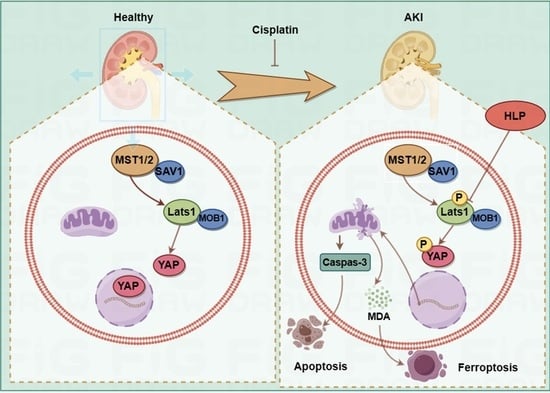
Hazel Leaf Polyphenol Extract Alleviated Cisplatin-Induced Acute Kidney Injury by Reducing Ferroptosis through Inhibiting Hippo Signaling
Abstract
1. Introduction
2. Results
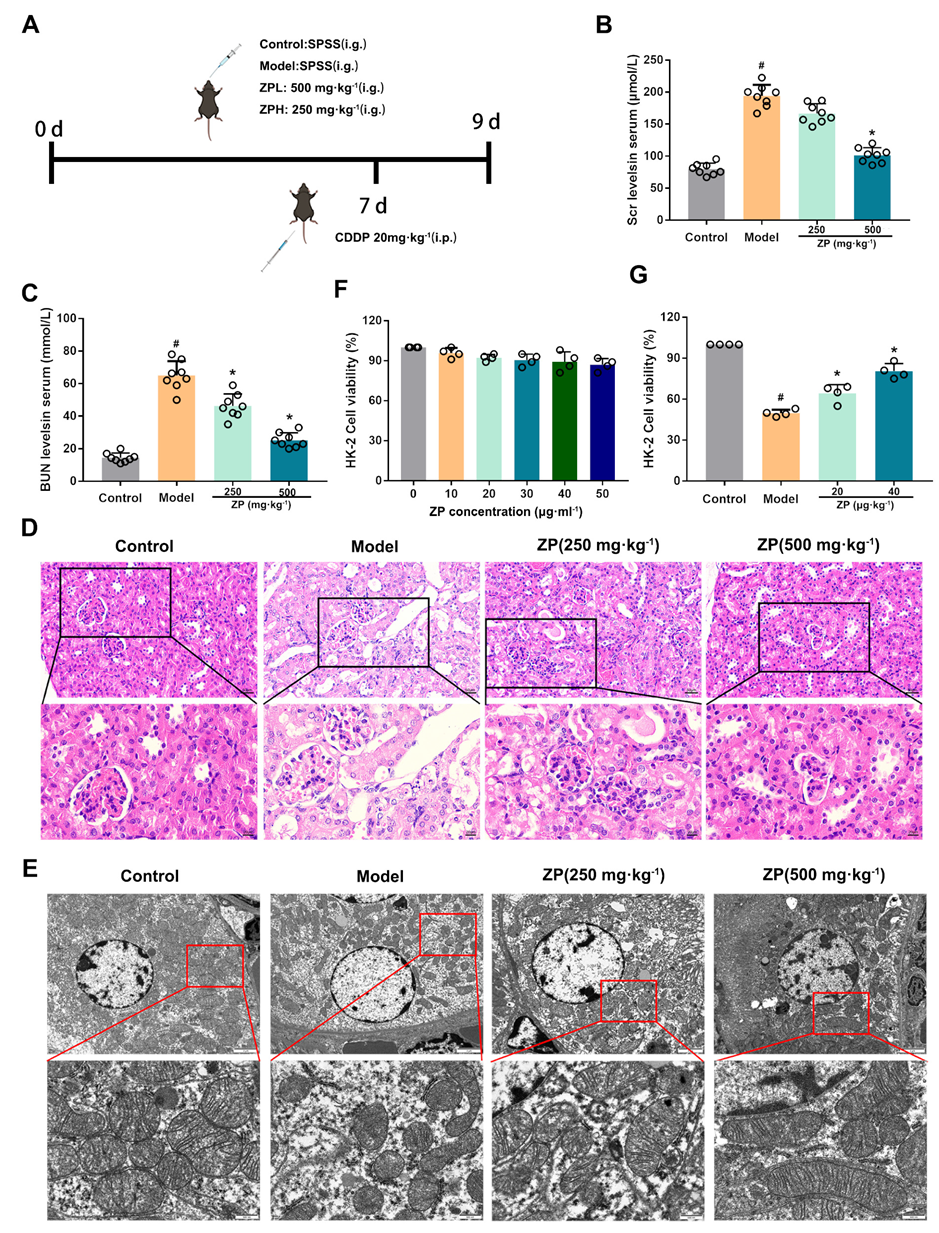
2.1. ZP Relieves Cisplatin-Induced AKI
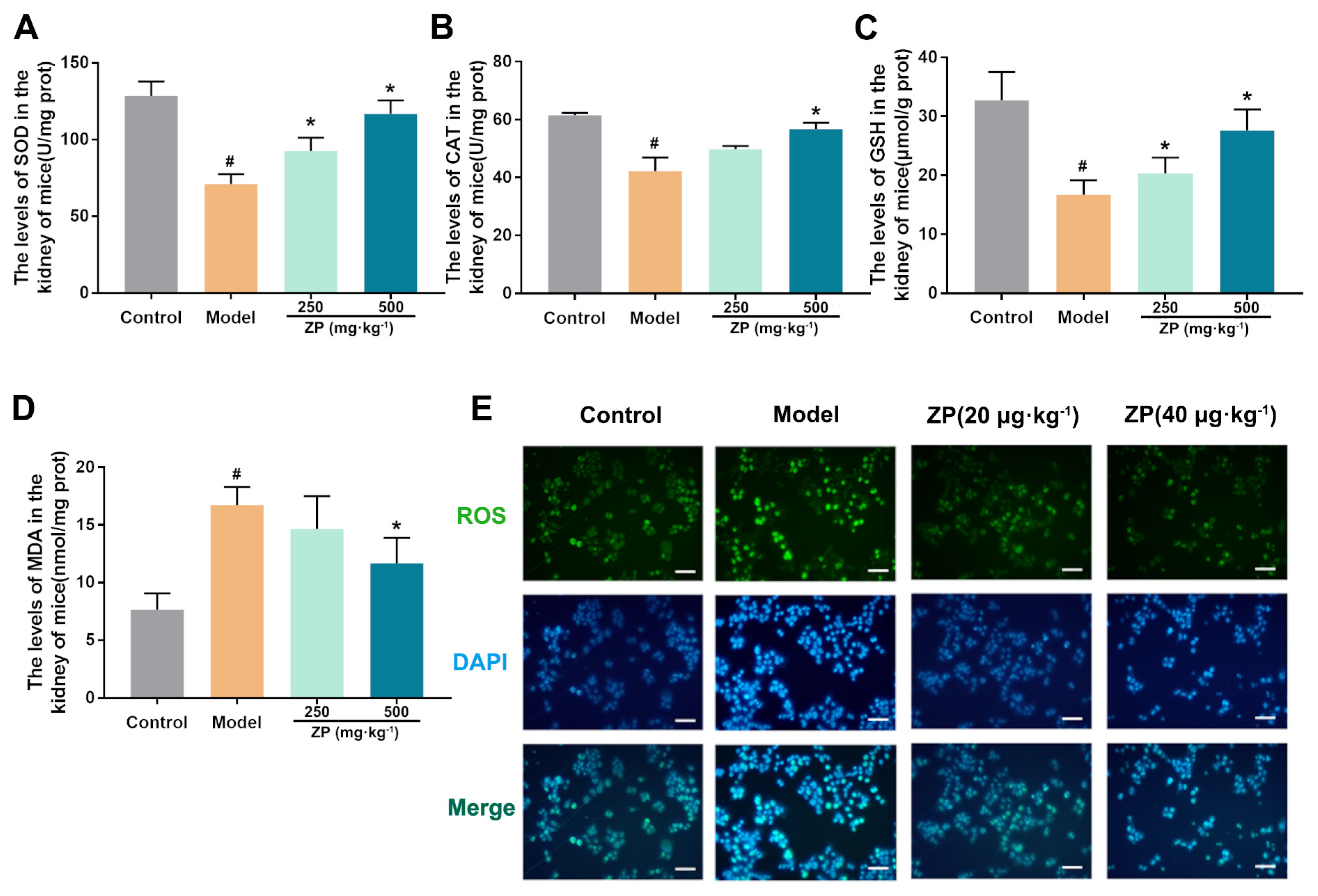
2.2. ZP Ameliorates Oxidative Damage in Mice Kidneys and HK-2 Cells
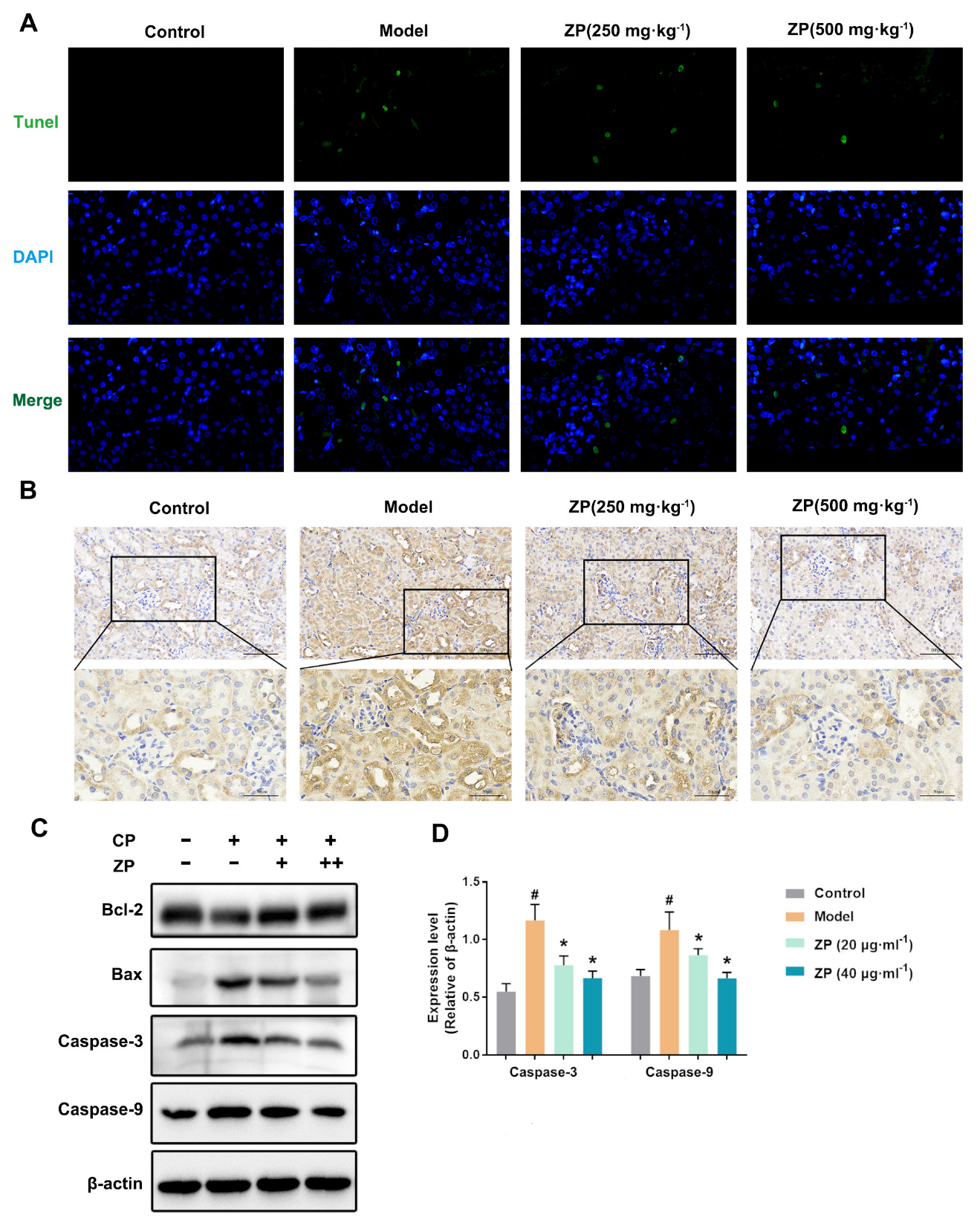
2.3. ZP Reduces Cisplatin-Induced Apoptosis in Mice Kidneys and HK-2 Cells
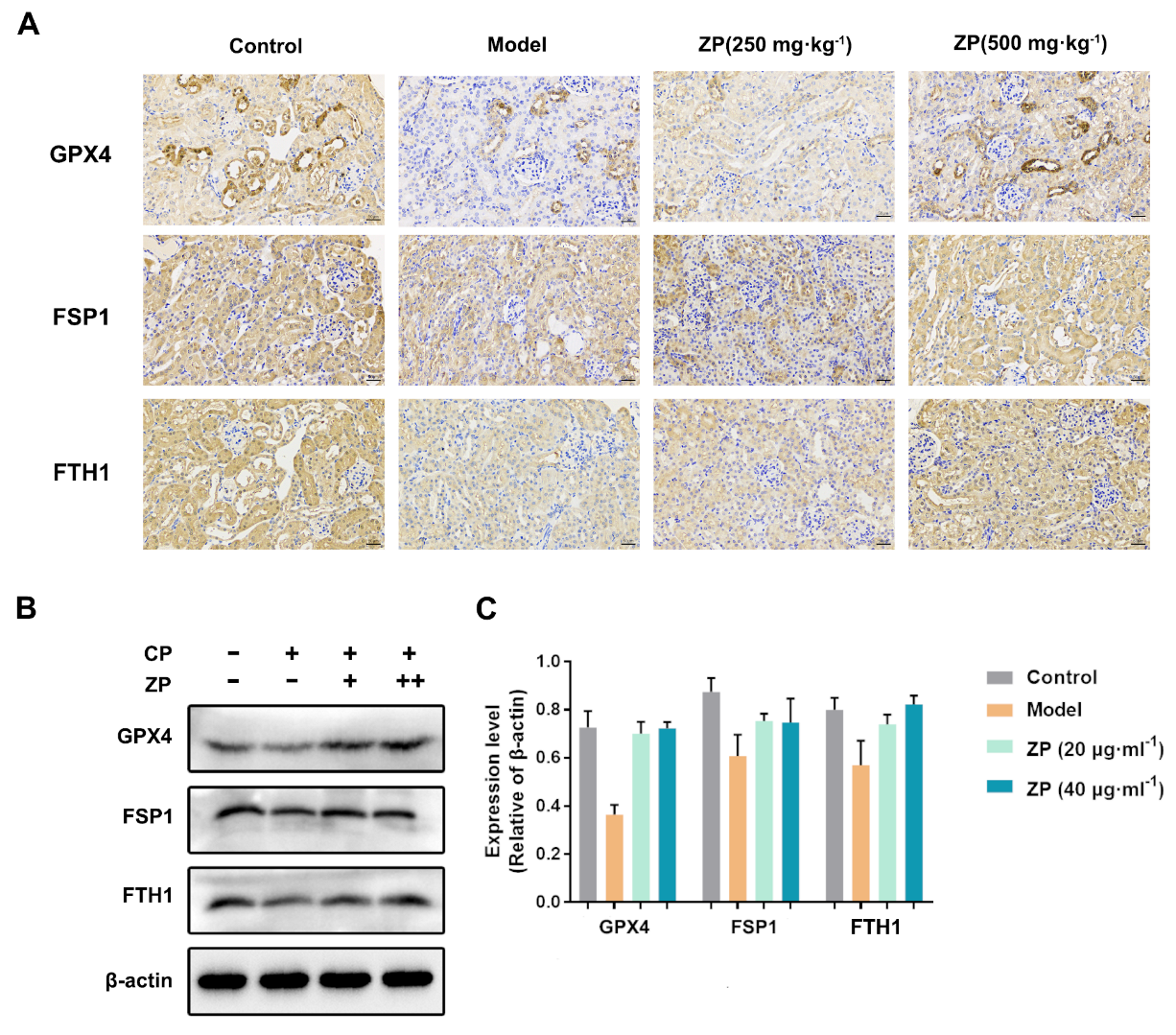
2.4. ZP Improves Cisplatin-Induced Ferroptosis in Mice Kidneys and HK-2 Cells
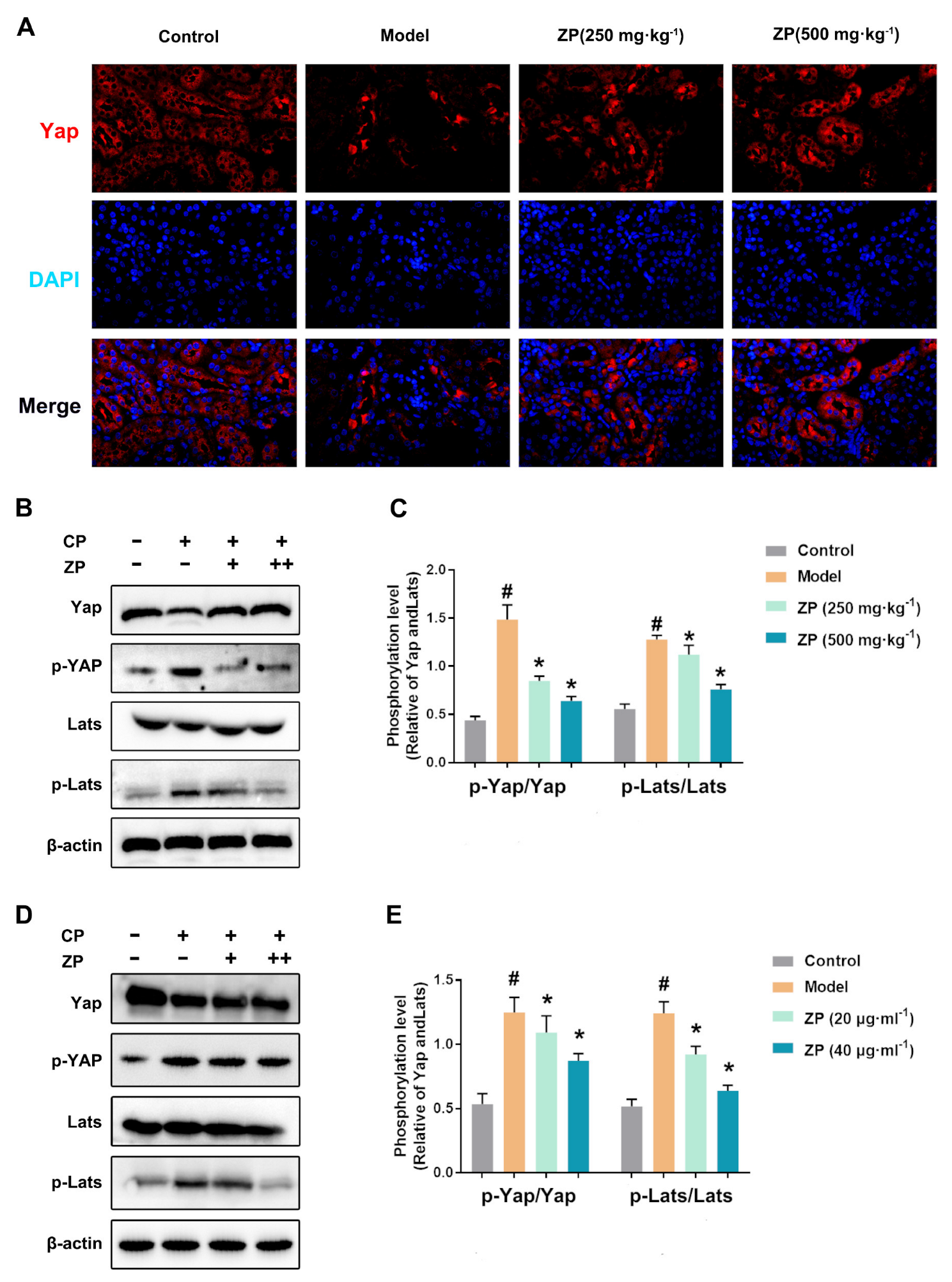
2.5. ZP Activates Hippo Signaling in Cisplatin-Treated Mice Kidneys and HK-2 Cells
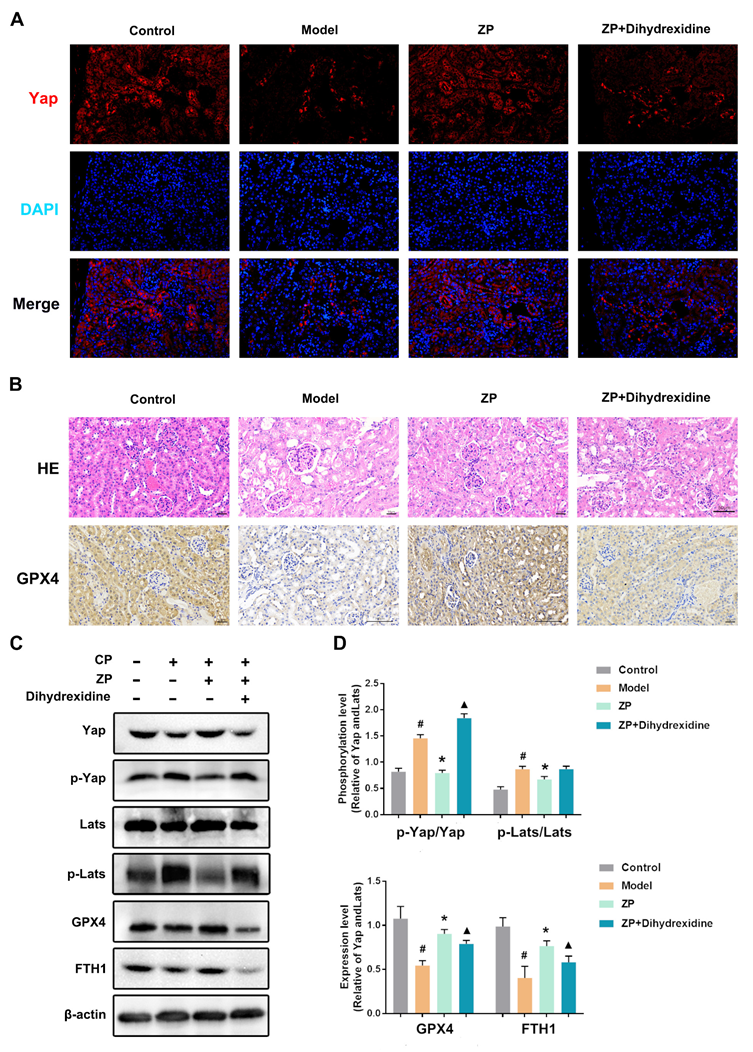
2.6. The Protective Effect of ZP on Mice Kidneys and HK-2 Cells Is Attenuated after YAP Phosphorylation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Preparation
4.3. Preparation of Animals and Samples Collection
4.4. Cell Culture
4.5. Transmission Electron Microscope and Hematoxylin–Eosin Staining (HE)
4.6. Biochemical Reagent Kit
4.7. ROS Detection
4.8. TUNEL Staining
4.9. Cell Viability Assay
4.10. Immunohistochemistry and Immunofluorescence
4.11. Western Blot
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K.D.; Goldstein, S.L.; Vijayan, A.; Parikh, C.R.; Kashani, K.; Okusa, M.D.; Agarwal, A.; Cerdá, J. AKI!Now Initiative: Recommendations for Awareness, Recognition, and Management of AKI. Clin. J. Am. Soc. Nephrol. CJASN 2020, 15, 1838–1847. [Google Scholar] [CrossRef]
- Deng, F.; Zheng, X.; Sharma, I.; Dai, Y.; Wang, Y.; Kanwar, Y.S. Regulated cell death in cisplatin-induced AKI: Relevance of myo-inositol metabolism. Am. J. Physiol. Ren. Physiol. 2021, 320, F578–F595. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.U.; Kim, D.G.; Choi, J.W.; Shin, J.Y.; Kweon, B.; Zhou, Z.; Lee, H.S.; Song, H.J.; Bae, G.S.; Park, S.J. Loganin Attenuates the Severity of Acute Kidney Injury Induced by Cisplatin through the Inhibition of ERK Activation in Mice. Int. J. Mol. Sci. 2021, 22, 1421. [Google Scholar] [CrossRef]
- Pan, M.; Wang, Z.; Wang, Y.; Jiang, X.; Fan, Y.; Gong, F.; Sun, Y.; Wang, D. Celastrol alleviated acute kidney injury by inhibition of ferroptosis through Nrf2/GPX4 pathway. Biomed. Pharmacother. 2023, 166, 115333. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli·, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Deng, F.; Sharma, I.; Dai, Y.; Yang, M.; Kanwar, Y.S. Myo-inositol oxygenase expression profile modulates pathogenic ferroptosis in the renal proximal tubule. J. Clin. Investig. 2019, 129, 5033–5049. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bi, J.; Ren, Y.; Du, Z.; Li, T.; Wang, T.; Zhang, L.; Wang, M.; Wei, S.; Lv, Y.; et al. Involvement of GPX4 in irisin’s protection against ischemia reperfusion-induced acute kidney injury. J. Cell Physiol. 2021, 236, 931–945. [Google Scholar] [CrossRef]
- Chen, C.; Wang, D.; Yu, Y.; Zhao, T.; Min, N.; Wu, Y.; Kang, L.; Zhao, Y.; Du, L.; Zhang, M.; et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021, 12, 65. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Yi, B.; Yang, S.; Liu, J.; Hu, J.; Wang, J.; Cao, K.; Zhang, W. VDR activation attenuate cisplatin induced AKI by inhibiting ferroptosis. Cell Death Dis. 2020, 11, 73. [Google Scholar] [CrossRef]
- Fu, M.; Hu, Y.; Lan, T.; Guan, K.L.; Luo, T.; Luo, M. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct. Target. Ther. 2022, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, P.X.; Wu, J.; Gao, Y.J.; Yin, M.X.; Lin, Y.; Yang, M.; Chen, D.P.; Sun, H.P.; Liu, Z.B.; et al. Involvement of the Hippo pathway in regeneration and fibrogenesis after ischaemic acute kidney injury: YAP is the key effector. Clin. Sci. 2016, 130, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Li, C.; Shao, G.; Li, J.; Xu, K.; Zhao, Z.; Zhang, Z.; Liu, J.; Wu, H. Hippo-YAP/MCP-1 mediated tubular maladaptive repair promote inflammation in renal failed recovery after ischemic AKI. Cell Death Dis. 2021, 12, 754. [Google Scholar] [CrossRef]
- Chen, J.; You, H.; Li, Y.; Xu, Y.; He, Q.; Harris, R.C. EGF Receptor-Dependent YAP Activation Is Important for Renal Recovery from AKI. J. Am. Soc. Nephrol. JASN 2018, 29, 2372–2385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qiu, S.; Wang, H.; Cui, J.; Tian, X.; Miao, Y.; Zhang, C.; Cao, L.; Ma, L.; Xu, X.; et al. Transcriptional Repression of Ferritin Light Chain Increases Ferroptosis Sensitivity in Lung Adenocarcinoma. Front. Cell Dev. Biol. 2021, 9, 719187. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Minikes, A.M.; Gao, M.; Bian, H.; Li, Y.; Stockwell, B.R.; Chen, Z.N.; Jiang, X. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature 2019, 572, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Calamelli, E.; Trozzo, A.; Di Blasi, E.; Serra, L.; Bottau, P. Hazelnut Allergy. Medicina 2021, 57, 67. [Google Scholar] [CrossRef]
- Alberti, Á.; Riethmüller, E.; Béni, S.; Kéry, Á. Evaluation of Radical Scavenging Activity of Sempervivum tectorum and Corylus avellana Extracts with Different Phenolic Composition. Nat. Prod. Commun. 2016, 11, 469–474. [Google Scholar] [PubMed]
- Uchikura, T.; Kitano, T.; Yoshimura, M.; Amakura, Y. Characterization of phenolic constituents in hazelnut kernels. Biosci. Biotechnol. Biochem. 2023, 87, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Karamać, M.; Amarowicz, R.; Shahidi, F. Antioxidant and antiradical activities in extracts of hazelnut kernel (Corylus avellana L.) and hazelnut green leafy cover. J. Agric. Food Chem. 2006, 54, 4826–4832. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Wang, Y.; Lv, G.; Lin, H.; Lin, Z. UPLC-MS/MS profiling, antioxidant and anti-inflammatory activities, and potential health benefits prediction of phenolic compounds in hazel leaf. Front. Nutr. 2023, 10, 1092071. [Google Scholar] [CrossRef]
- Motwani, S.S.; Kaur, S.S.; Kitchlu, A. Cisplatin Nephrotoxicity: Novel Insights Into Mechanisms and Preventative Strategies. Semin. Nephrol. 2022, 42, 151341. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, H.; Che, Y.; Jiang, X. Rasfonin promotes autophagy and apoptosis via upregulation of reactive oxygen species (ROS)/JNK pathway. Mycology 2016, 7, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.J.T.; Vlajkovic, S.M. Molecular Characteristics of Cisplatin-Induced Ototoxicity and Therapeutic Interventions. Int. J. Mol. Sci. 2023, 24, 16545. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; De Leon, M.; Devarajan, P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. J. Am. Soc. Nephrol. JASN 2002, 13, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Rendra, E.; Uhlig, S.; Moskal, I.; Thielemann, C.; Klüter, H.; Bieback, K. Adipose Stromal Cell-Derived Secretome Attenuates Cisplatin-Induced Injury In Vitro Surpassing the Intricate Interplay between Proximal Tubular Epithelial Cells and Macrophages. Cells 2024, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Lu, P.; Mo, X.; Yang, B.; Chen, T.; Yao, Y.; Xiong, T.; Yue, L.; Yang, X. Ferroptosis and metabolic syndrome and complications: Association, mechanism, and translational applications. Front. Endocrinol. 2023, 14, 1248934. [Google Scholar] [CrossRef]
- Hao, S.H.; Ma, X.D.; Xu, L.; Xie, J.D.; Feng, Z.H.; Chen, J.W.; Chen, R.X.; Wang, F.W.; Tang, Y.H.; Xie, D.; et al. Dual specific phosphatase 4 suppresses ferroptosis and enhances sorafenib resistance in hepatocellular carcinoma. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2024, 73, 101052. [Google Scholar] [CrossRef]
- Han, L.; Wang, Z.; Wang, D.; Gao, Z.; Hu, S.; Shi, D.; Shu, Y. Mechanisms and otoprotective strategies of programmed cell death on aminoglycoside-induced ototoxicity. Front. Cell Dev. Biol. 2023, 11, 1305433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, X.; Zheng, X.; Liang, X.; Wang, Z.; Zhang, J.; Zhao, X.; Zhuang, S.; Pan, Q.; Sun, F.; et al. Iron deficiency exacerbates cisplatin- or rhabdomyolysis-induced acute kidney injury through promoting iron-catalyzed oxidative damage. Free Radic. Biol. Med. 2021, 173, 81–96. [Google Scholar] [CrossRef]
- Zhang, K.; Long, M.; Dong, W.; Li, J.; Wang, X.; Liu, W.; Huang, Q.; Ping, Y.; Zou, H.; Song, R.; et al. Cadmium Induces Kidney Iron Deficiency and Chronic Kidney Injury by Interfering with the Iron Metabolism in Rats. Int. J. Mol. Sci. 2024, 25, 763. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Skalny, A.V.; Martins, A.C.; Sinitskii, A.I.; Farina, M.; Lu, R.; Barbosa, F., Jr.; Gluhcheva, Y.G.; Santamaria, A.; Tinkov, A.A. Ferroptosis as a mechanism of non-ferrous metal toxicity. Arch. Toxicol. 2022, 96, 2391–2417. [Google Scholar] [CrossRef]
- Cozza, G.; Rossetto, M.; Bosello-Travain, V.; Maiorino, M.; Roveri, A.; Toppo, S.; Zaccarin, M.; Zennaro, L.; Ursini, F. Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: The polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free Radic. Biol. Med. 2017, 112, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Hendricks, J.M.; Li, Z.; Magtanong, L.; Ford, B.; Tang, P.H.; Roberts, M.A.; Tong, B.; Maimone, T.J.; Zoncu, R.; et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 2019, 575, 688–692. [Google Scholar] [CrossRef]
- Yu, S.; Jing, L.; Yin, X.R.; Wang, M.C.; Chen, Y.M.; Guo, Y.; Nan, K.J.; Han, L.L. MiR-195 suppresses the metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by inhibiting YAP. Oncotarget 2017, 8, 99757–99771. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Chiara, L.; Conte, C.; Antonelli, G.; Lazzeri, E. Tubular Cell Cycle Response upon AKI: Revising Old and New Paradigms to Identify Novel Targets for CKD Prevention. Int. J. Mol. Sci. 2021, 22, 11093. [Google Scholar] [CrossRef]
- Xiang, J.; Jiang, M.; Du, X. The role of Hippo pathway in ferroptosis. Front. Oncol. 2022, 12, 1107505. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Tian, W.; Ge, C.; Su, Y.; Li, J.; Tian, H. AKR1C3 suppresses ferroptosis in hepatocellular carcinoma through regulation of YAP/SLC7A11 signaling pathway. Mol. Carcinog. 2023, 62, 833–844. [Google Scholar] [CrossRef]
- Shi, J.; Yu, T.; Song, K.; Du, S.; He, S.; Hu, X.; Li, X.; Li, H.; Dong, S.; Zhang, Y.; et al. Dexmedetomidine ameliorates endotoxin-induced acute lung injury in vivo and in vitro by preserving mitochondrial dynamic equilibrium through the HIF-1a/HO-1 signaling pathway. Redox Biol. 2021, 41, 101954. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhuang, X.; Lv, G.; Lin, Z.; Huang, X.; Zhao, J.; Lin, H.; Wang, Y. Ginsenoside CK Inhibits TGF-β-Induced Epithelial-Mesenchymal Transition in A549 Cell via SIRT1. Biomed. Res. Int. 2021, 2021, 9140191. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Retention Time/min | Concentration of Standard/μg/mL | Quantity Contained/mg/g |
|---|---|---|---|
| Kaempferol | 33.688 | 380 | 221.995 |
| Chlorogenic acid | 12.067 | 200 | 8.228 |
| Myricetin | 39.546 | 60 | 3.956 |
| Ellagic acid | 37.246 | 40 | 0.564 |
| Luteolin | 49.856 | 50 | 0.256 |
| Resveratrol | 35.988 | 200 | 0.214 |
| Caffeic acid | 16.262 | 20 | 0.111 |
| Gallic acid | 3.063 | 40 | 0.103 |
| p-Coumaric acid | 25.118 | 35.8 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Chang, H.; Jiang, F.; Zhang, W.; Yang, Q.; Wang, X.; Lv, G.; Lin, H.; Luo, H.; Lin, Z.; et al. Hazel Leaf Polyphenol Extract Alleviated Cisplatin-Induced Acute Kidney Injury by Reducing Ferroptosis through Inhibiting Hippo Signaling. Molecules 2024, 29, 1729. https://doi.org/10.3390/molecules29081729
Sun M, Chang H, Jiang F, Zhang W, Yang Q, Wang X, Lv G, Lin H, Luo H, Lin Z, et al. Hazel Leaf Polyphenol Extract Alleviated Cisplatin-Induced Acute Kidney Injury by Reducing Ferroptosis through Inhibiting Hippo Signaling. Molecules. 2024; 29(8):1729. https://doi.org/10.3390/molecules29081729
Chicago/Turabian StyleSun, Mingyang, He Chang, Fangyang Jiang, Wenjing Zhang, Qingxuan Yang, Xinhe Wang, Guangfu Lv, He Lin, Haoming Luo, Zhe Lin, and et al. 2024. "Hazel Leaf Polyphenol Extract Alleviated Cisplatin-Induced Acute Kidney Injury by Reducing Ferroptosis through Inhibiting Hippo Signaling" Molecules 29, no. 8: 1729. https://doi.org/10.3390/molecules29081729
APA StyleSun, M., Chang, H., Jiang, F., Zhang, W., Yang, Q., Wang, X., Lv, G., Lin, H., Luo, H., Lin, Z., & Wang, Y. (2024). Hazel Leaf Polyphenol Extract Alleviated Cisplatin-Induced Acute Kidney Injury by Reducing Ferroptosis through Inhibiting Hippo Signaling. Molecules, 29(8), 1729. https://doi.org/10.3390/molecules29081729