The Fast Quantification of Vitamin B12 in Milk Powder by High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
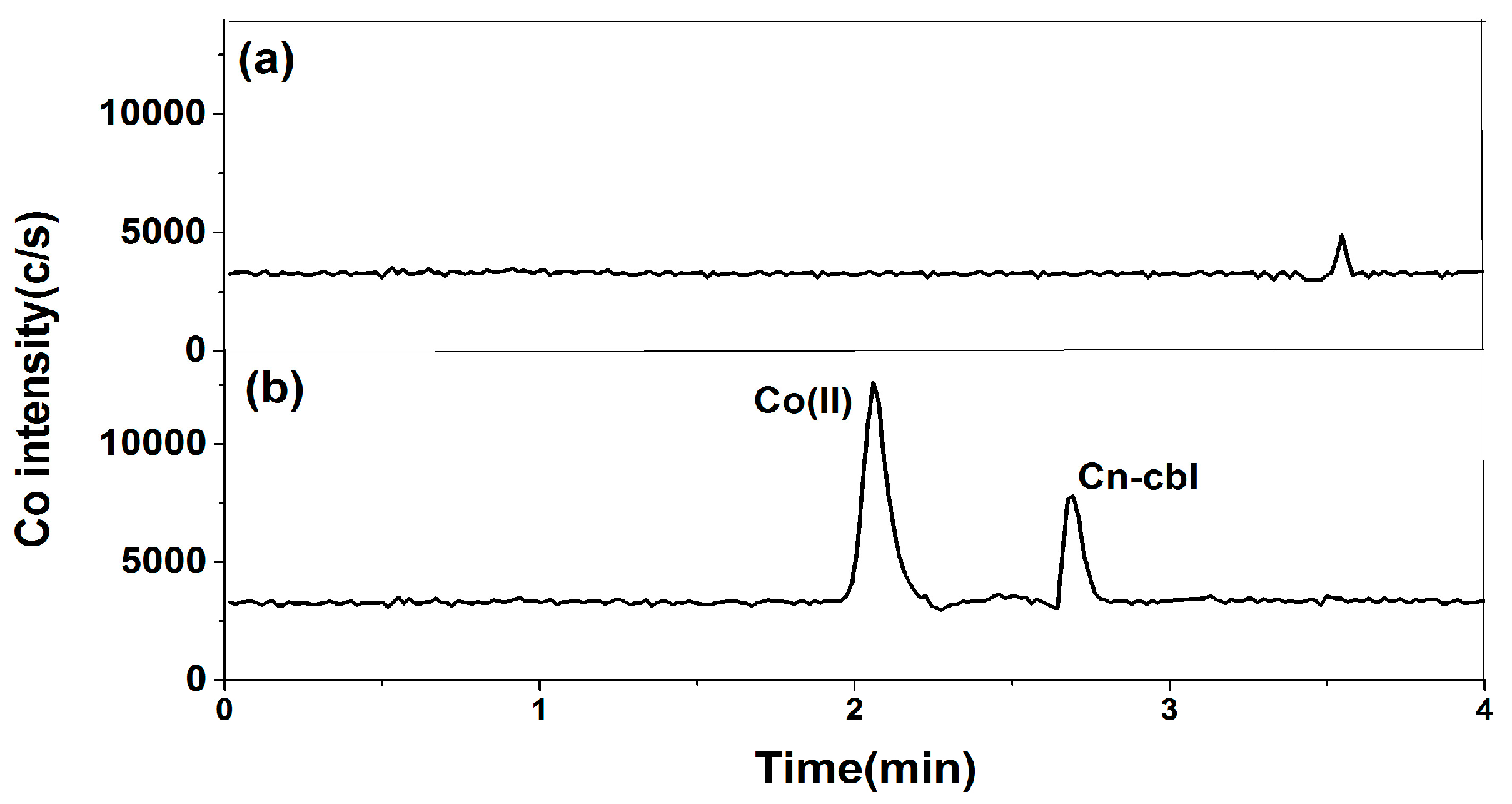
2.1. Optimization of Chromatographic Conditions
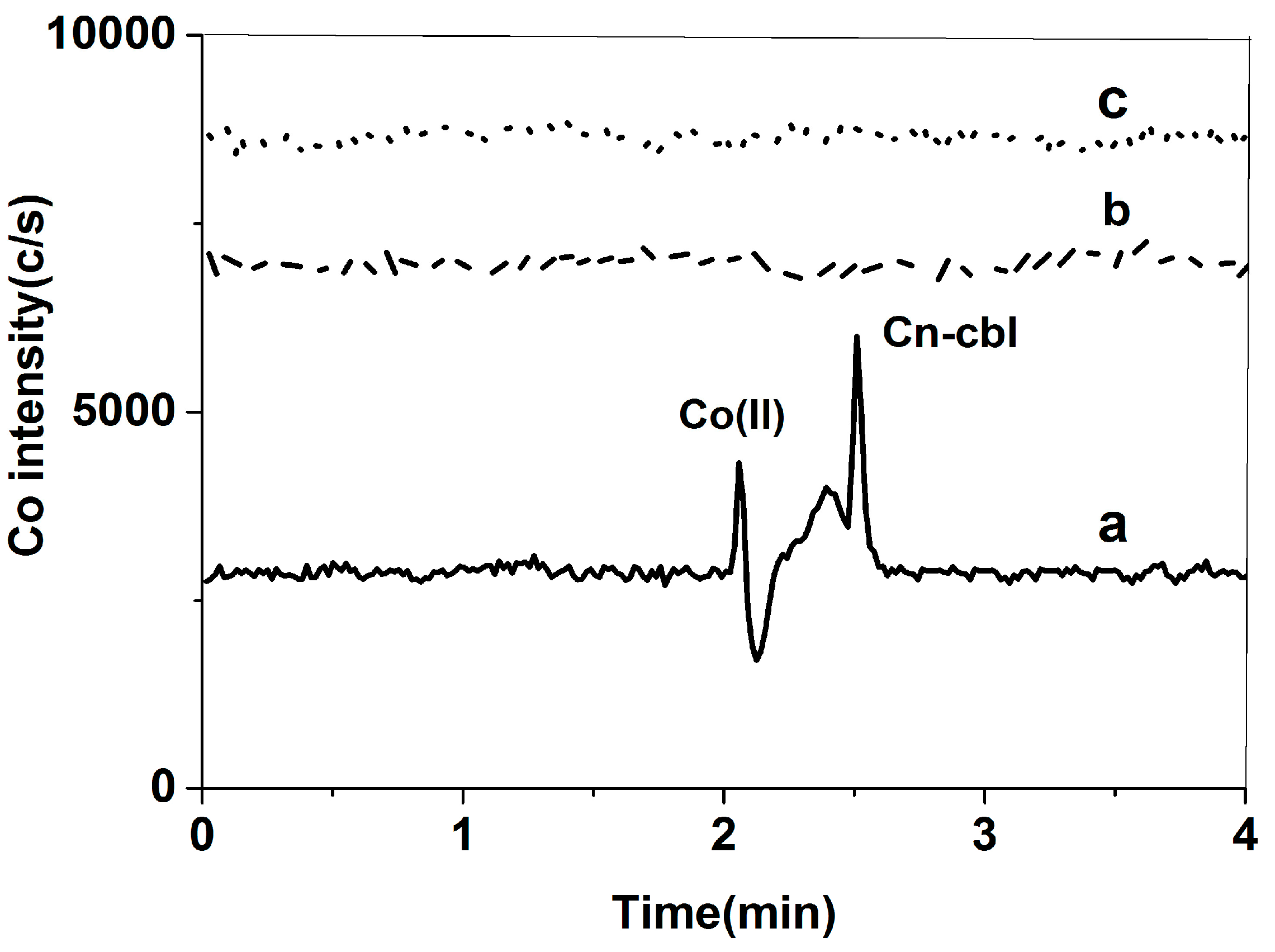
2.1.1. Selection of Chromatographic Column
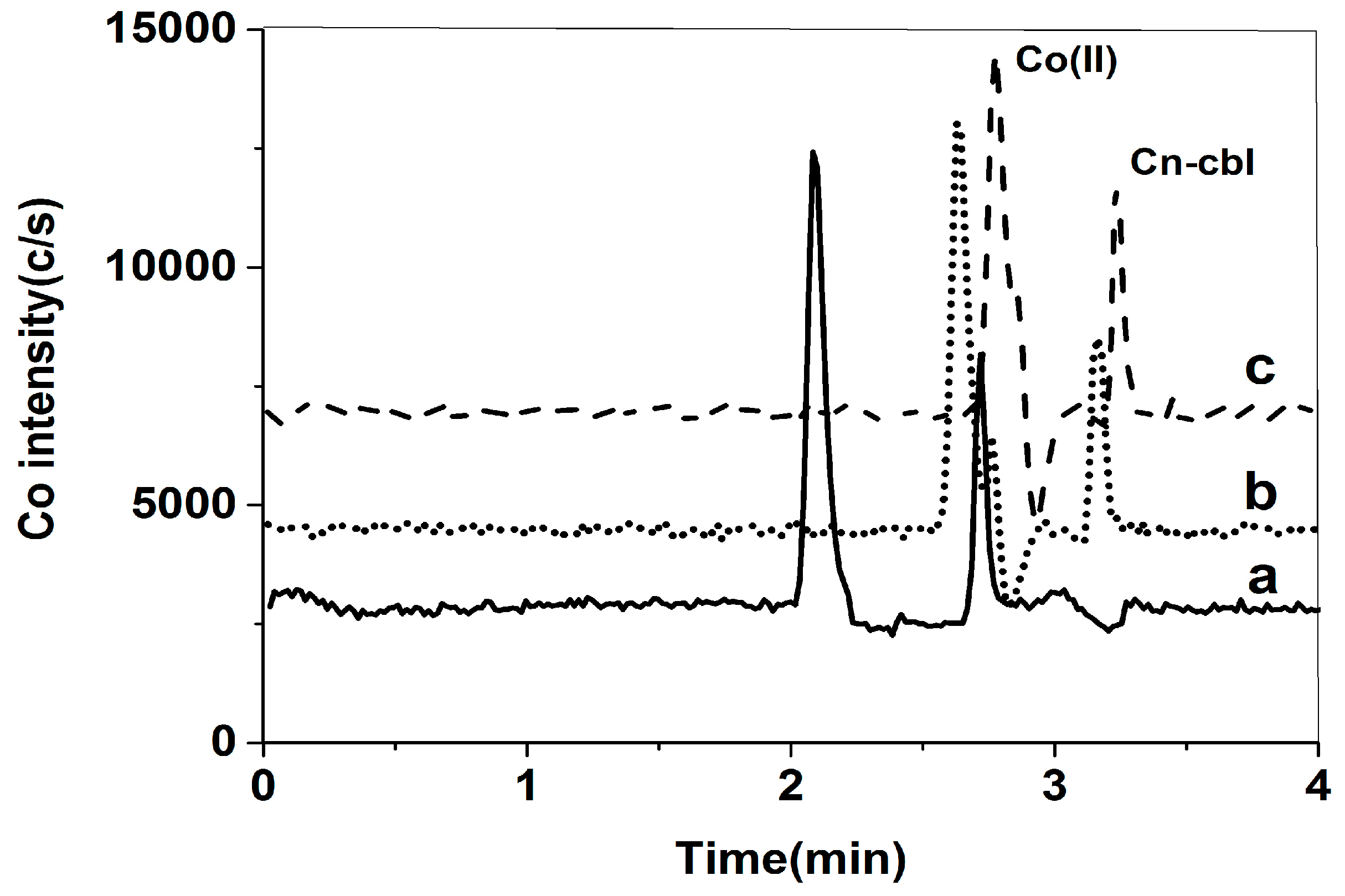
2.1.2. Selection of Mobile Phase
2.2. Optimization of Pretreatment Conditions
2.3. Figures of Merit
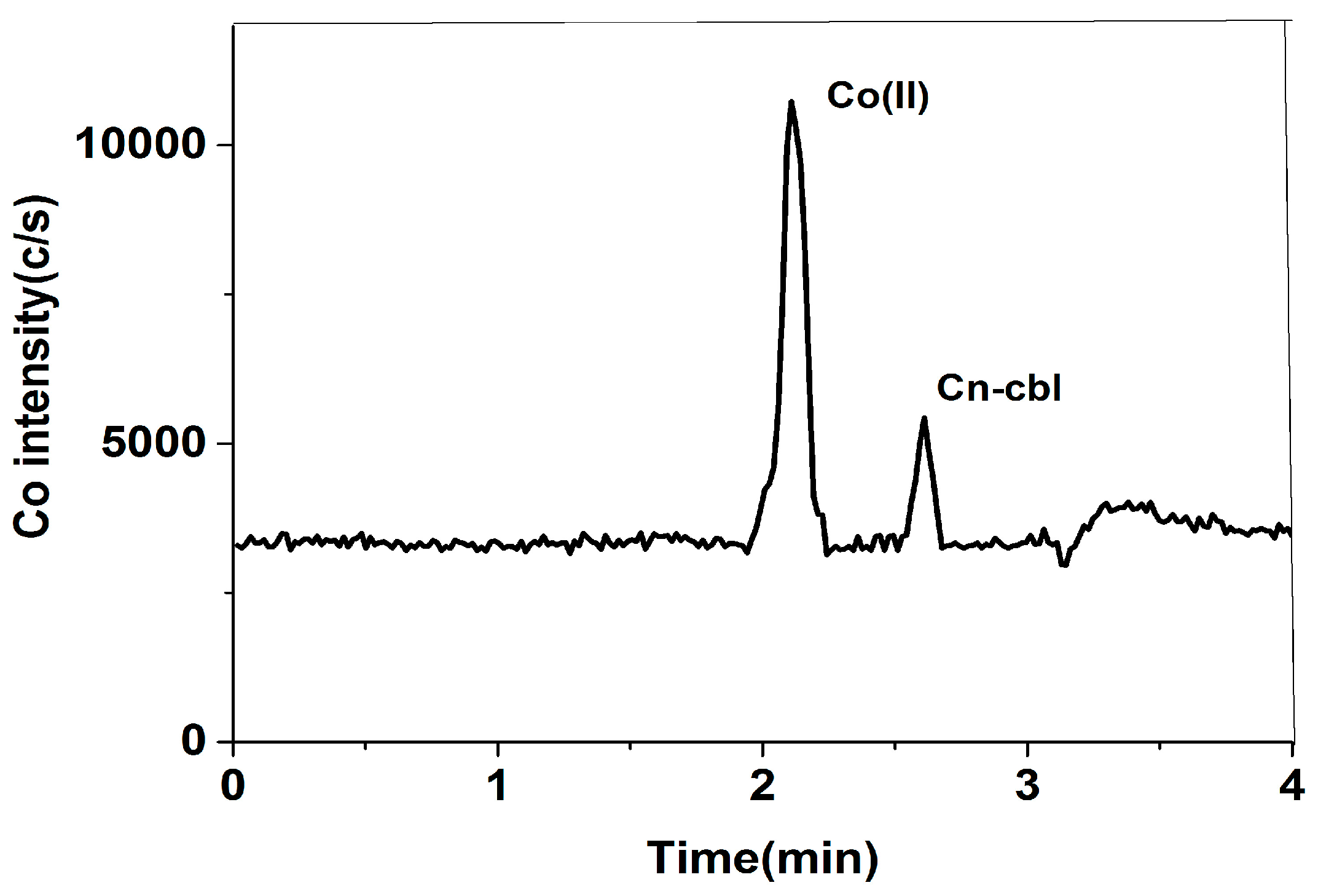
2.4. Accuracy of Method
2.5. Analysis of Real Samples
3. Materials and Methods
3.1. Sample Source
3.2. Instruments and Reagents
3.3. Chromatographic Conditions of HPLC
3.4. Mass Spectrometry Conditions of ICP-MS
3.5. Method Validation
3.6. Sample Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.Y.; Jiang, S.J.; Sahayam, A.C. Combined use of HPLC-ICP-MS and microwave-assisted extraction for the determination of cobalt compounds in nutritive supplements. Food Chem. 2014, 147, 215–219. [Google Scholar] [CrossRef]
- Bell, D. Metformin-induced vitamin B12 deficiency can cause or worsen distal symmetrical, autonomic and cardiac neuropathy in the patient with diabetes. Diabetes Obes. Metab. 2022, 24, 1423–1428. [Google Scholar] [CrossRef]
- Yu, Y.M.; So, S.; Khallouq, B.B. The effect of metformin on vitamin B12 level in pediatric patients. Ann. Pediatr. Endocrinol. Metab. 2022, 27, 223–228. [Google Scholar] [CrossRef]
- Schroder, T.H.; Quay, T.A.; Lamers, Y. Methylmalonic acid quantified in dried blood spots provides a precise, valid, and stable measure of functional vitamin B-12 status in healthy women. J. Nutr. 2014, 144, 1658–1663. [Google Scholar] [CrossRef]
- Nandyala, S.; Mohamed, A.; Bhargava, A.; Chaurasia, S.; Senthil, S.; Vaddavalli, P.K. Vitamin B12 deficiency in a large cohort of healthcare professionals across the network of an eyecare organization in India. Indian J. Ophthalmol. 2022, 70, 1718–1721. [Google Scholar]
- Karve, M.; Gholave, J.V. Solid-phase extraction and atomic absorption spectrometric determination of cobalt using an octadecyl bonded silica membrane disk modified with Cyanex 272. J. AOAC Int. 2011, 94, 627–633. [Google Scholar] [CrossRef]
- GB 10765-2021; National Standard for Infant Formula. National Health and Commission of PRC: Beijing, China, 2021.
- Joint FAO/WHO Codex Alimentarius Commission; World Health Organization; Food and Agriculture Organization of the United Nations. Standard for Canned Baby Foods; World Health Organization: Rome, Italy, 2017. [Google Scholar]
- GB/T 5009.217-2008; National Standard for Food Safety Determination of Vitamin B12 in Health Foods. Ministry of Health of the PRC: Beijing, China, 2008.
- Fan, D.; Zhang, Y.; Wu, H. Development of a simple and sensitive HPLC-DAD method for quantification of vitamin B12 fortified in infant food. Anal. Methods 2021, 13, 4920–4925. [Google Scholar] [CrossRef]
- Guggisberg, D.; Risse, M.C.; Hadorn, R. Determination of Vitamin B12 in meat products by RP-HPLC after enrichment and purification on an immunoaffinity column. Meat Sci. 2012, 90, 279–283. [Google Scholar] [CrossRef]
- Greibe, E.; Nexo, E. Forms and Amounts of Vitamin B12 in Infant Formula: A Pilot Study. PLoS ONE 2016, 11, e165458. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Domek, J.M.; Siddiqua, T.; Raqib, R.; Allen, L.H. Competitive chemiluminescent enzyme immunoassay for vitamin B12 analysis in human milk. Food Chem. 2014, 153, 60–65. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Zhang, C.; Wang, X.; Gu, Q. A riboswitch sensor to determine vitamin B12 in fermented foods. Food Chem. 2015, 175, 523–528. [Google Scholar] [CrossRef]
- Campos-Gimenez, E.; Fontannaz, P.; Trisconi, M.J.; Kilinc, T.; Gimenez, C.; Andrieux, P.; Nelson, M. Determination of vitamin B12 in infant formula and adult nutritionals by liquid chromatography/UV detection with immunoaffinity extraction: First Action 2011.08. J. AOAC Int. 2012, 95, 307–312. [Google Scholar] [CrossRef]
- Gimenez, E.C.; Martin, F. Vitamin B(12) (cyanocobalamin) in Infant Formula Adult/Pediatric Nutritional Formula by Liquid Chromatography with Ultraviolet Detection: Collaborative Study, Final Action 2014.02. J. AOAC Int. 2018, 101, 1112–1118. [Google Scholar] [CrossRef]
- Nshime, B.; Koedam, J.; Stanton, B.; Tran, Q.; Chen, P. Liquid Chromatography Method for the Simultaneous Quantification of Biotin and Vitamin B12 in Vitamin B Supplements. J. AOAC Int. 2019, 102, 445–450. [Google Scholar] [CrossRef]
- Matsumoto, T.; Takebayashi, J.; Ishimi, Y.; Ozawa, C.; Sano, A.; Hirota, T.; Endoh, K. Evaluation of cyanocobalamin in multivitamin tablets and their Standard Reference Material 3280 by HPLC with visible detection. J. AOAC Int. 2012, 95, 1609–1613. [Google Scholar] [CrossRef]
- AOAC. Vitamin B12—A review of analytical methods for use in food Government Chemist Programme Report. In National Measurement System; Report No. LGC/R/2014/378; AOAC: London, UK, 2015. [Google Scholar]
- Whitlock, L.L.; Melton, J.R.; Billings, T.J. Determination of Vitamin B12 in Dry Feeds by Atomic Absorption Spectrophotometry. J. Assoc. Off. Anal. Chem. 1976, 59, 580–581. [Google Scholar] [CrossRef]
- Francis, D.T.; Verma, S.; Bhatia, P.; Vaidya, P.C.; Sankhyan, N. Trace Element Status in Children with Infantile Tremor Syndrome: An Inductively Coupled Plasma-Mass Spectrometry Based Study. Indian J. Pediatr. 2020, 87, 221–223. [Google Scholar] [CrossRef]
- Szterk, A.; Roszko, M.; Malek, K.; Czerwonka, M.; Waszkiewicz-Robak, B. Application of the SPE reversed phase HPLC/MS technique to determine vitamin B12 bio-active forms in beef. Meat Sci. 2012, 91, 408–413. [Google Scholar] [CrossRef]
- Kesava Raju, C.S.; Yu, L.L.; Schiel, J.E.; Long, S.E. A simple and sensitive LC-ICP-MS method for the accurate determination of vitamin B12 in fortified breakfast cereals and multivitamin tablets. J. Anal. At. Spectrom. 2013, 28, 901. [Google Scholar] [CrossRef]
- Liu, Z.; Xue, A.; Chen, H.; Li, S. Quantitative determination of trace metals in single yeast cells by time-resolved ICP-MS using dissolved standards for calibration. Appl. Microbiol. Biotechnol. 2019, 103, 1475–1483. [Google Scholar] [CrossRef]
- Dubascoux, S.; Richoz, P.J.; Sylvain, P.; Nicolas, M.; Campos, G.E. Vitamin B12 quantification in human milk—Beyond current limitations using liquid chromatography and inductively coupled plasma—Mass spectrometry. Food Chem. 2021, 362, 130197. [Google Scholar] [CrossRef]
- Lipiec, E.; Ruzik, L.; Zhou, Y.; Jarosz, M.; Połeć-Pawlak, K. Study of chicken egg protein influence on bioavailability of vitamin B12 by SEC-ICP MS and ESI MS. J. Anal. At. Spectrom. 2011, 26, 608. [Google Scholar] [CrossRef]
- Quarles, C.J.; Jones, D.R.; Jarrett, J.M.; Shakirova, G.; Pan, Y.; Caldwell, K.L.; Jones, R.L. Analytical method for total chromium and nickel in urine using an inductively coupled plasma-universal cell technology-mass spectrometer (ICP-UCT-MS) in kinetic energy discrimination (KED) mode. J. Anal. At. Spectrom. 2014, 2014, 297–303. [Google Scholar] [CrossRef]
- Bajaj, A.O.; Parker, R.; Farnsworth, C.; Law, C.; Johnson-Davis, K.L. Method validation of multi-element panel in whole blood by inductively coupled plasma mass spectrometry (ICP-MS). J. Mass Spectrom. Adv. Clin. Lab. 2023, 27, 33–39. [Google Scholar] [CrossRef]
- Chen, L.; Shen, M.; Ma, A.; Han, W. Investigation of Trace Element Content in the Seeds, Pulp, and Peel of Mashui Oranges Using Microwave Digestion and ICP-MS Analysis. Biol. Trace Elem. Res. 2018, 182, 152–158. [Google Scholar] [CrossRef]
- Johnson-Davis, K.L.; Farnsworth, C.; Law, C.; Parker, R. Method validation for a multi-element panel in serum by inductively coupled plasma mass spectrometry (ICP-MS). Clin. Biochem. 2020, 82, 90–98. [Google Scholar] [CrossRef]
- GB 5009.9-2016; National Standard for Food Safety Determination of Starch in Foods. National Health and Commission of PRC: Beijing, China, 2016.
- Chassaigne, H.; Łobiński, R. Determination of Cobalamins and Cobinamides by Microbore Reversed-Phase HPLC with Spectrophotometric, Ion-Spray Ionization MS and Inductively Coupled Plasma MS Detection. Anal. Chim. Acta 1998, 359, 227–235. [Google Scholar] [CrossRef]
- Vyas, P.; O’Kane, A.A. Determination of vitamin B12 in fortified bovine milk-based infant formula powder, fortified soya-based infant formula powder, vitamin premix, and dietary supplements by surface plasmon resonance: Collaborative study. J. AOAC Int. 2011, 94, 1217–1226. [Google Scholar] [CrossRef]
- Bernardin, M.; Bessueille-Barbier, F.; Le Masle, A.; Lienemann, C.P.; Heinisch, S. Suitable interface for coupling liquid chromatography to inductively coupled plasma-mass spectrometry for the analysis of organic matrices. 1 Theoretical and experimental considerations on solute dispersion. J. Chromatogr. A 2018, 1565, 68–80. [Google Scholar] [CrossRef]
- El Deeb, S.; Abdelsamad, K.; Parr, M.K. Whiter and Greener RP-HPLC Method for Simultaneous Determination of Dorzolamide, Brinzolamide, and Timolol Using Isopropanol as a Sustainable Organic Solvent in the Mobile Phase. Separations 2024, 11, 83. [Google Scholar] [CrossRef]
- Marković, S.; Levstek, L.; Žigon, D.; Ščančar, J.; Milačič, R. Speciation and Bio-Imaging of Chromium in Taraxacum Officinale Using HPLC Post-Column ID-ICP-MS, High Resolution MS and Laser Ablation ICP-MS Techniques. Front. Chem. 2022, 10, 863387. [Google Scholar] [CrossRef] [PubMed]


| Four Experiences Designed | Concentration Found (μg/L) |
|---|---|
| Cyanobalamin standard with water | 0.03 |
| Cyanobalamin standard with extract solution | 0.02 |
| Sample extracted | 40.48 |
| Sample digested | 5.55 |
| Sample blank digested | 0.00 |
| Cobalt Species | Linear Equation | Linear Range (μg/L) | Correlation Coefficient (r2) | LOD (μg/kg) | LOQ (μg/kg) | RSD (%) |
|---|---|---|---|---|---|---|
| Free cobalt | y = 50,130x + 0.6239 | 0.05–1.0 | 0.9996 | 0.045 | 0.149 | 2.56% |
| Cyanocobalamin | y = 1863.6x + 194.79 | 1–20 | 0.9994 | 0.63 | 2.11 | 2.48% |
| GBW 10277 | ||
|---|---|---|
| Species | Found (μg/L) | Certified (μg/L) |
| cyanocobalamin | 30.3 ± 0.7 | 29.8 ± 3.6 |
| Sample | Background Value (μg/kg) | Added (μg/kg) | Concentration Found (μg/kg) | Mean Recovery (%) | RSDr (%) | |
|---|---|---|---|---|---|---|
| Milk powder | Co(II) | 2.1 | 0.5 | 2.5 ± 0.1 | 95.3 | 4.42 |
| 1.0 | 3.1 ± 0.1 | 99.6 | 3.10 | |||
| 4.0 | 6.2 ± 0.2 | 101.3 | 2.76 | |||
| Cyanocobalamin | 27.3 | 10 | 33.8 ± 2.1 | 90.6 | 4.66 | |
| 25 | 53.4 ± 1.5 | 102.2 | 4.01 | |||
| 50 | 79.7 ± 2.8 | 103.1 | 2.98 | |||
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | |
|---|---|---|---|---|---|---|
| Cyanocobalamin | 27.3 | 14.7 | 28.5 | 10.6 | 33.7 | 16.9 |
| Sum b | 29.4 | 16.1 | 30.3 | 11.3 | 37.1 | 19.5 |
| Digested c | 30.9 | 17.9 | 32.3 | 12.1 | 40.9 | 20.0 |
| The ratio of cobalt species and total cobalt (%) | 95.1 | 89.9 | 93.8 | 93.4 | 90.7 | 97.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhou, B.; Zheng, C. The Fast Quantification of Vitamin B12 in Milk Powder by High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. Molecules 2024, 29, 1795. https://doi.org/10.3390/molecules29081795
Yang Y, Zhou B, Zheng C. The Fast Quantification of Vitamin B12 in Milk Powder by High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. Molecules. 2024; 29(8):1795. https://doi.org/10.3390/molecules29081795
Chicago/Turabian StyleYang, Yue, Biao Zhou, and Chenyang Zheng. 2024. "The Fast Quantification of Vitamin B12 in Milk Powder by High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry" Molecules 29, no. 8: 1795. https://doi.org/10.3390/molecules29081795
APA StyleYang, Y., Zhou, B., & Zheng, C. (2024). The Fast Quantification of Vitamin B12 in Milk Powder by High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. Molecules, 29(8), 1795. https://doi.org/10.3390/molecules29081795






