Valorization of Purple Phototrophic Bacteria Biomass Resulting from Photo Fermentation Aimed at Biohydrogen Production
Abstract
:1. Introduction
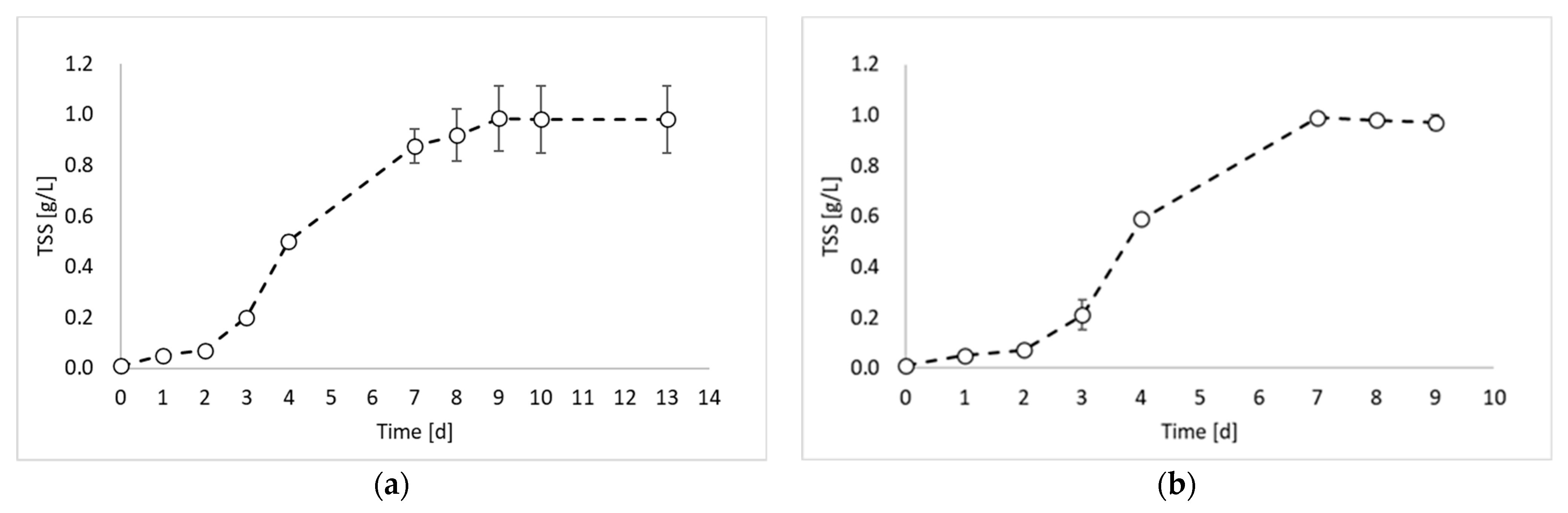
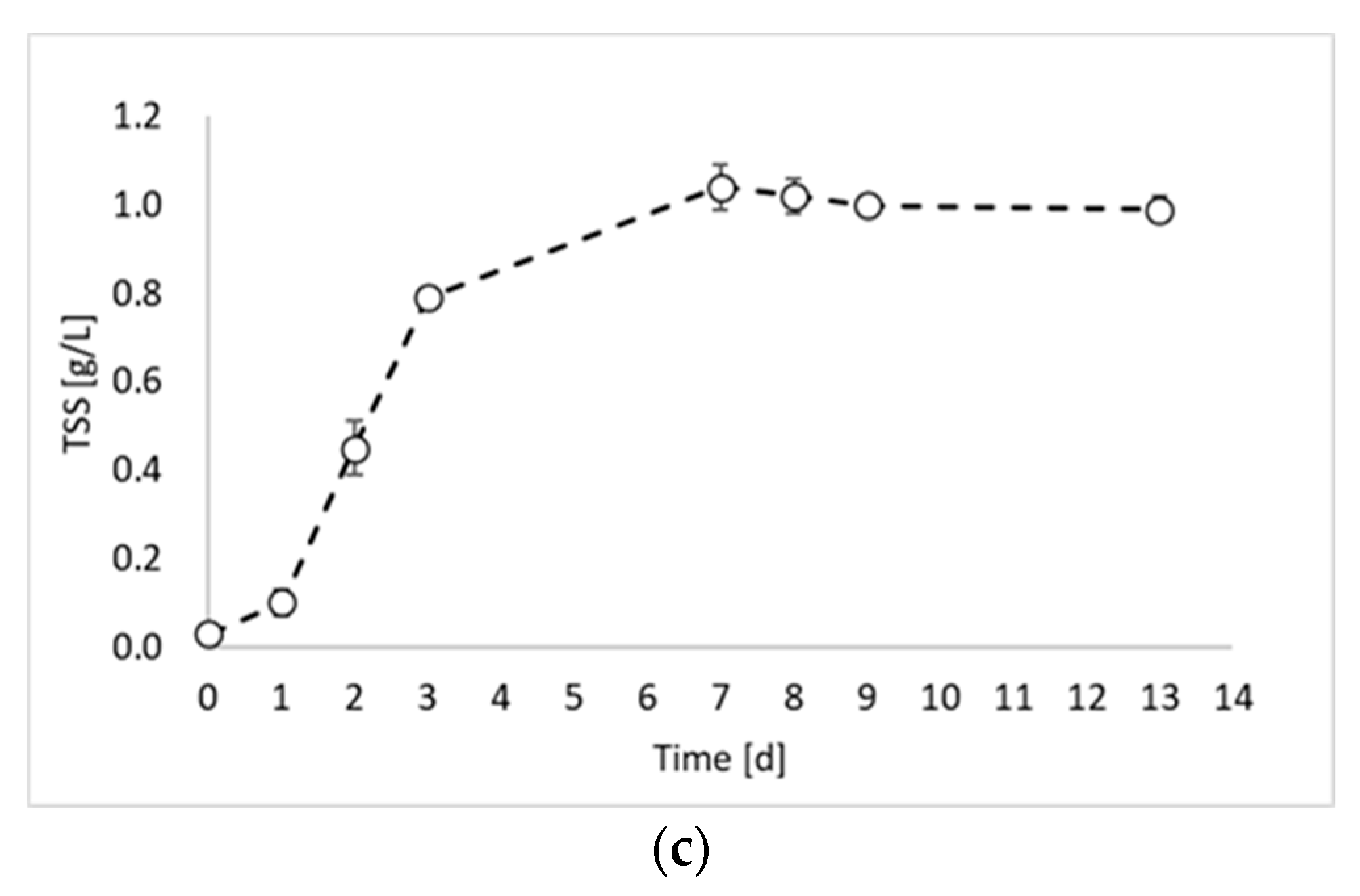
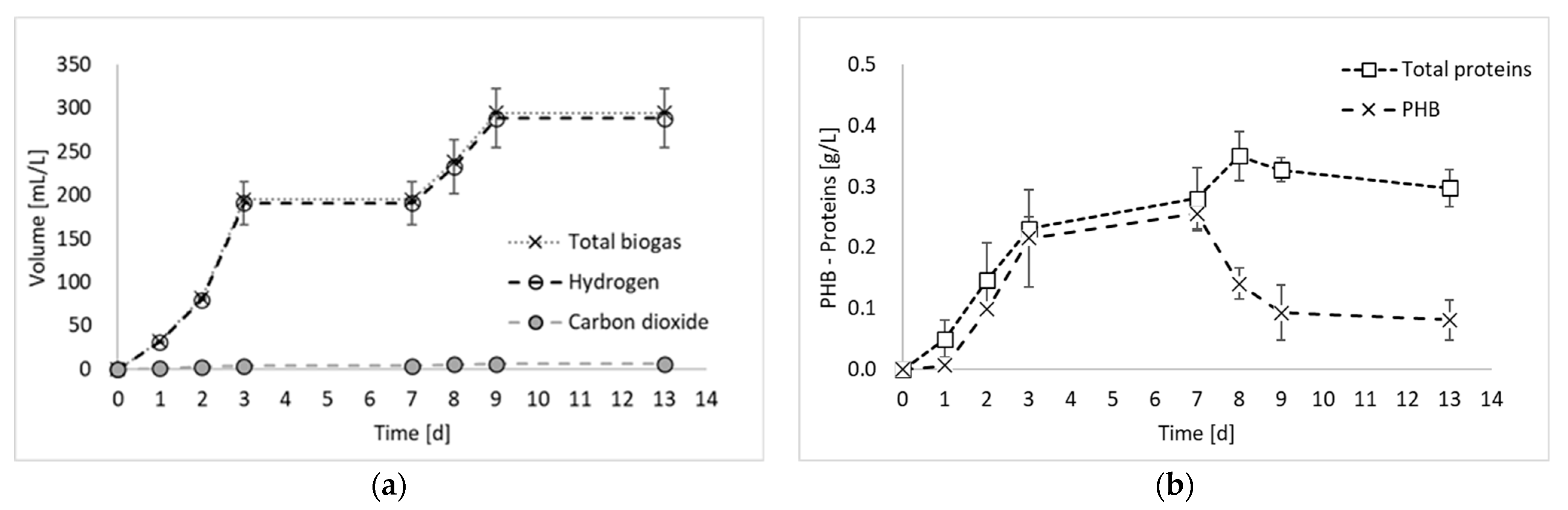
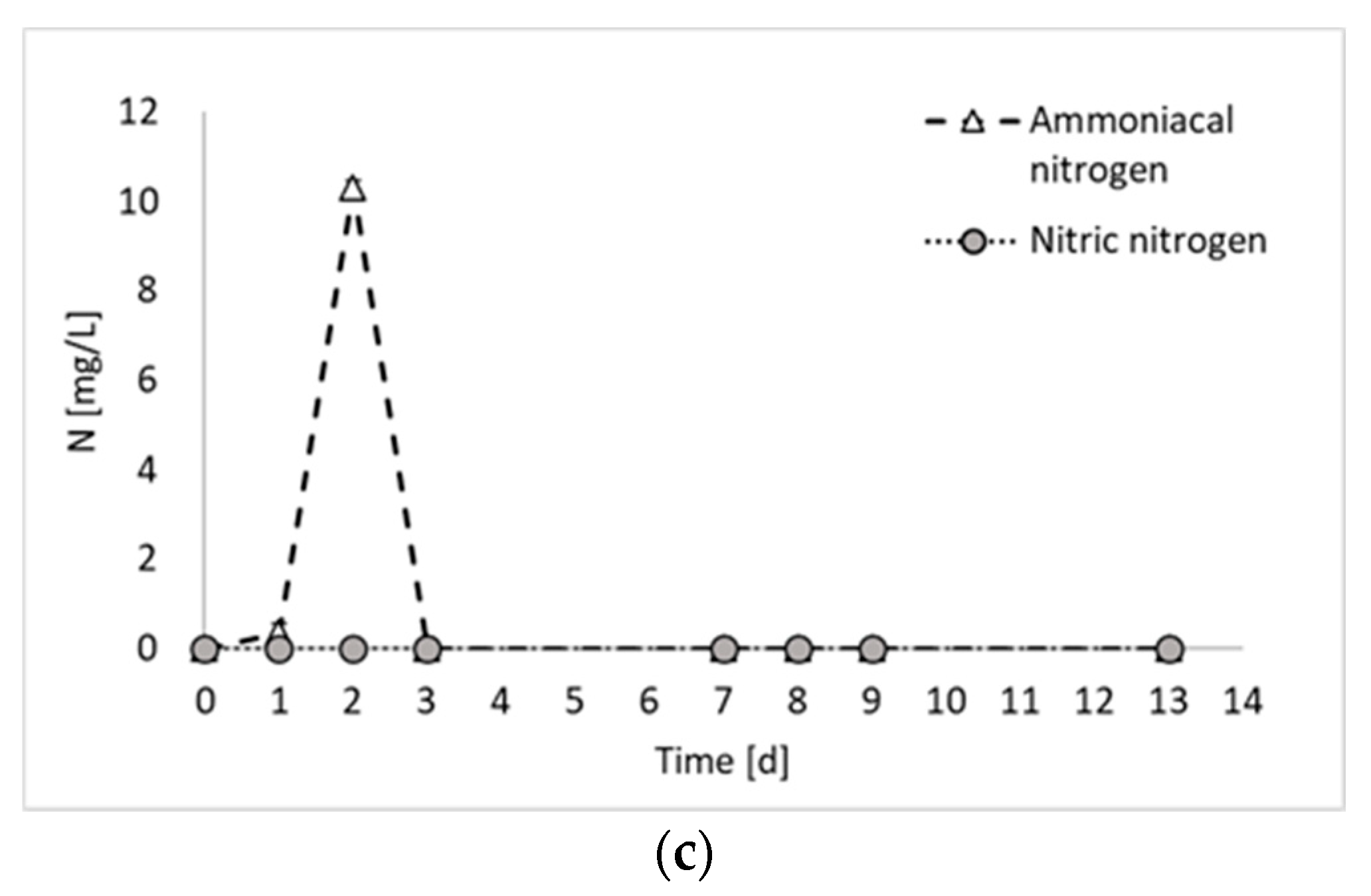
2. Results and Discussion
3. Materials and Methods
3.1. Substrate Composition and Inoculum Enrichment
- Biomass growth test, which was aimed at determining the time required for microorganisms to reach the exponential growth phase under photo fermentative conditions;
- Inoculum production test, which was performed using a sample from test 1 as an inoculum. This test was aimed at extracting an inoculum of microorganisms in the exponential growth phase (i.e., during the maximum biomass production rate in terms of gTSS/L h);
- Hydrogen production test, in which the inoculum obtained in the previous steps was employed for a final photo fermentation test, aimed at hydrogen production.
3.2. Experimental Setup
3.3. Analytical Procedures
3.4. COD and Nitrogen Balance: Economic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capson-Tojo, G.; Batstone, D.J.; Grassino, M.; Vlaeminck, S.E.; Puyol, D.; Verstraete, W.; Kleerebezem, R.; Oehmen, A.; Ghimire, A.; Pikaar, I.; et al. Purple Phototrophic Bacteria for Resource Recovery: Challenges and Opportunities. Biotechnol. Adv. 2020, 43, 107567. [Google Scholar] [CrossRef] [PubMed]
- Cerruti, M.; Ouboter, H.T.; Chasna, V.; van Loosdrecht, M.C.M.; Picioreanu, C.; Weissbrodt, D.G. Effects of Light/Dark Diel Cycles on the Photoorganoheterotrophic Metabolism of Rhodopseudomonas palustris for Differential Electron Allocation to PHAs and H2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Alloul, A.; Wuyts, S.; Lebeer, S.; Vlaeminck, S.E. Volatile Fatty Acids Impacting Phototrophic Growth Kinetics of Purple Bacteria: Paving the Way for Protein Production on Fermented Wastewater. Water Res. 2019, 152, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Esposito, G.; Luongo, V.; Pirozzi, F.; Frunzo, L.; Lens, P.N.L. Engineering Strategies for Enhancing Photofermentative Biohydrogen Production by Purple Nonsulfur Bacteria Using Dark Fermentation Effluents. In Microbial Fuels: Technologies and Applications; CRC Press: Boca Raton, FL, USA, 2017; pp. 275–314. [Google Scholar] [CrossRef]
- Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Photo-Fermentative Bacteria Used for Hydrogen Production. Appl. Sci. 2024, 14, 1191. [Google Scholar] [CrossRef]
- Montiel-Corona, V.; Buitrón, G. Polyhydroxyalkanoates from Organic Waste Streams Using Purple Non-Sulfur Bacteria. Bioresour. Technol. 2021, 323, 124610. [Google Scholar] [CrossRef] [PubMed]
- Wada, O.Z.; Vincent, A.S.; Mackey, H.R. Single-Cell Protein Production from Purple Non-Sulphur Bacteria-Based Wastewater Treatment. Rev. Environ. Sci. Biotechnol. 2022, 21, 931–956. [Google Scholar] [CrossRef]
- Policastro, G.; Luongo, V.; Fabbricino, M. Biohydrogen and Poly-β-Hydroxybutyrate Production by Winery Wastewater Photofermentation: Effect of Substrate Concentration and Nitrogen Source. J. Environ. Manag. 2020, 271, 111006. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Valentino, S.; Frunzo, L.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Concomitant Biohydrogen and Poly-β-Hydroxybutyrate Production from Dark Fermentation Effluents by Adapted Rhodobacter sphaeroides and Mixed Photofermentative Cultures. Bioresour. Technol. 2016, 217, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ooshima, H.; Takakuwa, S.; Katsuda, T.; Okuda, M.; Shirasawa, T.; Azuma, M.; Kato, J. Production of Hydrogen by a Hydrogenase-Deficient Mutant of Rhodobacter capsulatus. J. Ferment. Bioeng. 1998, 85, 470–475. [Google Scholar] [CrossRef]
- Policastro, G.; Giugliano, M.; Luongo, V.; Napolitano, R.; Fabbricino, M. Carbon Catabolite Repression Occurrence in Photo Fermentation of Ethanol-Rich Substrates. J. Environ. Manag. 2021, 297, 113371. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Das, D. Effect of Light Intensity and Initial PH during Hydrogen Production by an Integrated Dark and Photofermentation Process. Int. J. Hydrogen Energy 2009, 34, 7497–7501. [Google Scholar] [CrossRef]
- Johnson, K.; Jiang, Y.; Kleerebezem, R.; Muyzer, G.; Van Loosdrecht, M.C.M. Enrichment of a Mixed Bacterial Culture with a High Polyhydroxyalkanoate Storage Capacity. Biomacromolecules 2009, 10, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, Y.; Good, A.G. NAD(H)-Dependent Glutamate Dehydrogenase Is Essential for the Survival of Arabidopsis thaliana during Dark-Induced Carbon Starvation. J. Exp. Bot. 2008, 59, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, D.H.; Cha, J.; Lee, J.K. Effect of Carbon and Nitrogen Sources on Photo-Fermentative H2 Production Associated with Nitrogenase, Uptake Hydrogenase Activity, and PHB Accumulation in Rhodobacter sphaeroides KD131. Bioresour. Technol. 2012, 116, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Hillmer, P.; Gest, H. H2 Metabolism in the Photosynthetic Bacterium Rhodopseudomonas capsulata: H2 Production by Growing Cultures. J. Bacteriol. 1977, 129, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Rashid, N.; Onwusogh, U.; McKay, G.; Mackey, H.R. Effect of Nutrients Deficiency on Biofilm Formation and Single Cell Protein Production with a Purple Non-Sulphur Bacteria Enriched Culture. Biofilm 2023, 5, 100098. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Nur Fitriana, H.; Lee, S.Y.; Kim, M.S.; Moon, M.; Lee, W.H.; Lee, J.S.; Lee, S. Molecular Profiling and Optimization Studies for Growth and Phb Production Conditions in Rhodobacter sphaeroides. Energies 2020, 13, 6471. [Google Scholar] [CrossRef]
- Rashid, N.; Onwusogh, U.; Mackey, H.R. Exploring the Metabolic Features of Purple Non-Sulfur Bacteria for Waste Carbon Utilization and Single-Cell Protein Synthesis. Biomass Convers. Biorefinery 2022, 1–20. [Google Scholar] [CrossRef]
- Policastro, G.; Cesaro, A.; Fabbricino, M. Photo-Fermentative Hydrogen Production from Cheese Whey: Engineering of a Mixed Culture Process in a Semi-Continuous, Tubular Photo-Bioreactor. Int. J. Hydrogen Energy 2023, 48, 21038–21054. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pontoni, L.; d’Antonio, G.; Lens, P.N.L.; Esposito, G.; Pirozzi, F. Dark Fermentation of Complex Waste Biomass for Biohydrogen Production by Pretreated Thermophilic Anaerobic Digestate. J. Environ. Manag. 2015, 152, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Oehmen, A.; Keller-Lehmann, B.; Zeng, R.J.; Yuan, Z.; Keller, J. Optimisation of Poly-β-Hydroxyalkanoate Analysis Using Gas Chromatography for Enhanced Biological Phosphorus Removal Systems. J. Chromatogr. A 2005, 1070, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Waterborg, J.H. The Lowry Method for Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Springer Protocols Handbooks; Humana Press: Totowa, NJ, USA, 2009; pp. 7–10. [Google Scholar]
- Carlozzi, P.; Sacchi, A. Biomass Production and Studies on Rhodopseudomonas palustris Grown in an Outdoor, Temperature Controlled, Underwater Tubular Photobioreactor. J. Biotechnol. 2001, 88, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Akkoyunlu, B.; Gabarre, C.; Daly, S.; Casey, E.; Syron, E. Process Modelling for Industrial Scale Polyhydroxybutyrate Production Using Fructose, Formic Acid and CO2: Assessing Carbon Sources and Economic Viability. Bioresour. Technol. 2024, 393, 130139. [Google Scholar] [CrossRef] [PubMed]
- Fayaazuddin, T.; Prakash, P.; Shakena Fathima, T.; Dhanasekaran, D. Commercial Astaxanthin Production from Green Alga Haematococcus pluvialis; Springer: Singapore, 2023; ISBN 9789811950414. [Google Scholar]
- Hangri, S.; Derbal, K.; Policastro, G.; Panico, A.; Contestabile, P.; Pontoni, L.; Race, M.; Fabbricino, M. Combining Pretreatments and Co-Fermentation as Successful Approach to Improve Biohydrogen Production from Dairy Cow Manure. Environ. Res. 2024, 246, 118118. [Google Scholar] [CrossRef] [PubMed]

| Optimized Output | ||||
|---|---|---|---|---|
| Hydrogen | PHB | Proteins | Biomass Rate | |
| Day | 9 | 7 | 8 | 3 |
| Hydrogen volume (L/m3) | 288 ± 34 | 190 ± 24 | 233 ± 30 | 191 ± 25 |
| Energy H2 (kWh/m3) | 1.03 ± 0.12 | 0.68 ± 0.30 | 0.83 ± 0.40 | 0.68 ± 0.30 |
| Hydrogen rate (L/m3 d) | 32.07 ± 3.78 | 27.28 ± 3.49 | 29.15 ± 3.47 | 63.63 ± 8.30 |
| Energy rate (kWh/m3 d) | 0.11 ± 0.12 | 0.10 ± 0.08 | 0.10 ± 0.10 | 0.23 ± 0.10 |
| PHB (kg/m3) | 0.14 ± 0.04 | 0.26 ± 0.02 | 0.14 ± 0.02 | 0.13 ± 0.06 |
| PHB (%) | 0.09 ± 0.04 | 0.25 ± 0.02 | 0.14 ± 0.02 | 0.21 ± 0.02 |
| PHB rate (kg/m3 d) | 0.015 ± 0.004 | 0.036 ± 0.004 | 0.018 ± 0.004 | 0.045 ± 0.006 |
| Proteins (kg/m3) | 0.338 ± 0.010 | 0.280 ± 0.030 | 0.350 ± 0.034 | 0.147 ± 0.020 |
| Proteins (%) | 0.327 ± 0.012 | 0.270 ± 0.030 | 0.343 ± 0.034 | 0.230 ± 0.020 |
| Proteins rate (kg/m3 d) | 0.038 ± 0.002 | 0.040 ± 0.004 | 0.044 ± 0.004 | 0.049 ± 0.006 |
| Revenue_Energy ($/m3; $/m3 d) | 0.174 ± 0.020; 0.019 ± 0.002 | 0.115 ± 0.014; 0.016 ± 0.002 | 0.141 ± 0.018; 0.018 ± 0.002 | 0.115 ± 0.014; 0.038 ± 0.004 |
| Revenue_PHB ($/m3; $/m3 d) | 0.550 ± 0.178; 0.061 ± 0.020 | 1.022 ± 0.111; 0.146 ± 0.016 | 0.563 ± 0.103; 0.070 ± 0.012 | 0.540 ± 0.103; 0.180 ± 0.003 |
| Revenue_Proteins ($/m3; $/m3 d) | 2.63 ± 0.07; 0.292 ± 0.010 | 2.174 ± 0.18; 0.311 ± 0.034 | 2.719 ± 0.20; 0.340 ± 0.032 | 1.141 ± 0.20; 0.380 ± 0.060 |
| Total revenue ($/m3; $/m3 d) | 3.355 ± 0.266; 0.373 ± 0.029 | 3.31 ± 0.307; 0.473 ± 0.043 | 3.423 ± 0.322; 0.428 ± 0.040 | 1.796 ± 0.310; 0.599 ± 0.103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Policastro, G.; Cesaro, A.; Fabbricino, M. Valorization of Purple Phototrophic Bacteria Biomass Resulting from Photo Fermentation Aimed at Biohydrogen Production. Molecules 2024, 29, 1679. https://doi.org/10.3390/molecules29071679
Policastro G, Cesaro A, Fabbricino M. Valorization of Purple Phototrophic Bacteria Biomass Resulting from Photo Fermentation Aimed at Biohydrogen Production. Molecules. 2024; 29(7):1679. https://doi.org/10.3390/molecules29071679
Chicago/Turabian StylePolicastro, Grazia, Alessandra Cesaro, and Massimiliano Fabbricino. 2024. "Valorization of Purple Phototrophic Bacteria Biomass Resulting from Photo Fermentation Aimed at Biohydrogen Production" Molecules 29, no. 7: 1679. https://doi.org/10.3390/molecules29071679
APA StylePolicastro, G., Cesaro, A., & Fabbricino, M. (2024). Valorization of Purple Phototrophic Bacteria Biomass Resulting from Photo Fermentation Aimed at Biohydrogen Production. Molecules, 29(7), 1679. https://doi.org/10.3390/molecules29071679









