Secondary Atomization and Micro-Explosion Effect Induced by Surfactant and Nanoparticles on Enhancing the Combustion Performance of Al/JP-10/OA Nanofluid Fuel
Abstract
1. Introduction
2. Results and Discussion
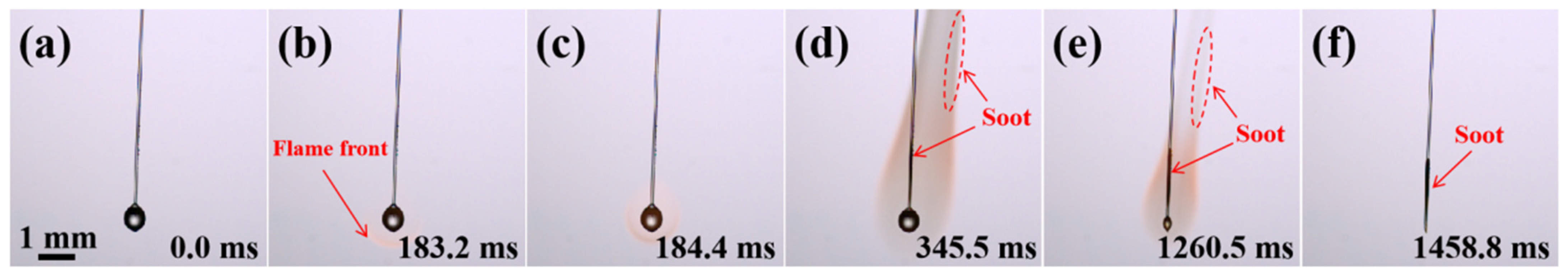
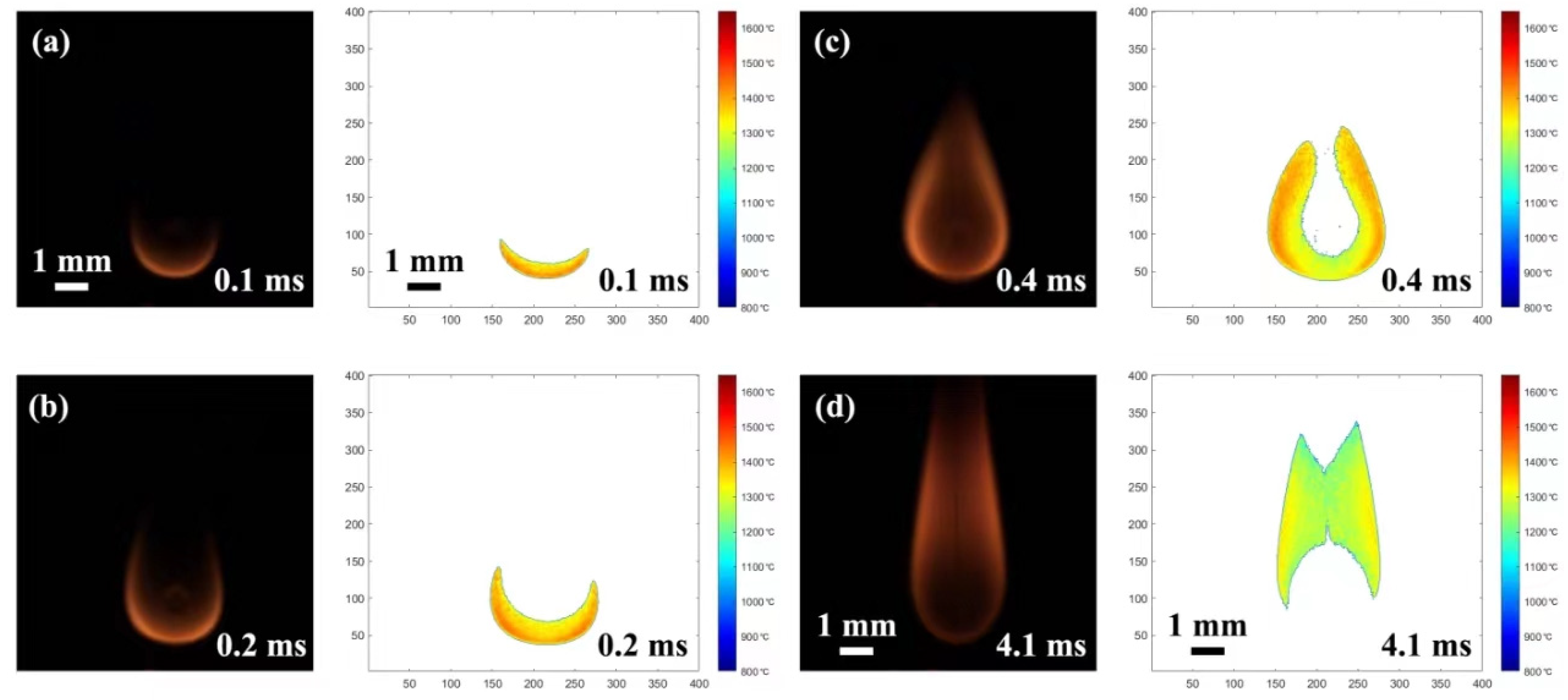
2.1. Combustion Evolution of Pure JP-10 Droplets
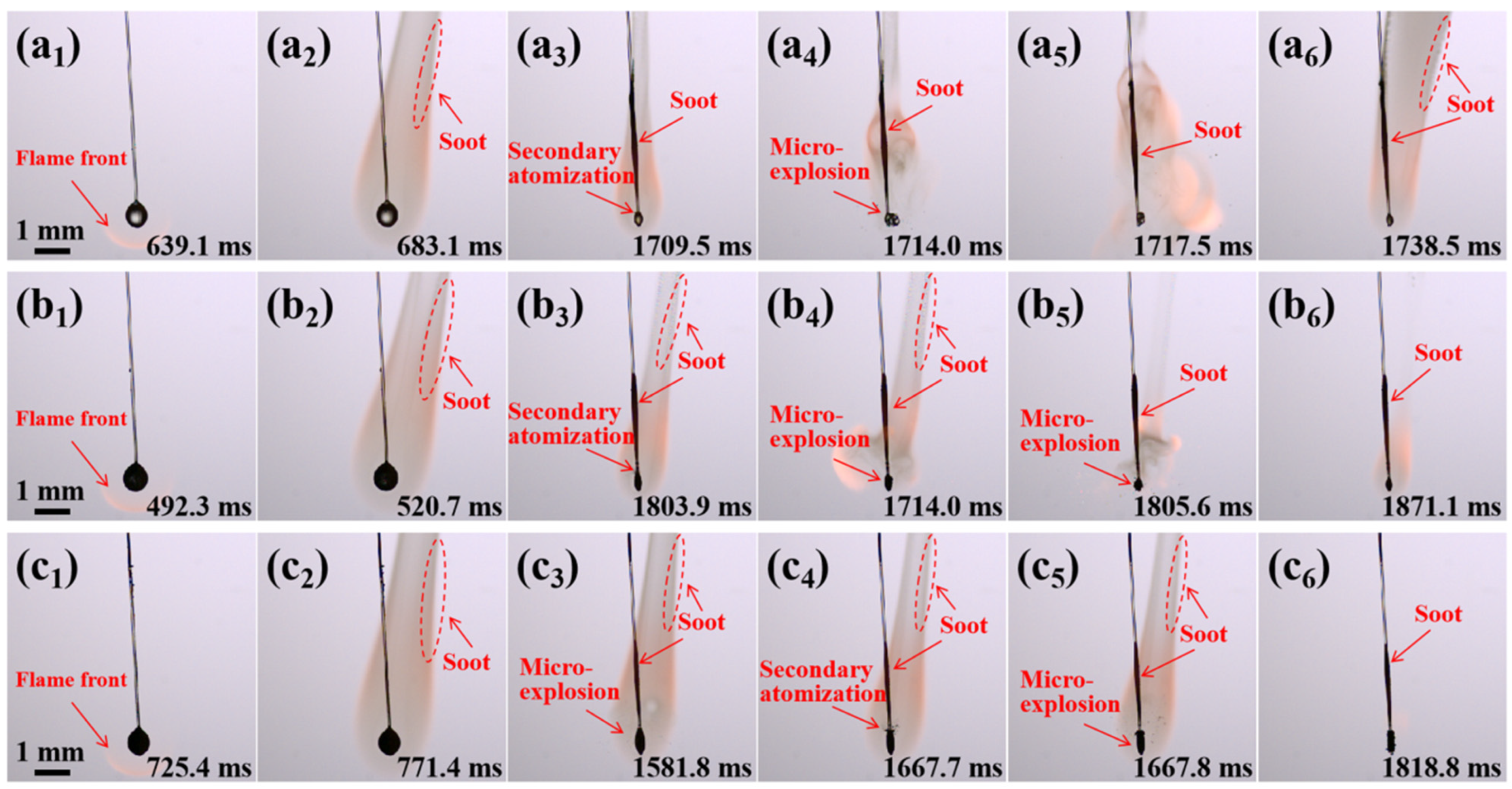
2.2. Combustion Evolution of JP-10/OA Droplets
2.3. Combustion Evolution of JP-10/Al Droplets
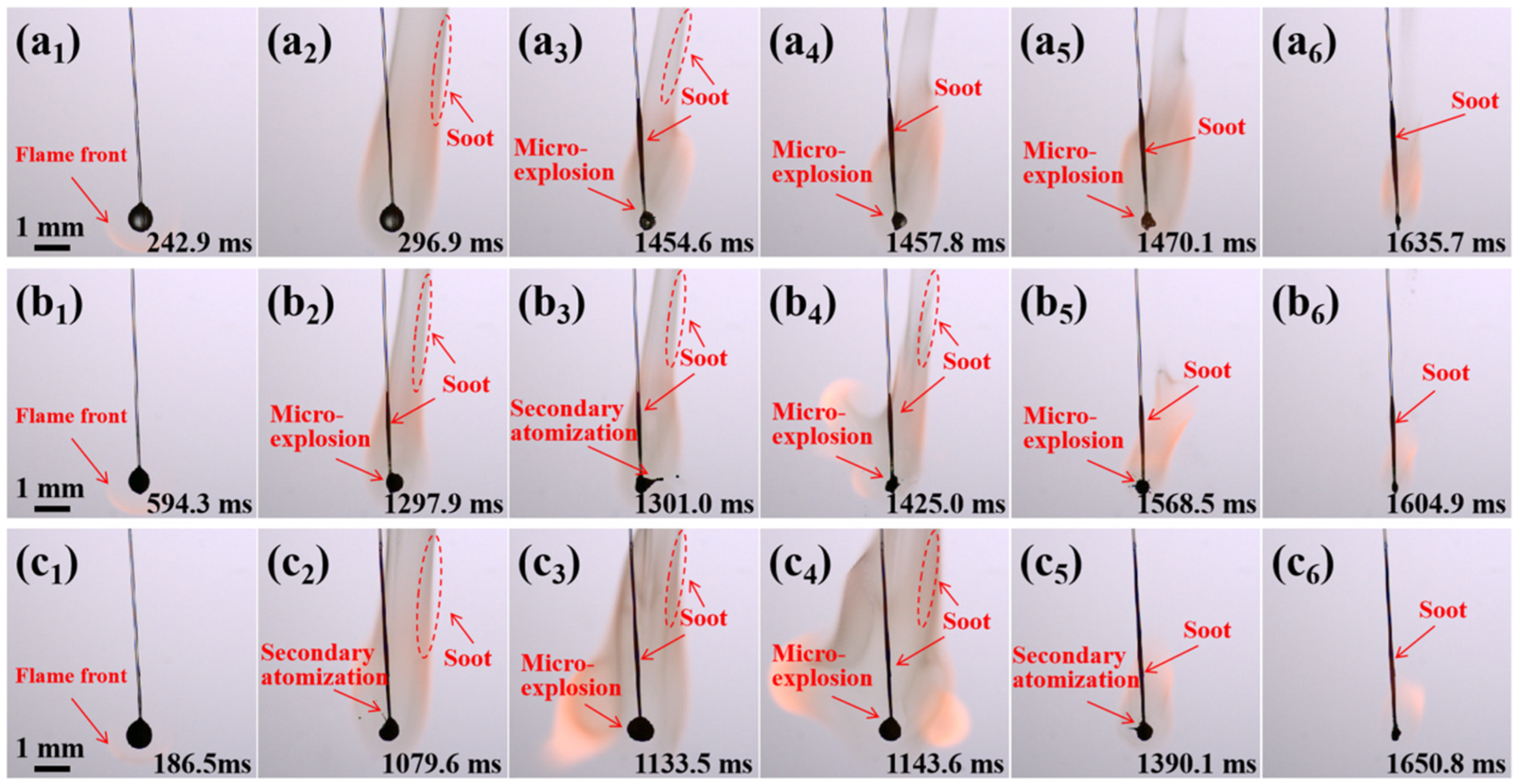
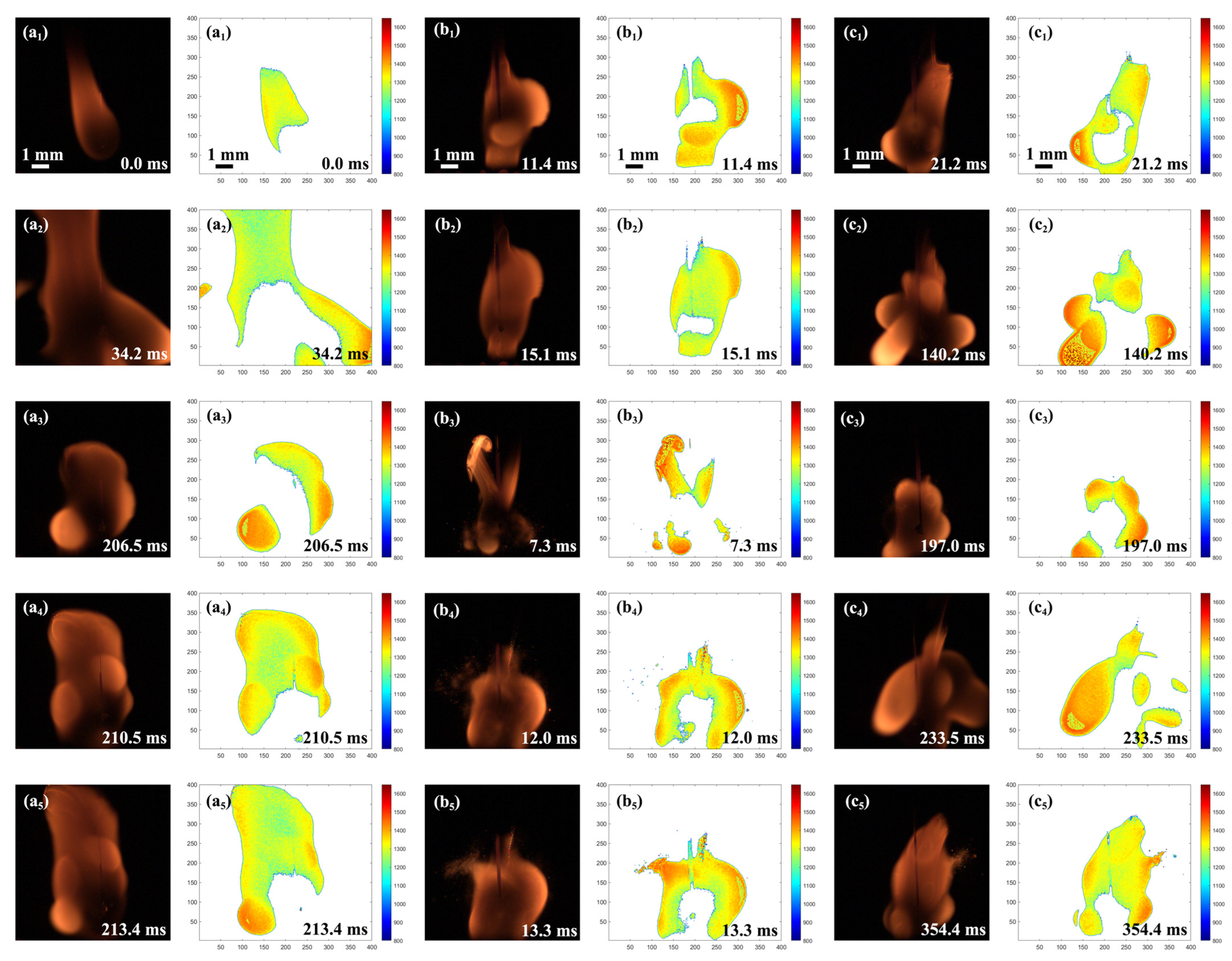
2.4. Combustion Evolution of Al/JP-10/OA Nanofluid Fuel Droplets
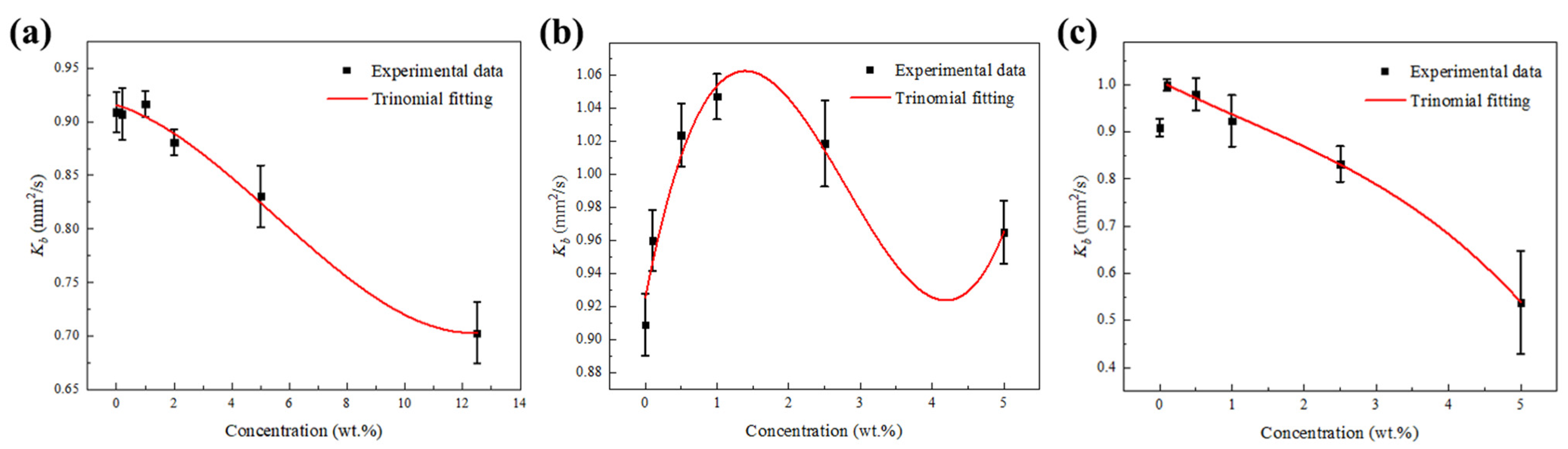
2.5. Combustion Rate Analysis
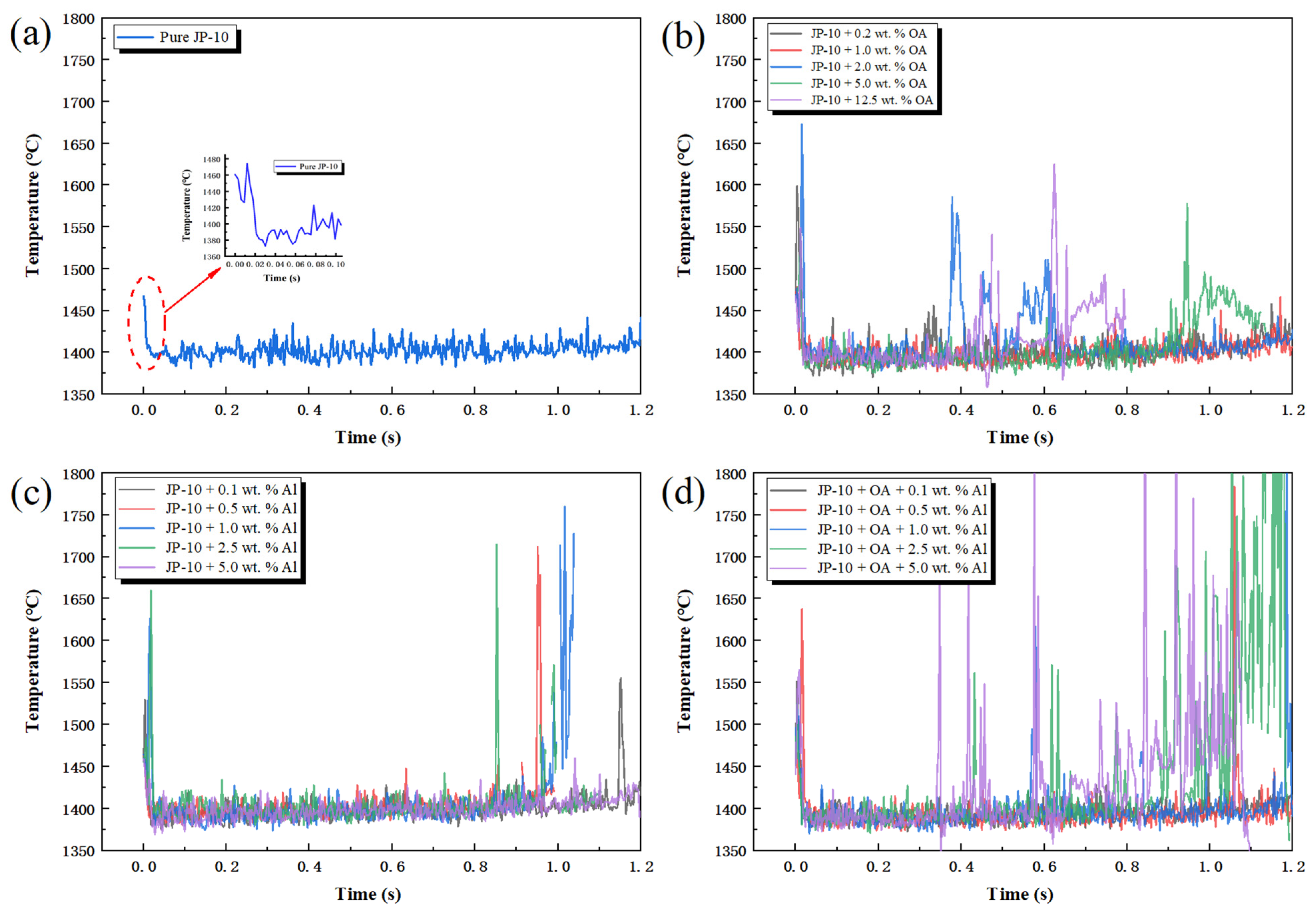
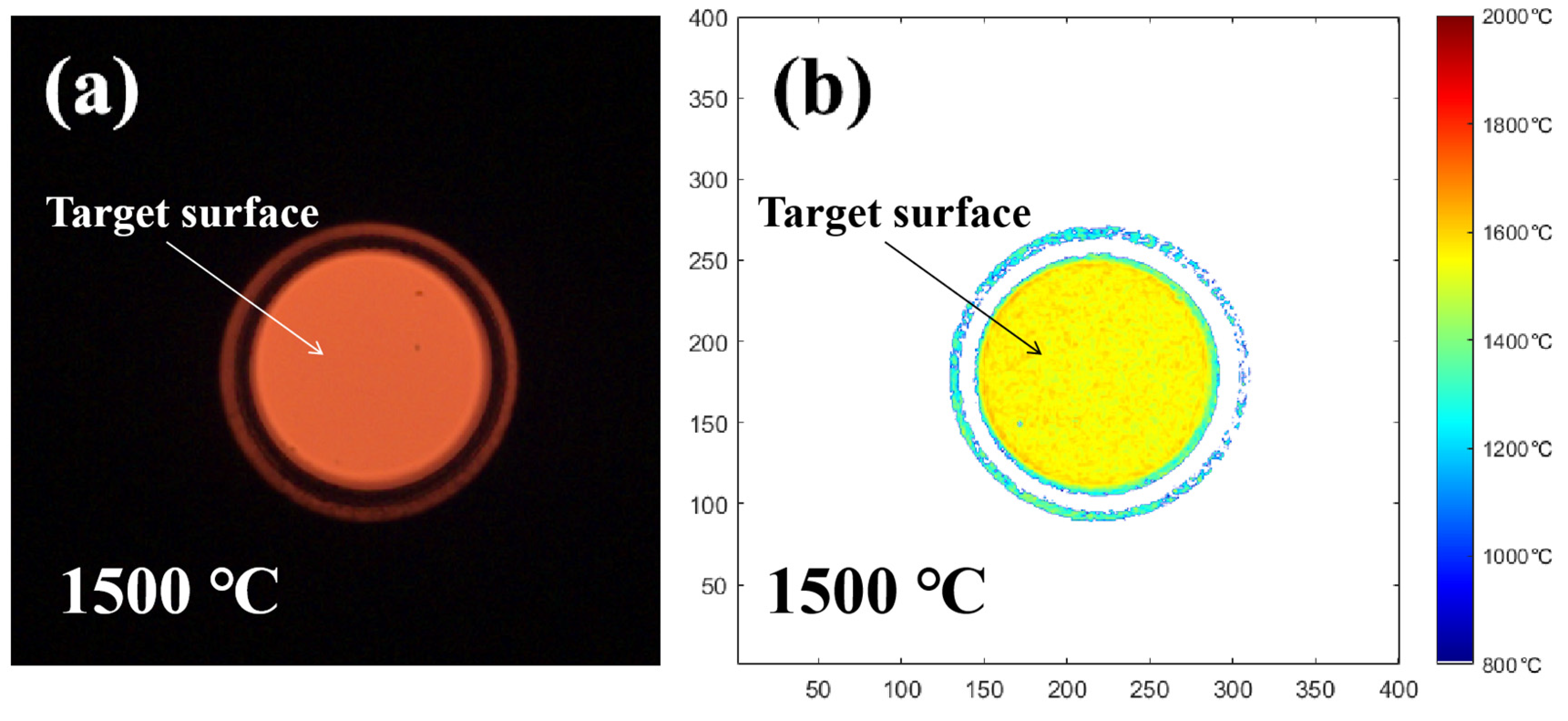
2.6. Flame Temperature Analysis
2.7. Secondary Atomization and Micro-Explosion Analysis
2.7.1. Evolution of Secondary Atomization and Micro-Explosion
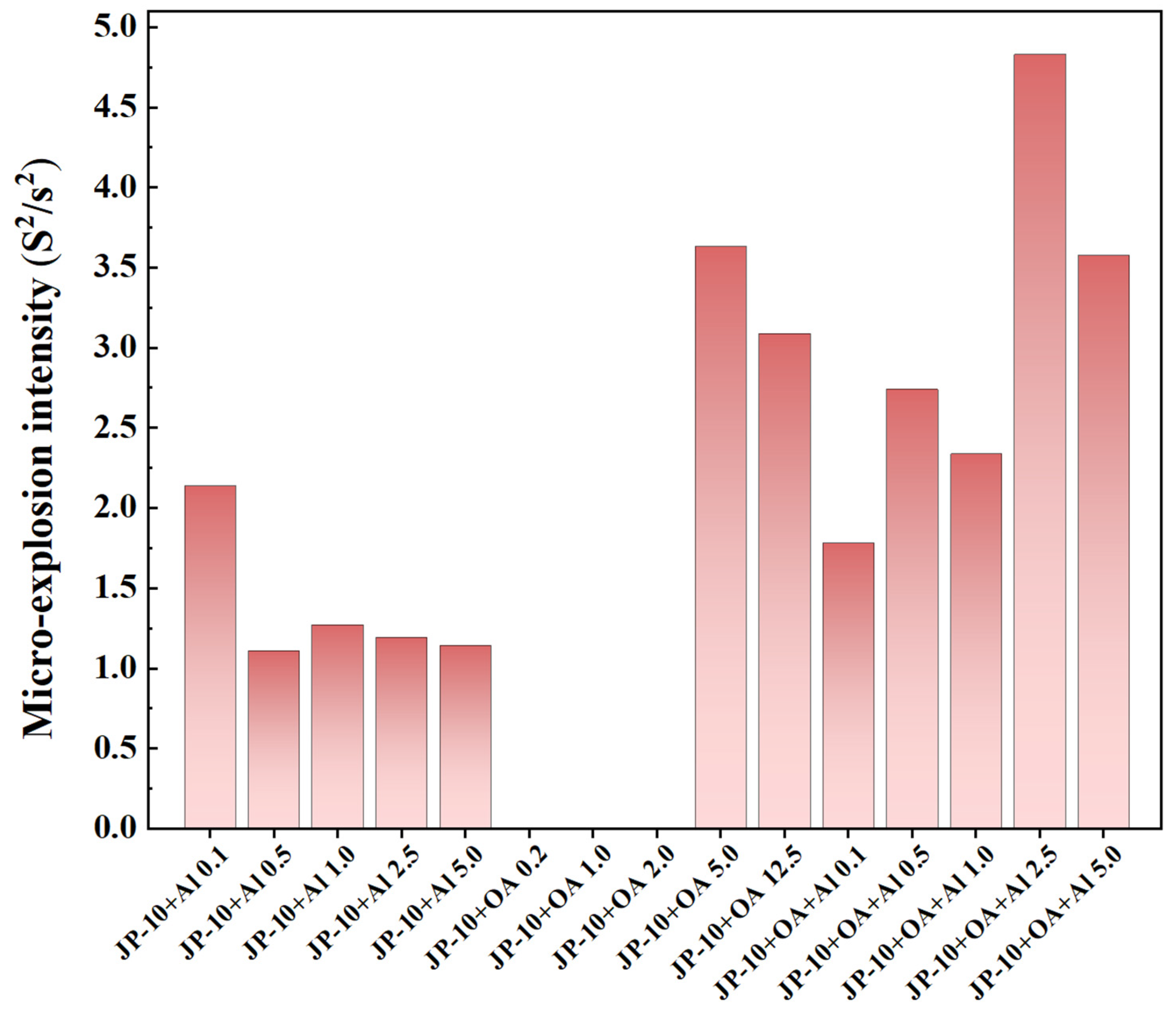
2.7.2. Intensity of Secondary Atomization and Micro-Explosion
2.7.3. Temperature Variation before and after Secondary Atomization and Micro-Explosion
3. Materials and Experimental Methods
3.1. Preparation of Al/JP-10/OA Nanofluid Fuels
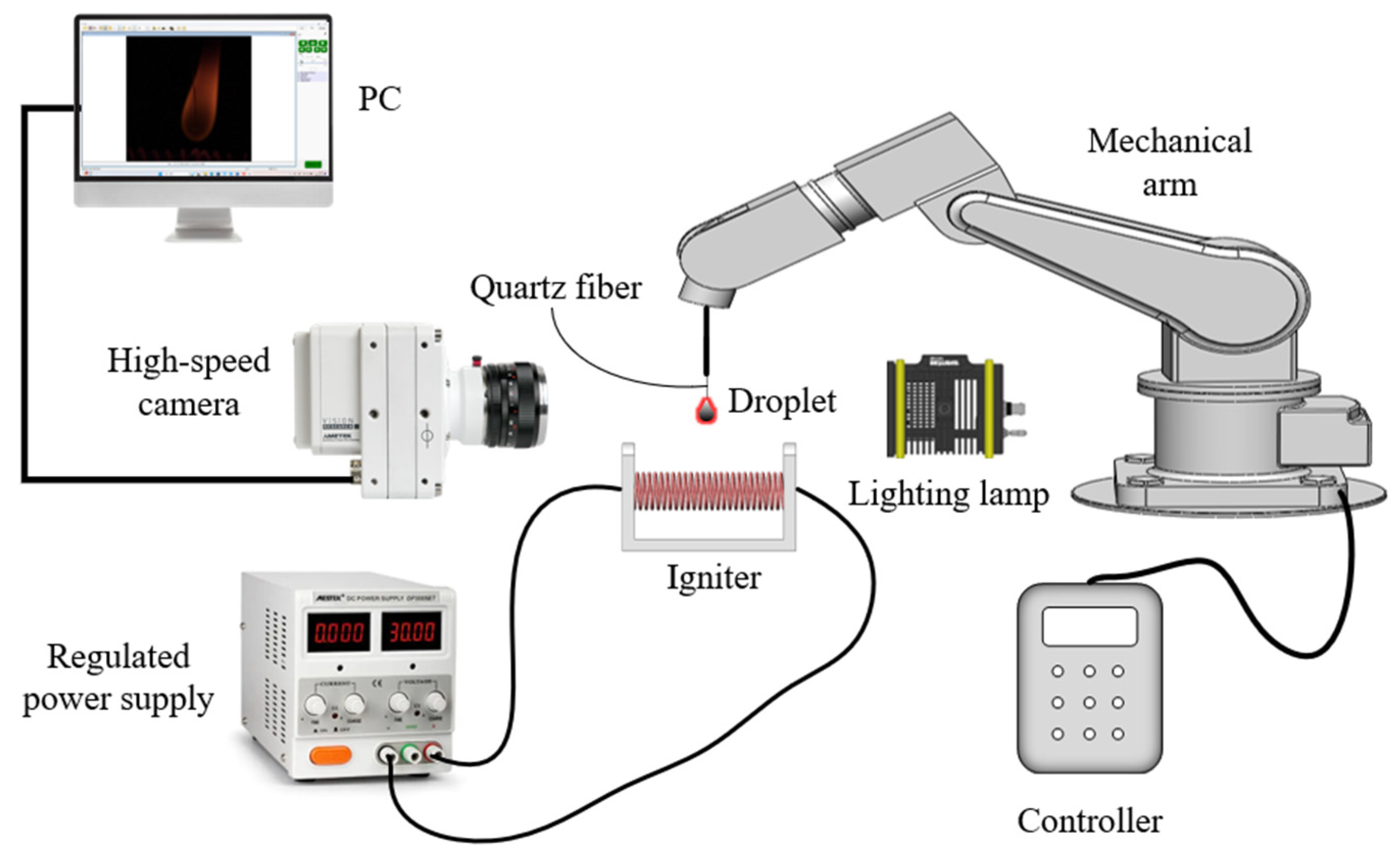
3.2. Experimental Setup for Combustion Characterization
4. Conclusions
- The combustion of pure JP-10 fuel droplets followed the d2 law for the whole process, while the JP-10/OA, JP-10/Al, and Al/JP-10/OA fuel droplets followed the d2 law only in the stable stage. The addition of OA surfactant inhibited evaporation and reduced the effective burning rate of JP-10. Adding OA surfactant at high concentrations resulted in a large reduction in the combustion rate of droplets (8.7% @ 5.0 wt.% OA; 22.7% @ 12.5 wt.% OA). The addition of Al nanoparticles promoted evaporation and increased the effective burning rate of JP-10. The addition of both OA surfactant and Al nanoparticles had a synergetic effect on the evaporation and burning rate. At the optimum ratio of OA to Al for the best suspension stability, there was a turning point from promotion to inhibition with the increase in the Al concentration, and the critical value was 1.0 wt.%. When the Al concentration was greater than 1.0 wt.%, the combustion rate of the Al/JP-10/OA nanofluid fuel droplets decreased with the increase in the Al concentration but was lower than the combustion rate of the JP-10 droplets (8.5% reduction @ 5.0 wt.% OA + 2.5 wt.% Al; 40.8% reduction @ 12.5 wt.% OA + 5.0 wt.% Al).
- The addition of OA surfactant could induce the occurrence of secondary atomization and micro-explosion of the droplets, but the critical concentration of OA needed to approximate or exceed 5.0 wt.%. The addition of Al nanoparticles at any concentration could result in the occurrence of secondary atomization and micro-explosion. The concentration of Al nanoparticles had a weak effect on the intensity of secondary atomization and micro-explosion. However, the addition of both of them greatly promoted secondary atomization and micro-explosion, even at dilute or dense concentrations.
- Secondary atomization and micro-explosion could break up the mother droplets into a large number of finer daughter droplets and distribute them in the flame zone, which made the base fuel, the surfactant, and the nanoparticles burn more completely and improved the combustion performance. The unsteady combustion induced by secondary atomization and micro-explosion elevated the transient temperature by hundreds of Kelvins (compared to the stable flame temperature (~1400 °C), the highest temperature in the secondary atomization and micro-explosion zones increased by 12.85%~41.36%) and widened the high-temperature flame zone, which is beneficial for energy release.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karczewski, M.; Chojnowski, J.; Szamrej, G. A Review of Low-CO2 Emission Fuels for a Dual-Fuel RCCI Engine. Energies 2021, 14, 5067. [Google Scholar] [CrossRef]
- Shi, E.; Yan, X.; Liang, H.; Chen, X.; Yu, J. Effects of nano-aluminum particle size on the explosion behaviors of alumi-num/methanol nanofluid fuel spray. Fuel 2024, 366, 131–333. [Google Scholar] [CrossRef]
- Emekwuru, N.; Sarıkoç, S. A case for oxygenated nanofluid fuels as alternative aviation fuels: Thermo-physical properties and effects on engine performance. Int. J. Sustain. Aviat. 2022, 8, 369–384. [Google Scholar] [CrossRef]
- Xiu, T.F.; Pan, L.; Zhang, X.; Zou, J.J. Influence of quadricyclane additive on ignition and combustion properties of high-density JP-10 fuel. Fuel 2020, 276, 118047. [Google Scholar] [CrossRef]
- Mao, Z.P.; Zhu, B.Z.; Sun, Y.L.; Chen, J.; Liu, J. Improving the ignition and combustion of JP10/boron-based nanofluid fuel droplets by the interaction of PTFE and boron. J. Therm. Anal. Calorim. 2023, 148, 4185–4194. [Google Scholar] [CrossRef]
- Yutko, B.M. Approaches to Representing Aircraft Fuel Efficiency Performance for the Purpose of a Commercial Aircraft Certification Standard. Master’s Thesis, The Pennsylvania State University, State College, PA, USA, 2011. [Google Scholar]
- Saxena, V.; Kumar, N.; Saxena, V. A comprehensive review on combustion and stability aspects of metal nanoparticles and its additive effect on diesel and biodiesel fuelled C.I. engine. Renew. Sustain. Energy Rev. 2017, 70, 563–588. [Google Scholar] [CrossRef]
- Huang, X.; Li, S. Ignition and combustion characteristics of jet fuel liquid film containing graphene powders at meso-scale. Fuel 2016, 177, 113–122. [Google Scholar] [CrossRef]
- Lujain, A.A.; Feroz, S.; Sundar, L.S. Thermal efficiency enhancement of mono and hybrid nanofluids in solar thermal applications—A review. Alex. Eng. J. 2023, 68, 365–404. [Google Scholar]
- Mehta, R.N.; Chakraborty, M.; Parikh, P.A. Nanofuels: Combustion, engine performance and emissions. Fuel 2014, 120, 91–97. [Google Scholar] [CrossRef]
- Gao, Y.; Ao, W.; Li, L.K.; Zhou, S.; He, W.; Liu, P.; Yan, Q. Catalyzed combustion of a nanofluid fuel droplet containing polydopamine-coated metastable intermixed composite n-Al/CuO. Aerosp. Sci. Technol. 2021, 118, 107005. [Google Scholar] [CrossRef]
- Venu, H.; Appavu, P. Al2O3 nano additives blended Polanga biodiesel as a potential alternative fuel for existing unmodified DI diesel engine. Fuel 2020, 279, 118518. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, X.; Zou, J.-J.; Zhang, X. Combustion of JP-10-Based Slurry with Nanosized Aluminum Additives. J. Propuls. Power 2016, 32, 1167–1177. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W.; Waheed, K. Effects of dense concentrations of aluminum nanoparticles on the evaporation behavior of kerosene droplet at elevated temperatures: The phenomenon of microexplosion. Exp. Therm. Fluid Sci. 2014, 56, 33–44. [Google Scholar] [CrossRef]
- Li, G.; Hou, B.; Wang, A.; Xin, X.; Cong, Y.; Wang, X.; Li, N.; Zhang, T. Making JP-10 superfuel affordable with lignocellulosic platform component. Angew. Chem. Int. Ed. 2019, 58, 12154–12158. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.-J.; Zeng, Z.-M.; Zhang, H.-Z. An experimental and kinetic modeling study of JP-10 combustion. Fuel 2022, 312, 122900. [Google Scholar] [CrossRef]
- Ye, L.; Li, S.; Huang, X. Effect of Nanoparticle Addition on Evaporation of Jet Fuel Liquid Films and Nanoparticle Deposition Patterns during Evaporation. Langmuir 2022, 38, 15973–15983. [Google Scholar] [CrossRef] [PubMed]
- Ojha, P.K.; Prabhudeva, P.; Karmakar, S.; Maurya, D. Combustion characteristics of JP-10 droplet loaded with Sub-micron boron particles. Exp. Therm. Fluid Sci. 2019, 109, 109900. [Google Scholar] [CrossRef]
- Jin, X.; Li, S.; Yang, Y.; Huang, X. Comparison on Laser Ignition and Combustion Characteristics of Nano- and Micron-Sized Aluminum. Combust. Sci. Technol. 2021, 193, 341–353. [Google Scholar] [CrossRef]
- Hou, F.; Li, S.; Wang, Y.; Huang, X. Laser-Induced Ignition and Combustion of Individual Aluminum Particles Below 10 μm by Microscopic High-Speed Cinematography. Processes 2020, 8, 280. [Google Scholar] [CrossRef]
- Yang, Q.; Li, S.; Ye, L.; Huang, X. Understanding of Contradiction on Concentration Effect on Stability, Physical Properties, Evaporation and Microexplosion Characteristics of Al/JP-10/Oleic Acid Nanofluid Fuel. Nanomaterials 2022, 12, 3446. [Google Scholar] [CrossRef]
- He, W.; Li, S.; Huang, X. Critical concentration effects of nanoparticle on combustion involved into secondary atomization and micro-explosion for B/JP-10/SP-80 nanofluid fuel. Fuel 2024, 358, 130262. [Google Scholar] [CrossRef]
- Kannan, G.; Karvembu, R.; Anand, R. Effect of metal based additive on performance emission and combustion characteristics of diesel engine fuelled with biodiesel. Appl. Energy 2011, 88, 3694–3703. [Google Scholar] [CrossRef]
- Gan, Y.; Qiao, L. Combustion characteristics of fuel droplets with addition of nano and micron-sized aluminum particles. Combust. Flame 2011, 158, 354–368. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ghasemi, A. Solidification heat transfer of nanofluid in existence of thermal radiation by means of FEM. Int. J. Heat Mass Transf. 2018, 123, 418–431. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W.; Waheed, K. Autoignition and combustion characteristics of kerosene droplets with dilute concentrations of aluminum nanoparticles at elevated temperatures. Combust. Flame 2015, 162, 774–787. [Google Scholar] [CrossRef]
- Gan, Y.; Qiao, L. Radiation-enhanced evaporation of ethanol fuel containing suspended metal nanoparticles. Int. J. Heat Mass Transf. 2012, 55, 5777–5782. [Google Scholar] [CrossRef]
- Yadav, A.K.; Nandakumar, K.; Srivastava, A.; Chowdhury, A. Combustion of rocket-grade kerosene droplets loaded with graphene nanoplatelets—A search for reasons behind optimum mass loadings. Combust. Flame 2019, 203, 1–13. [Google Scholar] [CrossRef]
- Ao, W.; Gao, Y.; Zhou, S.; Li, L.; He, W.; Liu, P.; Yan, Q. Enhancing the stability and combustion of a nanofluid fuel with polydopamine-coated aluminum nanoparticles. Chem. Eng. J. 2021, 418, 129527. [Google Scholar] [CrossRef]
- Lv, X.; Gao, Y.; Cui, Y.; Wang, C.; Zhang, G.; Wang, F.; Liu, P.; Ao, W. Study of ignition and combustion characteristics of kerosene-based nanofluid fuel containing n-Al/CuO thermite. Fuel 2023, 331, 125778. [Google Scholar] [CrossRef]
- Wong, S.-C.; Lin, A.-C.; Chi, H.-Y. Effects of surfactant on the evaporation, shell formation and disruptive behavior of slurry droplets. Symp. (Int.) Combust. 1991, 23, 1391–1397. [Google Scholar] [CrossRef]
- Du, L.; Liu, J.; Chen, B.; Sun, Z.; Yang, W. Combustion and energy release characteristics of LiF- and AP-modified B/JP-10 suspension fuels prepared using a two-solvent method. Combust. Flame 2023, 251, 112722. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, X.; Ju, D.; Wang, L.; Sun, C. Experimental study on the evaporation and micro-explosion characteristics of nanofuel droplet at dilute concentrations. Energy 2019, 183, 149–159. [Google Scholar] [CrossRef]



| No. | Sample’s Name | Compositions | ||
|---|---|---|---|---|
| JP-10 (wt.%) | OA (wt.%) | Al (wt.%) | ||
| 1 | JP-10 | 100.0 | 0.0 | 0.0 |
| 2 | JP-10+OA0.2 | 99.8 | 0.2 | 0.0 |
| 3 | JP-10+OA1.0 | 99.0 | 1.0 | 0.0 |
| 4 | JP-10+OA2.0 | 98.0 | 2.0 | 0.0 |
| 5 | JP-10+OA5.0 | 95.0 | 5.0 | 0.0 |
| 6 | JP-10+OA12.5 | 87.5 | 12.5 | 0.0 |
| 7 | JP-10+Al0.1 | 99.9 | 0.0 | 0.1 |
| 8 | JP-10+Al0.5 | 99.5 | 0.0 | 0.5 |
| 9 | JP-10+Al1.0 | 99.0 | 0.0 | 1.0 |
| 10 | JP-10+Al2.5 | 97.5 | 0.0 | 2.5 |
| 11 | JP-10+Al5.0 | 95.0 | 0.0 | 5.0 |
| 12 | JP-10+OA+Al0.1 | 99.7 | 0.2 | 0.1 |
| 13 | JP-10+OA+Al0.5 | 98.5 | 1.0 | 0.5 |
| 14 | JP-10+OA+Al1.0 | 97.0 | 2.0 | 1.0 |
| 15 | JP-10+OA+Al2.5 | 92.5 | 5.0 | 2.5 |
| 16 | JP-10+OA+Al5.0 | 82.2 | 12.5 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, Z.; Yang, Q.; Wang, Z.; Huang, X.; Luo, D. Secondary Atomization and Micro-Explosion Effect Induced by Surfactant and Nanoparticles on Enhancing the Combustion Performance of Al/JP-10/OA Nanofluid Fuel. Molecules 2024, 29, 1806. https://doi.org/10.3390/molecules29081806
Li S, Liu Z, Yang Q, Wang Z, Huang X, Luo D. Secondary Atomization and Micro-Explosion Effect Induced by Surfactant and Nanoparticles on Enhancing the Combustion Performance of Al/JP-10/OA Nanofluid Fuel. Molecules. 2024; 29(8):1806. https://doi.org/10.3390/molecules29081806
Chicago/Turabian StyleLi, Shengji, Zixuan Liu, Qianmei Yang, Zhangtao Wang, Xuefeng Huang, and Dan Luo. 2024. "Secondary Atomization and Micro-Explosion Effect Induced by Surfactant and Nanoparticles on Enhancing the Combustion Performance of Al/JP-10/OA Nanofluid Fuel" Molecules 29, no. 8: 1806. https://doi.org/10.3390/molecules29081806
APA StyleLi, S., Liu, Z., Yang, Q., Wang, Z., Huang, X., & Luo, D. (2024). Secondary Atomization and Micro-Explosion Effect Induced by Surfactant and Nanoparticles on Enhancing the Combustion Performance of Al/JP-10/OA Nanofluid Fuel. Molecules, 29(8), 1806. https://doi.org/10.3390/molecules29081806






