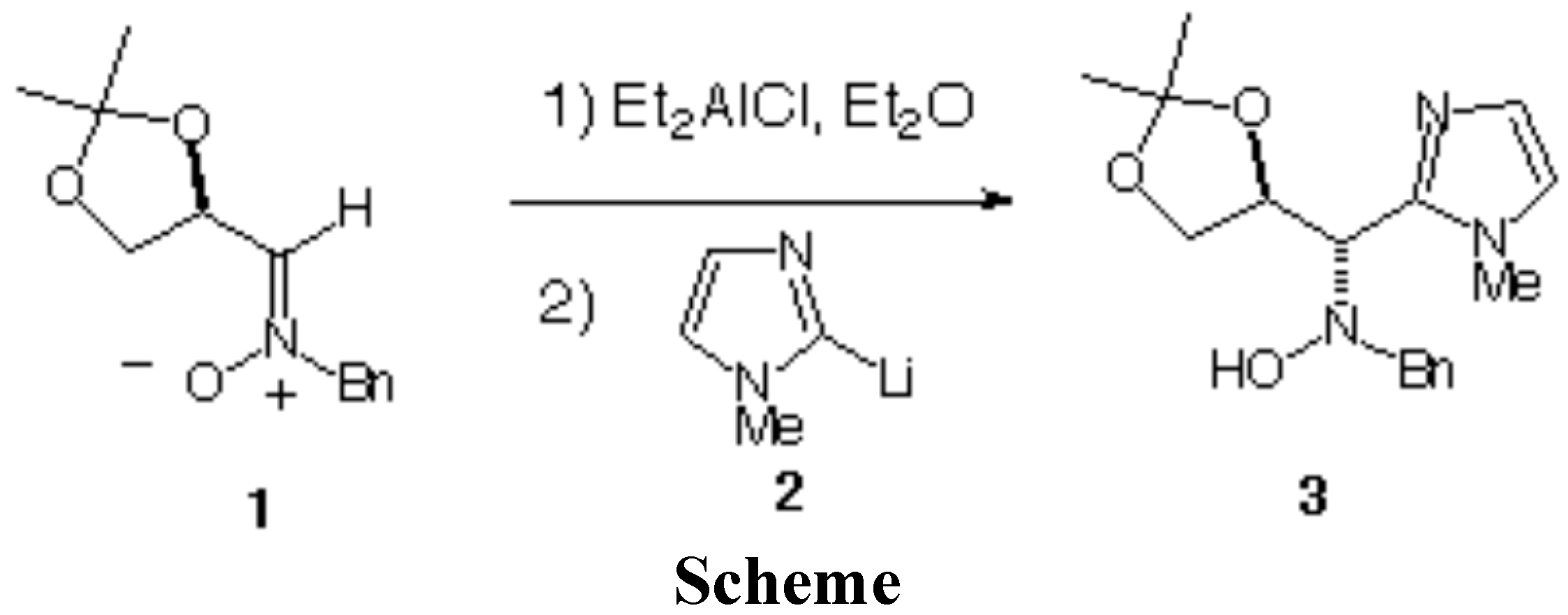
A cooled solution (-90°C) of 1-methylimidazole (0.25 g, 3 mmol) in THF (10 mL) was treated with butyllithium (2 mL of a 1.6 M solution in hexane, 3.2 mmol) under an inert atmopshere. The resulting solution was stirred at -20 °C for 20 min during which time the reaction mixture was cooled to -90 °C and treated via a syringe with a solution of nitrone 1 (0.235 g, 1 mmol) in Et2O (10 mL), previously treated with Et2AlCl (1 mL of a 1.0 M solution in hexanes, 1 mmol) at ambient temperature for 5 min. The rate of addition was adjusted so as to keep the internal temperature below -80°C. The reaction mixture was stirred for 2 h at -80 °C and then quenched with a 1 N aqueous solution of sodium hydroxyde (10 mL). The mixture was stirred at ambient temperature for 10 min and diluted with ethyl acetate (15 mL). The organic layer was separated, and the aqueous layer was extracted with ethyl acetate (3 x 10 ml). The combined organic extracts were washed with brine and dried over magnesium sulfate, and the solvent was evaporated under reduced pressure to give the crude mixture of diastereomeric hydroxylamines. The NMR analysis of this mixture revealed a diastereoselectivity of 79%. The absolute configuration of compound 3 was confirmed by the X-ray analysis of the corresponding syn isomer [1]. Purification by column chromatography (hexane/diethyl ether 80:20) on silica gel afforded 3 as a white solid (0.190 g, 60%).
Mp 118-120°C
TLC (hexane/diethyl ether 80:20) Rf 0.21
[a]D20 = -14.2 (c 0.88, CHCl3)
1H NMR (CDCl3) d 1.24 (s, 3H), 1.30 (s, 3H), 3.38 (s, 3H), 3.42 (d, 1H, J = 12.4 Hz), 3.57 (d, 1H, J = 9.3 Hz), 4.05 (dd, 1H, J = 5.7, 8.9 Hz), 4.09 (d, 1H, J = 12.4 Hz), 4.30 (dd, 1H, J = 6.5, 8.9 Hz), 4.75 (ddd, 1H, J = 5.8, 6.4, 9.4 Hz), 6.84 (d, 1H, J = 1.7 Hz), 7.04 (d, 1H, J = 1.7 Hz), 7.12 (bs, 1H, ex. D2O), 7.30-7.36 (m, 5H).
13C NMR (CDCl3) d 25.5, 25.6, 32.8, 59.7, 62.1, 66.7, 77.3, 108.1, 120.7, 126.8, 127.4, 128.3, 129.0, 136.7, 143.8.
Anal. Calcd. for C17H23N3O3: C, 64.33; H, 7.30; N, 13.24. Found: C, 64.45; H, 7.48; N, 13.51.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Merino, P.; Merchan, F. L.; Tejero, T. Acta Cryst. Sect. C 1995, 51, 2400–2401.
- Sample Availability: Available from the authors and MDPI.
© 1998 MDPI. All rights reserved. Molecules website http://www.mdpi.org/molecules/
