Biorefining Brazilian Green Propolis: An Eco-Friendly Approach Based on a Sequential High-Pressure Extraction for Recovering High-Added-Value Compounds
Abstract
:1. Introduction
2. Results
2.1. SFE Optimization
2.2. Biorefining Green Propolis: Sequential High-Pressure Extractions Approach
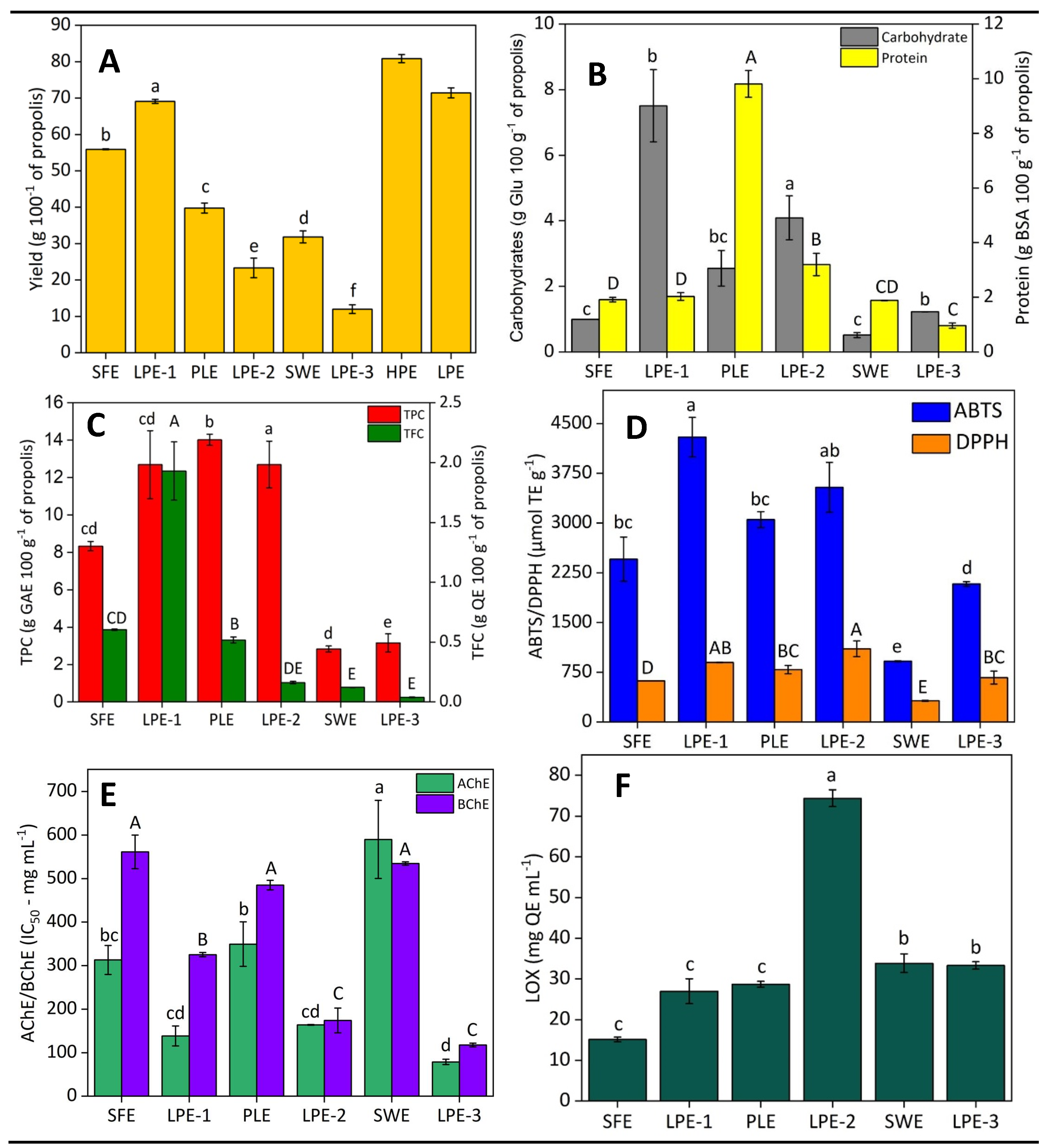

2.2.1. First Biorefinery Step: SFE and LPE–1
2.2.2. Second Biorefinery Step: PLE and LPE–2
2.2.3. Third Biorefinery Step: SWE and LPE–3
2.2.4. Global Process Evaluation
- SFE is enriched in lipids, lipophilic compounds, phenolics, terpenes, and waxes, making it ideal for cosmetics and skincare. It can also serve as an emollient and moisturizer, provide antioxidant and anti-inflammatory protection, offer fragrance and therapeutic effects, thicken products, and form a protective layer on the skin [1,38,39];
- PLE is abundant in phenolic compounds, flavonoids, carbohydrates, and proteins. It has potential uses in the food industry as a natural antioxidant and preservative, in cosmetics for anti-aging and moisturizing, in nutraceuticals for functional supplements, and in pharmaceuticals for preventive therapies. It can also be used in bioactive packaging to extend the shelf life of perishable products [1,10,38];
- SWE contains proteins and other less accessible compounds with moderate anti-inflammatory activity. It is suitable for pharmaceuticals as a topical treatment or supplement, for cosmetics to soothe and reduce redness in skincare products, and for the food industry and nutraceuticals as a source of proteins and bioactive compounds. Additionally, it has potential in biomedical applications, including tissue regeneration and therapeutic agents [11,21,39].
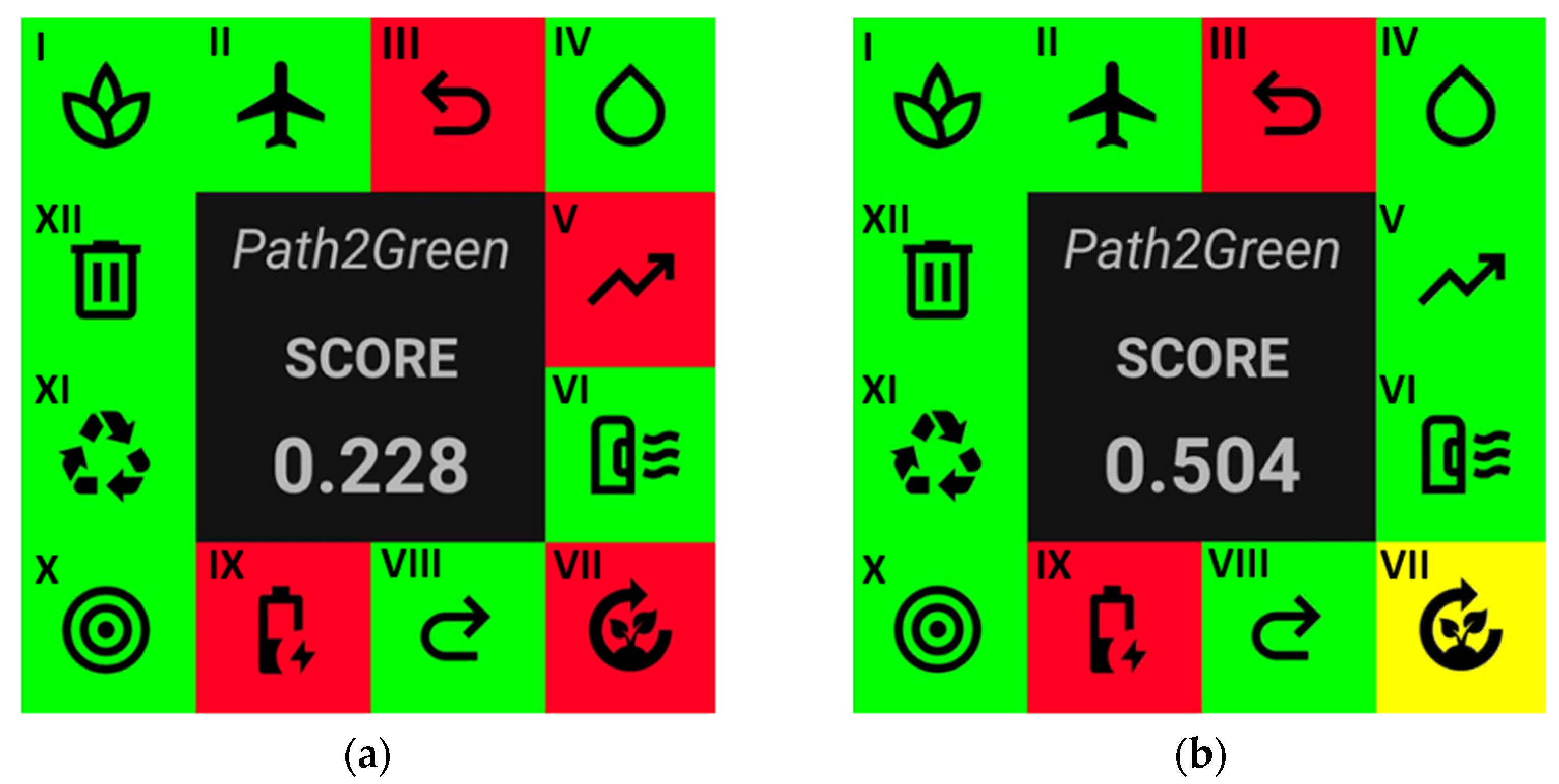
2.3. Greenness Assessment of the HPE and LPE Processes
3. Material and Methods
3.1. Propolis Biomass and Materials
3.2. Biorefining Green Propolis: Sequential High-Pressure Extraction Process
3.2.1. Supercritical Fluid Extraction (SFE)
3.2.2. Pressurized Liquid Extraction (PLE)
3.2.3. Subcritical Water Extraction (SWE)
3.2.4. Sequential Low-Pressure Extraction (LPE) Process
3.3. Extracts Characterization
3.3.1. Global Extraction Yield (%)
3.3.2. Total Phenolic Content (TPC)
3.3.3. Total Flavonoid Content (TFC)
3.3.4. Biological Activities
3.3.5. Total Carbohydrate and Protein Contents
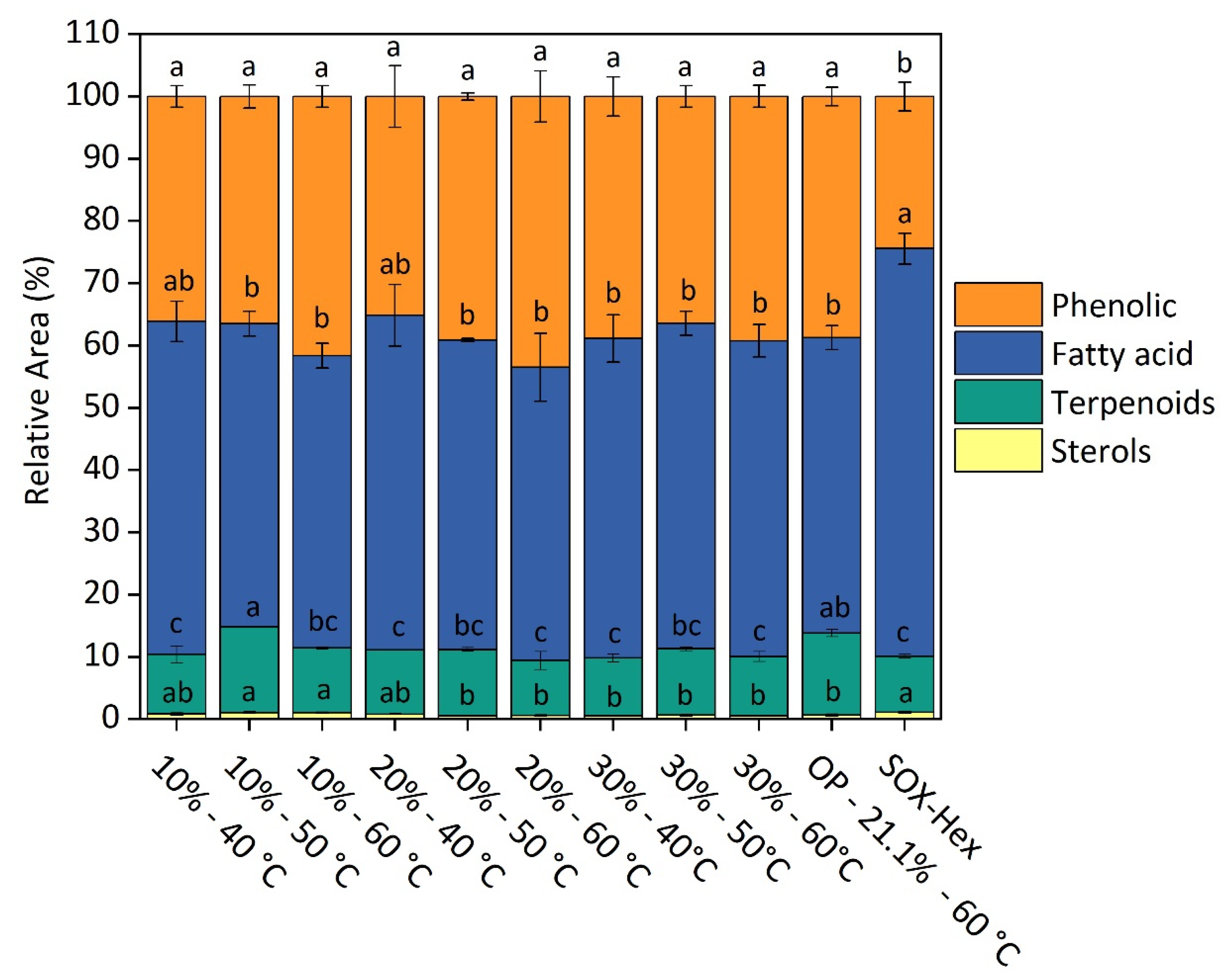
3.3.6. Chemical Characterization by Gas Chromatography–Mass Spectrometry
3.4. Greenness Assessment of HPE and LPE Processes
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pant, K.; Sharma, A.; Chopra, H.K.; Nanda, V. Impact of Biodiversification on Propolis Composition, Functionality, and Application in Foods as Natural Preservative: A Review. Food Control 2024, 155, 110097. [Google Scholar] [CrossRef]
- Lesmana, R.; Tandean, S.; Christoper, A.; Suwantika, A.A.; Wathoni, N.; Abdulah, R.; Fearnley, J.; Bankova, V.; Zulhendri, F. Propolis as an Autophagy Modulator in Relation to Its Roles in Redox Balance and Inflammation Regulation. Biomed. Pharmacother. 2024, 175, 116745. [Google Scholar] [CrossRef] [PubMed]
- Necip, A.; Demirtas, I.; Tayhan, S.E.; Işık, M.; Bilgin, S.; Turan, İ.F.; İpek, Y.; Beydemir, Ş. Isolation of Phenolic Compounds from Eco-friendly White Bee Propolis: Antioxidant, Wound-healing, and Anti-Alzheimer Effects. Food Sci. Nutr. 2024, 12, 1928–1939. [Google Scholar] [CrossRef]
- Oršolić, N. Allergic Inflammation: Effect of Propolis and Its Flavonoids. Molecules 2022, 27, 6694. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.M.S.; Guedes, J.A.C.; de Brito, E.S.; Mazzutti, S.; Ferreira, S.R.S. High-Pressure Biorefining of Ora-pro-Nobis (Pereskia aculeata). J. Supercrit. Fluids 2021, 181, 105514. [Google Scholar] [CrossRef]
- Zielinski, A.A.F.; Sanchez-Camargo, A.d.P.; Benvenutti, L.; Ferro, D.M.; Dias, J.L.; Ferreira, S.R.S. High-Pressure Fluid Technologies: Recent Approaches to the Production of Natural Pigments for Food and Pharmaceutical Applications. Trends Food Sci. Technol. 2021, 118, 850–869. [Google Scholar] [CrossRef]
- Sorita, G.D.; Leimann, F.V.; Ferreira, S.R.S. Biorefinery Approach: Is It an Upgrade Opportunity for Peanut by-Products? Trends Food Sci. Technol. 2020, 105, 56–69. [Google Scholar] [CrossRef]
- Abderrezag, N.; Bragagnolo, F.S.; Louaer, O.; Meniai, A.-H.; Cifuentes, A.; Ibáñez, E.; Mendiola, J.A. Optimization of Supercritical Fluid Extraction of Bioactive Compounds from Ammodaucus Leucotrichus Fruits by Using Multivariate Response Surface Methodology. J. Supercrit. Fluids 2024, 207, 106211. [Google Scholar] [CrossRef]
- Malenica, M.; Biesaga, M.; Pedisić, S.; Martinović, L.S. Stability of Propolis Phenolics during Ultrasound-Assisted Extraction Procedures. Foods 2024, 13, 2020. [Google Scholar] [CrossRef]
- Monroy, Y.M.; Rodrigues, R.A.F.; Rodrigues, M.V.N.; Sant’Ana, A.S.; Silva, B.S.; Cabral, F.A. Brazilian Green Propolis Extracts Obtained by Conventional Processes and by Processes at High Pressure with Supercritical Carbon Dioxide, Ethanol and Water. J. Supercrit. Fluids 2017, 130, 189–197. [Google Scholar] [CrossRef]
- Shin, S.-B.; Lee, J.-K.; Ko, M.-J. Application of a Subcritical Water Extraction Technique for Enhancing Extraction of Bioactive Compounds from Propolis. Sci. Rep. 2023, 1–16. [Google Scholar] [CrossRef]
- Rudke, A.R.; de Andrade, C.J.; Ferreira, S.R.S. Kappaphycus Alvarezii Macroalgae: An Unexplored and Valuable Biomass for Green Biorefinery Conversion. Trends Food Sci. Technol. 2020, 103, 214–224. [Google Scholar] [CrossRef]
- Sorita, G.D.; Favaro, S.P.; Ambrosi, A.; Di Luccio, M. Aqueous Extraction Processing: An Innovative and Sustainable Approach for Recovery of Unconventional Oils. Trends Food Sci. Technol. 2023, 133, 99–113. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep—Analytical Greenness Metric for Sample Preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- de Souza Mesquita, L.M.; Contieri, L.S.; e Silva, F.A.; Bagini, R.H.; Bragagnolo, F.S.; Strieder, M.M.; Sosa, F.H.B.; Schaeffer, N.; Freire, M.G.; Ventura, S.P.M.; et al. Path2Green: Introducing 12 Green Extraction Principles and a Novel Metric for Assessing Sustainability in Biomass Valorization. Green. Chem. 2024, 26, 10087–10106. [Google Scholar] [CrossRef]
- Fachri, B.A.; Sari, P.; Yuwanti, S.; Subroto, E. Experimental Study and Modeling on Supercritical CO2 Extraction of Indonesian Raw Propolis Using Response Surface Method: Influence of Pressure, Temperature and CO2 Mass Flowrate on Extraction Yield. Chem. Eng. Res. Des. 2020, 153, 452–462. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef] [PubMed]
- Gomes, K.O.; Messias da Silva, L.C.F.; dos Santos, R.D.; Prado, B.A.; da Silva Montes, P.; Silva Rodrigues, L.F.; de Araújo, M.O.; Bilac, C.A.; Freire, D.O.; Gris, E.F.; et al. Chemical Characterization and Antibacterial Activities of Brazilian Propolis Extracts from Apis mellifera Bees and Stingless Bees (Meliponini). PLoS ONE 2024, 19, e0307289. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Antunes, M.D.; Faleiro, M.L.; Miguel, M.G. Anti-acetylcholinesterase, Antidiabetic, Anti-inflammatory, Antityrosinase and Antixanthine Oxidase Activities of Moroccan Propolis. Int. J. Food Sci. Technol. 2016, 51, 1762–1773. [Google Scholar] [CrossRef]
- Osés, S.M.; Fernández-Muiño, M.A.; Rodríguez-Fernández, A.; Sancho, M.T.; Lázaro, R.; Bayarri, S. Phenolic Composition, Antiradical, Antimicrobial, and Anti-Inflammatory Activities of Propolis Extracts from North East Spain. J. Med. Food 2024, 27, 563–574. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Yanti, E.N.; Nisa, K.; Zulfa, A.F.; Batistuta, M.A. Bioactivity of Heterotrigona itama Propolis as Anti-Inflammatory: A Review. Biointerface Res. Appl. Chem. 2022, 13, 326. [Google Scholar] [CrossRef]
- Braun, S.; Jelača, S.; Laube, M.; George, S.; Hofmann, B.; Lönnecke, P.; Steinhilber, D.; Pietzsch, J.; Mijatović, S.; Maksimović-Ivanić, D.; et al. Synthesis and In Vitro Biological Evaluation of P-Carborane-Based Di-Tert-Butylphenol Analogs. Molecules 2023, 28, 4547. [Google Scholar] [CrossRef] [PubMed]
- Tandean, S.; Japardi, I.; Rusda, M.; Indharty, R.S.; Lelo, A.; Aman, R.A.; Amin, M.M.; Siahaan, A.M.P.; Eyanoer, P.C.; D’Prinzessin, C.A.; et al. Chemical Composition and Neuroprotective Properties of Indonesian Stingless Bee (Geniotrigona thoracica) Propolis Extract in an In-Vivo Model of Intracerebral Hemorrhage (ICH). Nutrients 2024, 16, 1880. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Shimazawa, M.; Mishima, S.; Hara, H. Water Extract of Propolis and Its Main Constituents, Caffeoylquinic Acid Derivatives, Exert Neuroprotective Effects via Antioxidant Actions. Life Sci. 2007, 80, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Cokdinleyen, M.; Alvarez-Rivera, G.; Tejera, J.L.G.; Mendiola, J.A.; Valdés, A.; Kara, H.; Ibáñez, E.; Cifuentes, A. Tetraselmis Chuii Edible Microalga as a New Source of Neuroprotective Compounds Obtained Using Fast Biosolvent Extraction. Int. J. Mol. Sci. 2024, 25, 3897. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibañez, E.; Cifuentes, A. In Vitro Neuroprotective Potential of Terpenes from Industrial Orange Juice By-Products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef]
- Ferro, D.M.; Mazzutti, S.; Vitali, L.; Oliveira Müller, C.M.; Ferreira, S.R.S. Integrated Extraction Approach to Increase the Recovery of Antioxidant Compounds from Sida rhombifolia Leaves. J. Supercrit. Fluids 2019, 149, 10–19. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Impact of the Use of Pressurized Liquids on the Extraction and Functionality of Proteins and Bioactives from Brewer’s Spent Grain. Food Chem. 2021, 359, 129874. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Dissanayaka, C.S. Phenolic-Protein Interactions: Insight from in-Silico Analyses—A Review. Food Prod. Process. Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Woźniak, M.; Sip, A.; Mrówczyńska, L.; Broniarczyk, J.; Waśkiewicz, A.; Ratajczak, I. Biological Activity and Chemical Composition of Propolis from Various Regions of Poland. Molecules 2022, 28, 141. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Modesto, Y.Y.; de Souza, P.D.Q.; Nascimento, F.C.d.A.; Pereira, R.R.; Converti, A.; Lynch, D.G.; Brasil, D.d.S.B.; da Silva, E.O.; Silva-Júnior, J.O.C.; et al. Characterization, Biocompatibility and Antioxidant Activity of Hydrogels Containing Propolis Extract as an Alternative Treatment in Wound Healing. Pharmaceuticals 2024, 17, 575. [Google Scholar] [CrossRef] [PubMed]
- Santana Neto, D.C.; Silva Paiva, T.; de Souza Tasso, I.; Matos Nascimento, K.; de Magalhães Cordeiro, Â.M.T.; de Albuquerque Meireles, B.R.L.; da Silva, F.A.P.; de Matos Jorge, L.M.; Maria Matos Jorge, R. A Comparison of the Antioxidant Properties of Two Different Brazilian Propolis. Microchem. J. 2024, 200, 110352. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activities of Antioxidant Peptides Obtained from Enzymatic Pea Protein Hydrolysates and Their Ultrafiltration Peptide Fractions. J. Food Biochem. 2022, 46, e14289. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical Characterization of Sulfated Polysaccharides from Gracilaria Gracilis and Ulva Lactuca and Their Radical Scavenging, Metal Chelating, and Cholinesterase Inhibitory Activities. Int. J. Food Prop. 2019, 22, 100–110. [Google Scholar] [CrossRef]
- Yassunaka Hata, N.N.; Surek, M.; Sartori, D.; Serrato, R.V.; Spinosa, W.A. Role of Acetic Acid Bacteria in Food and Beverages. Food Technol. Biotechnol. 2023, 61, 85–103. [Google Scholar] [CrossRef]
- Contieri, L.S.; de Souza Mesquita, L.M.; Sanches, V.L.; Chaves, J.; Pizani, R.S.; da Silva, L.C.; Viganó, J.; Ventura, S.P.M.; Rostagno, M.A. Recent Progress on the Recovery of Bioactive Compounds Obtained from Propolis as a Natural Resource: Processes, and Applications. Sep. Purif. Technol. 2022, 298, 121640. [Google Scholar] [CrossRef]
- Shin, S.B.; Lee, J.K.; Ko, M.J. Enhanced Extraction of Bioactive Compounds from Propolis (Apis mellifera L.) Using Subcritical Water. Sci. Rep. 2023, 13, 15038. [Google Scholar] [CrossRef] [PubMed]
- Putra, N.R.; Nur, D.; Ahmad, R.; Zaini, S.; Azizi, M.; Yunus, C.; Machmudah, S.; Syafiq, M.; Ruslan, H. Effect of Particle Size on Yield Extract and Antioxidant Activity of Peanut Skin Using Modified Supercritical Carbon Dioxide and Soxhlet Extraction. J. Food Process. Preserv. 2018, 42, e13689. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Christaki, S.; Biliaderis, C.G.; Chatzopoulou, P. Sustainable Recovery of Phenolic Compounds from Distilled Rosemary By-Product Using Green Extraction Methods: Optimization, Comparison, and Antioxidant Activity. Molecules 2023, 28, 6669. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, E.A.; Chea, J.D.; Yenkie, K.M. Systems Level Roadmap for Solvent Recovery and Reuse in Industries. iScience 2021, 24, 103114. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.; Alenzi, N.; Alenazi, F.; Tabassum, H.; Watson, D. Chemical Characterization of Saudi Propolis and Its Antiparasitic and Anticancer Properties. Sci. Rep. 2021, 11, 5390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zeng, Z.; Wu, Q.; Wang, X.; Qin, M.; Lei, S.; Cheng, S.; Xie, J. Loosely Coordinating Diluted Highly Concentrated Electrolyte toward −60 °C Li Metal Batteries. J. Energy Chem. 2024, 90, 380–387. [Google Scholar] [CrossRef]
- Gonzalo, G.; Alcántara, A.R.; Domínguez de María, P. Cyclopentyl Methyl Ether (CPME): A Versatile Eco-Friendly Solvent for Applications in Biotechnology and Biorefineries. ChemSusChem 2019, 12, 2083–2097. [Google Scholar] [CrossRef]
- Alaydi, H.; Downey, P.; McKeon-Bennett, M.; Beletskaya, T. Supercritical-CO2 Extraction, Identification and Quantification of Polyprenol as a Bioactive Ingredient from Irish Trees Species. Sci. Rep. 2021, 11, 7461. [Google Scholar] [CrossRef] [PubMed]
- Kosar, M.; Dorman, H.J.D.; Hiltunen, R. Effect of an Acid Treatment on the Phytochemical and Antioxidant Characteristics of Extracts from Selected Lamiaceae Species. Food Chem. 2005, 91, 525–533. [Google Scholar] [CrossRef]
- Dowd, L.E. Spectrophotometric Determination of Quercetin. Anal. Chem. 1959, 31, 1184–1187. [Google Scholar] [CrossRef]
- Mammen, D.; Daniel, M. A Critical Evaluation on the Reliability of Two Aluminum Chloride Chelation Methods for Quantification of Flavonoids. Food Chem. 2012, 135, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Whent, M.; Ping, T.; Kenworthy, W.; Yu, L. High-Throughput Assay for Detection of Soybean Lipoxygenase-1. J. Agric. Food Chem. 2010, 58, 12602–12607. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate Analysis by a Phenol–Sulfuric Acid Method in Microplate Format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]

| Responses | Predicted Values | Experimental Values | ||
|---|---|---|---|---|
| −95% | Optimum Values | +95% | ||
| Yield (%) | 51.0 | 56.7 | 62.4 | * 55.9 ± 0.1 |
| TPC (mg GAE g−1) | 147.0 | 166.7 | 186.4 | 149 ± 5 |
| ABTS (µmol TE g−1) | 2068.0 | 2496.0 | 2924.0 | 2455 ± 333 |
| DPPH (µmol TE g−1) | 598.2 | 634.7 | 671.3 | 620.0 ± 0.6 |
| LOX (mg QE g−1) | 14.9 | 21.5 | 28.0 | 15.2 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorita, G.D.; Caicedo Chacon, W.D.; Strieder, M.M.; Rodriguez-García, C.; Fritz, A.M.; Verruck, S.; Ayala Valencia, G.; Mendiola, J.A. Biorefining Brazilian Green Propolis: An Eco-Friendly Approach Based on a Sequential High-Pressure Extraction for Recovering High-Added-Value Compounds. Molecules 2025, 30, 189. https://doi.org/10.3390/molecules30010189
Sorita GD, Caicedo Chacon WD, Strieder MM, Rodriguez-García C, Fritz AM, Verruck S, Ayala Valencia G, Mendiola JA. Biorefining Brazilian Green Propolis: An Eco-Friendly Approach Based on a Sequential High-Pressure Extraction for Recovering High-Added-Value Compounds. Molecules. 2025; 30(1):189. https://doi.org/10.3390/molecules30010189
Chicago/Turabian StyleSorita, Guilherme Dallarmi, Wilson Daniel Caicedo Chacon, Monique Martins Strieder, Camilo Rodriguez-García, Alcilene Monteiro Fritz, Silvani Verruck, Germán Ayala Valencia, and José A. Mendiola. 2025. "Biorefining Brazilian Green Propolis: An Eco-Friendly Approach Based on a Sequential High-Pressure Extraction for Recovering High-Added-Value Compounds" Molecules 30, no. 1: 189. https://doi.org/10.3390/molecules30010189
APA StyleSorita, G. D., Caicedo Chacon, W. D., Strieder, M. M., Rodriguez-García, C., Fritz, A. M., Verruck, S., Ayala Valencia, G., & Mendiola, J. A. (2025). Biorefining Brazilian Green Propolis: An Eco-Friendly Approach Based on a Sequential High-Pressure Extraction for Recovering High-Added-Value Compounds. Molecules, 30(1), 189. https://doi.org/10.3390/molecules30010189









