Enhancement of Astaxanthin Bioaccessibility by Encapsulation in Liposomes: An In Vitro Study
Abstract
:1. Introduction
2. Results and Discussion
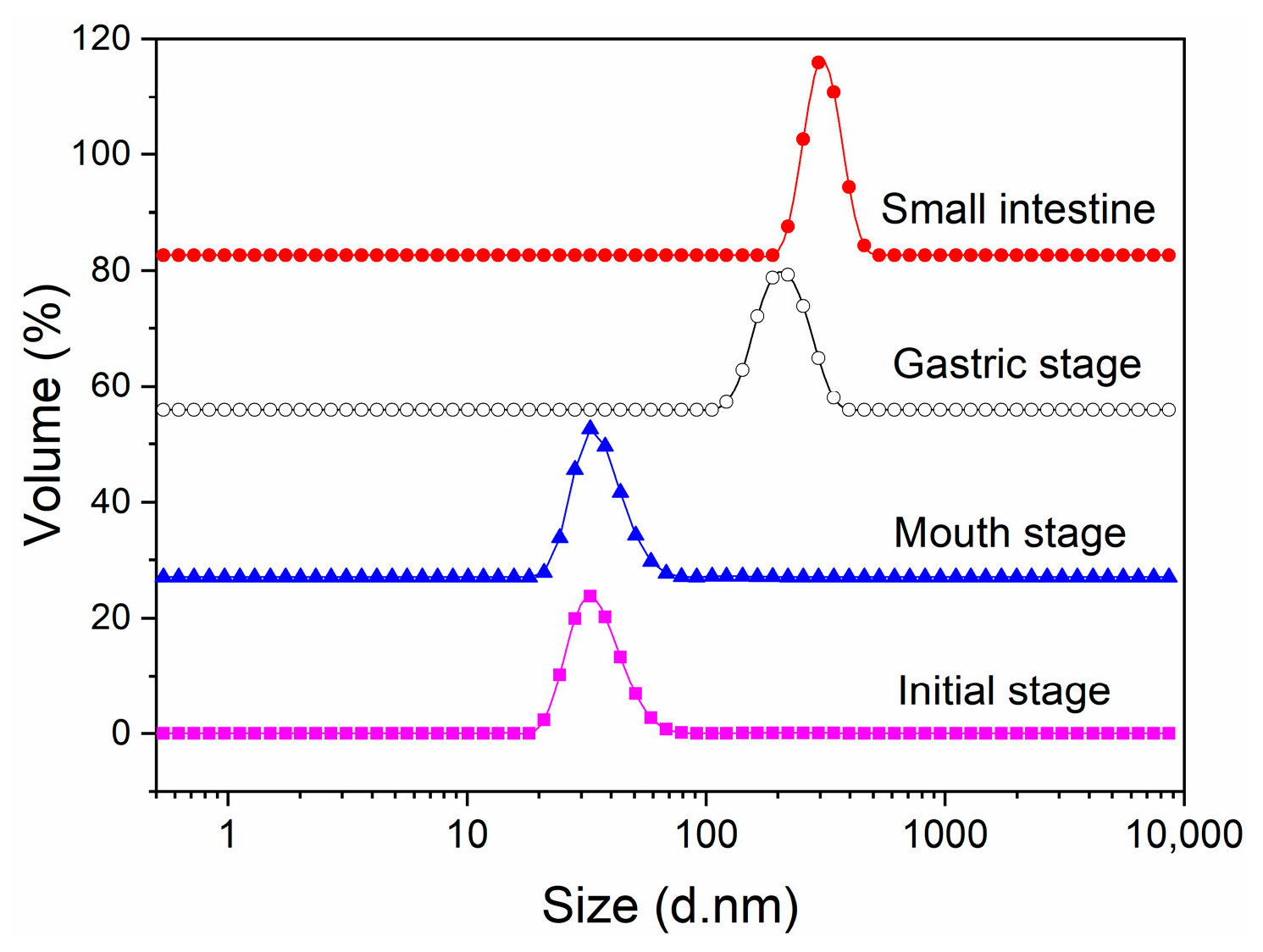
2.1. Changes in Particle Size and Size Distribution during In Vitro Digestion
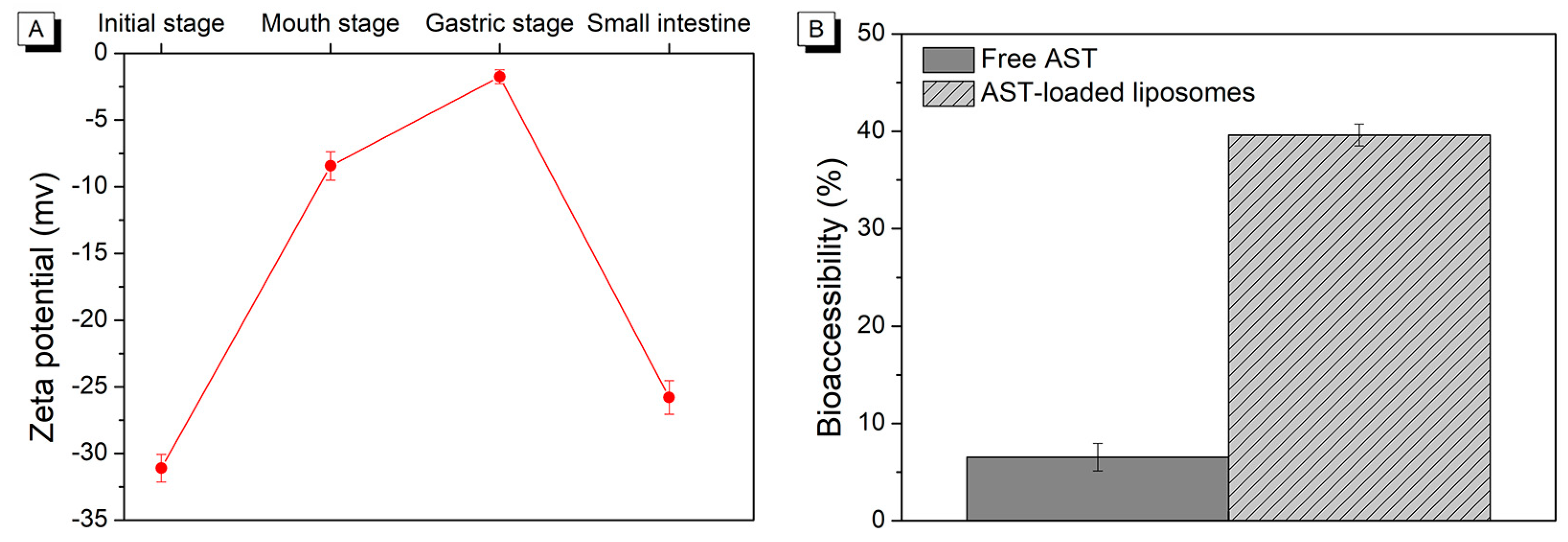
2.2. Changes in Zeta Potential during In Vitro Digestion
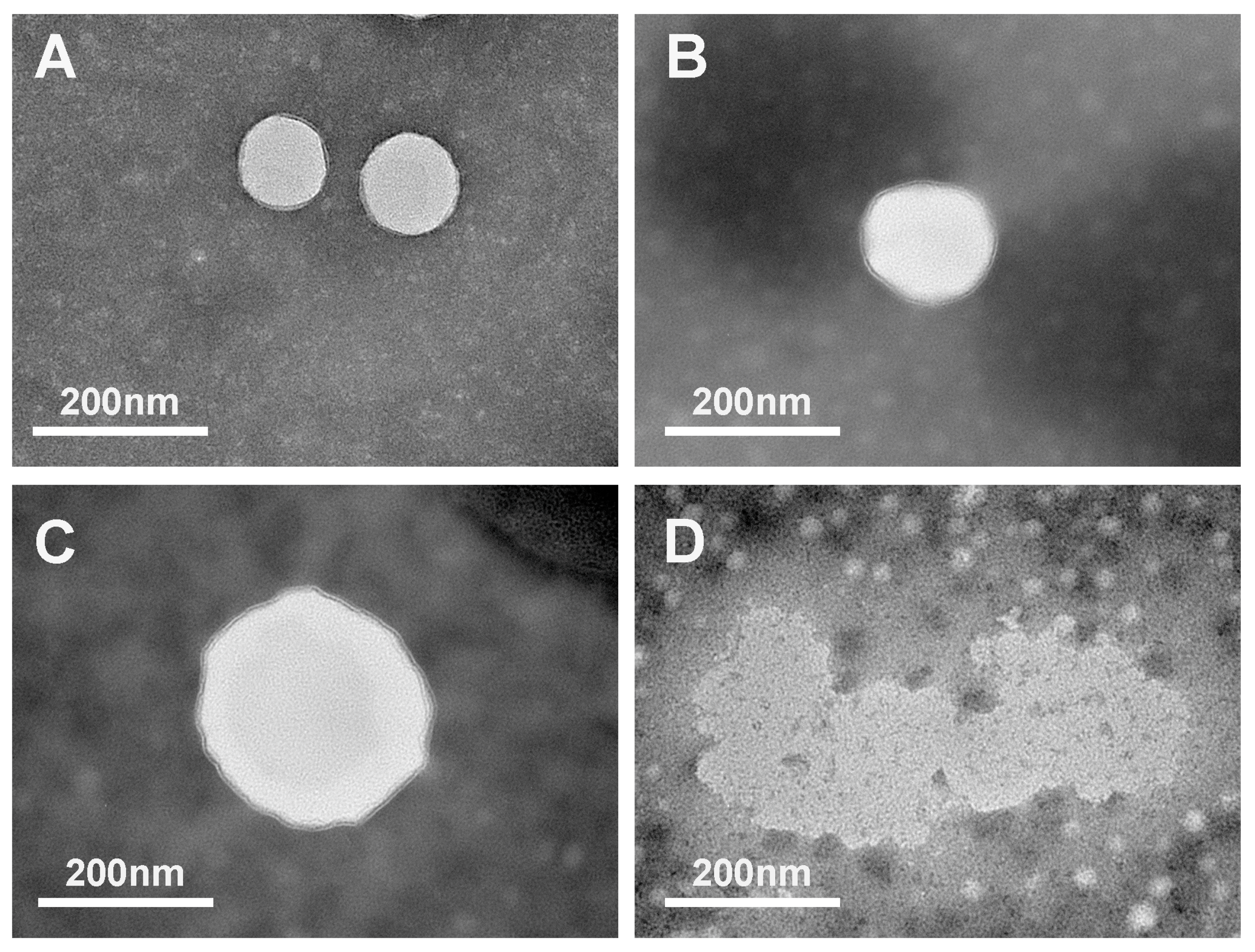
2.3. Changes in Microstructure during In Vitro Digestion
2.4. The Bioaccessibility of Astaxanthin
2.5. Changes in Color Characteristics during In Vitro Digestion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Astaxanthin-Loaded Liposomes
3.3. Simulated Gastrointestinal Digestion
3.4. Particle Size, Size Distribution, and Zeta Potential Measurements
3.5. Microstructure Study
3.6. Bioaccessibility Measurement
3.7. Color Measurement
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: Advances and outlook. Bioresour. Technol. 2021, 340, 125736. [Google Scholar] [CrossRef]
- Dethlefsen, M.W.; Hjermitslev, N.H.; Frosch, S.; Nielsen, M.E. Effect of storage on oxidative quality and stability of extruded astaxanthin-coated fish feed pellets. Anim. Feed Sci. Technol. 2016, 221, 157–166. [Google Scholar] [CrossRef]
- Martínez-Delgado, A.A.; Khandual, S.; Villanueva-Rodríguez, S.J. Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, H.; Li, X.; Secundo, F.; Mao, X. Preparation and characterization of phosphatidyl-agar oligosaccharide liposomes for astaxanthin encapsulation. Food Chem. 2023, 404, 134601. [Google Scholar] [CrossRef]
- Pu, J.; Bankston, J.D.; Sathivel, S. Developing microencapsulated flaxseed oil containing shrimp (Litopenaeus setiferus) astaxanthin using a pilot scale spray dryer. Biosyst. Eng. 2011, 108, 121–132. [Google Scholar] [CrossRef]
- Chen, X.; Chen, R.; Guo, Z.; Li, C.; Li, P. The preparation and stability of the inclusion complex of astaxanthin with β-cyclodextrin. Food Chem. 2007, 101, 1580–1584. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Z.; Xue, C. Recent research advances in astaxanthin delivery systems: Fabrication technologies, comparisons and applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 3497–3518. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Chu, L.L.; Xia, Q. Liposome-chitosan hydrogel bead delivery system for the encapsulation of linseed oil and quercetin: Preparation and in vitro characterization studies. LWT-Food Sci. Technol. 2020, 117, 108615. [Google Scholar] [CrossRef]
- Wang, M.; Li, Q.; Li, S.; Zhao, Y.; Jiang, X.; He, S.; Liu, J. Influence of Auricularia cornea Polysaccharide Coating on the Stability and Antioxidant Activity of Liposomes Ginsenoside Rh2. Foods 2023, 12, 3946. [Google Scholar] [CrossRef]
- Fan, W.; Jiang, X.; Li, Q.; Wang, J.; Lv, M.; Liu, J. Preparation of Phosphorylated Auricularia cornea var. Li. Polysaccharide Liposome Gel and Analysis of Its In Vitro Antioxidant Activity. Foods 2024, 13, 335. [Google Scholar] [CrossRef]
- Pallagi, E.; Jójárt-Laczkovich, O.; Németh, Z.; Szabó-Révész, P.; Csóka, I. Application of the QbD-based approach in the early development of liposomes for nasal administration. Int. J. Pharm. 2019, 562, 11–22. [Google Scholar] [CrossRef]
- Dos Reis, S.B.; de Oliveira Silva, J.; Garcia-Fossa, F.; Leite, E.A.; Malachias, A.; Pound-Lana, G.; Mosqueira, V.C.F.; Oliveira, M.C.; de Barros, A.L.B.; de Jesus, M.B.; et al. Desenvolvimento de carreador lipídico nanoestruturado contendo pool de siRNAs associados a agente antitumoral como uma abordagem terapêutica multifuncional para o câncer de pele. Biomed. Pharmacother. 2021, 134, 1–10. [Google Scholar]
- Pan, L.; Zhang, S.; Gu, K.; Zhang, N. Preparation of astaxanthin-loaded liposomes: Characterization, storage stability and antioxidant activity. CyTA-J. Food. 2018, 16, 607–618. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as vehicles for astaxanthin: Characterization, in vitro release evaluation and structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef]
- Lu, J.; Tu, P.; Feng, Y.; Li, N.; Xu, X.; Li, K.; Yao, Y.; Han, J.; Liu, W. Dietary interference on the oxidation and hydrolysis of liposomes during in vitro digestion. Int. J. Food Sci. Technol. 2020, 55, 729–741. [Google Scholar] [CrossRef]
- Li, Z.; Paulson, A.T.; Gill, T.A. Encapsulation of bioactive salmon protein hydrolysates with chitosan-coated liposomes. J. Funct. Food 2019, 19, 733–743. [Google Scholar] [CrossRef]
- Romero-Pérez, A.; García-García, E.; Zavaleta-Mancera, A.; Ramírez-Bribiesca, J.E.; Revilla-Vázquez, A.; Hernández-Calva, L.M.; López-Arellano, R.; Cruz-Monterrosa, R.G. Designing and evaluation of sodium selenite nanoparticles in vitro to improve selenium absorption in ruminants. Vet. Res. Commun. 2010, 34, 71–79. [Google Scholar] [CrossRef]
- Tian, M.; Han, J.; Ye, A.; Liu, W.; Xu, X.; Yao, Y.; Li, K.; Kong, Y.; Wei, F.; Zhou, W. Structural characterization and biological fate of lactoferrin-loaded liposomes during simulated infant digestion. J. Sci. Food Agric. 2019, 99, 2677–2684. [Google Scholar] [CrossRef]
- Mohan, A.; McClements, D.J.; Udenigwe, C.C. Encapsulation of bioactive whey peptides in soy lecithin-derived nanoliposomes: Influence of peptide molecular weight. Food Chem. 2016, 213, 143–148. [Google Scholar] [CrossRef]
- da Silva Malheiros, P.; Sant’Anna, V.; Micheletto, Y.M.S.; da Silveira, N.P. Brandelli, ANanovesicle encapsulation of antimicrobial peptide P34: Physicochemical characterization and mode of action on Listeria monocytogenes. J. Nanopart. Res. 2011, 13, 3545–3552. [Google Scholar] [CrossRef]
- Fan, M.; Xu, S.; Xia, S.; Zhang, X. Preparation of salidroside nano-liposomes by ethanol injection method and in vitro release study. Eur. Food Res. Technol. 2008, 227, 167–174. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion-and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. 2007, 51, 107–115. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Xia, S. Modulation of the carotenoid bioaccessibility through liposomal encapsulation. Colloid Surf. B-Biointerfaces 2014, 123, 692–700. [Google Scholar] [CrossRef]
- Li, Z.L.; Peng, S.F.; Chen, X.; Zhu, Y.Q.; Zou, L.Q.; Liu, W.; Liu, C.M. Pluronics modified liposomes for curcumin encapsulation: Sustained release, stability and bioaccessibility. Food Res. Int. 2018, 108, 246–253. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Gumus, C.E.; McClements, D.J. Lutein-enriched emulsion-based delivery systems: Influence of pH and temperature on physical and chemical stability. Food Chem. 2016, 196, 821–827. [Google Scholar] [CrossRef]
- Nasrollahzadeh, F.; Varidi, M.; Koocheki, A.; Hadizadeh, F. Effect of microwave and conventional heating on structural, functional and antioxidant properties of bovine serum albumin-maltodextrin conjugates through Maillard reaction. Food Res. Int. 2017, 100, 289–297. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; Mcclements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef]
- Song, N.; Tan, C.; Huang, M.; Liu, P.; Eric, K.; Zhang, X.; Xia, S.; Jia, C. Transglutaminase cross-linking effect on sensory characteristics and antioxidant activities of Maillard reaction products from soybean protein hydrolysates. Food Chem. 2013, 136, 144–151. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, X.; Fan, X.; Li, H.; Xu, B.; Li, X. Whey protein isolate coated liposomes as novel carrier systems for astaxanthin. Eur. J. Lipid Sci. Technol. 2020, 122, 1900325. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Zhu, Y.; Zou, L.; Li, Z.; Liu, W.; Cheng, C.; Gao, H.; Liu, C.; Liu, C. Gastrointestinal fate of fluid and gelled nutraceutical emulsions: Impact on proteolysis, lipolysis, and quercetin bioaccessibility. J. Agric. Food Chem. 2018, 66, 9087–9096. [Google Scholar] [CrossRef]
- Toniazzo, T.; Peres, M.S.; Ramos, A.P.; Pinho, S.C. Encapsulation of quercetin in liposomes by ethanol injection and physicochemical characterization of dispersions and lyophilized vesicles. Food Biosci. 2017, 19, 17–25. [Google Scholar] [CrossRef]
- Lee, M.H.; Jang, H.W.; Chun, Y.G.; Kim, T.E.; Lee, I.Y.; Kim, B.K. Influence of carrier oil on the physical stability and in vitro digestion of vitamin K lipid nanocarriers. Food Biosci. 2021, 43, 101254. [Google Scholar] [CrossRef]
- Tai, K.; Rappolt, M.; He, X.; Wei, Y.; Zhu, S.; Zhang, J.; Mao, L.; Gao, Y.; Yuan, F. Effect of β-sitosterol on the curcumin-loaded liposomes: Vesicle characteristics, physicochemical stability, in vitro release and bioavailability. Food Chem. 2019, 293, 92–102. [Google Scholar] [CrossRef]
- Sukyai, P.; Anongjanya, P.; Bunyahwuthakul, N.; Kongsin, K.; Harnkarnsujarit, N.; Sukatta, U.; Sothornvit, R.; Chollakup, R. Effect of cellulose nanocrystals from sugarcane bagasse on whey protein isolate-based films. Food Res. Int. 2018, 107, 528–535. [Google Scholar] [CrossRef]

| Sample | Digestion Stage | L* | a* | b* | ΔE* |
|---|---|---|---|---|---|
| Free AST | Initial stage | 13.13 a ± 0.03 | 3.13 d ± 0.03 | 5.15 d ± 0.04 | 82.31 d ± 0.03 |
| Mouth stage | 13.83 c ± 0.02 | 2.68 c ± 0.04 | 4.90 c ± 0.01 | 80.00 c ± 0.04 | |
| Gastric stage | 13.70 b ± 0.03 | 1.88 b ± 0.04 | 4.41 b ± 0.04 | 75.36 b ± 0.04 | |
| Small intestine | 15.88 d ± 0.03 | 1.50 a ± 0.03 | 3.97 a ± 0.04 | 74.00 a ± 0.04 | |
| AST-liposomes | Initial stage | 13.22 a ± 0.01 | 3.06 d ± 0.02 | 5.27 d ± 0.04 | 82.04 d ± 0.01 |
| Mouth stage | 13.65 c ± 0.02 | 2.85 c ± 0.03 | 4.57 c ± 0.03 | 81.59 b ± 0.02 | |
| Gastric stage | 13.53 b ± 0.01 | 2.41 b ± 0.03 | 4.03 b ± 0.04 | 81.07 c ± 0.01 | |
| Small intestine | 15.48 d ± 0.01 | 2.11 a ± 0.02 | 3.43 a ± 0.06 | 79.73 a ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Meng, H.; Li, J.; Liu, Z.; Zhang, D.; Liu, Z.; Zhao, Q.; Xu, F. Enhancement of Astaxanthin Bioaccessibility by Encapsulation in Liposomes: An In Vitro Study. Molecules 2024, 29, 1687. https://doi.org/10.3390/molecules29081687
Pan L, Meng H, Li J, Liu Z, Zhang D, Liu Z, Zhao Q, Xu F. Enhancement of Astaxanthin Bioaccessibility by Encapsulation in Liposomes: An In Vitro Study. Molecules. 2024; 29(8):1687. https://doi.org/10.3390/molecules29081687
Chicago/Turabian StylePan, Li, Haojie Meng, Jiaqi Li, Zongjie Liu, Dongsheng Zhang, Zhengyang Liu, Qian Zhao, and Fei Xu. 2024. "Enhancement of Astaxanthin Bioaccessibility by Encapsulation in Liposomes: An In Vitro Study" Molecules 29, no. 8: 1687. https://doi.org/10.3390/molecules29081687
APA StylePan, L., Meng, H., Li, J., Liu, Z., Zhang, D., Liu, Z., Zhao, Q., & Xu, F. (2024). Enhancement of Astaxanthin Bioaccessibility by Encapsulation in Liposomes: An In Vitro Study. Molecules, 29(8), 1687. https://doi.org/10.3390/molecules29081687





