.2326; found: 482.2298. NMR data of the major isomer deduced from the 1.7:1 mixture: 1H NMR: (700 MHz, CDCl3) δ 7.41–7.39 (m, 1H), 7.36 (d, J = 7.1 Hz, 2H), 7.34–7.32 (m, 2H), 7.29–7.27 (m, 3H), 7.19 (d, J = 7.6 Hz, 2H), 6.87–6.86 (m, 1H), 6.69 (q, J = 7.2 Hz, 1H), 6.50–6.43 (m, 2H), 6.02 (dd, J = 15.8, 8.8 Hz, 1H), 4.59 (d, J = 1.4 Hz, 1H), 4.58 (d, J = 6.7 Hz, 1H), 4.17 (d, J = 16.1 Hz, 1H), 3.79 (s, 3H), 3.45 (s, 3H), 2.51 (ddd, J = 13.8, 8.8, 4.9 Hz, 1H), 2.42 (t, J = 13.3 Hz, 1H), 2.27 (ddd, J = 12.7, 4.9, 1.5 Hz, 1H), 1.33 (s, 3H). 13C NMR: (176 MHz, CDCl3) δ 171.89, 169.06, 153.43, 138.70, 137.38, 133.20, 132.21, 131.31, 129.87, 128.79, 128.49, 127.53, 126.35, 125.55, 122.13, 118.67, 110.16, 80.16, 65.81, 57.61, 56.59, 52.94, 52.45, 52.03, 38.35, 27.74.
3.2.7. Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-7-methoxy-1-(4-methoxyphenyl)-8b-methyl-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3ca)
Colorless oil (34 mg, 83%, dr = 4:1) from N-benzylskatole 1c (20 mg, 79.58 μmol) and cyclopropane 2a (25.2 mg, 95.49 μmol). IR (KBr, cm−1) 𝜈: 2951, 2916, 2833, 1731, 1610, 1514, 1261, 1013. 1H NMR: (700 MHz, CDCl3) δ 7.30–7.27 (m, 4H), 7.24–7.18 (m, 4H), 7.13 (dd, J = 9.6, 2.9 Hz, 0.61H), 6.96 (d, J = 7.4 Hz, 1.33H), 6.87 (d, J = 8.6 Hz, 0.46H), 6.81 (d, J = 8.4 Hz, 1.45H), 6.58 (dd, J = 8.5, 2.7 Hz, 0.22H), 6.49 (dd, J = 8.6, 2.7 Hz, 0.72H), 6.32 (d, J = 8.6 Hz, 0.22H), 6.30 (d, J = 8.6 Hz, 0.68H), 6.28 (d, J = 2.7 Hz, 0.18H), 5.12 (d, J = 2.7 Hz, 0.70H), 4.69 (s, 0.22H), 4.60 (d, J = 16.2 Hz, 0.25H), 4.57 (s, 0.70H), 4.52 (d, J = 15.8 Hz, 0.80H), 4.23 (d, J = 16.1 Hz, 0.27H), 4.09 (d, J = 15.8 Hz, 0.81H), 3.82 (s, 0.67H), 3.79 (s, 2.25H), 3.77 (s, 1.94H), 3.68 (s, 0.56H), 3.48 (s, 1.97H), 3.40 (s, 0.57H), 3.34 (s, 2.22H), 2.97 (dd, J = 14.8, 4.6 Hz, 0.75H), 2.76 (dd, J = 14.8, 12.8 Hz, 0.82H), 2.71 (dd, J = 13.1, 5.7 Hz, 0.25H), 2.41–2.36 (m, 0.38H), 2.34–2.25 (m, 0.95H), 1.31 (s, 2.48H), 0.93 (s, 0.56H). 13C NMR: (176 MHz, CDCl3) δ 172.91, 172.07, 170.36, 169.20, 158.89, 158.77, 153.28, 152.76, 147.62, 145.34, 139.03, 138.91, 138.39, 134.37, 130.62, 130.49, 130.09, 130.02, 129.90, 129.76, 129.49, 129.15, 128.84, 128.44, 128.34, 127.81, 127.79, 127.54, 127.34, 127.04, 126.83, 124.98, 114.57, 114.15, 113.74, 113.48, 113.36, 113.30, 112.67, 111.34, 110.81, 110.41, 109.92, 83.14, 80.82, 65.43, 64.01, 58.30, 57.67, 56.02, 55.69, 55.47, 55.45, 55.42, 55.40, 53.41, 52.92, 52.91, 52.72, 52.58, 52.53, 52.44, 39.46, 36.67, 29.85, 27.92, 22.38, 14.27. HRMS (ESI): Calculated for C31H34NO6 [M + H]+ = 516.2381; found: 516.2376. NMR data of the major isomer deduced from the 4:1 mixture: 1H NMR: (700 MHz, CDCl3) δ 7.30–7.27 (m, 3H), 7.23–7.18 (m, 3H), 6.96 (d, J = 7.5 Hz, 2H), 6.81 (d, J = 8.4 Hz, 2H), 6.49 (dd, J = 8.6, 2.7 Hz, 1H), 6.30 (d, J = 8.6 Hz, 1H), 5.12 (d, J = 2.7 Hz, 1H), 4.56 (d, J = 1.5 Hz, 1H), 4.52 (d, J = 15.8 Hz, 1H), 4.09 (d, J = 15.8 Hz, 1H), 3.79 (s, 3H), 3.77 (s, 3H), 3.48 (s, 3H), 3.34 (s, 3H), 2.97 (dd, J = 14.8, 4.6 Hz, 1H), 2.76 (dd, J = 14.8, 12.7 Hz, 1H), 2.30 (ddt, J = 15.5, 4.3, 2.4 Hz, 1H), 1.31 (s, 3H). 13C NMR: (176 MHz, CDCl3) δ 172.07, 169.20, 158.89, 152.76, 147.62, 139.03, 134.37, 130.49, 130.09, 128.44, 128.34, 127.81, 127.79, 127.04, 114.57, 113.48, 113.36, 111.34, 110.81, 80.82, 65.43, 58.30, 57.67, 55.69, 55.47, 55.40, 52.91, 52.53, 52.44, 36.67, 29.85, 27.92. The spectroscopic data agreed with the literature [48].
3.2.8. Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-7-bromo-1-(4-methoxyphenyl)-8b-methyl-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3da)
Colorless oil (21.4 mg, 57%, dr = 4:1) from N-benzylskatole 1d (20 mg, 66.62 μmol) and cyclopropane 2a (21.1 mg, 79.95 μmol). IR (KBr, cm−1) 𝜈: 2927, 2917, 2848, 1731, 1610, 1514, 1260. 1H NMR: (700 MHz, CDCl3) δ 7.31–7.27 (m, 1.78H), 7.25–7.21 (m, 1.37H), 7.17–7.14 (m, 1.37H), 7.14–7.12 (m, 0.48H), 7.12–7.10 (m, 0.28H), 7.00 (dd, J = 8.4, 2.1 Hz, 0.70H), 6.96–6.92 (m, 1.13H), 6.90 (d, J = 8.6 Hz, 0.57H), 6.87–6.83 (m, 1.33H), 6.79 (d, J = 2.1 Hz, 0.28H), 6.76 (d, J = 8.7 Hz, 0.14H), 6.31 (d, J = 8.4 Hz, 0.21H), 6.26 (d, J = 8.5 Hz, 0.70H), 5.49 (d, J = 2.1 Hz, 0.74H), 4.68 (s, 0.20H), 4.62 (d, J = 1.5 Hz, 0.78H), 4.55 (d, J = 16.0 Hz, 0.77H), 4.32 (d, J = 16.1 Hz, 0.38H), 4.11 (d, J = 16.0 Hz, 0.76H), 3.84 (s, 0.72H), 3.83 (s, 1.93H), 3.81 (s, 0.71H), 3.77 (s, 1.93H), 3.52 (s, 1.87H), 3.47 (s, 0.55H), 2.95 (dd, J = 14.8, 4.7 Hz, 0.75H), 2.71 (dd, J = 14.8, 12.9 Hz, 0.79H), 2.65 (dd, J = 13.1, 5.8 Hz, 0.28H), 2.34–2.30 (m, 0.81H), 2.31–2.26 (m, 0.57H), 1.29 (s, 2.25H), 0.88 (s, 0.72H). 13C NMR: (176 MHz, CDCl3) δ 172.63, 171.81, 170.26, 169.07, 159.17, 158.89, 152.21, 149.66, 139.62, 138.09, 137.96, 135.39, 130.55, 130.51, 130.38, 130.03, 130.00, 129.78, 129.49, 129.06, 128.93, 128.84, 128.64, 128.60, 128.51, 127.79, 127.63, 127.54, 127.33, 127.17, 126.84, 125.90, 125.81, 124.98, 114.15, 113.71, 113.50, 110.98, 110.69, 110.04, 109.95, 82.01, 80.00, 65.25, 64.21, 57.73, 56.13, 55.61, 55.42, 54.89, 53.33, 53.04, 53.02, 53.00, 52.73, 52.71, 52.67, 52.65, 52.55, 52.51, 41.29, 39.16, 36.69, 32.08, 29.85, 29.81, 29.51, 27.71, 23.32, 22.84, 14.27, 1.17. HRMS (ESI): Calculated for C30H31BrNO5 [M + H]+ = 564.1380; found: 564.1361. NMR data of the major isomer deduced from the 4:1 mixture: 1H NMR: (700 MHz, CDCl3) δ 7.31–7.27 (m, 2H), 7.25–7.21 (m, 2H), 7.17–7.14 (m, 2H), 7.00 (dd, J = 8.4, 2.1 Hz, 1H), 6.96–6.92 (m, 1H), 6.87–6.83 (m, 2H), 6.26 (d, J = 8.5 Hz, 1H), 5.49 (d, J = 2.1 Hz, 1H), 4.62 (d, J = 1.5 Hz, 1H), 4.55 (d, J = 16.0 Hz, 1H), 4.11 (d, J = 16.0 Hz, 1H), 3.83 (s, 3H), 3.77 (s, 3H), 3.52 (s, 3H), 2.95 (dd, J = 14.8, 4.7 Hz, 1H), 2.71 (dd, J = 14.8, 12.9 Hz, 1H), 2.34–2.30 (m, 1H), 1.29 (s, 3H). 13C NMR: (176 MHz, CDCl3) δ 171.81, 169.07, 159.17, 152.21, 138.09, 135.39, 130.51, 130.03, 130.00, 129.78, 129.06, 128.60, 128.51, 127.79, 127.63, 127.33, 113.71, 113.50, 110.98, 109.95, 80.00, 65.25, 57.73, 56.12, 55.61, 53.00, 52.55, 52.51, 36.69, 29.85, 27.71. The spectroscopic data agreed with the literature [48].
3.2.9. Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-7-chloro-1-(4-methoxyphenyl)-8b-methyl-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3ea)
Colorless oil (23.6 mg, 58%, dr = 5:1) from N-benzylskatole 1e (20 mg, 78.2 μmol) and cyclopropane 2a (24.8 mg, 93.84 μmol). IR (KBr, cm−1) 𝜈: 3024, 2950, 2834, 1731, 1599, 1514, 1246. 1H NMR: (700 MHz, CDCl3) δ 7.32–7.24 (m, 4H), 7.24–7.16 (m, 3H), 7.04–7.01 (m, 0.29H), 6.92 (td, J = 7.7, 1.3 Hz, 1H), 6.88 (d, J = 8.6 Hz, 0.40H), 6.82–6.79 (m, 1.52H), 6.44 (d, J = 7.9 Hz, 0.19H), 6.40 (d, J = 7.9 Hz, 0.78H), 6.36 (td, J = 7.4, 1.0 Hz, 0.80H), 5.54 (dd, J = 7.5, 1.2 Hz, 0.81H), 4.70 (s, 0.21H), 4.65 (d, J = 16.3 Hz, 0.25H), 4.61 (d, J = 1.4 Hz, 0.65H), 4.59 (d, J = 16.1 Hz, 0.88H), 4.33 (d, J = 16.3 Hz, 0.25H), 4.16 (d, J = 16.1 Hz, 0.78H), 3.83 (s, 0.71H), 3.81 (s, 1.90H), 3.81 (s, 0.66H), 3.77 (s, 2.05H), 3.47 (s, 2.1H), 3.42 (s, 0.47H), 2.97 (dd, J = 14.8, 4.7 Hz, 0.80H), 2.75 (dd, J = 14.8, 12.8 Hz, 0.83H), 2.68 (dd, J = 13.1, 5.7 Hz, 0.24H), 2.31 (ddd, J = 12.6, 4.7, 1.5 Hz, 0.85H), 2.22 (t, J = 6.8 Hz, 0.14H), 1.34 (s, 2H), 0.93 (s, 0.60H). 13C NMR: (176 MHz, CDCl3) δ 172.86, 172.00, 170.38, 169.22, 158.79, 158.74, 153.48, 150.91, 138.80, 138.64, 137.20, 133.02, 130.83, 130.45, 130.10, 129.19, 128.47, 128.38, 127.87, 127.85, 127.77, 127.60, 127.06, 126.90, 125.97, 125.45, 124.98, 122.84, 118.41, 118.22, 113.50, 113.26, 109.69, 109.57, 82.37, 80.22, 65.31, 64.12, 57.59, 56.70, 55.41, 55.40, 55.15, 53.79, 53.41, 52.95, 52.92, 52.57, 52.56, 52.47, 39.37, 36.87, 29.85, 28.16, 22.97, 21.61. HRMS (ESI): Calculated for C30H31ClNO5 [M + H]+ = 520.1885; found: 520.1854. NMR data of the major isomer deduced from the 5:1 mixture: 1H NMR: (700 MHz, CDCl3) δ 7.32–7.24 (m, 3H), 7.22 (t, J = 7.3 Hz, 1H), 7.24–7.16 (m, 2H), 6.99–6.86 (m, 1H), 6.82–6.79 (m, 2H), 6.40 (d, J = 7.9 Hz, 1H), 6.36 (td, J = 7.4, 1.0 Hz, 1H), 5.54 (dd, J = 7.5, 1.2 Hz, 1H), 4.61 (d, J = 1.4 Hz, 1H), 4.59 (d, J = 16.1 Hz, 1H), 4.16 (d, J = 16.1 Hz, 1H), 3.81 (s, 3H), 3.77 (s, 3H), 3.47 (s, 3H), 2.97 (dd, J = 14.8, 4.7 Hz, 1H), 2.75 (dd, J = 14.8, 12.8 Hz, 1H), 2.31 (ddd, J = 12.6, 4.7, 1.5 Hz, 1H), 1.34 (s, 3H). 13C NMR: (176 MHz, CDCl3) δ 171.99, 169.22, 158.79, 153.48, 138.80, 133.02, 130.45, 130.10, 129.19, 128.47, 128.38, 127.87, 127.77, 127.60, 127.06, 125.97, 118.22, 113.50, 113.26, 109.69, 80.22, 65.31, 57.59, 56.70, 55.41, 52.92, 52.56, 52.47, 36.87, 28.16. The spectroscopic data agreed with the literature [48].
3.2.10. Dimethyl (1S*,3aR*,8bS*)-4-Benzyl-6-chloro-1-(4-methoxyphenyl)-8b-methyl-1,3a,4,8b-tetrahydrocyclopenta[b]indole-3,3(2H)-dicarboxylate (3fa)
Pale yellow oil (29.7 mg, 73%, dr = 8.6:1) from N-benzylskatole 1f (20 mg, 78.2 μmol) and cyclopropane 2a (24.8 mg, 93.84 μmol). IR (KBr, cm−1) 𝜈: 3000, 2978, 2952, 2919, 2847, 1731, 1594, 1514, 1247. 1H NMR: (700 MHz, CDCl3) δ 7.32–7.22 (m, 1.80H), 7.24 (t, J = 7.4 Hz, 1H), 7.18–7.15 (m, 2H), 7.12 (dd, J = 8.8, 2.6 Hz, 0.82H), 7.08–7.04 (m, 0.30H), 6.95 (d, J = 8.0 Hz, 1.34H), 6.89–6.87 (m, 0.28H), 6.83–6.78 (m, 1.87H), 6.62 (d, J = 1.3 Hz, 0.20H), 6.43 (s, 0.14H), 6.38 (d, J = 1.8 Hz, 0.82H), 6.31 (dd, J = 8.0, 1.9 Hz, 0.85H), 5.40 (d, J = 8.0 Hz, 0.82H), 4.69 (s, 0.14H), 4.64 (d, J = 1.4 Hz, 0.85H), 4.61 (d, J = 16.3 Hz, 0.21H), 4.56 (d, J = 16.1 Hz, 0.89H), 4.35 (d, J = 16.3 Hz, 0.20H), 4.13 (d, J = 16.1 Hz, 0.89H), 3.82 (s, 0.32H), 3.81 (s, 2.34H), 3.80 (s, 0.34H), 3.77 (s, 2.28H), 3.50 (s, 2.44H), 3.48 (s, 0.30H), 2.94 (dd, J = 14.8, 4.6 Hz, 0.88H), 2.72 (dd, J = 14.8, 12.8 Hz, 0.96H), 2.64 (dd, J = 13.1, 5.8 Hz, 0.20H), 2.31 (ddd, J = 12.8, 4.7, 1.5 Hz, 0.92H), 1.29 (s, 2.49H), 0.87 (s, 0.52H). 13C NMR: (176 MHz, CDCl3) δ 172.62, 171.80, 170.49, 170.24, 169.06, 167.32, 167.08, 159.06, 158.90, 154.43, 137.90, 137.77, 136.02, 133.74, 131.61, 130.55, 130.04, 130.02, 129.76, 129.49, 129.19, 129.04, 128.84, 128.64, 128.56, 128.38, 128.04, 127.79, 127.58, 127.54, 127.36, 127.22, 126.66, 126.61, 125.45, 124.98, 123.51, 118.12, 118.09, 114.15, 113.74, 113.62, 113.37, 109.57, 109.28, 82.02, 80.10, 65.12, 64.19, 57.65, 57.15, 55.70, 55.42, 55.41, 55.36, 54.53, 53.43, 53.02, 53.00, 52.90, 52.71, 52.66, 52.56, 52.40, 52.32, 50.12, 41.29, 39.17, 37.23, 36.82, 32.37, 29.85, 29.81, 27.92, 23.32, 19.43, 1.17. HRMS (ESI): Calculated for C30H31ClNO5 [M + H]+ = 520.1885; found: 520.1854. NMR data of the major isomer deduced from the 8.6:1 mixture: 1H NMR: (700 MHz, CDCl3) δ 7.32–7.22 (m, 2H), 7.18–7.15 (m, 1H), 7.12 (dd, J = 8.8, 2.6 Hz, 2H), 6.95 (d, J = 8.0 Hz, 1H), 6.83–6.78 (m, 1H), 6.38 (d, J = 1.8 Hz, 2H), 6.31 (dd, J = 8.0, 1.9 Hz, 1H), 5.40 (d, J = 8.0 Hz, 1H), 4.64 (d, J = 1.4 Hz, 1H), 4.56 (d, J = 16.1 Hz, 1H), 4.13 (d, J = 16.1 Hz, 1H), 3.81 (s, 3H), 3.77 (s, 3H), 3.50 (s, 3H), 2.94 (dd, J = 14.8, 4.6 Hz, 1H), 2.72 (dd, J = 14.8, 12.8 Hz, 1H), 2.31 (ddd, J = 12.8, 4.7, 1.5 Hz, 1H), 1.29 (s, 3H). 13C NMR: (176 MHz, CDCl3) δ 171.80, 169.06, 158.90, 154.43, 137.90, 133.74, 131.61, 130.04, 130.02, 129.76, 128.64, 127.58, 127.36, 126.66, 118.09, 113.74, 113.37, 109.57, 80.10, 65.12, 57.15, 55.70, 55.42, 55.41, 55.36, 53.00, 52.90, 52.71, 52.56, 52.40, 52.32, 37.23, 36.82, 32.37, 29.85, 27.92, 19.43.
4. Conclusions
In conclusion, the nickel-catalyzed regio- and diastereoselective formal [3+2] cycloaddition of N-benzylskatoles and donor–acceptor cyclopropanes was developed. The reaction tolerated different monosubstitutions in a series of indoles and cyclopropanes, providing yields of up to 93%, with dr 8.6:1 and complete regioselectivity. Of the synthesized compounds, we were able to determine six new cyclopenta[b]indoles and obtain five previously reported derivatives. Based on the results obtained and the bibliographic precedents, a reaction mechanism was proposed and studied via online reaction monitoring by ESI-MS. All the proposed intermediates were detected experimentally, allowing us to establish the active species of the catalytic cycle and the important role of rac-BINAP in the proposed mechanism. This method enables the rapid construction of the medicinally relevant cyclopenta[b]indole scaffold using an inexpensive nickel catalyst.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29071604/s1: Synthesis procedures and characterization of indoles 1a-m and donor–acceptor cyclopropanes 2a-I; Tables S1–S5: Copies of IR, 1H, 13C-NMR, 2D-NMR, and HRMS-ESI spectra. References [88,89,90,91,92,93,94,95,96,97,98,99,100] are cited in the supplementary materials.
Author Contributions
Conceptualization, R.N. and L.S.S.; methodology, R.N., V.Q., S.O., G.Z., F.A.A.R. and F.M.N.; software, R.N., V.Q., S.O., G.Z., F.A.A.R. and F.M.N.; formal analysis, R.N., F.V., A.C.-A. and L.S.S.; investigation, R.N., V.Q., M.C., S.O., G.Z., F.A.A.R. and L.S.S.; resources, R.N., F.V., F.M.N. and L.S.S.; writing—original draft preparation, R.N., M.C., F.V. and L.S.S.; writing—review and editing, R.N., M.C., F.V., A.C.-A. and L.S.S.; supervision, R.N., M.C., F.V., S.O., A.C.-A. and L.S.S.; project administration, R.N. and L.S.S.; funding acquisition, R.N., F.M.N. and L.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agencia Nacional Investigación y Desarrollo (ANID) Chile, with grants 3190118, 3210175, 1220618, and 1210576; ANID doctoral grant 21221447, Vicerrectoría de Investigación y Desarrollo Tecnológico (VRIDT-UCN, grant 20230803020-VRIDT-UCN); and Dirección General de Postgrado (DGP-UCN) of the Universidad Católica del Norte.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
This work received financial support from the Chilean Agencia Nacional de Investigación y Desarrollo ANID. R.N. and F.V. acknowledge ANID-FONDECYT Postdoctorado 3190118 and 3210175. R.N. acknowledges the Vicerrectoría de Investigación y Desarrollo Tecnológico (VRIDT-UCN, grant 20230803020-VRIDT-UCN), and V.Q. acknowledges the Dirección General de Postgrado (DGP-UCN) of the Universidad Católica del Norte, Chile. L.S.S. and F.M.N. also acknowledge FONDECYT-ANID (1220618 and 1210576) for its financial support. Finally, F.A.A.R. is thankful for an ANID doctoral grant (21221447).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kaushik, N.; Kaushik, N.; Attri, P.; Kumar, N.; Kim, C.; Verma, A.; Choi, E. Biomedical Importance of Indoles. Molecules 2013, 18, 6620–6662. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gavia, D.J.; Tang, Y. Biosynthesis of Fungal Indole Alkaloids. Nat. Prod. Rep. 2014, 31, 1474–1487. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine Indole Alkaloids: Potential New Drug Leads for the Control of Depression and Anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Ishikura, M.; Abe, T.; Choshi, T.; Hibino, S. Simple Indole Alkaloids and Those with a Nonrearranged Monoterpenoid Unit. Nat. Prod. Rep. 2015, 32, 1389–1471. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2002. [Google Scholar] [CrossRef]
- Fattorusso, E.; Taglialetela-Scafati, O. Modern Alkaloids: Structure, Isolation, Synthesis and Biology; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008. [Google Scholar] [CrossRef]
- Nakazawa, J.; Yajima, J.; Usui, T.; Ueki, M.; Takatsuki, A.; Imoto, M.; Toyoshima, Y.Y.; Osada, H. A Novel Action of Terpendole E on the Motor Activity of Mitotic Kinesin Eg5. Chem. Biol. 2003, 10, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Nagumo, Y.; Motoyama, T.; Hayashi, T.; Hirota, H.; Aono, H.; Kawatani, M.; Osada, H.; Usui, T. Structure-Activity Relationships of Terpendole E and Its Natural Derivatives. ChemistrySelect 2017, 2, 1533–1536. [Google Scholar] [CrossRef]
- Tiong, S.H.; Looi, C.Y.; Hazni, H.; Arya, A.; Paydar, M.; Wong, W.F.; Cheah, S.C.; Mustafa, M.R.; Awang, K. Antidiabetic and Antioxidant Properties of Alkaloids from Catharanthus roseus (L.) G. Don. Molecules 2013, 18, 9770–9784. [Google Scholar] [CrossRef] [PubMed]
- Omar, F.; Tareq, A.M.; Alqahtani, A.M.; Dhama, K.; Sayeed, M.A.; Emran, T.B.; Simal-Gandara, J. Plant-Based Indole Alkaloids: A Comprehensive Overview from a Pharmacological Perspective. Molecules 2021, 26, 2297. [Google Scholar] [CrossRef] [PubMed]
- Tillequin, F.; Koch, M.; Bert, M.; Sevenet, T. Plantes de Nouvelle Calédonie. LV. Isoborrévérine et Borrévérine, Alcaloïdes Bis-Indoliques de Flindersia Fournieri. J. Nat. Prod. 1979, 42, 92–95. [Google Scholar] [CrossRef]
- Maynart, G.; Pousset, J.L.; Mboup, S.; Denis, F. Antibacterial Effect of Borreverine, an Alkaloid Isolated from Borreria Verticillata (Rubiaceae). Comptes Rendus Seances Soc. Biol. Fil. 1980, 174, 925–928. [Google Scholar]
- Malona, J.A.; Colbourne, J.M.; Frontier, A.J. A General Method for the Catalytic Nazarov Cyclization of Heteroaromatic Compounds. Org. Lett. 2006, 8, 5661–5664. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Leonori, D. The First Calcium-Catalysed Nazarov Cyclisation. Chem. Commun. 2014, 50, 15171–15174. [Google Scholar] [CrossRef] [PubMed]
- Genesan, A.; Hoothcock, C.H. A Stereochemical Test of the Mechanism of Electrophilic Substitution in 3-Substituted Indoles. Tetrahedron Lett. 1993, 34, 439–440. [Google Scholar] [CrossRef]
- Kawahara, M.; Nishida, A.; Nakagawa, M. An Efficient Synthesis of Optically Active Physostigmine from Tryptophan via Alkylative Cyclization. Org. Lett. 2000, 2, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, A.; Petrini, M. Simplified Synthesis of 3-(1-Arylsulfonylalkyl) Indoles and Their Reaction with Reformatsky Reagents. J. Org. Chem. 2007, 72, 1863–1866. [Google Scholar] [CrossRef] [PubMed]
- Boal, B.W.; Schammel, A.W.; Garg, N.K. An Interrupted Fischer Indolization Approach toward Fused Indoline-Containing Natural Products. Org. Lett. 2009, 11, 3458–3461. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Webber, M.J.; List, B. The Catalytic Asymmetric Fischer Indolization. J. Am. Chem. Soc. 2011, 133, 18534–18537. [Google Scholar] [CrossRef]
- Ghosh, B.; Balhara, R.; Jindal, G.; Mukherjee, S. Catalytic Enantioselective Desymmetrizing Fischer Indolization through Dynamic Kinetic Resolution. Angew. Chem. Int. Ed. 2021, 60, 9086–9092. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.F.; Kim, S.G.; Sinz, C.J.; Xiao, W.J.; MacMillan, D.W.C. Enantioselective Organocatalytic Construction of Pyrroloindolines by a Cascade Addition-Cyclization Strategy: Synthesis of (-)-Flustramine B. Proc. Natl. Acad. Sci. USA 2004, 101, 5482–5487. [Google Scholar] [CrossRef]
- Xu, B.; Guo, Z.L.; Jin, W.Y.; Wang, Z.P.; Peng, Y.G.; Guo, Q.X. Multistep One-Pot Synthesis of Enantioenriched Polysubstituted Cyclopenta[b] Indoles. Angew. Chem. Int. Ed. 2012, 51, 1059–1062. [Google Scholar] [CrossRef]
- Repka, L.M.; Ni, J.; Reisman, S.E. Enantioselective Synthesis of Pyrroloindolines by a Formal [3 + 2] Cycloaddition Reaction. J. Am. Chem. Soc. 2010, 132, 14418–14420. [Google Scholar] [CrossRef]
- Han, B.; Xiao, Y.; Yao, Y.; Chen, Y. Lewis Acid Catalyzed Intramolecular Direct Ene Reaction of Indoles. Angew. Chem. 2010, 122, 10387–10389. [Google Scholar] [CrossRef]
- Baran, P.S.; Guerrero, C.A.; Ambhaikar, N.B.; Hafensteiner, B.D. Short, Enantioselective Total Synthesis of Stephacidin A. Angew. Chem. Int. Ed. 2005, 44, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Nazaré, M.; Schneider, C.; Lindenschmidt, A.; Will, D.W. A Flexible, Palladium-Catalyzed Indole and Azaindole Synthesis by Direct Annulation of Chloroanilines and Chloroaminopyridines with Ketones. Angew. Chem. Int. Ed. 2004, 43, 4526–4528. [Google Scholar] [CrossRef]
- Barluenga, J.; Jiménez-Aquino, A.; Aznar, F.; Valdés, C. Modular Synthesis of Indoles from Imines and O-Dihaloarenes or o-Chlorosulfonates by a Pd-Catalyzed Cascade Process. J. Am. Chem. Soc. 2009, 131, 4031–4041. [Google Scholar] [CrossRef]
- Vickerman, K.L.; Stanley, L.M. Catalytic, Enantioselective Synthesis of Polycyclic Nitrogen, Oxygen, and Sulfur Heterocycles via Rh-Catalyzed Alkene Hydroacylation. Org. Lett. 2017, 19, 5054–5057. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Cheng, Q.Q.; Deng, Y.; Arman, H.; Doyle, M.P. Highly Regio- and Enantioselective Formal [3 + 2]-Annulation of Indoles with Electrophilic Enol Carbene Intermediates. Org. Lett. 2016, 18, 4550–4553. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.B.; Tu, H.F.; You, S.L. Ligand-Enabled Ir-Catalyzed Intermolecular Diastereoselective and Enantioselective Allylic Alkylation of 3-Substituted Indoles. Chem. Sci. 2015, 6, 4525–4529. [Google Scholar] [CrossRef] [PubMed]
- Mietke, T.; Cruchter, T.; Larionov, V.A.; Faber, T.; Harms, K.; Meggers, E. Asymmetric Nazarov Cyclizations Catalyzed by Chiral-at-Metal Complexes. Adv. Synth. Catal. 2018, 360, 2093–2100. [Google Scholar] [CrossRef]
- Zi, W.; Wu, H.; Toste, F.D. Gold(I)-Catalyzed Dearomative Rautenstrauch Rearrangement: Enantioselective Access to Cyclopenta[ b ]Indoles. J. Am. Chem. Soc. 2015, 137, 3225–3228. [Google Scholar] [CrossRef]
- Scarpi, D.; Petrović, M.; Fiser, B.; Gómez-Bengoa, E.; Occhiato, E.G. Construction of Cyclopenta[b]Indol-1-Ones by a Tandem Gold(I)-Catalyzed Rearrangement/Nazarov Reaction and Application to the Synthesis of Bruceolline H. Org. Lett. 2016, 18, 3922–3925. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.Y.; Wei, Y.; Tang, X.Y.; Shi, M. Catalyst-Dependent Stereodivergent and Regioselective Synthesis of Indole-Fused Heterocycles through Formal Cycloadditions of Indolyl-Allenes. J. Am. Chem. Soc. 2015, 137, 8131–8137. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.Q.; Deng, Y.; Lankelma, M.; Doyle, M.P. Cycloaddition Reactions of Enoldiazo Compounds. Chem. Soc. Rev. 2017, 46, 5425–5443. [Google Scholar] [CrossRef] [PubMed]
- Awata, A.; Arai, T. PyBidine/Copper Catalyst: Asymmetric Exo’-Selective [3+2] Cycloaddition Using Imino Ester and Electrophilic Indole. Angew. Chem. Int. Ed. Engl. 2014, 53, 10462–10465. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Ehmke, V.; Michael O’Keefe, B.; Bringley, D.A. Palladium-Catalyzed Dearomative Trimethylenemethane Cycloaddition Reactions. J. Am. Chem. Soc. 2014, 136, 8213–8216. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Doyle, M.P. Asymmetric [3 + n]-Cycloaddition Reactions of Donor-Acceptor Cyclopropanes. ChemCatChem 2023, 15, e202301090. [Google Scholar] [CrossRef]
- Schneider, T.F.; Kaschel, J.; Werz, D.B. A New Golden Age for Donor-Acceptor Cyclopropanes. Angew. Chem.-Int. Ed. 2014, 53, 5504–5523. [Google Scholar] [CrossRef]
- Cavitt, M.A.; Phun, L.H.; France, S. Intramolecular Donor-Acceptor Cyclopropane Ring-Opening Cyclizations. Chem. Soc. Rev. 2014, 43, 804–818. [Google Scholar] [CrossRef]
- Meazza, M.; Guo, H.; Rios, R. Synthetic Applications of Vinyl Cyclopropane Opening. Org. Biomol. Chem. 2017, 15, 2479–2490. [Google Scholar] [CrossRef]
- Pirenne, V.; Muriel, B.; Waser, J. Catalytic Enantioselective Ring-Opening Reactions of Cyclopropanes. Chem. Rev. 2021, 121, 227–263. [Google Scholar] [CrossRef]
- Liu, J.; Liu, R.; Wei, Y.; Shi, M. Recent Developments in Cyclopropane Cycloaddition Reactions. Trends Chem. 2019, 1, 779–793. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, X.; Feng, X. Asymmetric Catalytic Reactions of Donor–Acceptor Cyclopropanes. Angew. Chem. Int. Ed. 2021, 60, 9192–9204. [Google Scholar] [CrossRef] [PubMed]
- Harrington, P.; Kerr, M.A. The High Pressure Reaction of Cyclopropanes with Indoles Catalyzed by Ytterbium Triflate. Tetrahedron Lett. 1997, 38, 5949–5952. [Google Scholar] [CrossRef]
- Kerr, M.A.; Keddy, R.G. The Annulation of 3-Alkylindoles with 1,1-Cyclopropanediesters. Tetrahedron Lett. 1999, 40, 5671–5675. [Google Scholar] [CrossRef]
- England, D.B.; Kuss, T.D.O.; Keddy, R.G.; Kerr, M.A. Cyclopentannulation of 3-Alkylindoles: A Synthesis of a Tetracyclic Subunit of the Kopsane Alkaloids. J. Org. Chem. 2001, 66, 4704–4709. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Xu, H.; Liao, S.; Xie, Z.; Tang, Y. Copper-Catalyzed Highly Enantioselective Cyclopentannulation of Indoles with Donor-Acceptor Cyclopropanes. J. Am. Chem. Soc. 2013, 135, 7851–7854. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Lopez, K.; Gurung, R.; Arman, H.; Doyle, M.P. Stereoretentive Catalytic [3 + 2]-Cycloaddition/Rearrangement/Decarboxylation Reactions of Indoles with Non-Racemic Donor-Acceptor Cyclopropanes. ACS Catal. 2023, 13, 1621–1629. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Tong, F.; Sun, B.B.; Fan, W.T.; Chen, J.B.; Hu, D.; Wang, X.W. Pd-Catalyzed Asymmetric Dearomative Cycloaddition for Construction of Optically Active Pyrroloindoline and Cyclopentaindoline Derivatives: Access to 3a-Aminopyrroloindolines. J. Org. Chem. 2018, 83, 2882–2891. [Google Scholar] [CrossRef] [PubMed]
- Laugeois, M.; Ling, J.; Férard, C.; Michelet, V.; Ratovelomanana-Vidal, V.; Vitale, M.R. Palladium(0)-Catalyzed Dearomative [3 + 2] Cycloaddition of 3-Nitroindoles with Vinylcyclopropanes: An Entry to Stereodefined 2,3-Fused Cyclopentannulated Indoline Derivatives. Org. Lett. 2017, 19, 2266–2269. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, Z.Q.; Gu, L.; Wan, X.; Mei, G.J.; Shi, F. Catalytic Asymmetric Dearomative [3 + 2] Cycloaddition of Electron-Deficient Indoles with All-Carbon 1,3-Dipoles. J. Org. Chem. 2018, 83, 2341–2348. [Google Scholar] [CrossRef]
- Trost, B.M.; Bai, W.J.; Hohn, C.; Bai, Y.; Cregg, J.J. Palladium-Catalyzed Asymmetric Allylic Alkylation of 3-Substituted 1 H-Indoles and Tryptophan Derivatives with Vinylcyclopropanes. J. Am. Chem. Soc. 2018, 140, 6710–6717. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Zhu, Y.M.; Yang, P.J.; Wang, S.; Wang, S.; Liu, Z.; Yang, G. Copper(I)-Catalyzed Kinetic Resolution of N-Sulfonylaziridines with Indoles: Efficient Construction of Pyrroloindolines. J. Am. Chem. Soc. 2015, 137, 10088–10091. [Google Scholar] [CrossRef]
- Huang, Y.M.; Zheng, C.W.; Pan, L.; Jin, Q.W.; Zhao, G. Palladium(II)-Catalyzed Formal [3 + 2] Cycloaddition of Aziridines with 3-Substituted Indoles: Synthesis of Enantioenriched Pyrroloindolines. J. Org. Chem. 2015, 80, 10710–10718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Wu, H.H.; Zhang, J. Ni(ClO4)2-Catalysed Regio- and Diastereoselective [3 + 2] Cycloaddition of Indoles and Aryl Oxiranyl-Dicarboxylates/Diketones: A Facile Access to Furo[3,4-b]Indoles. Chem. Commun. 2012, 48, 1817–1819. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R.M. Catalysis without Precious Metals; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2010. [Google Scholar]
- Wender, P.A.; Miller, B.L. Synthesis at the Molecular Frontier. Nature 2009, 460, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A. Toward the Ideal Synthesis and Molecular Function through Synthesis-Informed Design. Nat. Prod. Rep. 2014, 31, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Gaich, T.; Baran, P.S. Aiming for the Ideal Synthesis. J. Org. Chem. 2010, 75, 4657–4673. [Google Scholar] [CrossRef] [PubMed]
- Sibi, M.P.; Ma, Z.; Jasperse, C.P. Enantioselective addition of nitrones to activated cyclopropanes. J. Am. Chem. Soc. 2005, 127, 5764–5765. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.B.; Sun, X.L.; Tang, Y. Highly enantioselective and diastereoselective cycloaddition of cyclopropanes with nitrones and its application in the kinetic resolution of 2-substituted cyclopropane-1,1-dicarboxylates. Angew. Chem. Int. Ed. 2007, 46, 3918–3921. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Li, J.; Ling, L.; Liao, S.H.; Sun, X.L.; Li, Y.X.; Wang, L.J.; Tang, Y. Highly enantioselective [3 + 3] cycloaddition of aromatic azomethine imines with cyclopropanes directed by π-π Stacking interactions. Angew. Chem. Int. Ed. 2013, 52, 1452–1456. [Google Scholar] [CrossRef]
- Suo, J.J.; Liu, W.; Du, J.; Ding, C.H.; Hou, X.L. Diastereo- and Enantioselective Palladium-Catalyzed Dearomative [3 + 2] Cycloaddition of 3-Nitroindoles. Chem. Asian J. 2018, 13, 959–963. [Google Scholar] [CrossRef]
- Chen, W.; Xia, Y.; Lin, L.; Yuan, X.; Guo, S.; Liu, X.; Feng, X. Asymmetric Synthesis of Furo[3,4-b]Indoles by Catalytic [3+2] Cycloaddition of Indoles with Epoxides. Chem. Eur. J. 2015, 21, 15104–15107. [Google Scholar] [CrossRef]
- Dixon, N.E.; Jackson, W.G.; Lawrance, G.A.; Sargeson, A.M. Labile (Trifluoromethanesulfonato) Cobalt (III) Amine Complexes. Inorg. Chem. 1981, 20, 470–476. [Google Scholar] [CrossRef]
- Moran, J.; Smith, A.G.; Carris, R.M.; Johnson, S.; Krische, M.J. Polarity Inversion of Donor-Acceptor Cyclopropanes: Disubstituted. J. Am. Chem. Soc. 2011, 133, 18618–18621. [Google Scholar] [CrossRef] [PubMed]
- Nájera, C.; de Gracia Retamosa, M.; Sansano, J.M.; de Cozar, A.; Cossío, F.P. Enantioselective Synthesis of Polysubstituted Prolines by Binap-Silver-Catalyzed 1,3-Dipolar Cycloadditions. Tetrahedron Asymmetry 2008, 19, 2913–2923. [Google Scholar] [CrossRef]
- Clevenger, A.L.; Stolley, R.M.; Staudaher, N.D.; Al, N.; Rheingold, A.L.; Vanderlinden, R.T.; Louie, J. Comprehensive Study of the Reactions between Chelating Phosphines and Ni(Cod)2. Organometallics 2018, 37, 3259–3268. [Google Scholar] [CrossRef]
- Spielvogel, D.J.; Davis, W.M.; Buchwald, S.L. Preparation, Crystal Structure Analysis, and Catalytic Application of [(S)-BINAP]Ni(COD) and [(S)-BINAP]NiBr2. Organometallics 2002, 21, 3833–3836. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W.N.M.; Kamer, P.C.J.; Reek, J.N.H. The Bite Angle Makes the Catalyst. Pure Appl. Chem. 1999, 71, 1443–1452. [Google Scholar] [CrossRef]
- CCDC 2302398 Contains the Supplementary Crystallographic Data for Compound 3ac. These Data Can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre via. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 15 March 2024).
- Dawson, D.D.; Oswald, V.F.; Borovik, A.S.; Jarvo, E.R. Identification of the Active Catalyst for Nickel-Catalyzed Stereospecific Kumada Coupling Reactions of Ethers. Chem. Eur. J. 2020, 26, 3044–3048. [Google Scholar] [CrossRef]
- Evans, D.A.; Thomson, R.J.; Franco, F. Ni(II) Tol-BINAP-Catalyzed Enantioselective Michael Reactions of β-Ketoesters and Unsaturated N-Acylthiazolidinethiones. J. Am. Chem. Soc. 2005, 127, 10816–10817. [Google Scholar] [CrossRef]
- Pohlhaus, P.D.; Sanders, S.D.; Parsons, A.T.; Li, W.; Johnson, J.S. Scope and Mechanism for Lewis Acid-Catalyzed Cycloadditions of Aldehydes and Donor-Acceptor Cyclopropanes: Evidence for a Stereospecific Intimate Ion Pair Pathway. J. Am. Chem. Soc. 2008, 130, 8642–8650. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.J.; Johnson, J.S.; Parsons, A.T.; Pohlhaus, P.D.; Sanders, S.D. Complexity-Building Annulations of Strained Cycloalkanes and C=O π Bonds. J. Org. Chem. 2010, 75, 6317–6325. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.S. Reactive Intermediates: MS Investigations in Solution; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2009. [Google Scholar] [CrossRef]
- Santos, L.S.; Metzger, J.O. Study of Homogeneously Catalyzed Ziegler-Natta Polymerization of Ethene by ESI-MS. Angew. Chem.-Int. Ed. 2006, 45, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Sathish, M.; Nachtigall, F.M.; Santos, L.S. Enantioselective Imine Reduction of Dihydro-β-Carbolines by Fe-Thiosquaramide Catalyst. Org. Lett. 2022, 24, 7627–7631. [Google Scholar] [CrossRef] [PubMed]
- Gozzo, F.C.; Santos, L.S.; Augusti, R.; Consorti, C.S.; Dupont, J.; Eberlin, M.N. Gaseous Supramolecules of Imidazolium Ionic Liquids: “Magic” Numbers and Intrinsic Strengths of Hydrogen Bonds. Chem. Eur. J. 2004, 10, 6187–6193. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.E.; Cortés-Arriagada, D.; Trofymchuk, O.S.; Nachtigall, F.M.; Santos, L.S.; Rojas, R.S.; Toro-Labbé, A. Mechanistic Study of the Competitiveness between Branched and Linear Polyethylene Production on: N-Arylcyano-β-Diketiminate Nickel Hydride. Polym. Chem. 2020, 11, 6640–6649. [Google Scholar] [CrossRef]
- Trofymchuk, O.S.; Ortega, D.E.; Cortés-Arriagada, D.; Pereira, A.; Daniliuc, C.G.; Klitzke, C.F.; Santos, L.S.; Rojas, R.S. Neutral and Cationic Methallyl Nickel Complexes in Alkene Activation: A Combined DFT, ESI-MS and Chemometric Approach. Catal. Sci. Technol. 2021, 11, 7475–7485. [Google Scholar] [CrossRef]
- Escobar, M.A.; Trofymchuk, O.S.; Rodriguez, B.E.; Lopez-Lira, C.; Tapia, R.; Daniliuc, C.; Berke, H.; Nachtigall, F.M.; Santos, L.S.; Rojas, R.S. Lewis Acid Enhanced Ethene Dimerization and Alkene Isomerization-ESI-MS Identification of the Catalytically Active Pyridyldimethoxybenzimidazole Nickel(II) Hydride Species. ACS Catal. 2015, 5, 7338–7342. [Google Scholar] [CrossRef]
- Bao, M.; Doyle, M.P. Stereoretentive Catalytic [3 + 2]/[3 + 3]-Cycloaddition of Nonracemic Donor–Acceptor Cyclopropanes: Synthesis of Substituted Pyrrolidines and 1,2-Oxazinanes. Org. Lett. 2023, 25, 3029–3033. [Google Scholar] [CrossRef]
- Augustin, A.U.; Merz, J.L.; Jones, P.G.; Mlostoń, G.; Werz, D.B. (4 + 3)-Cycloaddition of Donor-Acceptor Cyclopropanes with Thiochalcones: A Diastereoselective Access to Tetrahydrothiepines. Org. Lett. 2019, 21, 9405–9409. [Google Scholar] [CrossRef]
- Chagarovskiy, A.O.; Ivanova, O.A.; Rakhmankulov, E.R.; Budynina, E.M.; Trushkov, I.V.; Melnikov, M.Y. Lewis Acid-Catalyzed Isomerization of 2-Arylcyclopropane-1,1- Dicarboxylates: A New Efficient Route to 2-Styrylmalonates. Adv. Synth. Catal. 2010, 352, 3179–3184. [Google Scholar] [CrossRef]
- Xie, M.S.; Zhao, G.F.; Qin, T.; Suo, Y.B.; Qu, G.R.; Guo, H.M. Thiourea Participation in [3 + 2] Cycloaddition with Donor-Acceptor Cyclopropanes: A Domino Process to 2-Amino-Dihydrothiophenes. Chem. Commun. 2019, 55, 1580–1583. [Google Scholar] [CrossRef]
- Pradhan, T.R.; Kim, H.W.; Park, J.K. Harnessing the Polarizability of Conjugated Alkynes toward [2 + 2] Cycloaddition, Alkenylation, and Ring Expansion of Indoles. Org. Lett. 2018, 20, 5286–5290. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Kanno, A.; Hanzawa, Y. Synthesis of 2,3-Disubstituted Indoles by a Rhodium-Catalyzed Aromatic Amino-Claisen Rearrangement of N-Propargyl Anilines. Angew. Chem.—Int. Ed. 2007, 46, 3931–3933. [Google Scholar] [CrossRef]
- Bergès, J.; García, B.; Muñiz, K. An Electrophilic Bromine Redox Catalysis for the Synthesis of Indole Alkaloid Building Blocks by Selective Aliphatic C−H Amination. Angew. Chem.—Int. Ed. 2018, 57, 15891–15895. [Google Scholar] [CrossRef]
- Varlet, T.; Bouchet, D.; Van Elslande, E.; Masson, G. Decatungstate-Photocatalyzed Dearomative Hydroacylation of Indoles: Direct Synthesis of 2-Acylindolines. Chem.—A Eur. J. 2022, 28, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mandal, T.; Chakraborti, G.; Dash, J. Reductive Aromatization of Oxindoles to 3-Substituted Indoles. Tetrahedron Lett. 2020, 61, 152109. [Google Scholar] [CrossRef]
- Yu, L.; Zhong, Y.; Yu, J.; Gan, L.; Cai, Z.; Wang, R.; Jiang, X. Highly Diastereoselective Oxa-[3+3] Cyclization with C,N-Cyclic Azomethine Imines: Via the Copper-Catalyzed Aerobic Oxygenated CC Bond of Indoles. Chem. Commun. 2018, 54, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.W.; Ko, T.Y.; Jang, M.J.; Jang, S.S. Silver(I)-Mediated C-H Amination of 2-Alkenylanilines: Unique Solvent-Dependent Migratory Aptitude. Adv. Synth. Catal. 2015, 357, 227–234. [Google Scholar] [CrossRef]
- Pan, S.; Ryu, N.; Shibata, T. Ir(I)-Catalyzed C-H Bond Alkylation of C2-Position of Indole with Alkenes: Selective Synthesis of Linear or Branched 2-Alkylindoles. J. Am. Chem. Soc. 2012, 134, 17474–17477. [Google Scholar] [CrossRef]
- Goudreau, R.; Marcoux, D.; Charette, B. General Method for the Synthesis of Phenyliodonium Ylides from Malonate Esters: Easy Access to 1, 1-Cyclopropane Diesters. J. Org. Chem. 2009, 74, 470–473. [Google Scholar] [CrossRef] [PubMed]
- Novikov, R.A.; Tarasova, A.V.; Korolev, V.A.; Timofeev, V.P.; Tomilov, Y.V. A New Type of Donor-Acceptor Cyclopropane Reactivity: The Generation of Formal 1,2- and 1,4-Dipoles. Angew. Chem.—Int. Ed. 2014, 53, 3187–3191. [Google Scholar] [CrossRef] [PubMed]
- Perreault, C.; Goudreau, S.R.; Zimmer, L.E.; Charette, A.B. Cycloadditions of Aromatic Azomethine Imines with 1,1-Cyclopropane Diesters. Org. Lett. 2008, 10, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, R.; Tiwari, D.P.; Saha, A.; Ghorai, M.K. Diastereoselective Synthesis of Functionalized Tetrahydrocarbazoles via a Domino-Ring Opening-Cyclization of Donor-Acceptor Cyclopropanes with Substituted 2-Vinylindoles. Org. Lett. 2014, 16, 3954–3957. [Google Scholar] [CrossRef]
- Parsons, A.T.; Campbell, M.J.; Johnson, J.S. Diastereoselective Synthesis of Tetrahydrofurans via Palladium(0)-Catalyzed [3 + 2] Cycloaddition of Vinylcyclopropanes and Aldehydes. Org. Lett. 2008, 10, 2541–2544. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).