Modulating the ESIPT Mechanism and Luminescence Characteristics of Two Reversible Fluorescent Probes by Solvent Polarity: A Novel Perspective
Abstract
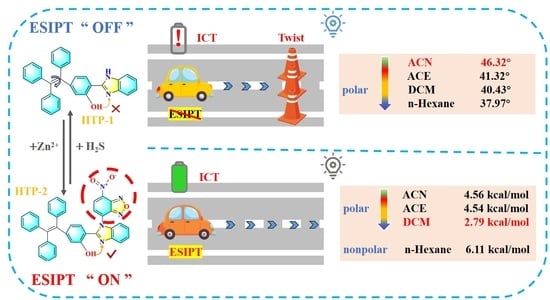
:1. Introduction
2. Results and Discussion
2.1. Geometric Parameters
2.2. Potential Energy Curves (PECs)
2.3. Infrared (IR) Spectra Analysis
2.4. Frontier Molecular Orbitals (FMOs)
2.5. Electronic Spectra and Hole-Electron Analysis
2.6. Reduced Density Gradient (RDG) Analysis
3. Theoretical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, P.W.; Han, K. Unraveling the Detailed Mechanism of Excited-State Proton Transfer. Acc. Chem. Res. 2018, 51, 1681–1690. [Google Scholar] [CrossRef]
- Chen, R.T.; Ren, Z.F.; Liang, Y.; Zhang, G.H.; Dittrich, T.; Liu, R.Z.; Liu, Y.; Zhao, Y.; Pang, S.; An, H.Y.; et al. Spatiotemporal imaging of charge transfer in photocatalyst particles. Nature 2022, 610, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Park, S.Y. Advanced organic optoelectronic materials: Harnessing excited-state intramolecular proton transfer (ESIPT) process. Adv. Mater. 2011, 23, 3615–3642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Liu, C. Computational Insights into Excited State Intramolecular Double Proton Transfer Behavior Associated with Atomic Electronegativity for Bis(2′-benzothiazolyl) hydroquinone. Molecules 2023, 28, 5951. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, M.; Li, W.Z.; Wang, Y.; Zhou, P.W. Theoretical Investigation on the “ON-OFF” Mechanism of a Fluorescent Probe for Thiophenols: Photoinduced Electron Transfer and Intramolecular Charge Transfer. Molecules 2023, 28, 6921. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Dong, B.; Liu, Y.; Lin, W. Fluorescent chemosensors manipulated by dual/ triple interplaying sensing mechanisms. Chem. Soc. Rev. 2016, 45, 6449–6461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; Northrop, B.; Han, K.; Stang, P. The effect of intermolecular hydrogen bonding on the fluorescence of a bimetallic platinum complex. J. Phys. Chem. A 2010, 114, 9007. [Google Scholar] [CrossRef]
- Zhu, L.X.; Li, Q.; Wan, Y.F.; Guo, M.L.; Yan, L.; Yin, H.; Shi, Y. Short-Range Charge Transfer in DNA Base Triplets: Real-Time Tracking of Coherent Fluctuation Electron Transfer. Molecules 2023, 28, 6802. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mu, H.Y.; Xin, C.; Cai, J.X.; Yuan, B.S.; Jin, G.Y. Turning ON/OFF the fluo-rescence of the ESIPT state by changing the hydrogen bond distance and ori-entation in quinoline-pyrazole derivatives. J. Mol. Struct. 2022, 1252, 132146. [Google Scholar] [CrossRef]
- Xin, X.; Zhao, Y.; Shi, W.; Zhao, G.J.; Li, Y.Q. Effects of Twisted Intramolecular Charge Transfer Behavior on Excited-State Intramolecular Proton Transfer Reactions of Methyl Benzoate Derivatives in Water Solution. J. Phys. Chem. A 2022, 126, 5126–5133. [Google Scholar] [CrossRef]
- Zhao, G.J.; Yang, Y.F.; Zhang, C.Y.; Song, Y.Z.; Li, Y.Q. The theoretical study of excited-state intramolecular proton transfer of N,N,-bis (salicylidene)-(2-(3″4′-diaminophenyl) benzothiazole). Spectrochim. J. Lumin. 2021, 230, 117741. [Google Scholar] [CrossRef]
- Jiang, G.S.; Tang, Z.; Han, H.Y.; Ding, J.X.; Zhou, P.W. Effects of Intermolecular Hydrogen Bonding and Solvation on Enol–Keto Tautomerism and Photophysics of Azomethine–BODIPY Dyads. J. Phys. Chem. B 2021, 125, 9296–9303. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.Y.; Sun, Y.H.; Gao, J.A.; Xin, C.; Zhao, H.F.; Jin, G.Y.; Li, H. Switching the ESIPT and TICT process of DP-HPPI via intermolecular hydrogen bonding. J. Mol. Struct. 2023, 1277, 134800. [Google Scholar] [CrossRef]
- Mu, H.Y.; Li, H.; Sun, C.F.; Gao, J.A.; Yang, M.; Xin, C.; Jin, G.Y. Different competition mechanism between ESPT and TICT process regulated by protic and aprotic solvent in DHP. J. Mol. Liq. 2023, 375, 121278. [Google Scholar] [CrossRef]
- Gawrys, P.; Morawski, O.; Banasiewicz, M.; Barboza, C.A. Magnifying the ESIPT process in tris(salicylideneanilines) via the steric effect-a pathway to the molecules with panchromatic fluorescence. Phys. Chem. Chem. Phys. 2023, 25, 12500–12514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.J.; Han, K.L. Early time hydrogen-bonding dynamics of photoexcited coumarin 102 in hydrogen-donating solvents: Theoretical study. J. Phys. Chem. A 2007, 111, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Oftadeh, M.; Barfarakh, Z.; Ravari, F. Luminescent excited-state intramolecular proton-transfer dyes based on 4-functionalized 6,6′-dimethyl-3,3′-dihydroxy-2,2′-bipyridine (BP(OH)2-Rs); DFT simulation study. J. Mol. Graph. Model. 2021, 107, 107948. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Interaction Region Indicator: A Simple Real Space Function Clearly Revealing Both Chemical Bonds and Weak Interactions. Chem.-Methods 2021, 1, 231–239. [Google Scholar] [CrossRef]
- Sun, L.C.; Chen, Y.C.; Sun, M.T. Exploring nonemissive excited-state intramolecular proton transfer by plasmon-enhanced hyper-raman scattering and two-photon excitation fluorescence. J. Phys. Chem. C 2022, 126, 487–492. [Google Scholar] [CrossRef]
- Mu, H.Y.; Li, D.; Gao, J.A.; Wang, Y.; Zhang, Y.F.; Jin, G.Y.; Li, H. Achieving the cooperative and stepwise regulation of ESIDPT process in AFBD by introducing different electronic groups. J. Mol. Struct. 2023, 1294, 136385. [Google Scholar] [CrossRef]
- Gao, J.A.; Mu, H.Y.; Zhen, Q.; Guan, X.Y.; Li, H. Accelerating the concerted double proton transfer process of 2,2′-bipyridine-3,3′-diol-5,5′-dicarboxylate acid ethyl ester (BPDC) molecule by centrosymmetric dual intermolecular hydrogen bonds. J. Mol. Struct. 2023, 1294, 136406. [Google Scholar] [CrossRef]
- Gao, J.A.; Zhang, Y.F.; Mu, H.Y.; Yang, M.; Guan, X.T.; Jin, G.Y.; Li, H. Paying Comprehensive Attention to the Temperature-Dependent Dual-Channel Excited-State Intramolecular Proton Transfer Mechanism of Fluorescence Ratio Probe BZ-DAM. Int. J. Mol. Sci. 2023, 24, 13899. [Google Scholar] [CrossRef]
- Lou, Z.R.; Zhou, X.Y.; Tang, Z.; Zhou, P.W. Theoretical Insights into the Excited State Decays of a Donor–Acceptor Dyad: Is the Twisted and Rehybridized Intramolecular Charge-Transfer State Involved? J. Phys. Chem. B 2020, 124, 4564–4572. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Cao, D.; Hu, T.; Ye, Y.; Jia, X.; Li, H.; Hu, X.; Dong, Y.; Ma, Y.; Wang, T. Novel Norrish type I flavonoid photoinitiator for safe LED light with high activity and low toxicity by inhibiting the ESIPT process. Dyes Pigment. 2021, 184, 108865. [Google Scholar] [CrossRef]
- Sun, C.F.; Su, X.; Zhou, Q.; Shi, Y. Regular tuning of the ESIPT reaction of 3-hydroxychromone-based derivatives by substitution of functional groups. Org. Chem. Front. 2019, 6, 3093–3100. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, L.X.; Wan, Y.; Wan, Y.F.; Gao, J.B.; Yin, H.; Shi, Y. Accelerating ultrafast processes in hydrogen-bonded complexes under pressure. Appl. Phys. Lett. 2023, 122, 061110. [Google Scholar] [CrossRef]
- Li, Y.Q.; Ma, Y.Z.; Yang, Y.F.; Shi, W.; Lan, R.F.; Guo, Q. Effects of different substituents of methyl 5-R-salicylates on the excited state intramolecular proton transfer process. Phys. Chem. Chem. Phys. 2018, 20, 4208–4215. [Google Scholar] [CrossRef]
- Yang, D.P.; Zhao, J.F.; Yang, G.; Song, N.H.; Zheng, R.; Wang, Y.S. A theoretical study about excited state behaviour for imide compound N-cyclohexyl-3-hydroxyphthalimide and 3,6-dihydroxy-N-cyclohexy lphthalimide. J. Mol. Liq. 2017, 241, 1003–1008. [Google Scholar] [CrossRef]
- Weller, A. Über die Fluoreszenz der Salizylsäure und verwandter Verbindungen. Naturwissenschaften 1955, 42, 175–176. [Google Scholar] [CrossRef]
- Li, H.; Han, J.H.; Zhao, H.F.; Liu, X.C.; Ma, L.N.; Sun, C.F.; Yin, H.; Shi, Y. Investigation of the Intermolecular Hydrogen Bonding Effects on the Intramolecular Charge Transfer Process of Coumarin 340 in Tetrahydrofuran Solvent. J. Clust. Sci. 2018, 29, 585–592. [Google Scholar] [CrossRef]
- Li, H.; Yin, H.; Liu, X.C.; Shi, Y.; Jin, M.X.; Ding, D.J. An experimental and theoretical study of solvent hydrogen-bond-donating capacity effects on ultrafast intramolecular charge transfer of LD 490. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 184, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.F.; de Souza, G.L.C. Water hydrogen-bonding effects on the ground and low-lying excited states of dipyridyl isomers. J. Mol. Liq. 2021, 338, 116767. [Google Scholar] [CrossRef]
- Chen, L.; Fu, P.Y.; Wang, H.P.; Pan, M. Excited-state intramolecular proton transfer (ESIPT) for optical sensing in solid state. Adv. Opt. Mater. 2021, 9, 2001952. [Google Scholar] [CrossRef]
- Zhai, H.S.; Zhu, M.Y.; Jia, X.L.; Liu, Y.; Guan, T.T.; Yang, Y.G.; Liu, Y.F. Theoretical investigation on the fluorescence properties and ESIPT mechanism of the Al3+ ion sensor 1-((2-hydroxynaphthalen-1-yl) methylene) urea (OCN). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 27, 121237. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Singh, I.; Goel, R.; Paul, K.; Luxami, V. Dual-channel ratiometric recognition of Al3+ and F ions through an ESIPT-ESICT signalling mechanism. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Yang, Y.G.; Liu, Y.F. Recognition mechanism of imidazo[1,5-α] pyridine-based fluorescence probe towards thiophenols with multi-mechanisms of PET and ESIPT. J. Photochem. Photobiol. A Chem. 2023, 437, 114477. [Google Scholar] [CrossRef]
- Trannoy, V.; Le’austic, A.; Gadan, S.; Guillot, R.; Allain, C.; Clavier, G.; Mazerat, S.; Geffffroy, B.; Yu, P. A highly efficient solution and solid state ESIPT fluorophore and its OLED application. New J. Chem. 2021, 45, 3014–3021. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, S.P.; Ma, Q.; Liao, S.H. Visible light-mediated ring-opening polymerization of lactones based on the excited state acidity of ESPT molecules. Polym. Chem. 2020, 11, 3709–3715. [Google Scholar] [CrossRef]
- Xiao, S.S.; Lou, Z.R.; Ji, D.B.; Zhao, J.F. Understanding solvent polarity dependent excited state behavior and ESIPT mechanism for 2-benzo[b]thiphen-3-yl-3-hydroxy-6-methoxy-chroman-4-one compound. Chem. Phys. Lett. 2021, 769, 138409. [Google Scholar] [CrossRef]
- Du, C.; Zhou, Q.; Zhang, M.X.; Song, P.; Ma, F.C. Excited-state intramolecular proton transfer of 6-amino-2-(2′-hydroxyphenyl) benzoxazole (6A-HBO) in different solvents. J. Phys. Org. Chem. 2019, 32, e3901. [Google Scholar] [CrossRef]
- Zhao, J.F.; Yang, Y.F.; Li, L.; Zhang, H.H. Theoretical Insights into Excited-State Stepwise Double Proton Transfer Associated with Solvent Polarity for 2-bis(benzothia-zolyl) naphthalene-Diol Compound. Chem. Sel. 2023, 8, e202301202. [Google Scholar] [CrossRef]
- Yi, S.Q.; Liu, H.L.; Chen, Z.; Fan, C.B.; Liu, G.; Pu, S.Z. Novel fluorescent probes based on NBD-substituted imidazole amino to sequentially detect H2S and Zn2+. Dyes Pigment. 2023, 214, 111211. [Google Scholar] [CrossRef]
- Shang, C.J.; Cao, Y.J.; Shao, Z.Q.; Sun, C.F.; Li, Y.Z. Tactfully unveiling the effect of solvent polarity on the ESIPT mechanism and photophysical property of the 3-hydroxylflavone derivative. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120496. [Google Scholar] [CrossRef]
- Yang, D.P.; Liu, W.; Kong, L.H.; Zhang, Q.L.; Liu, Y.F. Ultrafast excited state intramolecular proton transfer (ESIPT) mechanism for 2,6-bis(benzothiazolyl-2-yl)phenol: A theoretical investigation. Chem. Phys. Lett. 2020, 744, 137226. [Google Scholar] [CrossRef]
- Zhou, Q.; Du, C.; Yang, L.; Dai, Y.M.; Song, P. Mechanism for the excited-State multiple proton transfer process of dihydroxyanthraqui-none chromophores. J. Phys. Chem. A 2017, 121, 4645. [Google Scholar] [CrossRef]
- Zhao, G.J.; Han, K.L. Hydrogen bonding in the electronic excited state. Acc. Chem. Res. 2012, 45, 404. [Google Scholar] [CrossRef]
- Yang, D.P.; Yang, W.P.; Tian, Y.S.; Zheng, R. Regulating the excited state behaviors of 2-benzooxazol-2-yl-4,6-di-tert-butyl-phenol fluorophore by solvent polarity: A theoretical simulation. Chem. Phys. 2022, 558, 111513. [Google Scholar] [CrossRef]
- Li, C.Z.; Ma, C.; Li, D.L.; Liu, Y.F. Excited state intramolecular proton transfer (ESIPT) of 6-amino-2-(2′-hydroxyphenyl) benzoxazole in dichloromethane and methanol: A TD-DFT quantum chemical study. J. Lumin. 2016, 172, 29–33. [Google Scholar] [CrossRef]
- Liu, X.M.; Wang, Y.; Wang, Y.X.; Tao, Y.P.; Fei, X.; Tian, J.; Hou, Y.M. Solvent effect on the excited-state intramolecular double proton transfer of 1,3-bis(2-pyridylimino)-4,7-dihydroxyisoindole. Photochem. Photobiol. Sci. 2021, 20, 1183–1194. [Google Scholar] [CrossRef]
- Guan, Y.L.; Tang, Z.; Ju, L.P.; Zhao, J.F. Solvent polarity-dependent ESIPT behavior for 5-(benzothiazole-2-yl)-4-hydroxyisophthalaldehyde fluorophore: A theoretical study. J. Chin. Chem. Soc. 2022, 69, 1012–1019. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Yang, D.; Li, H.; Jiang, K.; Sun, J. Photoinduced excited state intramolecular proton transfer and spectral behaviors of aloesaponarin 1. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 151, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I. Molecular Orbitals and Organic Chemical Reactions, 2nd ed.; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Zhao, J.F.; Jin, B.; Tang, Z. Solvent-polarity-dependent conformation and ESIPT behaviors for 2-(benzimidazol-2-yl)-3-hydroxychromone: A novel dynamical mechanism. Phys. Chem. Chem. Phys. 2022, 24, 27660–27669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Li, Z.J.; Jin, B. Uncovering photo-induced hydrogen bonding interaction and proton transfer mechanism for the novel salicylaldehyde azine derivative with para-position electrophilic cyano group. J. Lumin. 2021, 238, 118231. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, revision B. 02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum: A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Contreras-Garcia, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.P.; Beratan, D.N.; Yang, W. NCIPLOT: A program for plotting non-covalent interaction regions. J. Chem. Theory Comput. 2011, 7, 625. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Chen, Q. An sp-hybridized all-carboatomic ring, cyclo 18 carbon: Electronic structure, electronic spectrum, and optical nonlinearity. Carbon 2020, 165, 461–467. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 27–38. [Google Scholar] [CrossRef]













| O1-H1 | N1-H1 | δ(O1-H1-N1) | C5-C1-C2-C3 | |||
|---|---|---|---|---|---|---|
| HTP-1 | ACN | S0 | 0.997 | 1.707 | 147.79 | 12.35 |
| S1 | 0.997 | 1.709 | 148.12 | 58.67 | ||
| ACE | S0 | 0.997 | 1.708 | 147.74 | 12.34 | |
| S1 | 0.997 | 1.711 | 148.07 | 53.66 | ||
| DCM | S0 | 0.997 | 1.711 | 147.63 | 12.32 | |
| S1 | 0.996 | 1.714 | 147.94 | 52.75 | ||
| n-Hexane | S0 | 0.995 | 1.726 | 147.08 | 12.24 | |
| S1 | 0.994 | 1.727 | 147.34 | 50.21 | ||
| HTP-2 | ACN | S0 | 0.990 | 1.775 | 145.68 | 12.56 |
| S1 | 0.999 | 1.687 | 146.60 | 20.22 | ||
| S1′ | 1.881 | 1.026 | 126.13 | 12.63 | ||
| ACE | S0 | 0.990 | 1.776 | 145.64 | 12.53 | |
| S1 | 1.000 | 1.685 | 146.63 | 20.24 | ||
| S1′ | 2.010 | 1.022 | 120.44 | 9.69 | ||
| DCM | S0 | 0.989 | 1.778 | 145.54 | 12.46 | |
| S1 | 1.000 | 1.682 | 146.71 | 20.32 | ||
| S1′ | 1.981 | 1.023 | 121.90 | 9.63 | ||
| n-Hexane | S0 | 0.988 | 1.789 | 145.10 | 12.23 | |
| S1 | 1.003 | 1.660 | 147.13 | 20.75 | ||
| S1′ | 1.886 | 1.027 | 126.50 | 9.90 |
| O1 | N1 | ||||
|---|---|---|---|---|---|
| S0 | S1 | S0 | S1 | ||
| HTP-1 | ACN | −0.727 | −0.729 | −0.577 | −0.576 |
| ACE | −0.726 | −0.728 | −0.576 | −0.575 | |
| DCM | −0.724 | −0.726 | −0.574 | −0.573 | |
| n-Hexane | −0.712 | −0.714 | −0.565 | −0.564 | |
| HTP-2 | ACN | −0.721 | −0.721 | −0.547 | −0.556 |
| ACE | −0.720 | −0.720 | −0.546 | −0.556 | |
| DCM | −0.718 | −0.718 | −0.544 | −0.555 | |
| n-Hexane | −0.709 | −0.710 | −0.536 | −0.553 | |
| Transition | λabs (nm) | f | Composition | CI (%) | ||
|---|---|---|---|---|---|---|
| HTP-1 | ACN | S0→S1 | 350.9 | 0.9149 | H→L | 68.84% |
| S0→S2 | 304.6 | 0.3238 | H-1→L | 57.83% | ||
| S0→S3 | 282.3 | 0.2764 | H→L+1 | 54.34% | ||
| ACE | S0→S1 | 351.2 | 0.9190 | H→L | 68.83% | |
| S0→S2 | 304.9 | 0.3238 | H-1→L | 57.93% | ||
| S0→S3 | 282.4 | 0.2784 | H→L+1 | 54.40% | ||
| DCM | S0→S1 | 352.3 | 0.9351 | H→L | 68.81% | |
| S0→S2 | 306.0 | 0.3278 | H-1→L | 58.17% | ||
| S0→S3 | 283.0 | 0.2853 | H→L+1 | 54.43% | ||
| n-Hexane | S0→S1 | 352.0 | 0.9315 | H→L | 68.77% | |
| S0→S2 | 308.2 | 0.2853 | H-1→L | 59.58% | ||
| S0→S3 | 283.7 | 0.3000 | H→L+1 | 56.36% |
| Transition | λabs (nm) | f | Composition | CI (%) | ||
|---|---|---|---|---|---|---|
| HTP-2 | ACN | S0→S1 | 515.6 | 0.0230 | H→L | 66.18% |
| S0→S2 | 413.1 | 0.0018 | H-1→L | 65.46% | ||
| S0→S3 | 393.2 | 0.1181 | H-3→L | 66.90% | ||
| S0→S4 | 374.6 | 0.0008 | H-2→L | 65.80% | ||
| S0→S5 | 352.2 | 0.8471 | H→L+2 | 65.34% | ||
| ACE | S0→S1 | 517.9 | 0.0230 | H→L | 66.19% | |
| S0→S2 | 414.7 | 0.0018 | H-1→L | 65.51% | ||
| S0→S3 | 393.8 | 0.1186 | H-3→L | 66.89% | ||
| S0→S4 | 375.6 | 0.0008 | H-2→L | 65.64% | ||
| S0→S5 | 352.5 | 0.8508 | H→L+2 | 65.52% | ||
| DCM | S0→S1 | 523.8 | 0.0233 | H→L | 66.21% | |
| S0→S2 | 418.9 | 0.0017 | H-1→L | 65.64% | ||
| S0→S3 | 395.6 | 0.1216 | H-3→L | 66.77% | ||
| S0→S4 | 378.0 | 0.0009 | H-2→L | 65.16% | ||
| S0→S5 | 353.7 | 0.8664 | H→L+2 | 65.85% | ||
| n-Hexane | S0→S1 | 542.7 | 0.0224 | H→L | 66.34% | |
| S0→S2 | 432.7 | 0.0016 | H-1→L | 66.11% | ||
| S0→S3 | 398.3 | 0.1166 | H-3→L | 62.45% | ||
| S0→S4 | 385.4 | 0.0025 | H-2→L | 62.72% | ||
| S0→S5 | 355.5 | 0.7547 | H→L+2 | 61.28% |
| D (Å) | Sr | H (Å) | T (Å) | |||
|---|---|---|---|---|---|---|
| HTP-1 | S1 | ACN | 0.214 | 0.89852 | 3.542 | −1.967 |
| ACE | 0.182 | 0.88927 | 3.569 | −1.880 | ||
| DCM | 0.176 | 0.88848 | 3.570 | −1.830 | ||
| n-Hexane | 0.155 | 0.86627 | 3.570 | −1.689 | ||
| HTP-2 | S1 | ACN | 5.979 | 0.13088 | 3.453 | 3.723 |
| ACE | 5.997 | 0.12876 | 3.450 | 3.749 | ||
| DCM | 6.042 | 0.12376 | 3.439 | 3.807 | ||
| n-Hexane | 6.208 | 0.10119 | 3.385 | 4.041 | ||
| S1′ | ACN | 5.091 | 0.13141 | 2.652 | 3.511 | |
| ACE | 4.682 | 0.15186 | 2.601 | 3.072 | ||
| DCM | 4.073 | 0.14849 | 2.588 | 3.102 | ||
| n-Hexane | 4.746 | 0.12309 | 2.528 | 3.192 | ||
| B3LYP | CAM-B3LYP | M06-2X | MPW1PW91 | MN15 | Exp. | |
|---|---|---|---|---|---|---|
| λabs | 374 | 314 | 326 | 364 | 351 | 348 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Mu, H.; Sun, Y.; Gao, J.; Zhu, X.; Li, H. Modulating the ESIPT Mechanism and Luminescence Characteristics of Two Reversible Fluorescent Probes by Solvent Polarity: A Novel Perspective. Molecules 2024, 29, 1629. https://doi.org/10.3390/molecules29071629
Wang Y, Mu H, Sun Y, Gao J, Zhu X, Li H. Modulating the ESIPT Mechanism and Luminescence Characteristics of Two Reversible Fluorescent Probes by Solvent Polarity: A Novel Perspective. Molecules. 2024; 29(7):1629. https://doi.org/10.3390/molecules29071629
Chicago/Turabian StyleWang, Yang, Hongyan Mu, Yuhang Sun, Jiaan Gao, Xiaodong Zhu, and Hui Li. 2024. "Modulating the ESIPT Mechanism and Luminescence Characteristics of Two Reversible Fluorescent Probes by Solvent Polarity: A Novel Perspective" Molecules 29, no. 7: 1629. https://doi.org/10.3390/molecules29071629
APA StyleWang, Y., Mu, H., Sun, Y., Gao, J., Zhu, X., & Li, H. (2024). Modulating the ESIPT Mechanism and Luminescence Characteristics of Two Reversible Fluorescent Probes by Solvent Polarity: A Novel Perspective. Molecules, 29(7), 1629. https://doi.org/10.3390/molecules29071629