No Evidence Was Found for the Presence of Terreolides, Terreumols or Saponaceolides H-S in the Fruiting Bodies of Tricholoma terreum (Basidiomycota, Agaricales)
Abstract
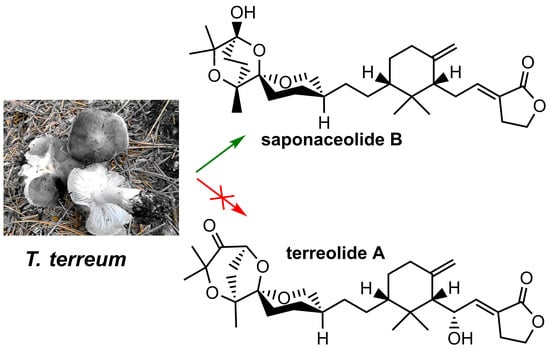
1. Introduction
2. Results and Discussion
2.1. DNA Analysis
2.2. Phytochemical Analysis
2.3. Saponaceolide B Chemical Stability
3. Materials and Methods
3.1. General
3.2. Fungal Material
3.3. Extraction of Tricholoma terreum Fruiting Bodies and Isolation of Saponaceolide B (1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, M.; Heilmann-Clausen, J. Fungi of Northern Europe; The Genus Tricholoma; Svampetryk: Tilst, Denmark, 2013; Volume 4. [Google Scholar]
- Geraci, C.; Piattelli, M.; Tringali, C. Applications of two-dimensional NMR in spectral assignments of the cytotoxic triterpene saponaceolide B. Magn. Reson. Chem. 1991, 29, 603–606. [Google Scholar] [CrossRef]
- De Bernardi, M.; Garlaschelli, L.; Toma, L.; Vidari, G.; Vita-Finzi, P. Fungal metabolites XXVI: The structure of saponaceolides B, C and D, new C-30 terpenoids from Tricholoma saponaceum. Tetrahedron 1991, 47, 7109–7116. [Google Scholar] [CrossRef]
- De Bernardi, M.; Garlaschelli, L.; Gattl, G.; Vidari, G.; Finzi, P.V. Fungal metabolites XXII: The unprecedented structure of saponaceolide a, a cytotoxic C-30 terpenoid from Tricholoma saponaceum. Tetrahedron 1988, 44, 235–240. [Google Scholar] [CrossRef]
- Gozzini, D.; Mellerio, G.G.; Gilardoni, G.; Clericuzio, M.; Vidari, G. New terpenoids from Tricholoma saponaceum. Nat. Prod. Commun. 2018, 13, 1097–1100. [Google Scholar] [CrossRef]
- Vidari, G.; Lanfranchi, G.; Sartori, P.; Serra, S. Saponaceolides: Differential cytotoxicity and enantioselective synthesis of the right-hand lactone moiety. Tetrahedron-Asymmetry 1995, 6, 2977–2990. [Google Scholar] [CrossRef]
- Yin, X.; Feng, T.; Li, Z.-H.; Dong, Z.-J.; Li, Y.; Liu, J.K. Highly oxygenated meroterpenoids from fruiting bodies of the mushroom Tricholoma terreum. J. Nat. Prod. 2013, 76, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Feng, T.; Shang, J.-H.; Zhao, Y.-L.; Wang, F.; Li, Z.-H.; Dong, Z.-J.; Luo, X.-D.; Liu, J.-K. Chemical and toxicological investigations of a previously unknown poisonous european mushroom Tricholoma terreum. Chem. Eur. J. 2014, 20, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; He, J.; Ai, H.-L.; Huang, R.; Li, Z.-H.; Liu, J.-K. Three new triterpenoids from european mushroom Tricholoma terreum. Nat. Prod. Bioprospect. 2015, 5, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Saviuc, P.; Danel, V. New syndromes in mushroom poisoning. Toxicol. Rev. 2006, 25, 199–209. [Google Scholar] [CrossRef]
- Boa, E. Wild Edible Fungi: A Global Overview of Their Use and Importance to People; Agriculture Organization of the United Nations (FAO): Rome, Italy, 2004. [Google Scholar]
- Davoli, P.; Floriani, M.; Assisi, F.; Kob, K.; Sitta, N. Comment on “chemical and toxicological investigations of a previously unknown poisonous european mushroom Tricholoma terreum”. Chem. Eur. J. 2016, 22, 5786–5788. [Google Scholar] [CrossRef]
- Yin, X.; Feng, T.; Li, Z.-H.; Liu, J.-K. Response to the “comment on chemical and toxicological investigations of a previously unknown poisonous european mushroom Tricholoma terreum”. Chem. Eur. J. 2016, 22, 5789–5792. [Google Scholar] [CrossRef] [PubMed]
- Heilmann-Clausen, J.; Christensen, M.; Frøslev, T.G.; Kjøller, R. Taxonomy of Tricholoma in northern europe based on its sequence data and morphological characters. Persoonia—Mol. Phylogeny Evol. Fungi 2017, 38, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Clericuzio, M.; Mellerio, G.G.; Finzi, P.V.; Vidari, G. Secondary metabolites isolated from Tricholoma species (basidiomycota, tricholomatacee): A review. Nat. Prod. Commun. 2018, 13, 1213–1224. [Google Scholar] [CrossRef]
- Díez, V.A.; Alvarez, A. Compositional and nutritional studies on two wild edible mushrooms from northwest spain. Food Chem. 2001, 75, 417–422. [Google Scholar] [CrossRef]
- Pedneault, K.; Angers, P.; Gosselin, A.; Tweddell, R.J. Fatty acid profiles of polar and neutral lipids of ten species of higher basidiomycetes indigenous to eastern canada. Mycol. Res. 2008, 112, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-C.; Choi, H.S.; Kim, J.-H.; Kim, S.-L.; Yun, B.-S.; Lee, D.-S. Coriolic acid (13-(S)-hydroxy-9Z,11E-octadecadienoic acid) from glasswort (Salicornia herbacea L.) suppresses breast cancer stem cell through the regulation of c-Myc. Molecules 2020, 25, 4950. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Peng, Y.-L.; He, J.; Li, Z.-H.; Liu, J.-K.; Feng, T. Structures, chemical conversions, and cytotoxicity of tricholopardins C and D, two Tricholoma triterpenoids from the wild mushroom Tricholoma pardinum. Nat. Prod. Bioprospect. 2021, 11, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Bergquist, K.-E.; Sterner, O. The isolation of a new isochromanone from injured fruit bodies of Tricholoma scalpturatum. Acta Chem. Scand. 1994, 48, 453–454. [Google Scholar] [CrossRef][Green Version]
- Rangsinth, P.; Sharika, R.; Pattarachotanant, N.; Duangjan, C.; Wongwan, C.; Sillapachaiyaporn, C.; Nilkhet, S.; Wongsirojkul, N.; Prasansuklab, A.; Tencomnao, T.; et al. Potential beneficial effects and pharmacological properties of ergosterol, a common bioactive compound in edible mushrooms. Foods 2023, 12, 2529. [Google Scholar] [CrossRef]
- Field, C.J.; Blewett, H.H.; Proctor, S.; Vine, D. Human health benefits of vaccenic acid. Appl. Physiol. Nutr. Metab. 2009, 34, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, M.; Legoy, M.D. Chemoenzymatic production of (+)-coriolic acid from trilinolein: Coupled synthesis and extraction. J. Am. Oil Chem. Soc. 1997, 74, 641–645. [Google Scholar] [CrossRef]





| Entry | Fatty Acids (%) 1 | Class of Fatty Acid (%) 1 |
|---|---|---|
| 1 | C15:0 (0.4) | Saturated (11.4) |
| 2 | C16:0 (9.9) | |
| 3 | C18:0 (1.1) | |
| 4 | C16:1 (0.5) | Monounsaturated (54.1) |
| 5 | C18:1 Δ9c (Oleic, 49.3) | |
| 6 | C18:1 Δ11c (Asclepic, 4.3) | |
| 7 | C18:1 Δ9c,12c (Linoleic, 34.5) | Polyunsaturated (34.5) |
| Time (min) | A % 1 | B % 2 |
|---|---|---|
| 0 | 70 | 30 |
| 4.5 | 70 | 30 |
| 10.0 | 40 | 60 |
| 13.0 | 20 | 80 |
| 19.0 | 10 | 90 |
| 22.0 | 0 | 100 |
| 31.0 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clericuzio, M.; Serra, S.; Vidari, G. No Evidence Was Found for the Presence of Terreolides, Terreumols or Saponaceolides H-S in the Fruiting Bodies of Tricholoma terreum (Basidiomycota, Agaricales). Molecules 2024, 29, 1794. https://doi.org/10.3390/molecules29081794
Clericuzio M, Serra S, Vidari G. No Evidence Was Found for the Presence of Terreolides, Terreumols or Saponaceolides H-S in the Fruiting Bodies of Tricholoma terreum (Basidiomycota, Agaricales). Molecules. 2024; 29(8):1794. https://doi.org/10.3390/molecules29081794
Chicago/Turabian StyleClericuzio, Marco, Stefano Serra, and Giovanni Vidari. 2024. "No Evidence Was Found for the Presence of Terreolides, Terreumols or Saponaceolides H-S in the Fruiting Bodies of Tricholoma terreum (Basidiomycota, Agaricales)" Molecules 29, no. 8: 1794. https://doi.org/10.3390/molecules29081794
APA StyleClericuzio, M., Serra, S., & Vidari, G. (2024). No Evidence Was Found for the Presence of Terreolides, Terreumols or Saponaceolides H-S in the Fruiting Bodies of Tricholoma terreum (Basidiomycota, Agaricales). Molecules, 29(8), 1794. https://doi.org/10.3390/molecules29081794