A Review on Current Aspects of Curcumin-Based Effects in Relation to Neurodegenerative, Neuroinflammatory and Cerebrovascular Diseases
Abstract
:1. Introduction
2. Curcumin’s Metabolism and Bioavailability Related to Brain Physiology
2.1. Curcumin Metabolism
- Encapsulation in surface-modified poly (amidoamine) (PAMAM) dendrimers: Curcumin has been encapsulated in fourth-generation PAMAM dendrimers, which are surface-modified to improve BBB penetration. In vitro studies employing therapeutic quantities of encapsulated curcumin demonstrated significant reductions in the viability of glioblastoma cells from different species [26].
- Solid dispersion: Solid dispersion involves dispersing drugs in highly soluble carriers to enhance solubilisation, particularly for insoluble drugs like curcumin. Methods like hot melt extrusion, solvent evaporation, and spray drying are utilised to achieve fine-grain dispersion and improve solubility [27,28].
- Ultrasound-induced BBB opening: This is a non-invasive technique that avoids implants, making them attractive to patients. It enables targeted drug delivery to specific, widely distributed brain regions, with adjustable parameters like ultrasound settings and microbubble dosing. The method has been tested in various species, including rodents, large animals, and humans, targeting areas such as the hippocampus, striatum, and tumour tissues. While safe for repeated use, FUS is particularly suitable for therapies with infrequent dosing, like gene therapy, to reduce chronic exposure risks [29]. One application involves delivering curcumin to treat Parkinson’s disease (PD) in mice, using lipid-PLGA nanobubbles and low-intensity ultrasound to enhance effectiveness [30].
- Carrier-molecule conjugates: Another method proposes enhancing the ability of neurologically active compounds to penetrate the blood–nerve barrier (BNB) or BBB by administering conjugates comprising the active compound linked to carrier molecules with substantial permeability coefficients across the BNB and BBB [31].
2.2. Curcumin’s Bioavailability in the Central Nervous System
3. Curcumin and Neuro-Vascular Pathologies
3.1. Curcumin Effects in Brain Ischemia and Stroke
- (i)
- Anti-inflammatory and antioxidant effects [59]: Curcumin exhibits potent anti-inflammatory characteristics by suppressing the activation of inflammatory pathways and diminishing the production of pro-inflammatory mediators like IL-1β (interleukin-1 beta), IL-6 (interleukin-6), MCAP-1 and TNF-α with a prominent role of the IL-1β in hypoxia-related detrimental effects [60] as well as in the progression of ischemic brain injury, contributing to neuronal damage and exacerbating stroke outcomes. Curcumin’s ability to attenuate neuroinflammation [61] may help mitigate secondary brain injury and promote tissue repair following ischemic stroke by Akt/Nrf2 pathway stimulation, upregulation of the brain-derived neurotrophic factor (BDNF) expression, and suppression of the NAD(P)H: Quinone oxidoreductase 1 (NQO1) induced by brain hypoxia [62,63,64]. By scavenging free radicals and avoiding lipid peroxidation, curcumin also functions as a strong antioxidant [65] and upregulates endogenous antioxidant enzymes like GPx1, GPx4, CAT, and SOD1 [66,67]. By reducing oxidative damage, curcumin may protect neurons from ischemic injury and promote neuronal survival.
- (ii)
- Anti-apoptotic effects: Apoptosis is a prominent feature of ischemic stroke pathology, leading to neuronal loss and tissue damage [68]. Curcumin has been shown to modulate apoptotic pathways by downregulating the expression of pro-apoptotic [69] and upregulating the expression of the anti-apoptotic proteins [70], thereby promoting cell survival and reducing neuronal death and glial activation in ischemic conditions in the daily administration of ~ 2g/kg diet during 2 months [71]. Brain pro-apoptotic factors inhibited by curcumin are caspase-3, Fas and its ligand (FasL), Bax, Bcl2 [72,73] with notable differences as compared to cancer cells or other tissues where the brain anti-apoptotic signalling due to curcumin was observed as pro-apoptotic behaviour like PI2K/Akt inhibition, Fas/FasL upregulation of Bax/Bcl2 increasing [74,75,76]. Moreover, recent studies observed that curcumin inhibited proteasomal degradation and reduced apoptosis [77,78] but these findings must be subjected to more experimental data considering their action in neoplastic processes as a pro-tumoural agent.
- (iii)
- Neuroprotection against excitotoxicity, resulting from excessive glutamate release and subsequent calcium influx, contributes to neuronal injury in ischemic stroke [82]. Curcumin has been reported to modulate glutamate receptors, prevent calcium influx, and attenuate excitotoxic cell death by regulating excitotoxic signalling pathways [83]. Some authors observed the upregulation influence of curcumin on brain-derived neurotrophic factor after neuron exposure to 10 µM of sodium glutamate which was followed by decreased cell viability and improved cell apoptosis. Curcumin pretreatment of neurons resulted in a dose- and time-dependent reversal of BDNF expression and cell survival. Nevertheless, the survival-promoting impact of curcumin has been abolished when neurons are exposed to a Trk receptor inhibitor, which is known to suppress BDNF activation. Additionally, K252a, a Trk receptor inhibitor, inhibited curcumin’s upregulation of BDNF mRNA and protein. When combined, these findings implied that the BDNF/TrkB signalling pathway may be the mechanism via which curcumin exerts its neuroprotective effects [84].
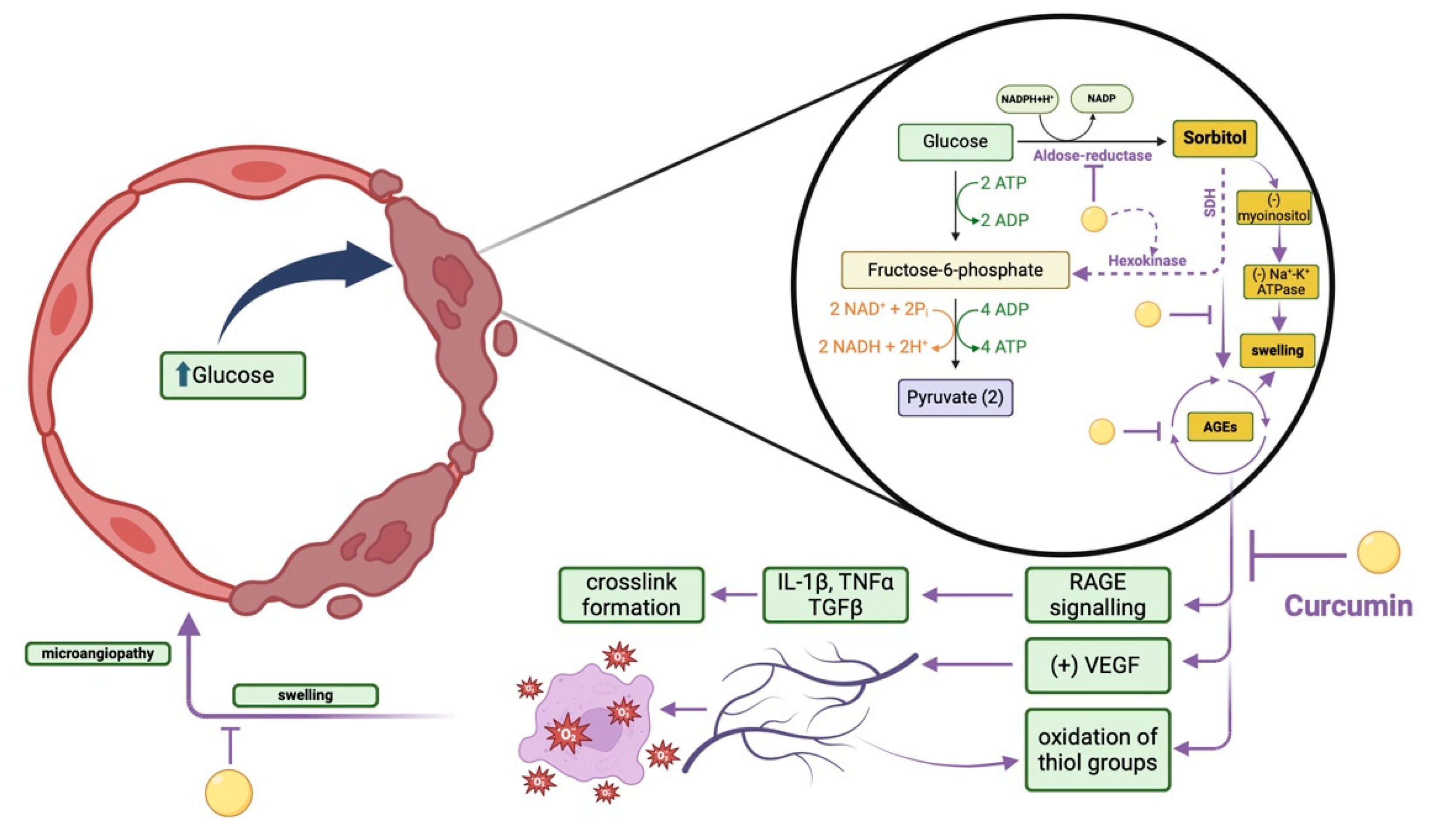
3.2. Curcumin Effects on Brain Microangiopathy
4. Curcumin in Neurodegeneration, Neuroinflammation and Schizophrenia
4.1. Curcumin Effects in Alzheimer’s Disease
4.1.1. Inhibition of Aβ Formation
4.1.2. Copper Chelation
4.1.3. Cholesterol-Lowering Effects
4.1.4. Anti-Inflammatory Activity
- (i)
- Aβ clearance: Curcumin has been shown to modulate the aggregation and clearance of Aβ peptides, which are thought to be essential in AD pathogenesis. Curcumin has been described to hinder the formation of Aβ fibrils, destabilise preformed fibrils, and promote the clearance of Aβ aggregates by enhancing microglial phagocytosis and proteasomal degradation pathways [142,143,144].
- (ii)
- Tau protein modification: Curcumin exhibits anti-tau properties by inhibiting the hyperphosphorylation of tau protein, a process linked with the formation of NFTs in AD brains [145]. Curcumin has been shown to inhibit kinases participating in tau phosphorylation, like glycogen synthase kinase-3β (GSK-3β), and promote tau dephosphorylation, thereby attenuating tau pathology and neuronal dysfunction [146].
- (iii)
- Anti-inflammatory effects: Chronic neuroinflammation is a hallmark feature of AD, having a role in neuronal impairment and cognitive deterioration. Curcumin possesses powerful anti-inflammatory properties by hindering the activation of microglia, and the resident immune cells of the brain, and suppressing the fabrication of pro-inflammatory cytokines and chemokines, including IL-1β, TNF-α, and IL-6 [147,148,149].
- (iv)
- Antioxidant Activity: Oxidative stress is essential in the pathogenesis of AD, contributing to neuronal destruction and the progression of neurodegeneration. Curcumin acts as an effective antioxidant by scavenging free radicals, inhibiting lipid peroxidation, and upregulating endogenous antioxidant enzymes, like superoxide dismutase (SOD) and catalase (CAT), thereby reducing oxidative damage and protecting neurons from oxidative stress [150,151].
- (v)
- Protection and synaptic function: Curcumin exerts neuroprotective effects by preserving neuronal viability, enhancing synaptic plasticity, and promoting neuronal survival. Curcumin has been shown to shield neurons against excitotoxicity, mitochondrial dysfunction, and apoptosis, while also promoting the expression of neurotrophic factors, like brain-derived neurotrophic factor (BDNF), which play a critical role in synaptic function and neuronal survival [153,154].
- (vi)
- (BBB) Integrity: Disruption of the BBB contributes to neuroinflammation and neuronal damage in AD. Curcumin has been reported to preserve BBB integrity by reducing endothelial permeability, inhibiting matrix metalloproteinases (MMP2, MMP9) activity, and modulating tight junction proteins, thereby limiting the pass of peripheral immune cells and inflammatory mediators into the brain parenchyma [155,156,157].
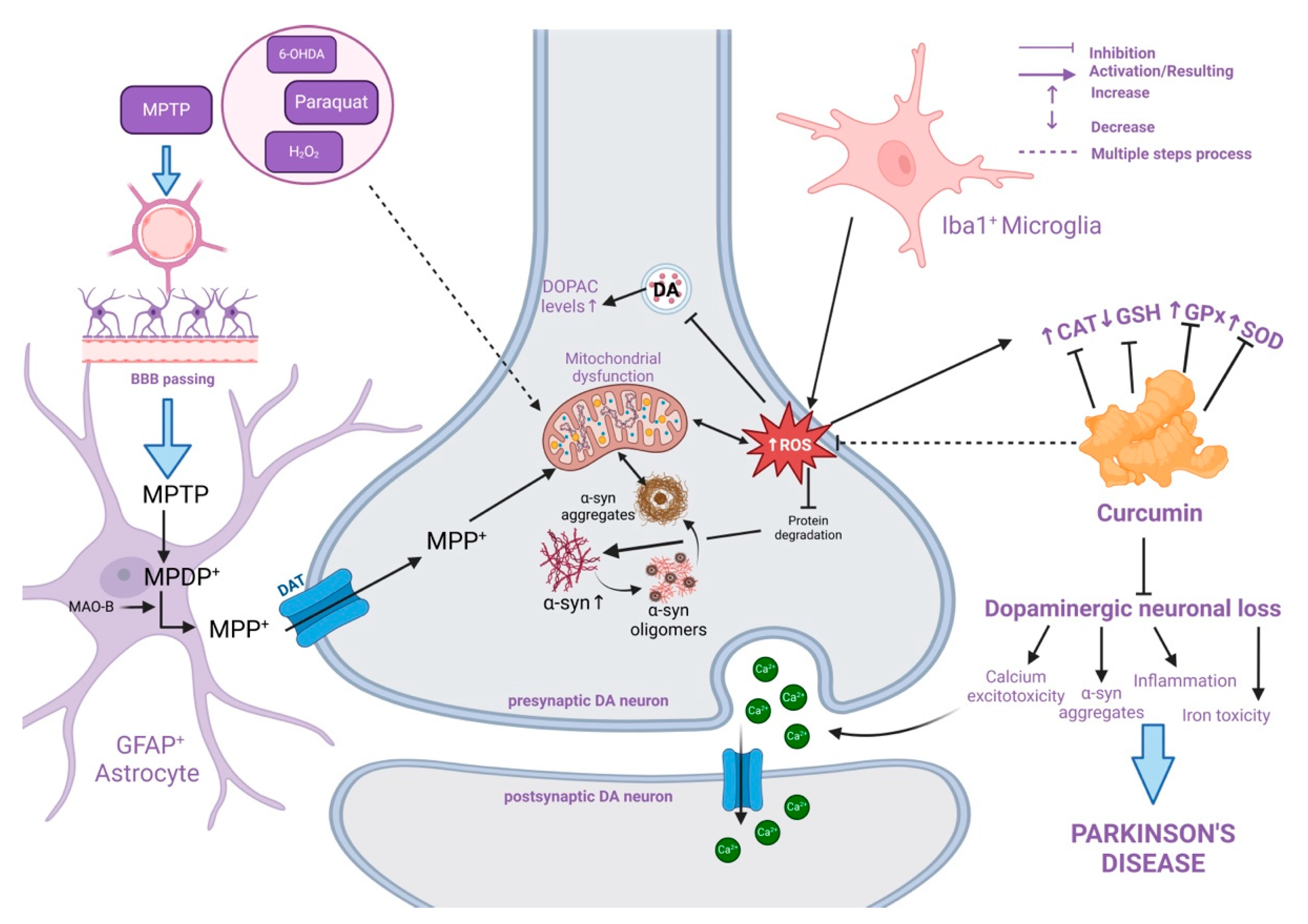
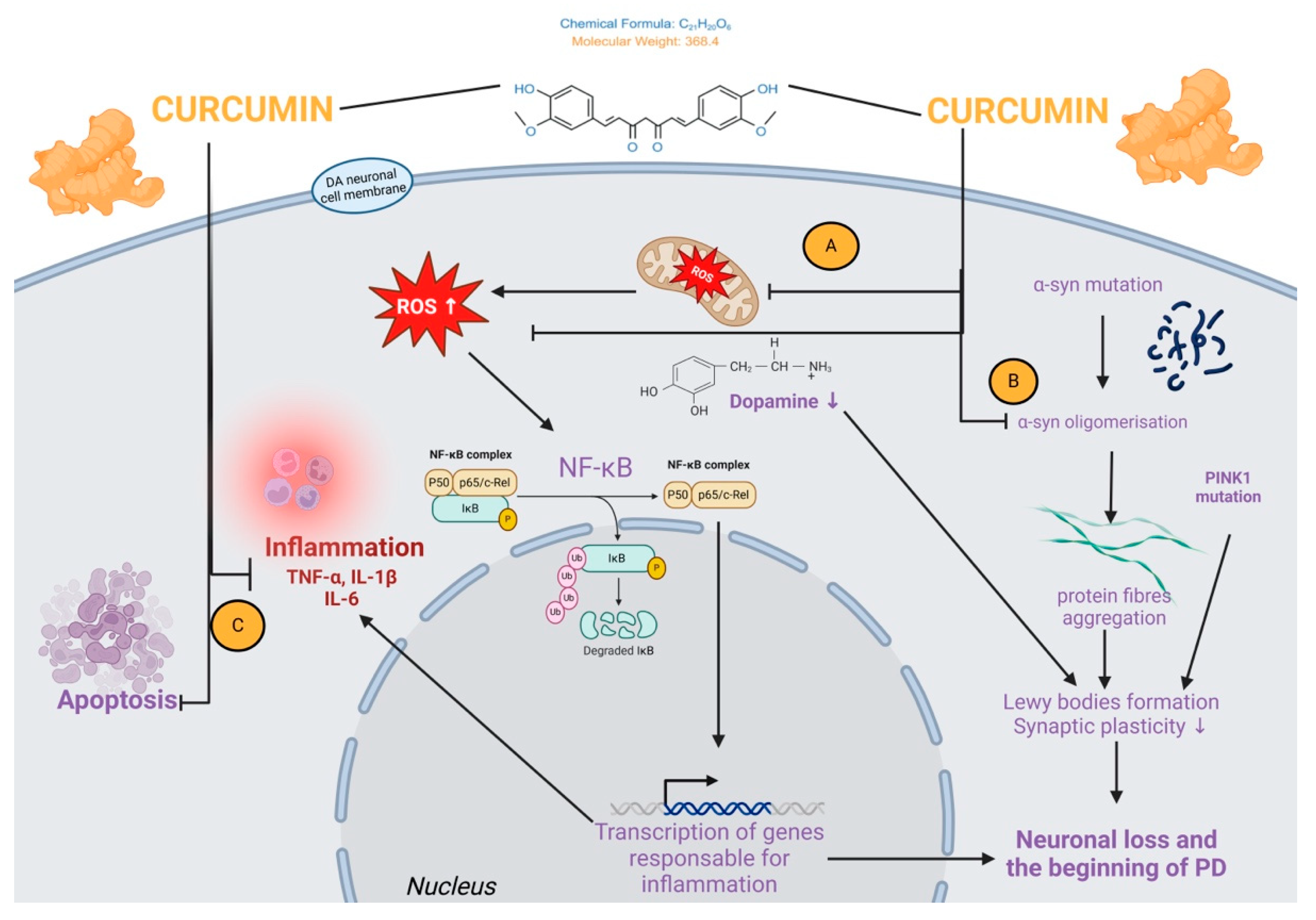
4.2. Curcumin Effects in Parkinson’s Disease
Experimental Models for PD
- (I)
- Paraquat and Rotenone
- (II)
- 6-OH-Dopamine
- (III)
- MPTP
- (IV)
- Lipopolysaccharide
4.3. Curcumin and Schizophrenia
5. Curcumin Effects on Inflammation Caused by Diabetic Neuropathy
6. Curcumin Effects in Metal-Induced Neurotoxicity
7. Current Challenges and Prospects Concerning Curcumin-Based Therapies
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
List of Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| AD | Alzheimer’s Disease |
| AGEs | advanced glycated endo-products |
| Aβ | amyloid-beta |
| b.w. | body weight |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| Cas-3 | caspase 3 |
| CAT | catalase |
| CGM | curcuma-galactomannosides |
| COX-2 | cyclooxygenase-2 |
| DAT | dopamine transporter |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| eNOS | endothelial nitric oxide synthase |
| GFAP+ | glial fibrillary acidic protein |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| GSK-3β | glycogen synthase kinase-3β |
| i.m. | intramuscular |
| i.p. | intraperitoneal |
| Iba1+ | ionised calcium-binding adaptor molecule 1 |
| IᴋB | inhibitor of NF-ᴋB |
| IL-1β | interleukin-1 beta |
| IL-6 | interleukin-6 |
| IRF | interferon regulatory factors |
| LDL | low-density lipoproteins |
| LPS | lipopolysaccharides |
| MAO | monoamine oxidase |
| MAO-B- | monoamine oxidase B |
| MAPK- | mitogen-activated protein kinase |
| MCAP-1- | monocyte chemoattractant protein-1 |
| MDA- | malondialdehyde |
| MMP-9- | matrix metalloproteinase 9 |
| MPDP+ | 1-methyl -4-phenyl-2,3-dihydropyridine |
| MPP+ | 1-methyl-4-phenylpyridine |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MTHFR | methylene tetrahydrofolate reductase |
| NDDs | neurodevelopmental diseases |
| NF-ᴋB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NFTs | neurofibrillary tangles |
| NLRP3 | NOD-like receptor pyrin domain-containing 3 |
| NMDA-N | methyl-D-aspartate |
| NO | nitric oxide |
| NQO1 | quinone oxidoreductase 1 |
| Nrf2 | nuclear factor-erythroid 2-related factor 2 |
| P50 p65/c-Rel | family of NF-ᴋB dimers |
| PAMAM | encapsulation in surface-modified polyamidoamine |
| PD | Parkinson’s Disease |
| PI3K | phosphatidylinositol 3-kinase |
| PINK1 | phosphatase and tensin homolog-induced kinase 1 |
| PPAR-γ | peroxisome proliferator-activated receptor-γ |
| Px | peroxidase |
| RAGE | receptor for AGE |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| SN | substantia nigra |
| SOD | superoxide dismutase |
| SREBPs | sterol regulatory element-binding proteins |
| TC | tetrahydrocurcumin |
| TGFβ | transforming growth factor-β |
| TH | tyrosine hydroxylase |
| TNF-α | tumour necrosis factor-α |
| TrkB | tropomyosin-related kinase receptor type B |
| VEGF | vascular endothelial growth factor |
References
- Davis, P.H.; Hachinski, V. Epidemiology of cerebrovascular disease. In Neuroepidemiology. A Tribute to Bruce Schoenberg, 1st ed.; Anderson, D.W., Schoenberg, D.G., Eds.; CRC Press: Boca Raton, FL, USA, 1991; p. 28. ISBN 9780429277276. [Google Scholar] [CrossRef]
- Biller, J.; Love, B.B. Ischemic cerebrovascular disease. In Neurology in Clinical Practice, 4th ed.; Bradley, W.G., Daroff, R.B., Fenichel, G.M., Jankovic, J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2004; Volume 1, pp. 1197–1250. ISBN 9997625889. [Google Scholar]
- An, S.J.; Kim, T.J.; Yoon, B.W. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: An update. J. Stroke 2017, 19, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Claas, S.A.; Arnett, D.K. The role of healthy lifestyle in the primordial prevention of cardiovascular disease. Curr. Cardiol. Rep. 2016, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Plant-based foods and prevention of cardiovascular disease: An overview. Am. J. Clin. Nutr. 2003, 78, 544S–551S. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T. A plant-based diet and stroke. J. Geriatr. Cardiol. JGC 2017, 14, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Jeyarani, S.; Choephel, T.; Manisha, C.; Antony, J. Recent Plant Based Remedies for Alzheimer’s Disease, Parkinson’s Disease and Cerebral Ischemic Stroke. Res. J. Pharm. Technol. 2019, 12, 3951–3959. [Google Scholar] [CrossRef]
- Baden, M.Y.; Shan, Z.; Wang, F.; Li, Y.; Manson, J.E.; Rimm, E.B.; Rexrode, K.M. Quality of plant-based diet and risk of total, ischemic, and hemorrhagic stroke. Neurology 2021, 96, e1940–e1953. [Google Scholar] [CrossRef]
- Bavarsad, K.; Barreto, G.E.; Hadjzadeh, M.A.; Sahebkar, A. Protective Effects of Curcumin against Ischemia-Reperfusion Injury in the Nervous System. Mol. Neurobiol. 2019, 56, 1391–1404. [Google Scholar] [CrossRef]
- Venkatesan, N.; Punithavathi, D.; Arumugam, V. Curcumin prevents adriamycin nephrotoxicity in rats. Br. J. Pharmacol. 2000, 129, 231–234. [Google Scholar] [CrossRef]
- Zhao, F.; Gong, Y.; Hu, Y.; Lu, M.; Wang, J.; Dong, J.; Chen, D.; Chen, L.; Fu, F.; Qiu, F. Curcumin and its major metabolites inhibit the inflammatory response induced by lipopolysaccharide: Translocation of nuclear factor-κB as potential target. Mol. Med. Rep. 2015, 11, 3087–3093. [Google Scholar] [CrossRef]
- Potter, W.Z. New era for novel CNS drug development. Neuropsychopharmacology 2012, 37, 278–280. [Google Scholar] [CrossRef]
- Zhongfa, L.; Chiu, M.; Wang, J.; Chen, W.; Yen, W.; Fan-Havard, P.; Yee, L.D.; Chan, K.K. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother. Pharmacol. 2012, 69, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef]
- Mythri, R.B.; Balusamy, J.; Pradhan, N.; Andersen, J.; Bharath, S. Mitochondrial complex I inhibition in Parkinson’s disease: How can curcumin protect mitochondria? Antioxid. Redox Signal. 2007, 9, 399–408. [Google Scholar] [CrossRef]
- Ma, Z.; Shayeganpour, A.; Brocks, D.R.; Lavasanifar, A.; Samuel, J. Highperformance liquid chromatography analysis of curcumin in rat plasma: Application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomed. Chromatogr. 2007, 21, 546–552. [Google Scholar] [CrossRef]
- Mohanty, C.; Sahoo, S.K. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 2010, 31, 6597–6611. [Google Scholar] [CrossRef]
- Yang, X.; Song, D.; Chen, L.; Xiao, H.; Ma, X.; Jiang, Q.; Cheng, O. Curcumin Promotes Neurogenesis of Hippocampal Dentate Gyrus via Wnt/β-Catenin Signal Pathway Following Cerebral Ischemia in Mice. Brain Res. 2021, 1751, 147197. [Google Scholar] [CrossRef]
- Katz, R.; Tomoaia-Cotisel, M. Lipophilic-Polycationic Delivery Systems. U.S. Patent 6.005.004, 21 December 1999. [Google Scholar]
- Jiang, J.; Wang, W.; Sun, Y.J.; Hu, M.; Li, F.; Zhu, D.Y. Neuroprotective Effect of Curcumin on Focal Cerebral Ischemic Rats by Preventing Blood-Brain Barrier Damage. Eur. J. Pharmacol. 2007, 561, 54–62. [Google Scholar] [CrossRef]
- Krishnakumar, I.M.; Abhilash, R.; Dinesh, K.; Ramadasan, K.; Balu, M. An enhanced bioavailable formulation of curcumin using fenugreek-derived soluble dietary fibre. J. Funct. Foods 2012, 4, 348–357. [Google Scholar] [CrossRef]
- Tsai, Y.-M.; Chien, C.-F.; Lin, L.-C.; Tsai, T.-H. Curcumin and its nano formulation: The kinetics of tissue distribution and blood brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.S.; Lui, E.; Majeed, M.; Vishwanatha, J.K.; Ranjan, A.P.; Maitra, A.; Pramanik, D.; Smith, J.A.; Helson, L. Differential distribution of intravenous curcumin formulations in the rat brain. Anticancer Res. 2011, 31, 907–911. [Google Scholar] [PubMed]
- Gallien, J.; Srinageshwar, B.; Gallo, K.; Holtgrefe, G.; Koneru, S.; Otero, P.S.; Bueno, C.A.; Mosher, J.; Roh, A.; Kohtz, D.S.; et al. Curcumin Loaded Dendrimers Specifically Reduce Viability of Glioblastoma Cell Lines. Molecules 2021, 26, 6050. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.T.; Potter, C.B.; Walker, G.M. Downstream processing of a ternary amorphous solid dispersion: The impacts of spray drying and hot melt extrusion on powder flow, compression and dissolution. Int. J. Pharm. 2018, 544, 242–253. [Google Scholar] [CrossRef]
- Poduslo, J.F.; Geoffrey, L. Method to Enhance Permeability of the Blood/Brain Blood/Nerve Barriers to Therapeutic Agents. U.S. Patent 5604198, 23 September 1997. [Google Scholar]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Y.; Liu, Z.; Cai, F.; Niu, W.; Song, L.; Liang, H.; Su, Z.; Yu, B.; Yan, F. Brain Delivery of Curcumin through Low-Intensity Ultrasound-Induced Blood–Brain Barrier Opening via Lipid-PLGA Nanobubbles. Int. J. Nanomed. 2021, 16, 7433–7447. [Google Scholar] [CrossRef]
- Huber, J.D.; Egleton, R.D.; Davis, T.P. Molecular Physiology and Pathophysiology of Tight Junctions in the Blood-Brain Barrier. Trends Neurosci. 2001, 24, 719–725. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, Maintenance and Disruption of the Blood-Brain Barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Alahmari, A. Blood-Brain Barrier Overview: Structural and Functional Correlation. Neural Plast. 2021, 2021, 6564585. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Barreto, E.G.; Henney, C.N.; Majeed, M.; Sahebkar, A. Neuroprotection by curcumin: A review on brain delivery strategies. IJP 2020, 585, 11947. [Google Scholar] [CrossRef]
- Cao, C.; Zhou, J.; Wu, X.; Qian, Y.; Hong, Y.; Mu, J.; Jin, L.; Zhu, C.; Li, S. Activation of CRHR1 contributes to cerebral endothelial barrier impairment via cPLA2 phosphorylation in experimental ischemic stroke. Cell Signal 2020, 66, 109467. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.I.; Beg, S.; Samad, A.; Baboota, S.; Kohli, K.; Ali, J.; Ahuja, A.; Akbar, M. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010, 40, 385–403. [Google Scholar] [CrossRef]
- Bueŭvić Popović, V.; Karahmet Farhat, E.; Banjari, I.; Jeličić Kadić, A.; Puljak, L. Bioavailability of Oral Curcumin in Systematic Reviews: A Methodological Study. Pharmaceuticals 2024, 17, 164. [Google Scholar] [CrossRef]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879. [Google Scholar] [CrossRef]
- De Oliveira, T.V.; Stein, R.; De Andrade, D.F.; Beck, R.C.R. Preclinical Studies of the Antitumor Effect of Curcumin-loaded Polymeric Nanocapsules: A Systematic Review and Meta-analysis. Phytother. Res. 2022, 36, 3202–3214. [Google Scholar] [CrossRef]
- Beltzig, L.; Frumkina, A.; Schwarzenbach, C.; Kaina, B. Cytotoxic, Genotoxic and Senolytic Potential of Native and Micellar Curcumin. Nutrients 2021, 13, 2385. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef]
- Sundaram, J.R.; Poore, C.P.; Sulaimee, N.H.B.; Pareek, T.; Cheong, W.F.; Wenk, M.R.; Pant, H.C.; Frautschy, S.A.; Low, C.-M.; Kesavapany, S. Curcumin Ameliorates Neuroinflammation, Neurodegeneration, and Memory Deficits in P25 Transgenic Mouse Model That Bears Hallmarks of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 1429–1442. [Google Scholar] [CrossRef]
- Park, C.-H.; Song, J.H.; Kim, S.-N.; Lee, J.H.; Lee, H.-J.; Kang, K.S.; Lim, H.-H. Neuroprotective Effects of Tetrahydrocurcumin against Glutamate-Induced Oxidative Stress in Hippocampal HT22 Cells. Molecules 2020, 25, 144. [Google Scholar] [CrossRef]
- Josifovska, S.; Panov, S.; Hadzi-Petrushev, N.; Mitrokhin, V.; Kamkin, A.; Stojchevski, R.; Avtanski, D.; Mladenov, M. Positive Tetrahydrocurcumin-Associated Brain-Related Metabolomic Implications. Molecules 2023, 28, 3734. [Google Scholar] [CrossRef] [PubMed]
- Sethi, P.; Jyoti, A.; Hussain, E.; Sharma, D. Curcumin attenuates aluminium induced functional neurotoxicity in rats. Pharmacol. Biochem. Behav. 2009, 93, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, S.; Morasso, C.; Stivaktakis, P.; Pandini, C.; Tinelli, V.; Tsatsakis, A.; Prosperi, D.; Hickey, M.; Corsi, F.; Cereda, C. Curcumin formulations and trials: What’s new in neurological diseases. Molecules 2020, 25, 5389. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.-T.; Dong, S.-Q.; Wang, S.-S.; Chen, M.; Li, C.-F.; Geng, D.; Zhu, J.-X.; Liu, Q.; Cheng, J. Curcumin attenuates cognitive impairment by enhancing autophagy in chemotherapy. Neurobiol. Dis. 2020, 136, 104715. [Google Scholar] [CrossRef]
- Maheshwari, K.R.; Singh, K.A.; Gaddipati, J.; Srimal, C.R. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.K.; Frautschy, S.; Cole, G. The Curry Spice Curcumin Reduces Oxidative Damage and Amyloid Pathology in an Alzheimer Transgenic Mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef]
- Krishnakumar, I.; Maliakel, A.; Gopakumar, G.; Kumar, D.; Maliakel, B.; Kuttan, R. Improved blood–brain-barrier permeability and tissue distribution following the oral administration of a food-grade formulation of curcumin with fenugreek fibre. J. Funct. Foods 2015, 14, 215–225. [Google Scholar] [CrossRef]
- Fan, F.; Lei, M. Mechanisms Underlying Curcumin-Induced Neuroprotection in Cerebral Ischemia. Front. Pharmacol. 2022, 13, 893118. [Google Scholar] [CrossRef]
- Srivastava, P.; Dhuriya, Y.K.; Gupta, R.; Shukla, R.K.; Yadav, R.S.; Dwivedi, H.N.; Pant, A.B.; Khanna, V.K. Protective Effect of Curcumin by Modulating BDNF/DARPP32/ CREB in Arsenic-Induced Alterations in Dopaminergic Signaling in Rat Corpus Striatum. Mol. Neurobiol. 2018, 55, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Ramaholimihaso, T.; Bouazzaoui, F.; Kaladjian, A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence-A Narrative Review. Front. Psychiatry 2020, 11, 572533. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Giacomucci, G.; Panaro, M.; Ruggiero, M.; Trotta, T.; Monda, V.; Pizzolorusso, I.; Lofrumento, D.D.; Porro, C.; Messina, G. New promising therapeutic avenues of curcumin in brain diseases. Molecules 2021, 27, 236. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Ghasemi, F.; Barreto, E.G.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of curcumin on mitochondria in neurodegenerative diseases. BioFactors 2020, 46, 5–20. [Google Scholar] [CrossRef]
- Dong, H.J.; Shang, C.Z.; Peng, D.W.; Xu, J.; Xu, P.X.; Zhan, L.; Wang, P. Curcumin attenuates ischemia-like injury-induced IL-1β elevation in brain microvascular endothelial cells via inhibiting MAPK pathways and nuclear factor-κB activation. Neurol. Sci. 2014, 35, 1387–1392. [Google Scholar] [CrossRef]
- Briones-Valdivieso, C.; Briones, F.; Orellana-Urzúa, S.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Novel Multi-Antioxidant Approach for Ischemic Stroke Therapy Targeting the Role of Oxidative Stress. Biomedicines 2024, 12, 501. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Wang, X.; Yu, S.; Li, L.; Wu, X.; Chen, Y.; Zhao, J.; Zhao, Y. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS ONE 2013, 8, e59843. [Google Scholar] [CrossRef]
- Sun, G.; Miao, Z.; Ye, Y.; Zhao, P.; Fan, L.; Bao, Z.; Tu, Y.; Li, C.; Chao, H.; Xu, X.; et al. Curcumin alleviates neuroinflammation, enhances hippocampal neurogenesis, and improves spatial memory after traumatic brain injury. Brain Res. Bull. 2020, 162, 84–93. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Asher, G.; Reiss, V.; Shaul, Y.; Sachs, L.; Lotem, J. Inhibition of NAD (P) H: Quinone oxidoreductase 1 activity and induction of p53 degradation by the natural phenolic compound curcumin. Proc. Natl. Acad. Sci. USA 2005, 102, 5535–5540. [Google Scholar] [CrossRef]
- Panahi, Y.; Ahmadi, Y.; Teymouri, M.; Johnston, T.P.; Sahebkar, A. Curcumin as a potential candidate for treating hyperlipidemia: A review of cellular and metabolic mechanisms. J. Cell. Physiol. 2018, 233, 141–152. [Google Scholar] [CrossRef]
- Bhowmick, S.; D’Mello, V.; Caruso, D.; Abdul-Muneer, P.M. Traumatic brain injury-induced downregulation of Nrf2 activates inflammatory response and apoptotic cell death. J. Mol. Med. 2019, 97, 1627–1641. [Google Scholar] [CrossRef] [PubMed]
- Meshkibaf, M.H.; Maleknia, M.; Noroozi, S. Effect of curcumin on gene expression and protein level of methionine sulfoxide reductase A (MSRA), SOD, CAT and GPx in Freund’s adjuvant inflammation-induced male rats. J. Inflamm. Res. 2019, 12, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective effects of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2007; pp. 197–212. [Google Scholar] [CrossRef]
- Manica, D.; da Silva, G.B.; da Silva, A.P.; Marafon, F.; Maciel, S.F.V.d.O.; Bagatini, M.D.; Moreno, M. Curcumin promotes apoptosis of human melanoma cells by caspase 3. Cell Biochem. Funct. 2023, 41, 1295–1304. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, A.Y.; Simonyi, A.; Jensen, M.D.; Shelat, P.B.; Rottinghaus, G.E.; MacDonald, R.S.; Miller, D.K.; Lubahn, D.E.; Weisman, G.A.; et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J. Neurosci. Res. 2005, 82, 138–148. [Google Scholar] [CrossRef]
- Goel, A.; Jhurani, S.; Aggarwal, B.B. Multi-targeted therapy by curcumin: How spicy is it? Mol. Nutr. Food Res. 2008, 52, 1010–1030. [Google Scholar] [CrossRef]
- Yu, L.; Fan, Y.; Ye, G.; Li, J.; Feng, X.; Lin, K.; Dong, M.; Wang, Z. Curcumin inhibits apoptosis and brain edema induced by hypoxia-hypercapnia brain damage in rat models. Am. J. Med. Sci. 2015, 349, 521–525. [Google Scholar] [CrossRef]
- Xie, C.J.; Gu, A.P.; Cai, J.; Wu, Y.; Chen, R.C. Curcumin protects neural cells against ischemic injury in N2a cells and mouse brain with ischemic stroke. Brain Behav. 2018, 8, e00921. [Google Scholar] [CrossRef]
- Choudhuri, T.; Pal, S.; Agwarwal, M.L.; Das, T.; Sa, G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS Lett. 2002, 512, 334–340. [Google Scholar] [CrossRef]
- Keçeci, M.; Ferah, M.A.; Khoshvaghti, H.; Karaçetin, S. Curcumin regulates inflammation and apoptosis through PARP-1 and NF-κB in ethanol-induced gastric ulcer model. Indian J. Exp. Biol. (IJEB) 2024, 62, 83–92. [Google Scholar] [CrossRef]
- Mortezaee, K.; Salehi, E.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Najafi, M.; Farhood, B.; Rosengren, R.J.; Sahebkar, A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019, 234, 12537–12550. [Google Scholar] [CrossRef]
- Shu, J.C.; He, Y.J.; Lv, X.; Ye, G.R.; Wang, L.X. Curcumin prevents liver fibrosis by inducing apoptosis and suppressing activation of hepatic stellate cells. J. Nat. Med. 2009, 63, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.; Leibel, R.; Tortoriello, D.V. Proteasome inhibitors, including curcumin, improve pancreatic β-cell function and insulin sensitivity in diabetic mice. Nutr. Diabetes 2016, 6, e205. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.X.; Wang, Y.; Qin, Z.H. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A. Maria Stefania Sinicropi Neuroprotective Effects of Curcumin in Neurodegenerative Diseases. Foods 2024, 13, 1774. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The Beneficial Role of Curcumin on Inflammation, Diabetes and Neurodegenerative Disease: A Recent Update. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Y.; Zhang, S.; Li, J.; Zheng, Y.; Fan, X. The Natural (Poly)Phenols as Modulators of Microglia Polarization via TLR4/NF-ΚB Pathway Exert Anti-Inflammatory Activity in Ischemic Stroke. Eur. J. Pharmacol. 2022, 914, 174660. [Google Scholar] [CrossRef]
- Sakul, A.A.; Balcikanli, Z.; Ozsoy, N.A.; Orhan, C.; Sahin, N.; Tuzcu, M.; Sahin, K. A highly bioavailable curcumin formulation ameliorates inflammation cytokines and neurotrophic factors in mice with traumatic brain injury. Chem. Biol. Drug Des. 2024, 103, e14439. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.B.; Li, Y.H.; Xu, Y.; Wu, H.L.; Li, X.J. Curcumin protects against glutamate excitotoxicity in rat cerebral cortical neurons by increasing brain-derived neurotrophic factor level and activating TrkB. Brain Res. 2008, 1210, 84–91. [Google Scholar] [CrossRef]
- Beuker, C.; Strecker, J.K.; Rawal, R.; Schmidt-Pogoda, A.; Ruck, T.; Wiendl, H.; Klotz, L.; Schabitz, W.R.; Sommer, C.J.; Minnerup, H.; et al. Immune cell infiltration into the brain after ischemic stroke in humans compared to mice and rats: A systematic review and meta-analysis. Transl. Stroke Res. 2021, 12, 976–990. [Google Scholar] [CrossRef]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef]
- Fan, Z.; Jing, H.; Yao, J.; Li, Y.; Hu, X.; Shao, H.; Shen, G.; Pan, J.; Luo, F.; Tian, X. The Protective Effects of Curcumin on Experimental Acute Liver Lesion Induced by Intestinal Ischemia-Reperfusion through Inhibiting the Pathway of NF-κB in a Rat Model. Oxidative Med. Cell. Longev. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benameur, T.; Frota Gaban, S.V.; Giacomucci, G.; Filannino, F.M.; Trotta, T.; Polito, R.; Messina, G.; Porro, C.; Panaro, M.A. The Effects of Curcumin on Inflammasome: Latest Update. Molecules 2023, 28, 742. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.-F.; Zhang, X.; Liu, Q.; Xie, Y.; Chen, Z.; Chen, J.; Li, Y.; Guo, H.; Sun, R.; Hong, Y.; et al. Microglial TREM-1 Receptor Mediates Neuroinflammatory Injury via Interaction with SYK in Experimental Ischemic Stroke. Cell Death Dis. 2019, 10, 555. [Google Scholar] [CrossRef]
- Ran, Y.; Su, W.; Gao, F.; Ding, Z.; Yang, S.; Ye, L.; Chen, X.; Tian, G.; Xi, J.; Liu, Z. Curcumin Ameliorates White Matter Injury after Ischemic Stroke by Inhibiting Microglia/Macrophage Pyroptosis through NF-ΚB Suppression and NLRP3 Inflammasome Inhibition. Oxidative Med. Cell. Longev. 2021, 2021, 1552127. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, J.; Li, S.-Y. Nano-Curcumin Simultaneously Protects the Blood–Brain Barrier and Reduces M1 Microglial Activation during Cerebral Ischemia–Reperfusion Injury. ACS Appl. Mater. Interfaces 2019, 11, 3763–3770. [Google Scholar] [CrossRef]
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The role of selected pro-inflammatory cytokines in pathogenesis of ischemic stroke. Clin. Interv. Aging 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Welsh, P.; Lowe, G.D.; Chalmers, J.; Campbell, D.J.; Rumley, A.; Neal, B.C.; MacMahon, S.W.; Woodward, M. Associations of proinflammatory cytokines with the risk of recurrent stroke. Stroke 2008, 39, 2226–2230. [Google Scholar] [CrossRef]
- Datta, A.; Sarmah, D.; Mounica, L.; Kaur, H.; Kesharwani, R.; Verma, G.; Veeresh, P.; Kotian, V.; Kalia, K.; Borah, A.; et al. Cell death pathways in ischemic stroke and targeted pharmacotherapy. Transl. Stroke Res. 2020, 11, 1185–1202. [Google Scholar] [CrossRef]
- Wu, F.; Lin, Y.; Xiao, L.; Chen, Q.; Lin, F.; Li, R. Administration with curcumin alleviates spinal cord ischemia-reperfusion injury by regulating anti-oxidative stress and microglia activation-mediated neuroinflammation via Nrf2/NF-κB axis. Vitr. Cell. Dev. Biol.-Anim. 2024, 60, 172–182. [Google Scholar] [CrossRef]
- Ghasemi, F.; Bagheri, H.; Barreto, G.E.; Read, M.I.; Sahebkar, A. Effects of curcumin on microglial cells. Neurotox. Res. 2019, 36, 12–26. [Google Scholar] [CrossRef]
- Moustapha, A.; Pérétout, P.A.; Rainey, N.E.; Sureau, F.; Geze, M.; Petit, J.M.; Petit, P.X. Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discov. 2015, 1, 15017. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin prevents reperfusion injury following ischemic stroke in rats via inhibition of NF-κB, ICAM-1, MMP-9 and caspase-3 expression. Mol. Med. Rep. 2017, 16, 4710–4720. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dai, M.; Wang, Y.; Wang, W.; Sun, Q.; Yang, G.Y.; Bian, L. Neuroprotection and sensorimotor functional improvement by curcumin after intracerebral hemorrhage in mice. J. Neurotrauma 2011, 28, 2513–2521. [Google Scholar] [CrossRef]
- Benameur, T.; Panaro, M.A.; Ruggiero, M.; Messina, G.; Messina, A.; Polito, R.; Trotta, T.; Pizzolorusso, I.; Porro, C. Neuroprotection induced by curcumin. In Natural Molecules in Neuroprotection and Neurotoxicity; Academic Press: Cambridge, MA, USA, 2024; pp. 1441–1463. [Google Scholar] [CrossRef]
- Reeder, B.J. Globin Associated Oxidative Stress. Antioxidants 2023, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp. Neurol. 2006, 197, 309–317. [Google Scholar] [CrossRef]
- Bhavani, T.; Gautam, A. Expression analysis of synaptic plasticity genes in curcumin-treated amnesic mice. Mater. Today Proc. 2023, 73, 307–311. [Google Scholar] [CrossRef]
- Sankrityayan, H.; Majumdar, A.S. Curcumin and folic acid abrogated methotrexate induced vascular endothelial dysfunction. Can. J. Physiol. Pharmacol. 2016, 94, 89–96. [Google Scholar] [CrossRef]
- Cheriki, M.; Habibian, M.; Moosavi, S.J. Curcumin Attenuates Brain Aging by Reducing Apoptosis and Oxidative Stress. Metab. Brain Dis. 2024, 39, 833–840. [Google Scholar] [CrossRef]
- Abdolahi, M.; Jafarieh, A.; Sarraf, P.; Sedighiyan, M.; Yousefi, A.; Tafakhori, A.; Abdollahi, H.; Salehinia, F.; Djalali, M. The Neuromodulatory Effects of ω-3 Fatty Acids and Nano-Curcumin on the COX-2/ INOS Network in Migraines: A Clinical Trial Study from Gene Expression to Clinical Symptoms. Endocr. Metab. Immune Disord.-Drug Targets 2019, 19, 874–884. [Google Scholar] [CrossRef]
- Abdolahi, M.; Sarraf, P.; Javanbakht, M.H.; Honarvar, N.M.; Hatami, M.; Soveyd, N.; Tafakhori, A.; Sedighiyan, M.; Djalali, M.; Jafarieh, A.; et al. A Novel Combination of ω-3 Fatty Acids and Nano-Curcumin Modulates Interleukin-6 Gene Expression and High Sensitivity C-Reactive Protein Serum Levels in Patients with Migraine: A Randomized Clinical Trial Study. CNS Neurol. Disord.-Drug Targets 2018, 17, 430–438. [Google Scholar] [CrossRef]
- Abdolahi, M.; Karimi, E.; Sarraf, P.; Tafakhori, A.; Siri, G.; Salehinia, F.; Sedighiyan, M.; Asanjarani, B.; Badeli, M.; Abdollahi, H.; et al. The Omega-3 and Nano-Curcumin Effects on Vascular Cell Adhesion Molecule (VCAM) in Episodic Migraine Patients: A Randomized Clinical Trial. BMC Res. Notes 2021, 14, 283. [Google Scholar] [CrossRef] [PubMed]
- Chico, L.; Ienco, E.C.; Bisordi, C.; Lo Gerfo, A.; Petrozzi, L.; Petrucci, A.; Mancuso, M.; Siciliano, G. Amyotrophic Lateral Sclerosis and Oxidative Stress: A Double-Blind Therapeutic Trial after Curcumin Supplementation. CNS Neurol. Disord. Drug Targets 2018, 17, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Djalali, M.; Abdolahi, M.; Hosseini, R.; Miraghajani, M.; Mohammadi, H.; Djalali, M. The Effects of Nano-Curcumin Supplementation on Th1/Th17 Balance in Migraine Patients: A Randomized Controlled Clinical Trial. Complement. Ther. Clin. Pract. 2020, 41, 101256. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Hou, Z.; Zhong, L.; Zhang, Q. Effect of Curcumin on the Induction of Glutathione S-Transferases and NADP(H):Quinone Oxidoreductase and Its Possible Mechanism of Action. PubMed 2007, 42, 376–380. [Google Scholar]
- Abdolahi, M.; Tafakhori, A.; Togha, M.; Okhovat, A.A.; Siassi, F.; Eshraghian, M.R.; Sedighiyan, M.; Djalali, M.; Mohammadzadeh Honarvar, N.; Djalali, M. The Synergistic Effects of ω-3 Fatty Acids and Nano-Curcumin Supplementation on Tumor Necrosis Factor (TNF)-α Gene Expression and Serum Level in Migraine Patients. Immunogenetics 2017, 69, 371–378. [Google Scholar] [CrossRef]
- Ahmadi, M.; Agah, E.; Nafissi, S.; Jaafari, M.R.; Harirchian, M.H.; Sarraf, P.; Faghihi-Kashani, S.; Hosseini, S.J.; Ghoreishi, A.; Aghamollaii, V.; et al. Safety and Efficacy of Nanocurcumin as Add-on Therapy to Riluzole in Patients with Amyotrophic Lateral Sclerosis: A Pilot Randomized Clinical Trial. Neurotherapeutics 2018, 15, 430–438. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.-K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients with Alzheimer Disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Djalali, M.; Abdolahi, M.; Hosseini, R.; Miraghajani, M.; Mohammadi, H.; Djalali, M. The Effects of Nano-Curcumin Supplementation on Th2/Tregulatory Axis in Migraine Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Int. J. Neurosci. 2021, 133, 169–175. [Google Scholar] [CrossRef]
- Dolati, S.; Aghebati-Maleki, L.; Ahmadi, M.; Marofi, F.; Babaloo, Z.; Ayramloo, H.; Jafarisavari, Z.; Oskouei, H.; Afkham, A.; Younesi, V.; et al. Nanocurcumin Restores Aberrant MiRNA Expression Profile in Multiple Sclerosis, Randomized, Double-Blind, Placebo-Controlled Trial. J. Cell. Physiol. 2018, 233, 5222–5230. [Google Scholar] [CrossRef]
- Dolati, S.; Ahmadi, M.; Aghebti-Maleki, L.; Nikmaram, A.; Marofi, F.; Rikhtegar, R.; Ayromlou, H.; Yousefi, M. Nanocurcumin Is a Potential Novel Therapy for Multiple Sclerosis by Influencing Inflammatory Mediators. Pharmacol. Rep. 2018, 70, 1158–1167. [Google Scholar] [CrossRef]
- Dolati, S.; Babaloo, Z.; Ayromlou, H.; Ahmadi, M.; Rikhtegar, R.; Rostamzadeh, D.; Roshangar, L.; Nouri, M.; Mehdizadeh, A.; Younesi, V.; et al. Nanocurcumin Improves Regulatory T-Cell Frequency and Function in Patients with Multiple Sclerosis. J. Neuroimmunol. 2019, 327, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Dützmann, S.; Schiborr, C.; Kocher, A.; Pilatus, U.; Hattingen, E.; Weissenberger, J.; Geßler, F.; Quick-Weller, J.; Franz, K.; Seifert, V.; et al. Intratumoral Concentrations and Effects of Orally Administered Micellar Curcuminoids in Glioblastoma Patients. Nutr. Cancer 2016, 68, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Honarvar, N.M.; Soveid, N.; Abdolahi, M.; Djalali, M.; Hatami, M.; Karzar, N.H. Anti-Neuroinflammatory Properties of N-3 Fatty Acids and Nano- Curcumin on Migraine Patients from Cellular to Clinical Insight: A Randomized, Double-Blind and Placebo-Controlled Trial. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Sarraf, P.; Javanbakht, M.H.; Foroushani, A.R.; Ranji-Burachaloo, S.; Djalali, M. The Synergistic Effects of Nano-Curcumin and Coenzyme Q10 Supplementation in Migraine Prophylaxis: A Randomized, Placebo-Controlled, Double-Blind Trial. Nutr. Neurosci. 2019, 24, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral Curcumin for Alzheimer’s Disease: Tolerability and Efficacy in a 24-Week Randomized, Double Blind, Placebo-Controlled Study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef]
- Soveyd, N.; Abdolahi, M.; Djalali, M.; Hatami, M.; Tafakhori, A.; Sarraf, P.; Honarvar, N.M. The Combined Effects of ω -3 Fatty Acids and Nano-Curcumin Supplementation on Intercellular Adhesion Molecule-1 (ICAM-1) Gene Expression and Serum Levels in Migraine Patients. CNS Neurol. Disord.-Drug Targets 2018, 16, 1120–1126. [Google Scholar] [CrossRef]
- Mohseni, M.; Sahebkar, A.; Askari, G.; Johnston, T.P.; Alikiaii, B.; Bagherniya, M. The Clinical Use of Curcumin on Neurological Disorders: An Updated Systematic Review of Clinical Trials. Phytother. Res. 2021, 35, 6862–6882. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, H.; Wang, P.; Zhao, Y.; Li, Q.; Wei, X.; Cui, Y.; Sun, J.; Shang, Q.; Liu, D.; et al. Dietary curcumin ameliorates aging-related cerebrovascular dysfunction through the AMPK/uncoupling protein 2 pathway. Cell. Physiol. Biochem. 2013, 32, 1167–1177. [Google Scholar] [CrossRef]
- Mohammadian Haftcheshmeh, S.; Karimzadeh, M.R.; Azhdari, S.; Vahedi, P.; Abdollahi, E.; Momtazi-Borojeni, A.A. Modulatory effects of curcumin on the atherogenic activities of inflammatory monocytes: Evidence from in vitro and animal models of human atherosclerosis. BioFactors 2020, 46, 341–355. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P. Neuroprotective effects of curcumin in cerebral ischemia: Cellular and molecular mechanisms. ACS Chem. Neurosci. 2021, 12, 2562–2572. [Google Scholar] [CrossRef]
- Wakade, C.; King, M.D.; Laird, M.D.; Alleyne Jr, C.H.; Dhandapani, K.M. Curcumin attenuates vascular inflammation and cerebral vasospasm after subarachnoid hemorrhage in mice. Antioxid. Redox Signal. 2009, 11, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.O.; Kostiev, F.I.; Borisov, O.V.; Dekhtiar, Y.M.; Vit, V.V.; Molchaniuk, N.I.; Mikheytseva, I.M.; Kolomiichuk, S.G.; Ahmed, A. Effect of experimental type 2 diabetes complicated by pyelonephritis on ultrastructural changes in the choroid, retina and nephrons. J. Ophthalmol. Ukr. 2022, 3, 32–38. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Butler, A.E.; Jamialahmadi, T.; Gumpricht, E.; Sahebkar, A. The beneficial effects of curcumin on lipids: Possible effects on dyslipidemia-induced cardiovascular complications. Curr. Med. Chem. 2024, 31, 6957–6970. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Bose, S.; Kataria, T.; Tyagi, A.; Singla, K.; Sharma, S.; Ghosh, S.; Jha, C.B. Curcumin as a Potential Phytoconstituent used for Cancer Treatment: An Overview. Nat. Prod. J. 2024, 14, e160823219803. [Google Scholar] [CrossRef]
- Muthenna, P.; Suryanarayana, P.; Gunda, S.K.; Petrash, J.M.; Reddy, G.B. Inhibition of aldose reductase by dietary antioxidant curcumin: Mechanism of inhibition, specificity and significance. FEBS Lett. 2009, 583, 3637–3642. [Google Scholar] [CrossRef]
- Ramaswami, G.; Chai, H.; Yao, Q.; Lin, P.H.; Lumsden, A.B.; Chen, C. Curcumin Blocks Homocysteine-Induced Endothelial Dysfunction in Porcine Coronary Arteries. J. Vasc. Surg. 2004, 40, 1216–1222. [Google Scholar] [CrossRef]
- Clarke, R.; Halsey, J.; Bennett, D.; Lewington, S. Homocysteine and Vascular Disease: Review of Published Results of the Homocysteine-Lowering Trials. J. Inherit. Metab. Dis. 2010, 34, 83–91. [Google Scholar] [CrossRef]
- ROBINSON, K. Homocysteine, B Vitamins, and Risk of Cardiovascular Disease. Heart 2000, 83, 127–130. [Google Scholar] [CrossRef]
- Froldi, G.; Ragazzi, E. Selected Plant-Derived Polyphenols as Potential Therapeutic Agents for Peripheral Artery Disease: Molecular Mechanisms, Efficacy and Safety. Molecules 2022, 27, 7110. [Google Scholar] [CrossRef]
- Yang, X.; Thomas, D.P.; Zhang, X.; Culver, B.W.; Alexander, B.M.; Murdoch, W.J.; Rao, M.N.A.; Tulis, D.A.; Ren, J.; Sreejayan, N. Curcumin Inhibits Platelet-Derived Growth Factor–Stimulated Vascular Smooth Muscle Cell Function and Injury-Induced Neointima Formation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 85–90. [Google Scholar] [CrossRef]
- Tang, M.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Picciano, A.L.; Vaden, T.D. Complexation between Cu(II) and Curcumin in the Presence of Two Different Segments of Amyloid β. Biophys. Chem. 2013, 184, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, Y. Hypocholesterolemic Effects of Curcumin via Up-Regulation of Cholesterol 7a-Hydroxylase in Rats Fed a High Fat Diet. Nutr. Res. Pract. 2010, 4, 191. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zheng, Z.; Li, J.; Xiao, Z.; Qi, W.; Zhang, A.; Wu, Q.; Fang, Y. Curcumin Inhibits Aβ-Induced Microglial Inflammatory Responses in Vitro: Involvement of ERK1/2 and P38 Signaling Pathways. Neurosci. Lett. 2015, 594, 105–110. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on Alzheimer’s disease pathophysiology and its management: An update. Pharmacol. Rep. 2015, 67, 195–203. [Google Scholar] [CrossRef]
- Thapa, A.; Jett, S.D.; Chi, E.Y. Curcumin attenuates amyloid-β aggregate toxicity and modulates amyloid-β aggregation pathway. ACS Chem. Neurosci. 2016, 7, 56–68. [Google Scholar] [CrossRef]
- Kotani, R.; Urano, Y.; Sugimoto, H.; Noguchi, N. Decrease of amyloid-β levels by curcumin derivative via modulation of amyloid-β protein precursor trafficking. J. Alzheimer’s Dis. 2017, 56, 529–542. [Google Scholar] [CrossRef]
- Manap, A.S.A.; Madhavan, P.; Vijayabalan, S.; Chia, A.; Fukui, K. Explicating anti-amyloidogenic role of curcumin and piperine via amyloid beta (Aβ) explicit pathway: Recovery and reversal paradigm effects. Peer J. 2020, 8, e10003. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Cocco, L.; Ratti, S.; Martelli, A.M.; Candido, S.; Libra, M.; Montalto, G.; et al. Regulation of GSK-3 activity by curcumin, berberine and resveratrol: Potential effects on multiple diseases. Adv. Biol. Regul. 2017, 65, 77–88. [Google Scholar] [CrossRef]
- Ray, B.; Lahiri, D.K. Neuroinflammation in Alzheimer’s disease: Different molecular targets and potential therapeutic agents including curcumin. Curr. Opin. Pharmacol. 2009, 9, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Zahedipour, F.; Hosseini, S.A.; Henney, N.C.; Barreto, G.E.; Sahebkar, A. Phytochemicals as inhibitors of tumor necrosis factor alpha and neuroinflammatory responses in neurodegenerative diseases. Neural Regen. Res. 2022, 17, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A. Effects of curcumin on neuroinflammation in animal models and in patients with Alzheimer disease. In Therapeutic Potentials of Curcumin for Alzheimer Disease, 1st ed.; Springer: Cham, Switzerland, 2016; pp. 259–296. [Google Scholar] [CrossRef]
- Chainoglou, E.; Hadjipavlou-Litina, D. Curcumin in health and diseases: Alzheimer’s disease and curcumin analogues, derivatives, and hybrids. Int. J. Mol. Sci. 2020, 21, 1975. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, T.; Awuah, W.A.; Mikhailova, T.; Kalmanovich, J.; Mehta, A.; Ng, J.C.; Ashraf, G.M. Antioxidant, anti-inflammatory and epigenetic potential of curcumin in Alzheimer’s disease. BioFactors 2024, 50, 693–708. [Google Scholar] [CrossRef]
- Chin, D.; Huebbe, P.; Pallauf, K.; Rimbach, G. Neuroprotective properties of curcumin in Alzheimer’s disease-merits and limitations. Curr. Med. Chem. 2013, 20, 3955–3985. [Google Scholar] [CrossRef]
- Hoppe, J.B.; Haag, M.; Whalley, B.J.; Salbego, C.G.; Cimarosti, H. Curcumin protects organotypic hippocampal slice cultures from Aβ1–42-induced synaptic toxicity. Toxicol. Vitr. 2013, 27, 2325–2330. [Google Scholar] [CrossRef]
- Dong, S.; Zeng, Q.; Mitchell, E.S.; Xiu, J.; Duan, Y.; Li, C.; Tiwari, J.K.; Hu, Y.; Cao, X.; Zhao, Z. Curcumin Enhances Neurogenesis and Cognition in Aged Rats: Implications for Transcriptional Interactions Related to Growth and Synaptic Plasticity. PLoS ONE 2012, 7, e31211. [Google Scholar] [CrossRef]
- SoukhakLari, R.; Moezi, L.; Pirsalami, F.; Moosavi, M. The effect of BSA-Based Curcumin Nanoparticles on memory and hippocampal MMP-2, MMP-9, and MAPKs in adult mice. J. Mol. Neurosci. 2018, 65, 319–326. [Google Scholar] [CrossRef]
- Hagl, S.; Heinrich, M.; Kocher, A.; Schiborr, C.; Frank, J.; Eckert, G.P. Curcumin micelles improve mitochondrial function in a mouse model of Alzheimer’s disease. J. Prev. Alzheimer’s Dis. 2014, 1, 80–83. [Google Scholar] [CrossRef]
- Cheng, K.K.; Yeung, C.F.; Ho, S.W.; Chow, S.F.; Chow, A.H.; Baum, L. Highly stabilized curcumin nanoparticles tested in an in vitro blood–brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013, 15, 324–336. [Google Scholar] [CrossRef]
- Kakkar, V.; Kumari, P.; Kaur, J.; Chholta, S. Curcumin Nanoformulations in Neurodegenerative Diseases. In Curcumin and Neurodegenerative Diseases: From Traditional to Translational Medicines; Springer Nature: Singapore, 2024; pp. 379–402. [Google Scholar]
- Wynn, J.K.; Green, M.F.; Hellemann, G.; Karunaratne, K.; Davis, M.C.; Marder, S.R. The effects of curcumin on brain-derived neurotrophic factor and cognition in schizophrenia: A randomized controlled study. Schizophr. Res. 2018, 195, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Laabdar, W.; Elgot, A.; Gamrani, H. The protective effect of curcumin on dopaminergic system after chronic aluminium intoxication: Possible link with Parkinson’s disease. Park. Relat. Disord. 2016, 22, e188. [Google Scholar] [CrossRef]
- Bartels, A.L.; Leenders, K.L. Parkinson’s disease: The syndrome, the pathogenesis and pathophysiology. Cortex 2009, 45, 915–921. [Google Scholar] [CrossRef]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef]
- Mythri, R.B.; Bharath, M.M. Curcumin: A potential neuroprotective agent in Parkinson’s disease. Curr. Pharm. Des. 2012, 18, 91–99. [Google Scholar] [CrossRef]
- Singletary, K. Turmeric: An Overview of Potential Health Benefits. Nutr. Today 2010, 45, 216–225. [Google Scholar] [CrossRef]
- Sadan, O.; Bahat-Stromza, M.; Barhum, Y.; Levy, Y.S.; Pisnevsky, A.; Peretz, H.; Bar Ilan, A.; Bulvik, S.; Shemesh, N.; Krepel, D.; et al. Protective Effects of Neurotrophic Factor–Secreting Cells in a 6-OHDA Rat Model of Parkinson Disease. Stem Cells Dev. 2009, 18, 1179–1190. [Google Scholar] [CrossRef]
- Rajeswari, A.; Sabesan, M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology 2008, 16, 96–99. [Google Scholar] [CrossRef]
- Rhodes, S.L.; Ritz, B. Genetics of iron regulation and the possible role of iron in Parkinson’s disease. Neurobiol. Dis. 2008, 32, 183–195. [Google Scholar] [CrossRef]
- Lv, H.; Liu, J.; Wang, L.; Zhang, H.; Yu, S.; Li, Z.; Jiang, F.; Niu, Y.; Yuan, J.; Cui, X.; et al. Ameliorating Effects of Combined Curcumin and Desferrioxamine on 6-OHDA-Induced Rat Mode of Parkinson’s Disease. Cell Biochem. Biophys. 2014, 70, 1433–1438. [Google Scholar] [CrossRef]
- Wang, M.S.; Boddapati, S.; Emadi, S.; Sierks, M. Curcumin reduces α-synuclein induced cytotoxicity in Parkinson’s disease cell model. BMC Neurosci. 2010, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Jaisin, Y.; Thampithak, A.; Meesarapee, B.; Ratanachamnong, P.; Suksamrarn, A.; Phivthong-Ngam, L.; Phumala-Morales, N.; Chongthammakun, S.; Govitrapong, P.; Sanvarinda, Y. Curcumin I protect the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis. Neurosci. Lett. 2011, 489, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Farooqui, T. Chapter 18—Therapeutic Potentials of Curcumin in Parkinson’s Disease. In Curcumin for Neurological and Psychiatric Disorders; Academic Press: Cambridge, MA, USA, 2019; pp. 333–344. [Google Scholar] [CrossRef]
- van der Merwe, C.; van Dyk, H.C.; Engelbrecht, L.; van der Westhuizen, F.C.; Kinnear, C.; Loos, B.; Bardien, S. Curcumin Rescues a PINK1 Knock Down SH-SY5Y Cellular Model of Parkinson’s Disease from Mitochondrial Dysfunction and Cell Death. Mol. Neurobiol. 2017, 54, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Wang, R.; Bhakta, V.; Driver, Z.; Vadim, Y.; Kiritoshi, T.; Ji, G.; Neugebauer, V.; Shen, C.-L. Turmeric Bioactive Compounds Alleviate Spinal Nerve Ligation-Induced Neuropathic Pain by Suppressing Glial Activation and Improving Mitochondrial Function in Spinal Cord and Amygdala. Nutrients 2023, 15, 4403. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.H.; Yang, X.; Hu, J.Q.; Zhu, Y.Z.; Yan, P.Y.; Xie, Y. Novel nano-drug delivery system for natural products and their application. Pharmacol. Res. 2024, 201, 107100. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Y.; Li, X.; Ross, C.; Smith, W. Curcumin protects against A53T alpha-synuclein-induced toxicity in a PC12 inducible cell model for Parkinsonism. Pharmacol. Res. 2011, 63, 439–444. [Google Scholar] [CrossRef]
- Jiang, T.F.; Zhang, Y.J.; Zhou, H.Y.; Wang, H.M.; Tian, L.P.; Liu, J.; Ding, J.Q.; Chen, S.D. Curcumin Ameliorates the Neurodegenerative Pathology in A53T α-synuclein Cell Model of Parkinson’s Disease Through the Downregulation of mTOR/p70S6K Signaling and the Recovery of Macroautophagy. J. Neuroimmune Pharmacol. 2013, 8, 356–369. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, C.; Iyaswamy, A.; Krishnamoorthi, S.; Sreenivasmurthy, S.G.; Liu, J.; Wang, Z.; Tong, B.C.-K.; Song, J.; Lu, J.; et al. Balancing mTOR Signaling and Autophagy in the Treatment of Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 728. [Google Scholar] [CrossRef]
- He, H.J.; Xiong, X.; Zhou, S.; Zhang, X.R.; Zhao, X.; Chen, L.; Xie, C.L. Neuroprotective effects of curcumin via autophagy induction in 6-hydroxydopamine Parkinson’s models. Neurochem. Int. 2022, 155, 105297. [Google Scholar] [CrossRef]
- Abrahams, S.; Haylett, W.L.; Johnson, G.; Carr, J.A.; Bardien, S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience 2019, 406, 1–21. [Google Scholar] [CrossRef]
- Ramkumar, M.; Rajasankar, S.; Gobi, V.V.; Dhanalakshmi, C.; Manivasagam, T.; Thenmozhi, A.J.; Essa, M.M.; Kalandar, A.; Chidambaram, R. Neuroprotective effect of Demethoxycurcumin, a natural derivative of Curcumin on rotenone induced neurotoxicity in SH-SY 5Y Neuroblastoma cells. BMC Complement. Altern. Med. 2017, 17, 217. [Google Scholar] [CrossRef] [PubMed]
- Jaroonwitchawan, T.; Chaicharoenaudomrung, N.; Namkaew, J.; Noisa, P. Curcumin attenuates paraquat-induced cell death in human neuroblastoma cells through modulating oxidative stress and autophagy. Neurosci. Lett. 2017, 636, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS protection efficiency and free radical-scavenging activity of curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Atsumi, T.; Ishihara, M.; Kadoma, Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer. Res. 2004, 24, 563–569. [Google Scholar]
- Xiong, N.; Long, X.; Xiong, J.; Jia, M.; Chen, C.; Huang, H.; Ghoorah, D.; Kong, X.; Lin, Z.; Wang, T. Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson’s disease models. Crit. Rev. Toxicol. 2012, 42, 613–632. [Google Scholar] [CrossRef]
- Innos, J.; Hickey, M.A. Using rotenone to model Parkinson’s disease in mice: A review of the role of pharmacokinetics. Chem. Res. Toxicol. 2021, 34, 1223–1239. [Google Scholar] [CrossRef]
- Alvarez-Fischer, D.; Henze, C.; Strenzke, C.; Westrich, J.; Ferger, B.; Höglinger, G.U.; Oertel, W.H.; Hartmann, A. Characterization of the striatal 6-OHDA model of Parkinson’s disease in wild type and α-synuclein-deleted mice. Exp. Neurol. 2008, 210, 182–193. [Google Scholar] [CrossRef]
- Mustapha, M.; Taib, C.N.M. MPTP-induced mouse model of Parkinson’s disease: A promising direction for therapeutic strategies. Bosn. J. Basic Med. Sci. 2021, 21, 422–433. [Google Scholar] [CrossRef]
- Singh, A.; Tripathi, P.; Yadawa, A.K.; Singh, S. Promising Polyphenols in Parkinson’s Disease Therapeutics. Neurochem. Res. 2020, 45, 1731–1745. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Read, M.I.; Barreto, G.E.; Sahebkar, A. Neuroprotective effects of curcumin through autophagy modulation. IUBMB Life 2020, 72, 652–664. [Google Scholar] [CrossRef]
- Fukutomi, R.; Ohishi, T.; Koyama, Y.; Pervin, M.; Nakamura, Y.; Isemura, M. Beneficial Effects of Epigallocatechin-3-O-Gallate, Chlorogenic Acid, Resveratrol, and Curcumin on Neurodegenerative Diseases. Molecules 2021, 26, 415. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Singh, S.P. NF-κB-Mediated Neuroinflammation in Parkinson’s Disease and Potential Therapeutic Effect of Polyphenols. Neurotox. Res. 2020, 37, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Thompson, M.; Xu, Y.H. Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab. Investig. 2016, 96, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nishio, K.; Ogawa, Y.; Kinumi, T.; Yoshida, Y.; Masuo, Y.; Niki, E. Molecular mechanisms of 6-hydroxydopamine-induced cytotoxicity in PC12 cells: Involvement of hydrogen peroxide-dependent and-independent action. Free. Radic. Biol. Med. 2007, 42, 675–685. [Google Scholar] [CrossRef]
- Vila, M. Neuromelanin, aging, and neuronal vulnerability in Parkinson’s disease. Mov. Disord. 2019, 34, 1440–1451. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Haller, D. Functional diversity of flavonoids in the inhibition of the proinflammatory NF-κB, IRF, and Akt signaling pathways in murine intestinal epithelial cells. J. Nutr. 2006, 136, 664–671. [Google Scholar] [CrossRef]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The Pathology of Parkinson’s Disease and Potential Benefit of Dietary Polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Farhadi, F.; Barreto, G.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on neurological diseases: Focus on astrocytes. Pharmacol. Rep. 2020, 72, 769–782. [Google Scholar] [CrossRef]
- Kujawska, M.; Jodynis-Liebert, J. Polyphenols in Parkinson’s Disease: A Systematic Review of In Vivo Studies. Nutrients. 2018, 10, 642. [Google Scholar] [CrossRef]
- Tripanichkul, W.; Jaroensuppaperch, E. Curcumin Protects Nigrostriatal Dopaminergic Neurons and Reduces Glial Activation in 6-Hydroxydopamine Hemiparkinsonian Mice Model. Int. J. Neurosci. 2012, 122, 263–270. [Google Scholar] [CrossRef]
- Singh, P.K.; Kotia, V.; Ghosh, D.; Mohite, G.M.; Kumar, A.; Maji, S.K. Curcumin modulates α-synuclein aggregation and toxicity. ACS Chem. Neurosci. 2013, 4, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Khuwaja, G.; Khan, M.M.; Ishrat, T.; Ahmad, A.; Raza, S.S.; Ashafaq, M.; Javed, H.; Khan, M.B.; Khan, A.; Kumar, V.; et al. Neuroprotective effects of curcumin on 6-hydroxydopamine-induced Parkinsonism in rats: Behavioral, neurochemical and immunohistochemical studies. Brain Res. 2011, 1368, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.C.; Jackson-Lewis, V.; Vila, M.; Tieu, K.; Teismann, P.; Vadseth, C.; Choi, D.-K.; Ischiropoulos, H.; Przedborski, S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002, 22, 1763–1771. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, S.; Nehru, B. Curcumin protects dopaminergic neurons against inflammation-mediated damage and improves motor dysfunction induced by single intranigral lipopolysaccharide injection. Inflammopharmacology 2017, 25, 351–368. [Google Scholar] [CrossRef]
- Mobahat, M.; Sadroddiny, E.; Nooshabadi, V.T.; Ebrahimi-Barough, S.; Goodarzi, A.; Malekshahi, Z.V.; Ai, J. Curcumin-loaded human endometrial stem cells derived exosomes as an effective carrier to suppress alpha-synuclein aggregates in 6OHDA-induced Parkinson’s disease mouse model. Cell Tissue Bank. 2023, 24, 75–91. [Google Scholar] [CrossRef]
- Essawy, A.E.; Matta, C.A.; Nabil, B.; Elkader, H.T.A.E.A.; Alhasani, R.H.; Soffar, A.A. Neuroprotective Effect of Curcumin on the Rat Model of Parkinson’s Disease Induced by Rotenone via Modulating Tyrosine Hydroxylase and Dopa Decarboxylase Expression Levels. Neurochem. J. 2023, 17, 457–466. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin formulations for better bioavailability: What we learned from clinical trials thus far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Sharma, N.; Nehru, B. Curcumin affords neuroprotection and inhibits α-synuclein aggregation in lipopolysaccharide-induced Parkinson’s disease model. Inflammopharmacology 2018, 26, 349–360. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, D.; Yang, Z.; Hu, X.; Qian, S.; Liu, J.; Wilson, B.; Block, M.; Hong, J.S. Curcumin protects dopaminergic neuron against LPS induced neurotoxicity in primary rat neuron/glia culture. Neurochem. Res. 2008, 33, 2044–2053. [Google Scholar] [CrossRef]
- Sian, J.; Dexter, D.T.; Lees, A.J.; Daniel, S.; Agid, Y.; Javoy-Agid, F.; Jenner, P.; Marsden, C.D. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1994, 36, 348–355. [Google Scholar] [CrossRef]
- Kalsoom, I.; Wang, Y.; Li, B.; Wen, H. Research Progress of α-Synuclein Aggregation Inhibitors for Potential Parkinson’s Disease Treatment. Mini Rev. Med. Chem. 2023, 23, 1959–1974. [Google Scholar] [CrossRef] [PubMed]
- Bássoli, R.M.F.; Audi, D.; Ramalho, B.J.; Audi, M.; Quesada, K.R.; Barbalho, S.M. The Effects of Curcumin on Neurodegenerative Diseases: A Systematic Review. J. Herb. Med. 2023, 42, 100771. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.; He, X.; Wang, Y.; Gao, S.; Ren, L.; Shi, Y. Curcumin exerts anti-inflammatory and antioxidative properties in 1-methyl-4-phenylpyridinium ion (MPP (+))-stimulated mesencephalic astrocytes by interference with TLR4 and downstream signaling pathway. Cell Stress Chaperones 2016, 21, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Amanollahi, M.; Jameie, M.; Rezaei, N. Neuroinflammation as a potential therapeutic target in neuroimmunological diseases. In Translational Neuroimmunology; Academic Press: Cambridge, MA, USA, 2023; Volume 7, pp. 475–504. [Google Scholar] [CrossRef]
- Hajipour, S.; Vastegani, S.M.; Sarkaki, A.; Basir, Z.; Navabi, S.P.; Farbood, Y.; Khoshnam, S.E. Curcumin attenuates memory impairments and long-term potentiation deficits by damping hippocampal inflammatory cytokines in lipopolysaccharide-challenged rats. Metab. Brain Dis. 2023, 38, 1379–1388. [Google Scholar] [CrossRef]
- Lombardi, N.; Crescioli, G.; Maggini, V.; Ippoliti, I.; Menniti-Ippolito, F.; Gallo, E.; Brilli, V.; Lanzi, C.; Mannaioni, G.; Firenzuoli, F.; et al. Acute Liver Injury Following Turmeric Use in Tuscany: An Analysis of the Italian Phytovigilance Database and Systematic Review of Case Reports. Br. J. Clin. Pharmacol. 2020, 87, 741–753. [Google Scholar] [CrossRef]
- Zhao, H.-L.; Song, C.H.; Chai, O.H. Negative Effects of Curcumin on Liver Injury Induced by Alcohol. Phytother. Res. 2012, 26, 1857–1863. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Calderón-Montaño, J.M.; Salvador, J.; Robles, A.; López-Lázaro, M. The Dark Side of Curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Franco-Robles, E.; Campos-Cervantes, A.; Murillo-Ortiz, B.O.; Segovia, J.; López-Briones, S.; Vergara, P.; Perez- Vasquez, V.; Solis-Ortiz, M.S.; Ramírez-Emiliano, J. Effects of curcumin on brain-derived neurotrophic factor levels and oxidative damage in obesity and diabetes. Appl. Physiol. Nutr. Metab. 2014, 39, 211–218. [Google Scholar] [CrossRef]
- Radbakhsh, S.; Butler, A.E.; Moallem, S.A.; Sahebkar, A. The Effects of Curcumin on Brain-Derived Neurotrophic Factor Expression in Neurodegenerative Disorders. Curr. Med. Chem. 2024, 31, 5937–5952. [Google Scholar] [CrossRef]
- Nebrisi, E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef]
- Kristiansen, L.V.; Patel, S.A.; Haroutunian, V.; Meador-Woodruff, J.H. Expression of the NR2B-NMDA Receptor Subunit and Its Tbr-1/CINAP Regulatory Proteins in Postmortem Brain Suggest Altered Receptor Processing in Schizophrenia. Synapse 2010, 64, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Chang, P.; Lu, S.-Y.; Zheng, B.-W.; Jiang, Z.-F. Protection of Curcumin against Amyloid-β-Induced Cell Damage and Death Involves the Prevention from NMDA Receptor-Mediated Intracellular Ca2+ Elevation. J. Recept. Signal Transduct. 2015, 35, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Jayanarayanan, S.; Smijin, S.; Peeyush, K.; Anju, T.; Paulose, C. NMDA and AMPA Receptor Mediated Excitotoxicity in Cerebral Cortex of Streptozotocin Induced Diabetic Rat: Ameliorating Effects of Curcumin. Chem. -Biol. Interact. 2012, 201, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, D.J.; Marquez, A.; Calcutt, N.A.; Schubert, D. A novel curcumin derivative for the treatment of diabetic neuropathy. Neuropharmacology 2018, 129, 26–35. [Google Scholar] [CrossRef]
- Dewanjee, S.; Das, S.; Das, A.K.; Bhattacharjee, N.; Dihingia, A.; Dua, T.K.; Kalita, J.; Manna, P. Molecular mechanism of diabetic neuropathy and its pharmacotherapeutic targets. Eur. J. Pharmacol. 2018, 833, 472–523. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Liu, D.B.; Liu, H.Y.; Hou, W.G.; Dong, Y.S. Curcumin attenuates diabetic neuropathic pain by downregulating TNF-α in a rat model. Int. J. Med. Sci. 2013, 10, 377–381. [Google Scholar] [CrossRef]
- Rashid, K.; Chowdhury, S.; Ghosh, S.; Sil, P.C. Curcumin attenuates oxidative stress induced NFκB mediated inflammation and endoplasmic reticulum dependent apoptosis of splenocytes in diabetes. Biochem. Pharmacol. 2017, 143, 140–155. [Google Scholar] [CrossRef]
- Ma, J.; Yu, H.; Liu, J.; Chen, Y.; Wang, Q.; Xiang, L. Curcumin promotes nerve regeneration and functional recovery after sciatic nerve crush injury in diabetic rats. Neurosci. Lett. 2016, 610, 139–143. [Google Scholar] [CrossRef]
- Zhang, W.X.; Lin, Z.Q.; Sun, A.L.; Shi, Y.Y.; Hong, Q.X.; Zhao, G.F. Curcumin ameliorates the experimental diabetic peripheral neuropathy through promotion of NGF expression in rats. Chem. Biodivers. 2022, 19, e202200029. [Google Scholar] [CrossRef]
- Sun, J.; Chen, F.; Braun, C.; Zhou, Y.Q.; Rittner, H.; Tian, Y.K.; Cai, X.Y.; Ye, D.W. Role of curcumin in the management of pathological pain. Phytomedicine 2018, 48, 129–140. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.H.; Sim, J.H.; Park, J.; Choi, S.-S.; Leem, J.G. Effects of Curcumin Treatment in a Diabetic Neuropathic Pain Model of Rats: Involvement of C-Jun N-Terminal Kinase Located in the Astrocytes and Neurons of the Dorsal Root Ganglion. Pain Res. Manag. 2021, 18, 8787231. [Google Scholar] [CrossRef] [PubMed]
- Parsamanesh, N.; Moossavi, M.; Bahrami, A.; Butler, A.E.; Sahebkar, A. Therapeutic potential of curcumin in diabetic complications. Pharmacol. Res. 2018, 136, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Suzuki, K.; Veeraveedu, P.T.; Arumugam, S.; Lakshmanan, A.P.; Sone, H.; Watanabe, K. Molecular understanding of curcumin in diabetic nephropathy. Drug Discov. Today 2013, 18, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tian, Y.; Liu, X.; Bu, Y.; Shui, J.; Yin, Y. Research progress of traditional Chinese medicine monomer in treating diabetic peripheral neuropathy: A review. Medicine 2024, 103, e37767. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, B.; Ke, T.; Li, S.; Yang, X.; Aschner, M.; Chen, P. Mechanisms of metal-induced mitochondrial dysfunction in neurological disorders. Toxics 2021, 9, 142. [Google Scholar] [CrossRef]
- Vellingiri, B.; Suriyanarayanan, A.; Selvaraj, P.; Abraham, K.S.; Pasha, M.Y.; Winster, H.; Venkatesan, D. Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere 2022, 301, 134625. [Google Scholar] [CrossRef]
- Li, B.; Xia, M.; Zorec, R.; Parpura, V.; Verkhratsky, A. Astrocytes in heavy metal neurotoxicity and neurodegeneration. Brain Res. 2021, 1752, 147234. [Google Scholar] [CrossRef]
- Wei, R.; Wei, P.; Yuan, H.; Yi, X.; Aschner, M.; Jiang, Y.M.; Li, S.J. Inflammation in Metal-Induced Neurological Disorders and Neurodegenerative Diseases. Biol. Trace Elem. Res. 2024, 202, 4459–4481. [Google Scholar] [CrossRef]
- Carmona, A.; Roudeau, S.; Ortega, R. Molecular mechanisms of environmental metal neurotoxicity: A focus on the interactions of metals with synapse structure and function. Toxics 2021, 9, 198. [Google Scholar] [CrossRef]
- Mailman, R.B.; Mayleben, M.; Lawler, C.P. Effects of toxic metals on neurotransmitters. In Toxicology of Metals; CRC Press: Boca Raton, FL, USA, 2023; Volume 1, pp. 627–638. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. On the mechanisms of heavy metal-induced neurotoxicity: Amelioration by plant products. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2021, 91, 743–751. [Google Scholar] [CrossRef]
- Harish, G.; Venkateshappa, C.; Mythri, R.B.; Dubey, S.K.; Mishra, K.; Singh, N.; Bharath, M.S. Bioconjugates of curcumin display improved protection against glutathione depletion mediated oxidative stress in a dopaminergic neuronal cell line: Implications for Parkinson’s disease. Bioorganic Med. Chem. 2021, 18, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Singh, S.; Agrawal, A.; Siddiqi, N.J.; Sharma, B. Phytochemicals mediated remediation of neurotoxicity induced by heavy metals. Biochem. Res. Int. 2015, 2015, 534769. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Moniruzzaman, M.; Chin, S.; Sureshbabu, A.; Karthikeyan, A.; Do, K.; Min, T. A review of the role of curcumin in metal induced toxicity. Antioxidants 2023, 12, 243. [Google Scholar] [CrossRef]
- Mehrandish, R.; Rahimian, A.; Shahriary, A. Heavy metals detoxification: A review of herbal compounds for chelation therapy in heavy metals toxicity. J. Herbmed Pharmacol. 2019, 8, 69–77. [Google Scholar] [CrossRef]
- Kabeer, A.; Mailafiya, M.M.; Danmaigoro, A.; Rahim, E.A.; bu Bakar, M.Z.A. Therapeutic potential of curcumin against lead-induced toxicity: A review. Biomed. Res. Ther. 2019, 6, 3053–3066. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Jaruga, E.; Sokal, A.; Chrul, S.; Bartosz, G. Apoptosis-Independent Alterations in Membrane Dynamics Induced by Curcumin. Exp. Cell Res. 1998, 245, 303–312. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, L.; Zhang, S.; Sun, P.-C.; Ding, C.-F.; Chu, Y.-Q.; Zhou, P. Interaction of Curcumin with Al(III) and Its Complex Structures Based on Experiments and Theoretical Calculations. J. Mol. Struct. 2011, 1004, 163–173. [Google Scholar] [CrossRef]
- Vajragupta, O.; Boonchoong, P.; Watanabe, H.; Tohda, M.; Kummasud, N.; Sumanont, Y. Manganese complexes of curcumin and its derivatives: Evaluation for the radical scavenging ability and neuroprotective activity. Free. Radic. Biol. Med. 2003, 35, 1632–1644. [Google Scholar] [CrossRef]
- Sarawi, W.S.; Ahlam, M.A.; Laila, M.F.; Hatun, A.A.; Awatif, B.A.; Amjad, S.A.; Areej, M.A.; Iman, H.H.; Ayman, M.M. Curcumin and nano-curcumin mitigate copper neurotoxicity by modulating oxidative stress, inflammation, and Akt/GSK-3β signaling. Molecules 2021, 26, 5591. [Google Scholar] [CrossRef] [PubMed]
- Klinger, N.V.; Sandeep, M. Therapeutic potential of curcumin for the treatment of brain tumors. Oxidative Med. Cell. Longev. 2016, 2016, 9324085. [Google Scholar] [CrossRef] [PubMed]
- Racz, L.Z.; Tomoaia-Cotisel, M.; Racz, C.P.; Bulieris, P.; Grosu, I.; Porav, S.; Ciorîță, A.; Filip, X.; Martin, F.; Serban, G.; et al. Curcumin-Whey Protein Solid Dispersion System with Improved Solubility and Cancer Cell Inhibitory Effect. Stud. Univ. Babes-Bolyai Chem. 2021, 66, 209–224. [Google Scholar] [CrossRef]
- Racz, L.Z.; Paltinean, G.A.; Petean, I.; Tomoaia, G.; Pop, L.C.; Arghir, G.; Levei, E.; Mocanu, A.; Racz, C.P.; Tomoaia-Cotisel, M. Curcumin and Whey Protein Binding and Structural Characteristics of Their Complex Evidenced by Atomic Force Microscopy. Stud. Univ. Babes-Bolyai. Chem. 2022, 67, 61–74. [Google Scholar] [CrossRef]
- Racz, L.Z.; Racz, C.P.; Horovitz, O.; Tomoaia, G.; Mocanu, A.; Kacso, I.; Sarkozi, M.; Dan, M.; Porav, S.; Borodi, G.; et al. Complexation of Curcumin using Whey Proteins to Enhance Aqueous Solubility, Stability and Antioxidant Property. Stud. Univ. Babes-Bolyai. Chem. 2022, 67, 75–99. [Google Scholar] [CrossRef]
- Racz, C.-P.; Racz, L.Z.; Floare, C.G.; Tomoaia, G.; Horovitz, O.; Riga, S.; Kacso, I.; Borodi, G.; Sarkozi, M.; Mocanu, A.; et al. Curcumin and Whey Protein Concentrate Binding: Thermodynamic and Structural Approach. Food. Hydrocoll. 2023, 139, 1088547. [Google Scholar] [CrossRef]
- Karlstetter, M.; Lippe, E.; Walczak, Y.; Moehle, C.; Aslanidis, A.; Mirza, M.; Langmann, T. Curcumin Is a Potent Modulator of Microglial Gene Expression and Migration. J. Neuroinflam. 2011, 8, 125. [Google Scholar] [CrossRef]
- Ullah, F.; Gamage, R.; Sen, M.K.; Gyengesi, E. The Effects of Modified Curcumin Preparations on Glial Morphology in Aging and Neuroinflammation. Neurochem. Res. 2022, 47, 813–824. [Google Scholar] [CrossRef]
- Bernardo, A.; Plumitallo, C.; De Nuccio, C.; Visentin, S.; Minghetti, L. Curcumin Promotes Oligodendrocyte Differentiation and Their Protection against TNF-α through the Activation of the Nuclear Receptor PPAR-γ. Sci. Rep. 2021, 11, 4952. [Google Scholar] [CrossRef]
- Yu, L.-H.; Morimura, T.; Numata, Y.; Yamamoto, R.; Inoue, N.; Antalfy, B.; Goto, Y.-I.; Deguchi, K.; Osaka, H.; Inoue, K. Effect of Curcumin in a Mouse Model of Pelizaeus–Merzbacher Disease. Mol. Genet. Metab. 2012, 106, 108–114. [Google Scholar] [CrossRef]
- Seady, M.; Schirmbeck, G.; Taday, J.; Fróes, F.T.; Baú, J.V.; Jantsch, J.; Guedes, R.P.; Gonçalves, C.-A.; Leite, M.C. Curcumin Attenuates Neuroinflammatory Damage Induced by LPS: Implications for the Role of S100B. J. Nutr. Biochem. 2024, 135, 109768. [Google Scholar] [CrossRef]


| Mechanism/Effect | Physiological Effects | |
|---|---|---|
| Anti-inflammatory | Inhibits pro-inflammatory cytokines (TNF-α, IL-1β) and NF-κB signalling. | Reduces chronic neuroinflammation, a key factor in AD |
| Antioxidant | Scavenges free radicals, enhances glutathione, SOD, and catalase activity | Protects neurons from oxidative damage in PD |
| Amyloid aggregation inhibition | Binds to Aβ, preventing their formation and facilitating disaggregation | Mitigates the hallmark pathology of AD |
| Mitochondrial protection | Preserves mitochondrial membrane integrity, reduces oxidative stress within mitochondria | Prevents energy deficits and apoptotic signalling, critical in PD |
| Metal chelation | Binds metals like iron and copper, reducing metal-induced oxidative damage | Decreases oxidative stress in AD and PD |
| Autophagy modulation | Enhances autophagic processes, promoting clearance of damaged proteins | Prevents accumulation of toxic aggregates in diseases like AD and PD |
| Neurogenesis stimulation | Increases BDNF expression | Supports synaptic plasticity and neuronal survival in various neurodegenerative contexts |
| Apoptosis inhibition | Downregulates caspase-3 expression | Prevents neuronal loss in diseases such as PD |
| Curcumin Effects in Cerebral and Cerebrovascular Homeostasis |
|---|
| reduced neuronal death and promoting the microglial activation in ischemic stroke [95,96,97] |
| increased Iba1 microglial activation in haemorrhagic and ischemic stroke [210,211,212] |
| inhibition of the Cas-3, Fas/FasL, Bax and Bcl2 [72,73] |
| proteasome protection [77,78] |
| increasing proteasomal degradation of Aβ aggregates [142,143,144] |
| increasing the mRNA and protein for TrkB and BDNF [84] |
| reduction in IL-1β in haemorrhagic stroke [61] |
| reducing the NMDA-mediated excitotoxicity in stroke [82,83] |
| decreasing the VEGF expression [129,130] |
| inhibiting matrix metalloproteinases (MMP2, MMP9) [98] |
| regulating DA receptor expression and signalling [218] |
| downregulation of phospho-mTOR and phosphor-p70S6K [201,202,203] |
| NQO1 inhibition, Nrf2 activation, increasing mRNA, and catalytic activity of the CAT, SOD, and GPx1 [62,63,64,66,67,68] |
| free radical scavenging [49,50,51] |
| MCAP-1 and TNF-α inhibition in stroke [60] |
| reducing the oxidative stress-induced mitochondrial dysfunction [9,21,57] |
| counteracting the MPTP-induced dopaminergic lesion, increasing the DA levels in the frontal cortex and striatum; and inhibiting the MAO [160,161] |
| spatial learning and memory improvement [175] |
| total glutathione levels increasing [179,182] |
| decreased the sorbitol level by inhibition of the aldose-reductase activity [131] |
| reducing the myoinositol formation in endothelial and glial cells [131] |
| inhibition of the AGEs synthesis in endothelial cells [213] |
| decreasing the cell swelling by a general inhibition of the polyol pathway [226] |
| reducing apoptosis via JNK, ERK, and PKA [9,21,57,68,77,78,95,97,128,153,229] |
| reducing BBB leakage [129,130] |
| decreasing α-syn aggregation [210] |
| preventing the neurofibrillary tangle formation [141,145] |
| promoting nerve regeneration by NGF upregulation [230,231] |
| increasing NO bioavailability by eNOS protection [125,232] |
| decreasing iNOS activity [200,201,202,214] |
| reducing heavy metals-induced neurotoxicity [246,247,251] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldoveanu, C.-A.; Tomoaia-Cotisel, M.; Sevastre-Berghian, A.; Tomoaia, G.; Mocanu, A.; Pal-Racz, C.; Toma, V.-A.; Roman, I.; Ujica, M.-A.; Pop, L.-C. A Review on Current Aspects of Curcumin-Based Effects in Relation to Neurodegenerative, Neuroinflammatory and Cerebrovascular Diseases. Molecules 2025, 30, 43. https://doi.org/10.3390/molecules30010043
Moldoveanu C-A, Tomoaia-Cotisel M, Sevastre-Berghian A, Tomoaia G, Mocanu A, Pal-Racz C, Toma V-A, Roman I, Ujica M-A, Pop L-C. A Review on Current Aspects of Curcumin-Based Effects in Relation to Neurodegenerative, Neuroinflammatory and Cerebrovascular Diseases. Molecules. 2025; 30(1):43. https://doi.org/10.3390/molecules30010043
Chicago/Turabian StyleMoldoveanu, Claudia-Andreea, Maria Tomoaia-Cotisel, Alexandra Sevastre-Berghian, Gheorghe Tomoaia, Aurora Mocanu, Csaba Pal-Racz, Vlad-Alexandru Toma, Ioana Roman, Madalina-Anca Ujica, and Lucian-Cristian Pop. 2025. "A Review on Current Aspects of Curcumin-Based Effects in Relation to Neurodegenerative, Neuroinflammatory and Cerebrovascular Diseases" Molecules 30, no. 1: 43. https://doi.org/10.3390/molecules30010043
APA StyleMoldoveanu, C.-A., Tomoaia-Cotisel, M., Sevastre-Berghian, A., Tomoaia, G., Mocanu, A., Pal-Racz, C., Toma, V.-A., Roman, I., Ujica, M.-A., & Pop, L.-C. (2025). A Review on Current Aspects of Curcumin-Based Effects in Relation to Neurodegenerative, Neuroinflammatory and Cerebrovascular Diseases. Molecules, 30(1), 43. https://doi.org/10.3390/molecules30010043









