Abstract
Electrocatalytic CO2 reduction reaction (CO2RR) is a crucial technology for achieving carbon cycling and renewable energy conversion, yet it faces challenges such as complex reaction pathways, competition for intermediate adsorption, and low product selectivity. In recent years, molecular modification has emerged as a promising strategy. By adjusting the surface properties of catalysts, molecular modification alters the electronic structure, steric hindrance, promotes the adsorption of reactants, stabilizes intermediates, modifies the hydrophilic–hydrophobic environment, and regulates pH, thereby significantly enhancing the conversion efficiency and selectivity of CO2RR. This paper systematically reviews the modification strategies and mechanisms of molecularly modified materials in CO2RR. By summarizing and analyzing the existing literature, this review provides new perspectives and insights for future research on molecularly modified materials in electrocatalytic CO2 reduction.
1. Introduction
With the intensification of global climate change and the energy crisis, the development of effective carbon dioxide (CO2) reduction technologies has become one of the most pressing challenges faced by contemporary society [1]. Among various CO2 conversion methods, electrocatalytic CO2 reduction (CO2RR) has gained significant attention due to its mild reaction conditions, high energy efficiency, ease of control, and potential for being driven by renewable energy sources [2]. CO2RR can convert CO2 into high-value chemicals, such as CO (an important basic chemical raw material), HCOOH (an important organic acid and hydrogen storage carrier), CH4 (an essential fuel and chemical feedstock), CH3OH (an emerging fuel), C2H4 (a basic petrochemical raw material), and C2H5OH (an important fuel, solvent, and chemical), making it a key technology for achieving carbon neutrality [3,4,5,6]. However, CO2 molecules possess high chemical stability, and their electrocatalytic reduction typically requires high overpotentials [7]. Additionally, the reaction often involves multiple proton-coupled electron transfer steps, and even C-C coupling, which complicates the reaction pathway. Moreover, the thermodynamic redox potentials of various CO2RR products are not significantly different from each other and are close to that of the hydrogen evolution reaction (HER). These make it particularly challenging to improve the conversion efficiency of CO2 and further enhance the selectivity for a single product [8,9,10]. To address these issues, in CO2RR, it is essential to develop advanced catalysts to steer the reaction pathway and thereby achieve high selectivity of a single product.
With the development of CO2RR technology, traditional catalysts (such as metals/alloys, metal oxides, metal–organic frameworks, and covalent organic frameworks) have achieved significant progress in CO2RR. However, these catalysts still suffer from certain limitations, including insufficient active sites, poor stability, and particularly low selectivity for a single product [11]. Currently, molecular modification strategies have emerged as a focal point in CO2RR research, as they can effectively regulate the surface properties of catalyst materials. By introducing functional molecules onto the catalyst surface, adjusting the electronic structure of active sites, modulating the local reaction environment, and enhancing the adsorption of specific intermediates through hydrogen-bond-like interactions, the reaction can be guided toward the desired direction [12,13]. In contrast, molecular modification can significantly enhance the activation of carbon dioxide and product selectivity, making it a highly promising strategy for improving catalytic efficiency.
In recent years, as research deepens, molecular modification has become an important area in CO2RR research due to their structural flexibility and tunable functionality. The central objective is to address the key challenges in CO2RR by enhancing the selectivity for a specific product, suppressing competitive HER. Molecularly modified strategies (such as functionalizing catalyst surfaces with thiols, amino acids, pyridine salts, imidazole compounds, or polymers) aim to optimize key reaction steps by introducing specific electronic effects (regulating the electron density of metal centers) and spatial effects (microenvironmental regulation), thereby improving the target product yield of CO2RR reactions [14,15]. Particularly, the combination of metals/metal compounds and organic molecules is considered an effective way to achieve the above-mentioned regulation, thereby promoting the selective production of target products [16,17]. Moreover, the development of molecular modification polymer-based materials has played an important role in enhancing the stability and reaction efficiency of catalysts. The advantages and importance of molecular modification in CO2RR are evident in several aspects. The potential advantages of molecularly modified CO2RR lie in the precision of its design. Firstly, molecular modification enables the precise design of the surface properties of the catalyst, allowing for more effective adsorption and activation of CO2 molecules, thereby improving the reaction rate. Secondly, molecular modification aids in the selective regulation of the adsorption and conversion of intermediates in the CO2 reduction process, reducing side reactions and increasing the selectivity and yield of the desired products. Additionally, molecularly modified materials offer excellent tunability and diversity. Researchers can further optimize catalyst performance by altering the structure of organic molecules, the types of functional groups, and the arrangement of ligands, to adapt to different reaction conditions and target product requirements. Current molecular modification strategies mainly include metal–organic molecule bonding modification, weak interaction modification, and polymer-based modification. As an innovative catalyst optimization strategy, molecular modification has gradually become an indispensable part of CO2RR research. This paper will systematically review the main strategies of molecular modification and the mechanisms of molecularly modified materials in electrocatalytic CO2 reduction and explore how the design of molecularly modified materials can be further improved to enhance the performance of CO2RR, thus advancing the breakthroughs in CO2 resource utilization technologies.
2. Major Strategies of Molecular Modification
Molecular modification can regulate the electronic structure and geometric configuration of the catalyst surface and its interaction with the reactants, which in turn affects the adsorption, activation, and subsequent conversion pathways of CO2. To date, a variety of molecularly modified catalysts have been developed for CO2RR, including metal-based materials modified with organic small molecules, metal–organic frameworks (MOFs) and covalent organic frameworks (COFs) with functionalized ligands, and polymer modification materials, etc. Their strategies have been summarized for the molecular modification, such as metal–organic bonding, weak interactions, and modification with polymer-based materials.
2.1. Metal–Organic Molecular Bonding Modification
The most common approach for metal–organic molecular bonding is the connection of organic molecules to the surface of metal catalysts via metal-sulfur/nitrogen/carbon (M-S/N/C) bonds. Typical organic molecules employed for this strategy include thiols, amines, pyridines, etc. These molecules form stable bonds with metals, thereby modulating the metal′s electron density, surface morphology, and catalytic activity.
The most commonly used organic molecules are thiols, which primarily anchor to the catalyst surface through covalent bonding or chemical adsorption of the thiol groups. Once the-SH groups form bonds with the metal, the electrocatalytic CO2RR performance of the catalyst is effectively tuned. For example, through self-assembly in organic solution, 4-mercaptopyridine (SPy) molecules can be immobilized on the surface of Cu nanoparticles, to provide rational control over the product distribution. Fourier transform infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS) verified the successful immobilization, and density functional theory (DFT) calculations showed that the S atom preferentially binds to the two Cu atoms, with the ring slightly deviating from the Cu surface (Figure 1a) [18]. The presence of SPy energetically hinders the formation of the intermediate *OCOH, thereby blocking the CO production pathway, while the intermediate *COOH in the formic acid generation pathway remains unaffected, promoting formic acid production. Similarly, by using the interaction between sulfur and metals, thiols or sulfur-containing molecules can be modified onto the surface of other metal substrates, such as Au [19] and Ag [20], or used to stabilize nanoclusters [21,22]. The modified organic molecules on metals can modulate the supply of protons, the binding energy of key intermediates, etc., and thus enhance the selectivity of the targe product.
In addition to metals, thiol molecules can also be modified onto the surface of metal oxides to enhance CO2RR performance by modulating the metal–organic interface. Recently, Wang et al. [23] systematically examined and analyzed the structure–activity relationship with 180 molecular modifiers using an automated electrocatalysis platform and machine learning techniques. It was found that organic modifiers, such as 1,8-octanedithiol, formed metal–organic interphases over 10 nm thick on the surface of the CuOx catalysts, instead of the traditional monolayer adsorption. These thick interphases significantly altered the interfacial structure of the catalyst, affecting the adsorption and conversion of CO2RR intermediates, thereby enhancing the selectivity of ethanol and C2+ products (Figure 1b). This strategy is also applicable to the development of electrocatalytic CO reduction (CORR) catalysts. Both SPy-modified Cu2O nanocubes [24] and thiol-modified Cu catalysts [25] display high FE in CORR to acetate.
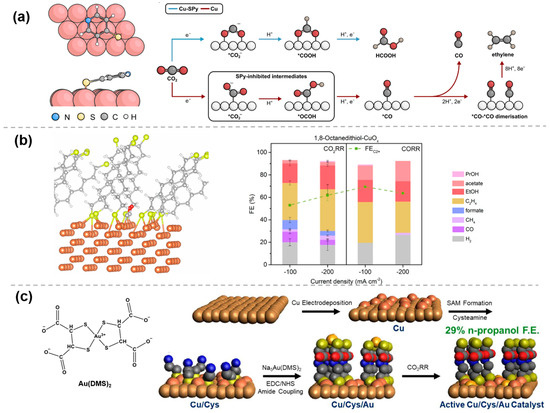
Figure 1.
Modification of metal and organic molecules by M−S bonding: (a) Cu−SPy [18], with permission from John Wiley and Sons, copyright 2022; (b) 1,8−octanedithiol−CuOx [23], with permission from Springer Nature, copyright 2025; and (c) Cu/Cys/Au catalyst [26], with permission from John Wiley and Sons, copyright 2025.
Figure 1.
Modification of metal and organic molecules by M−S bonding: (a) Cu−SPy [18], with permission from John Wiley and Sons, copyright 2022; (b) 1,8−octanedithiol−CuOx [23], with permission from Springer Nature, copyright 2025; and (c) Cu/Cys/Au catalyst [26], with permission from John Wiley and Sons, copyright 2025.
Further, through the fine surface engineering modulation, the second metal nanoparticles can be loaded on the surface of the self-assembled monolayers, which facilitates highly selective C-C coupling via the synergistic effect of the bimetals as well as the uniformly distributed bimetallic active sites. For example, Barilex et al. [26] proposed a Cu/Cys/Au catalyst structure that significantly increased FE for n-propanol production. To fabricate this catalyst, a rough Cu layer was first electrodeposited on polycrystalline copper foil to enhance surface area. Subsequently, a cysteamine (Cys) self-assembled monolayer (SAM) was anchored to the electrodeposited Cu layer via Cu-thiolate bonds. Then, a Au3+ complex of dimercaptosuccinate (DMS) was attached to the Cys SAM through an amide coupling reaction. During the initial stage of CO2RR, Au3+ was reduced to Au and electrodeposited on the Cu surface, forming the final active Cu/Cys/Au catalyst (Figure 1c). At −1.2 V vs. reversible hydrogen electrode (RHE), the FE for n-propanol reached 29.1%, showing a significant improvement compared to traditional Au-Cu electrocatalysts.
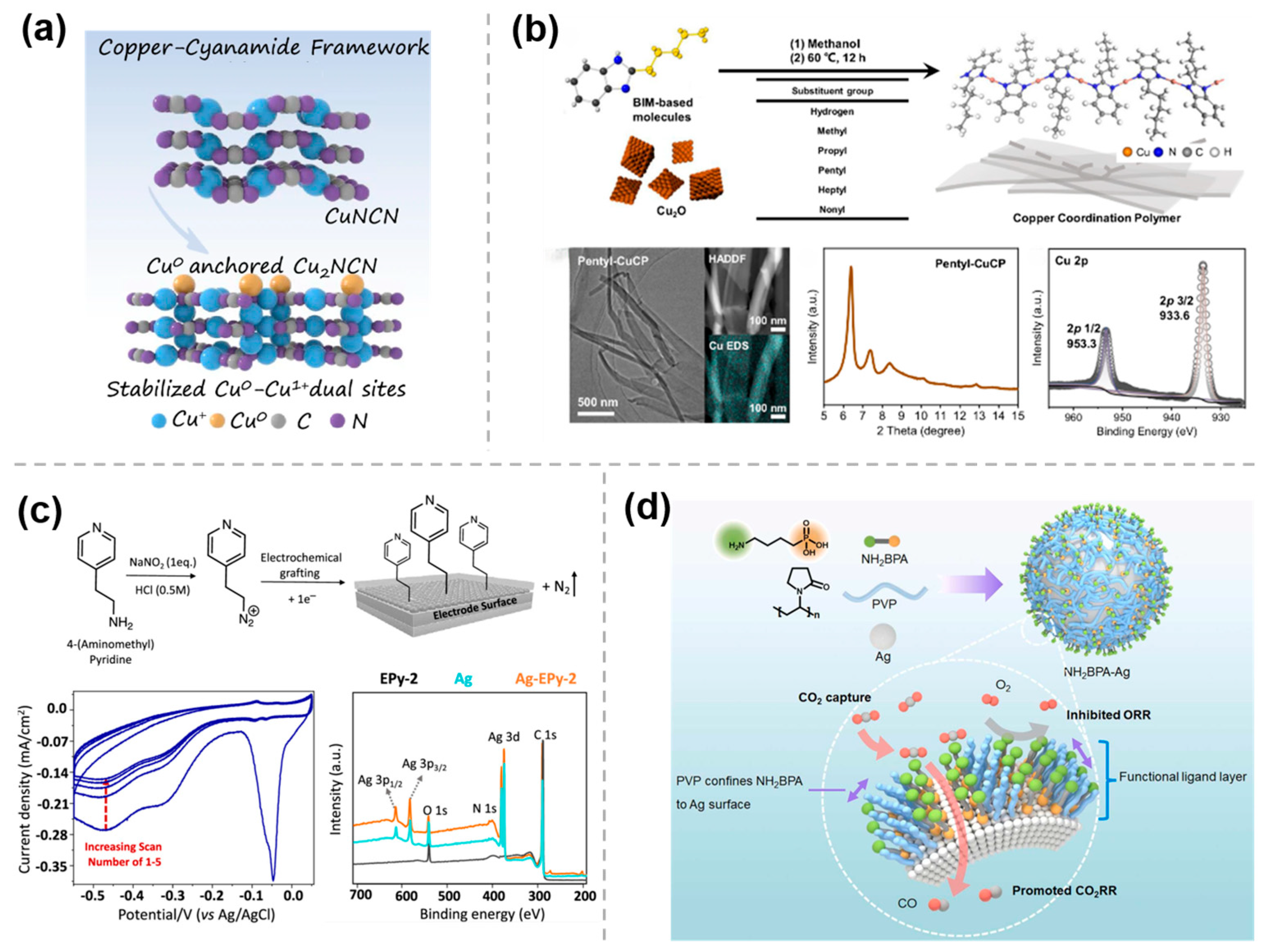
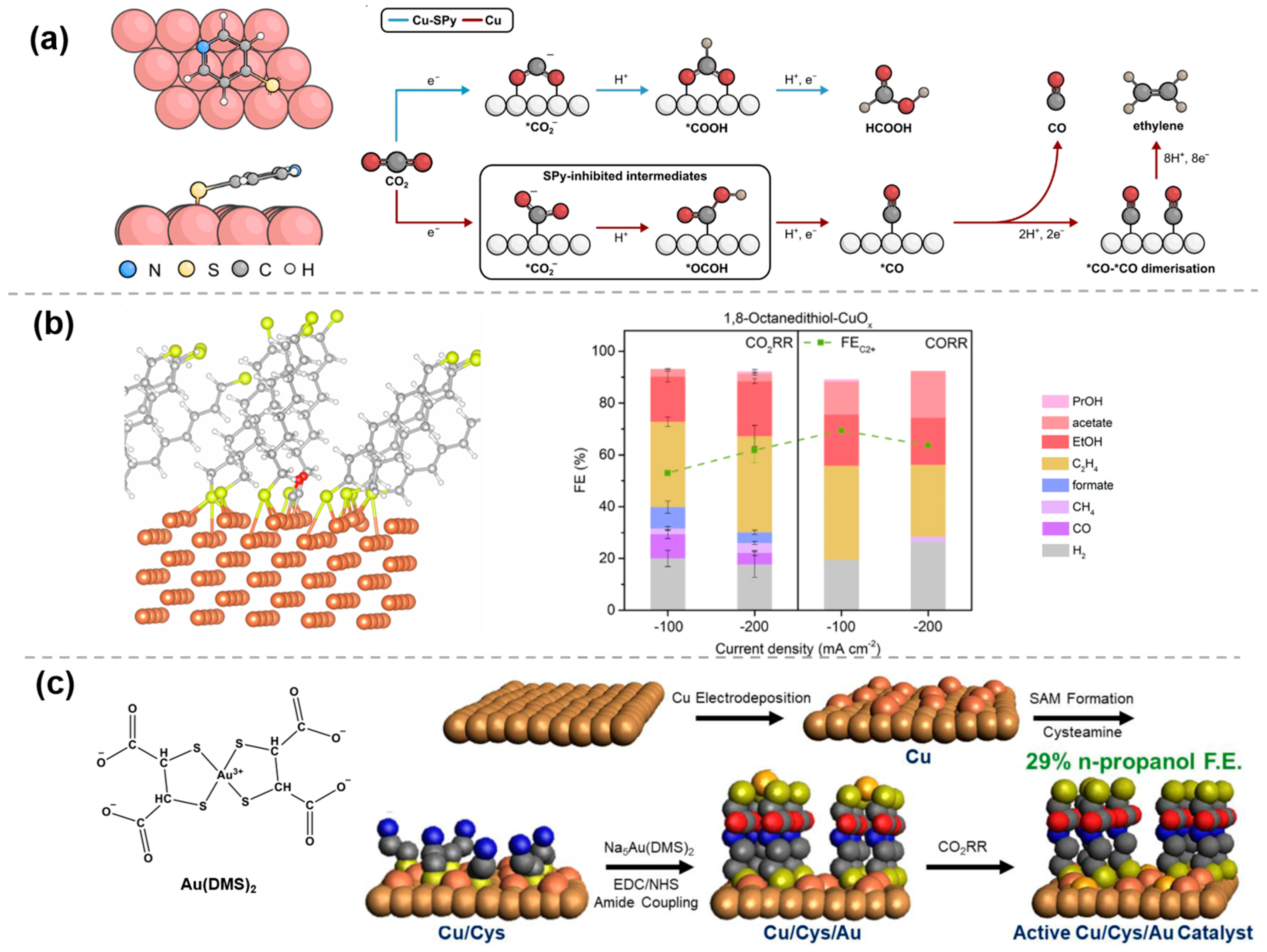
The M-N bond has been extensively employed in organic chemistry to synthesize a wide range of organometallic complexes with rich structural diversity and versatile properties. Moreover, it can also serve as a fundamental structural motif for the preparation of organic molecule-modified materials, which has been shown to significantly enhance the catalytic performance of the resulting materials. To improve the selectivity and yield of multi-carbon products, Yan et al. [27] prepared a cyanamide coordinated isolated Cu framework (Cuδ+NCN), using a modified liquid-phase precipitation method. In this catalyst, the linear [NCN]2− anions in the Cuδ+NCN open framework stabilize the Cu0-Cu1+ ensembles by strong covalent interactions and the fast electrons transfer, while Cuδ+ is linked to the nitrogen atom of [NCN]2− and oxygen atom through Cu-N/C/O bonding to form a stable localized charge environment (Figure 2a). This distinctive coordination mode is key to achieving high ethylene selectivity (FE > 75%) and long-term stability (80 h). Furthermore, Wang et al. [28] prepared a series of benzimidazole (BIM)-based Cu coordination polymers (CuCPs) as precatalysts by reacting BIM derivatives with Cu2O at 60 °C (Figure 2b) to enhance CO2RR to C2+ products in acidic environments. The decrease or disappearance of the Cu2O peaks in X-ray diffraction (XRD) patterns, the disappearance of the Cu-O stretching mode peak in FTIR, and the merging of the N 1S spectra into a single peak in XPS, confirmed the formation of Cu-N bond and the successful preparation of CuCPs. By varying the length of the substituent at the two-position of BIM ligands, a volcano-liked trend in the FE for C2+ products was observed. Notably, pentyl-CuCP exhibited the highest FE for C2+ products, reaching 73.4 ± 2.9% at a current density of 260 mA cm−2.
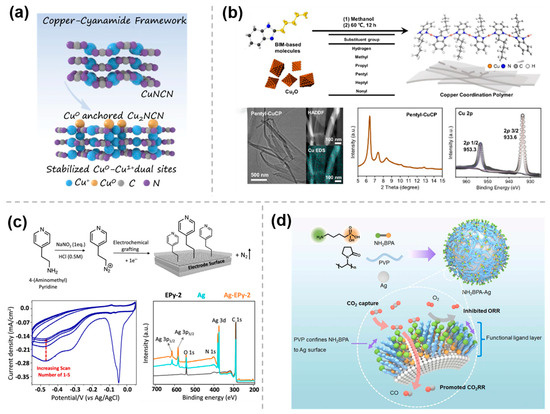
Figure 2.
Modification of metal and organic molecules by M−N/C/O bonding: (a) Cuδ+NCN [27], with permission from Springer Nature, copyright 2024; (b) pentyl−CuCP [28], with permission from Springer Nature, copyright 2024; (c) Ag−EPy [29], with permission from American Chemical Society, copyright 2022; and (d) NH2BPA−Ag [30], with permission from Elsevier, copyright 2024.
In addition, organic molecules can be immobilized on metal and carbon substrates using electrografting techniques involving diazotization, allowing organic molecular modifications on the electrode surface. As an instance, after the diazotization of aminopyridine derivatives in HCl solution by using NaNO2 to form pyridines-N2+, in situ reduction in the diazonium cation can be carried out on the surface of the Ag electrode by cyclic voltammetry (CV). The presence of characteristic irreversible reduction peaks in the CV curves indicates that the reduction in diazonium salt generates an aryl radical intermediate, which subsequently bonds covalently to the electrode surface and releases N2 gas. As shown in Figure 2c, the cathodic peaks shifted to more negative potentials and the current density decreased during cycles 2 to 5, confirming the successful electrografting of pyridine onto the electrode surface, which was further verified by XPS. The catalyst (Ag-EPy) prepared by this method, with pyridine grafted onto the Ag electrode, shows a significant improvement in product selectivity during the CO2 conversion process compared to a single catalyst. A 10-fold activity enhancement is observed at −0.7 V vs. RHE, and demonstrable higher partial current densities for CO are observed, indicating that a cocatalytic effect can be achieved through the integration of the two different catalytic structures [29].
In addition, a facile hydrothermal reaction can also be employed for molecular modification of metal surfaces. Kang et al. [30] applied this approach for constructing a controllable local environment at the molecular scale by grafting amine-functionalized ligand layers onto Ag nanoparticles (NPs) (Figure 2d). In this strategy, 4-aminobutylphosphonic acid (NH2BPA) binds to the Ag surface with its phosphonic acid group, while the carbonyl oxygen atoms of the polyvinylpyrrolidone (PVP) molecule also bind to Ag surface atoms preventing the growth of NPs and forming a thin organic layer on the Ag NPs confining NH2BPA to the catalyst surface. The results show that the amino groups significantly facilitate CO2 adsorption on the nanoparticle surface, while the phosphonic acid groups inhibit the oxygen reduction reaction, and thus the prepared NH2BPA-Ag can be used for electroreduction of simulated flue gas with high FE of CO.
2.2. Weak Interaction Modification
In addition to the most common metal–organic molecular bonding, many catalysts are modified through weak interactions such as π-π interactions. The π-π interaction modification strategy primarily relies on the interaction between organic molecules with π-electrons, such as aromatic rings and the catalyst. This interaction can modulate the electronic density on the catalyst surface, promote CO2 molecule adsorption, or stabilize intermediates, thereby enhancing the reaction selectivity and catalytic efficiency of CO2RR.
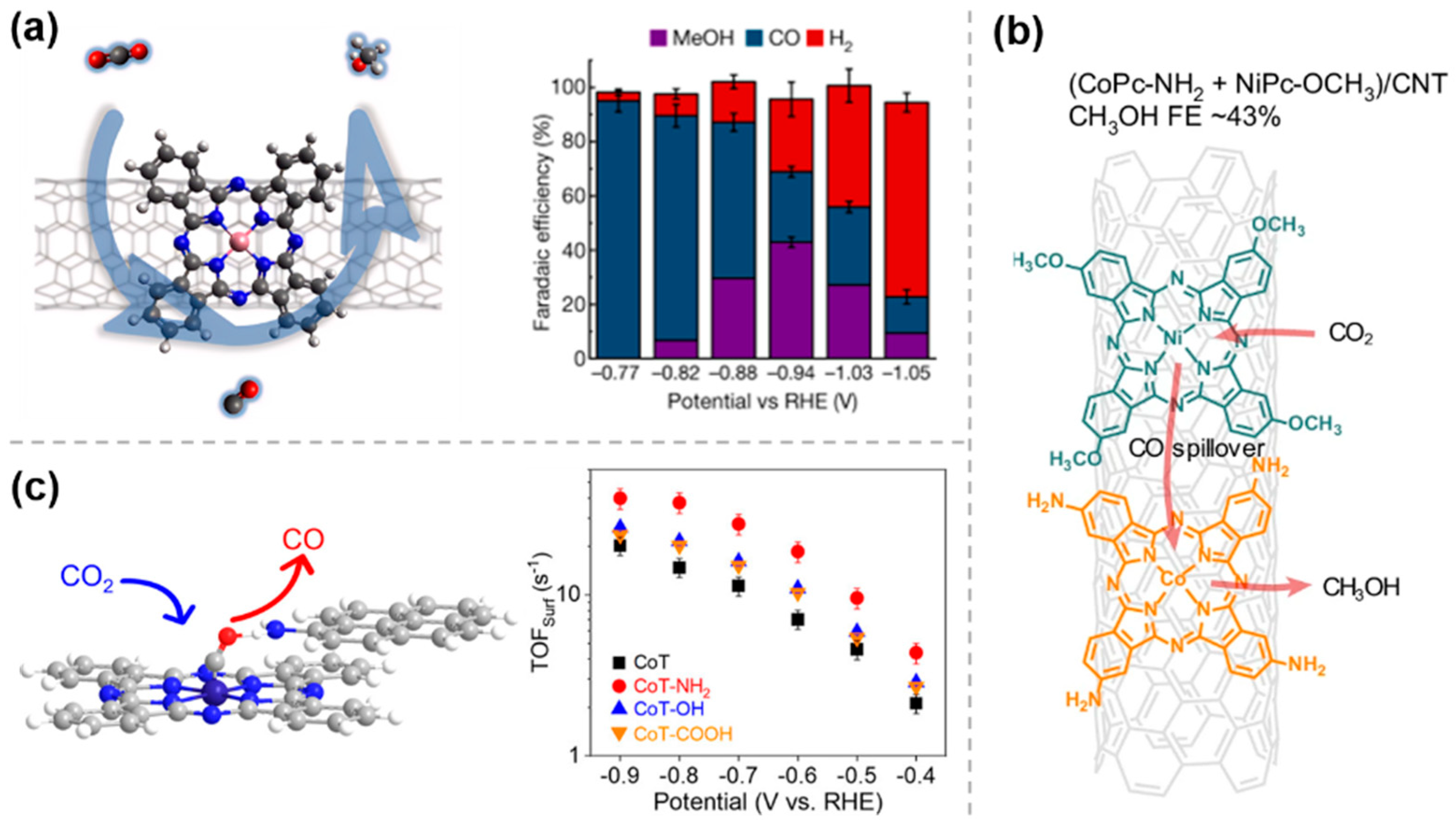
Currently, the compounds used for the modification of catalysts through weak interaction are mainly based on phthalocyanine catalysts. Regarding the current problem of poor selectivity in the generation of methanol through CO2RR, in 2019, Wang et al. [31] utilized this strategy by uniformly dispersing molecular catalysts such as cobalt phthalocyanine (CoPc) on carbon nanotubes (CNTs) enabled the efficient electrocatalytic reduction of CO2 to methanol via a domino process with CO as an intermediate (Figure 3a). Based on this discovery, subsequent studies have explored various modifications of CNTs with CoPc-like materials for CO2 electrocatalytic reduction. For example, in order to further improve the efficiency of methanol production from CO2RR, the same group [32] reported in early 2025 on a strategy to integrate a second catalytic site into a CoPc-NH2/CNT electrocatalyst (Figure 3b). The nickel tetramethoxyphthalocyanine (NiPc-OCH3) molecules in this cascade catalyst produce a large amount of CO via CO2 reduction, which then overflows to the nearby methanol-active CoPc-NH2 sites for further reduction, thereby promoting CO2RR to methanol. These studies demonstrate that efficient immobilization of molecular catalysts can be achieved by π-π interactions, which enhance the selectivity of CO2RR.
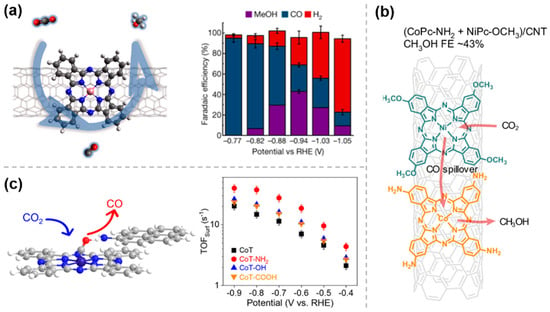
Figure 3.
Weak interaction modification by π−π conjugation: (a) CoPc/CNTs [31], with permission from Springer Nature, copyright 2019; (b) (CoPc−NH2 + NiPc−OCH3)/CNT [32], with permission from Springer Nature, copyright 2025; and (c) CoT−NH2 [33], with permission from American Chemical Society, copyright 2025.
Similarly, 1-aminopyrene molecules can be noncovalently anchored onto a nanosized CoPc nanotube (CoT) via simple π-π interactions, leading to the construction of hangman structures (Figure 3c) [33]. This structure facilitates electron transfer kinetics in both the inner and outer layers of the complex interface and enhances the chemisorption of *COOH and *CO intermediates through the interaction of Co sites and side chain amine groups in a bridging geometry. It therefore improves the intrinsic CO2RR activity of CoT and its selectivity for CO.
2.3. Polymer-Based Material Modification
Polymers are macromolecular compounds composed of repeating structural units, characterized by their diverse structures, tunable properties, and widespread applications in various fields such as materials science, engineering, and biotechnology. They can be used not only as modifiers for the electrocatalysts to increase surface hydrophobicity and optimize the CO2RR process, but also as substrate materials, especially conductive polymers, to receive functionalized modifications from other organic molecules, thereby improving their electrocatalytic performance. The modification of polymer-based material can be primarily achieved through various techniques, including spray-coating, in situ polymerization, and co-electrodeposition.
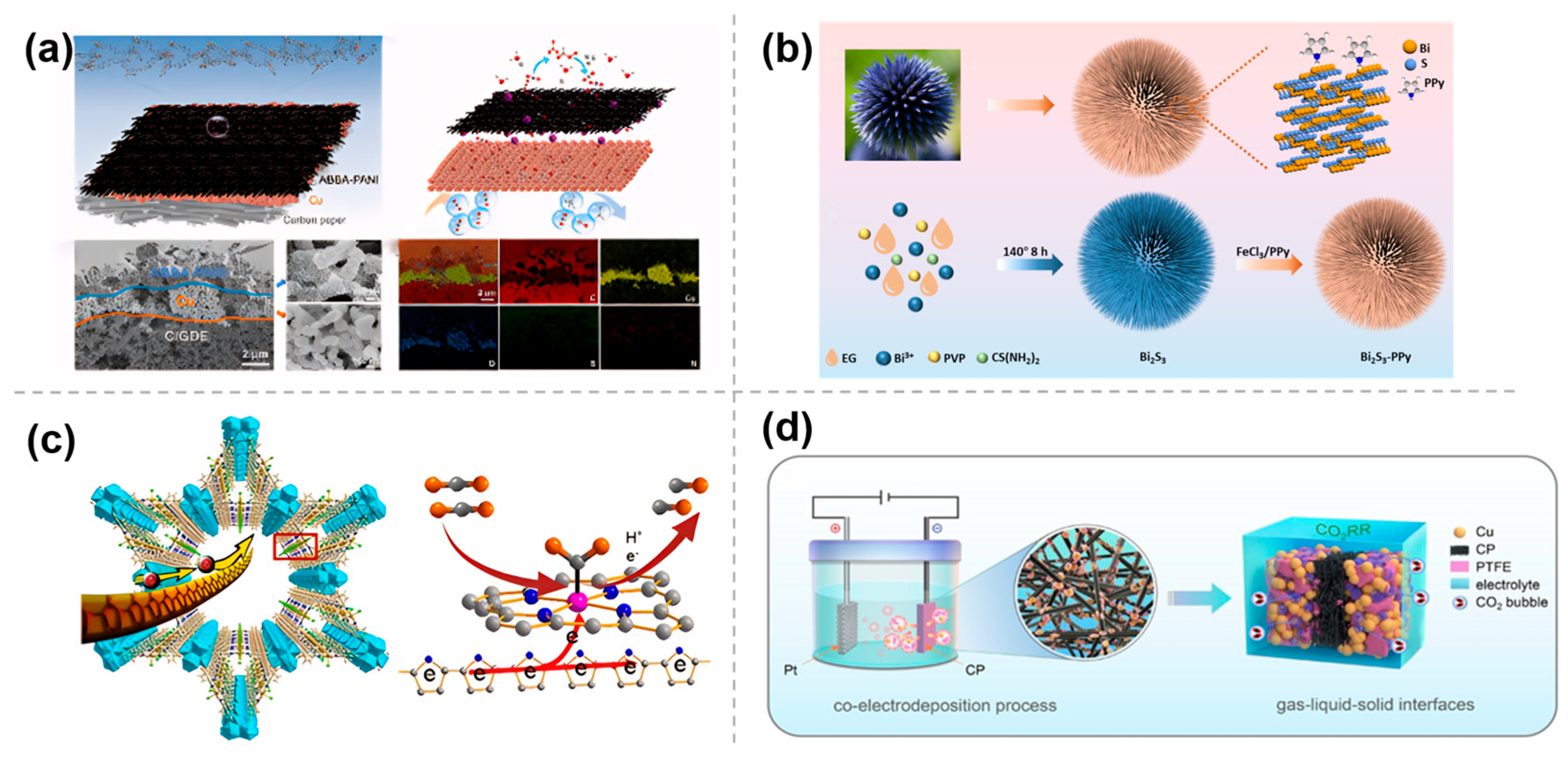
The spray-coating method allows the easy modification of various pre-prepared polymer molecules onto the substrate material and also facilitates the evaluation of the effect of the polymer layer on the CO2RR performance of the catalytic layer. For example, Huang et al. [34] created an ideal microenvironment for CO2RR between the Cu catalyst layer and the electrolyte by using polyaniline (PANI) modified by different proton acids (Figure 4a). This polymer layer modulated the local hydrophobicity, facilitated the adsorption and actitation of CO2, and also enhanced charge transfer and ion transport at the electrochemical reaction interface, thus significantly improving the acidic CO2RR kinetics. In the case of amino benzenesulfonic acid-PANI (ABSA-PANI), the prepared Cu/ABSA-PANI catalysts exhibited both excellent electronic conductivity and ion conductivity, resulting in reduced ohmic losses at the cathode and lower overpotential, achieving FE of 81% for C2+ products with a partial current density of 486 mA cm−2.
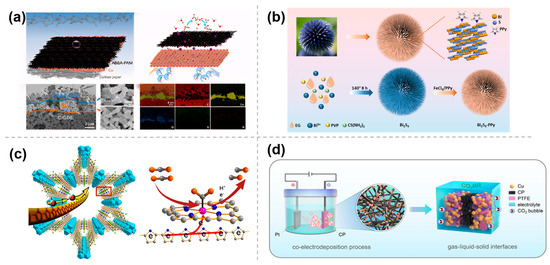
Figure 4.
Modification by polymer-based materials: (a) Cu/ABSA−PANI [34], with permission from John Wiley and Sons, copyright 2025; (b) Bi2S3−PPy [35], with permission from RSC Publishing, copyright 2025; (c) PPy@MOF−545−Co [36], with permission from American Chemical Society, copyright 2021; and (d) Cu−PTFE/CP [37], with permission from John Wiley and Sons, copyright 2023.
Alternatively, the polymer can also be prepared by in situ polymerization on the electrocatalyst surface. Chen et al. [35] constructed a novel Bi2S3-polymer polypyrrole (PPy) composite electrocatalyst with rich S vacancies through in situ vapor-phase polymerization with pyrrole on the surface of a 3D hierarchical urchin-like Bi2S3 nanoflower (Figure 4b). The unique nanostructure morphology maximized the exposure of active sites, thereby increasing the contact area with CO2 and improving the adsorption of the reactionintermediate *OCHO, resulting in excellent selectivity for formate. Moreover, the PPy layer on the surface of the Bi2S3-PPy composite can effectively prevent the loss of S elements and enhance the structural stability of Bi2S3. In a separate study, Xin et al. [36] inserted the conductive polymer PPy into the channels of MOF-545-Co via in situ polymerization, creating a new composite catalyst, PPy@MOF-545-Co (Figure 4c). In this structure, PPy molecules act like electric cables within the MOF channels, facilitating electron transfer to the Co-TCPP center, which in turn exhibits excellent electrocatalytic performance for CO2RR.
In addition, the polymer can be cemented onto the electrode surface by co-electrodeposition to modulate the interfacial microenvironment, especially the construction of hydrophobic microenvironment to overcome the limitation of CO2 solubility in aqueous electrolytes. Han et al. [37] reported a direct and facile strategy for polytetrafluoroethylene (PTFE) treatment to construct electrocatalysts by one-step co-electrodeposition of Cu and PTFE on carbon paper. The copper-based electrodes with superhydrophobic surface microenvironment exhibited high ethylene selectivity (Figure 4d). The improved performance was attributed to the formation of a gas–liquid–solid microenvironment, which accelerated mass transfer, increased the local concentration of CO2 near the catalyst surface, and improved the adsorption of CO2 for the reaction.
3. Effects of Molecularly Modified Materials in CO2RR
CO2RR is a process involving multiple proton-coupled electron transfers, encompassing both electrochemical and chemical reactions. The number of transferred electrons for the main products of CO2RR ranges from 2 to 18. This process involves numerous intermediates that significantly influence the reaction pathways and final products. Through molecular modification, the microenvironment of the catalyst surface can be regulated, and new active sites can be created on the catalyst surface, which can enhance the adsorption and activation of CO2, lower the reaction barriers, and thereby improve the efficiency of the reduction reaction and the selectivity of the target products. The effects involve multiple factors, including but not limited to the following: the electronic effects of the catalyst, steric hindrance, promoting reactant adsorption and stabilizing intermediates, the hydrophobic environment, and the regulation of pH. The catalytic role of molecularly modified materials in CO2RR is mainly reflected in the following aspects.
3.1. Electronic Effects
Organic molecules can influence the electronic environment of the catalyst surface, thereby affecting the electron transfer process and altering the catalytic performance. The realization of electronic effects depends on the function of different functional groups in the organic molecules. For example, organic molecules containing electron-donating groups (such as amino, thiol, etc.) can increase the electron density of the catalyst surface by direct electron donation, thereby enhancing the interaction between the catalyst and CO2 molecules and promoting effective CO2 adsorption. In contrast, certain functional groups with strong electron-withdrawing properties (such as fluoro, carboxyl, etc.) can significantly reduce the electron density on the catalyst surface. The electronic effect not only affects the stability of reaction intermediates but also modifies the reaction pathway to some extent.
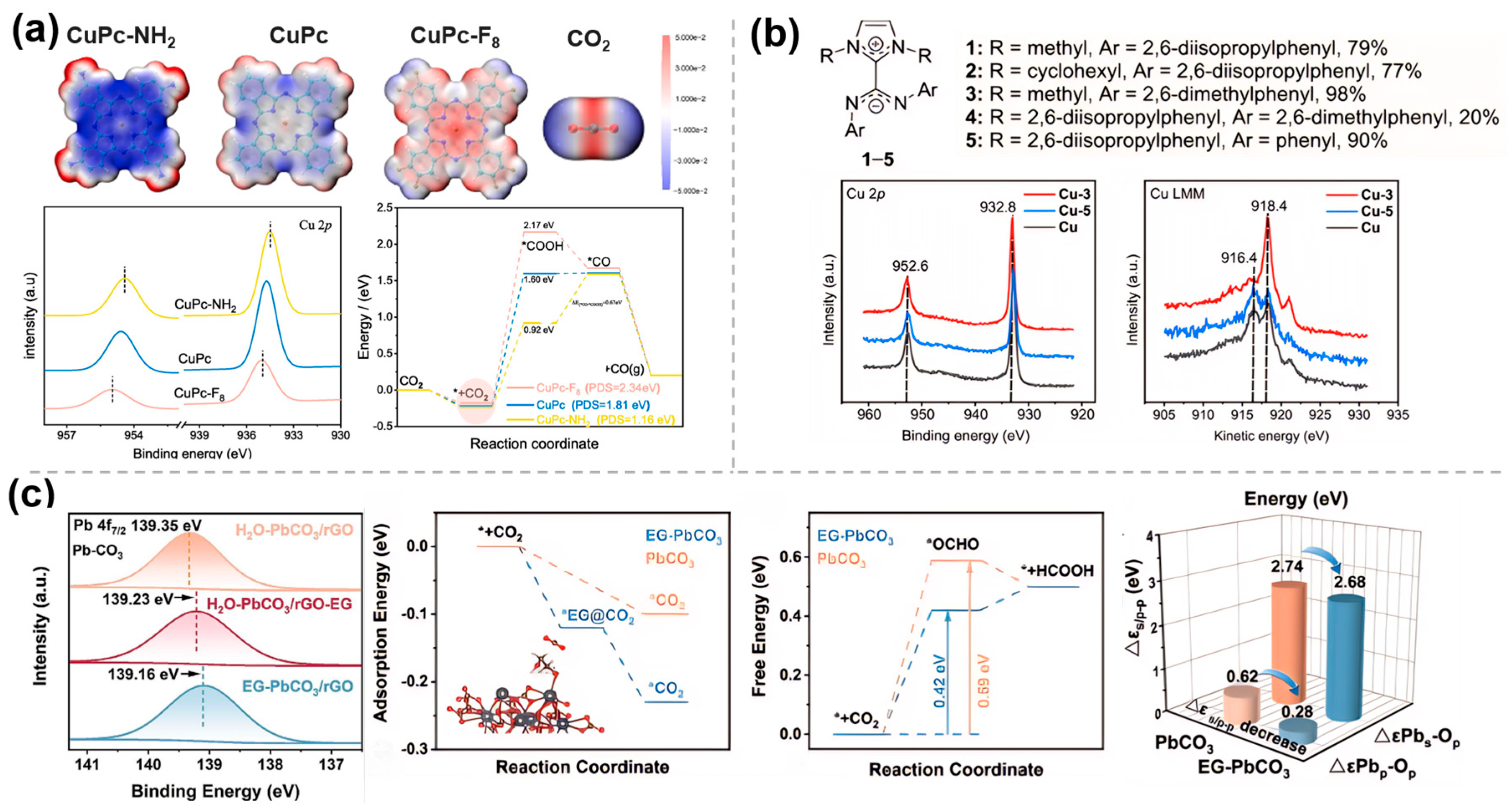
As mentioned in Section 2.2, molecular catalysts, such as phthalocyanine catalysts, can effectively catalyze CO2RR. Using copper phthalocyanine (CuPc), Peng et al. [38] investigated the effect of the electron-induced action of ligands on the electrocatalytic performance of CO2RR by combining electrochemical analysis, in situ characterization, and theoretical calculations (Figure 5a). The results show that the introduction of electron-donating groups (e.g., -NH2) increases the contribution of ligand groups to the density near the high electron-occupied states, decreases the width of the HOMO energy gap between CuPc-NH2 and CO2, and thus decreases the energy barrier formation of *COOH, and thus the FE of CO is close to 100%. On the contrary, the electron-inducing effect of electron-withdrawing groups (e.g., -F) leads to a decrease in the electron density around the Cu center and an increase in the positive electrostatic potential around the Cu-N4 site, which facilitates the formation of formic acid by adsorption at the oxygen end of *OCHO. Similarly, the electron donating/withdrawing effects of ligands have been exploited to induce changes in the electronic density at the metal centers of molecular catalysts, such as Cu/CH2NH2/SWCNTs [39] and MOF (ZIF-7) [40], thereby achieving precise control over catalytic activity, product selectivity, and reaction pathways.
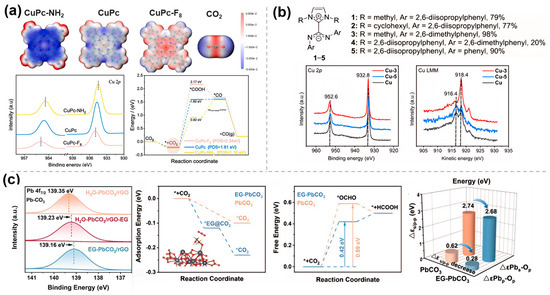
Figure 5.
Electronic effects of molecularly modified materials: (a) CuPc−NH2 [38], with permission from Elsevier, copyright 2025; (b) Cu−NHC−CDI [41], with permission from American Chemical Society, copyright 2024; and (c) EG−PbCO3/rGO [42], with permission from Elsevier, copyright 2025.
To improve the intrinsic activity of Cu-based materials towards multi-carbon hydrocarbons, Rosas-Hernández et al. [41] exploited the modification of Cu catalysts with N-heterocyclic carbene–carbon diimide (NHC-CDI). XPS measurements indicated that the NHC-CDI ligand 3 (Figure 5b) modified the Cu surface, adjusting its electronic structure, increasing the Cu0 concentration, and reducing the Cu+ content, thereby enhancing the electron density. Surface charge transfer was assessed using the modified pulse voltammetry method, revealing that the NHC-CDI ligand donates electrons to the Cu surface, stabilizing charged intermediates (e.g., *CO−) and enhancing surface charge accumulation. The NHC-CDI modification significantly improved the selectivity and current density for multi-carbon products through electronic effects.
Ren et al. [42] successfully synthesized ethylene glycol-functionalized lead carbonate/ reduced graphene oxide composite (EG-PbCO3/rGO) to increase the catalytic conversion rate of Pb-based catalysts. XPS results (Figure 5c) showed that EG modification caused a negative shift in Pb 4f binding energy (Δ = 0.12 eV), with electrons transferred from EG to Pb, thereby reducing its oxidation state. DFT calculations indicated that EG regulated the d-band center of Pb, enhancing orbital hybridization with the *OCHO intermediate and reducing the reaction energy barrier (ΔG decreased by 0.17 eV). Furthermore, EG minimized the difference between the Pb s/p bands and the O p-band, promoting interfacial electron transfer and enhancing catalytic activity.
3.2. Steric Hindrance Effects
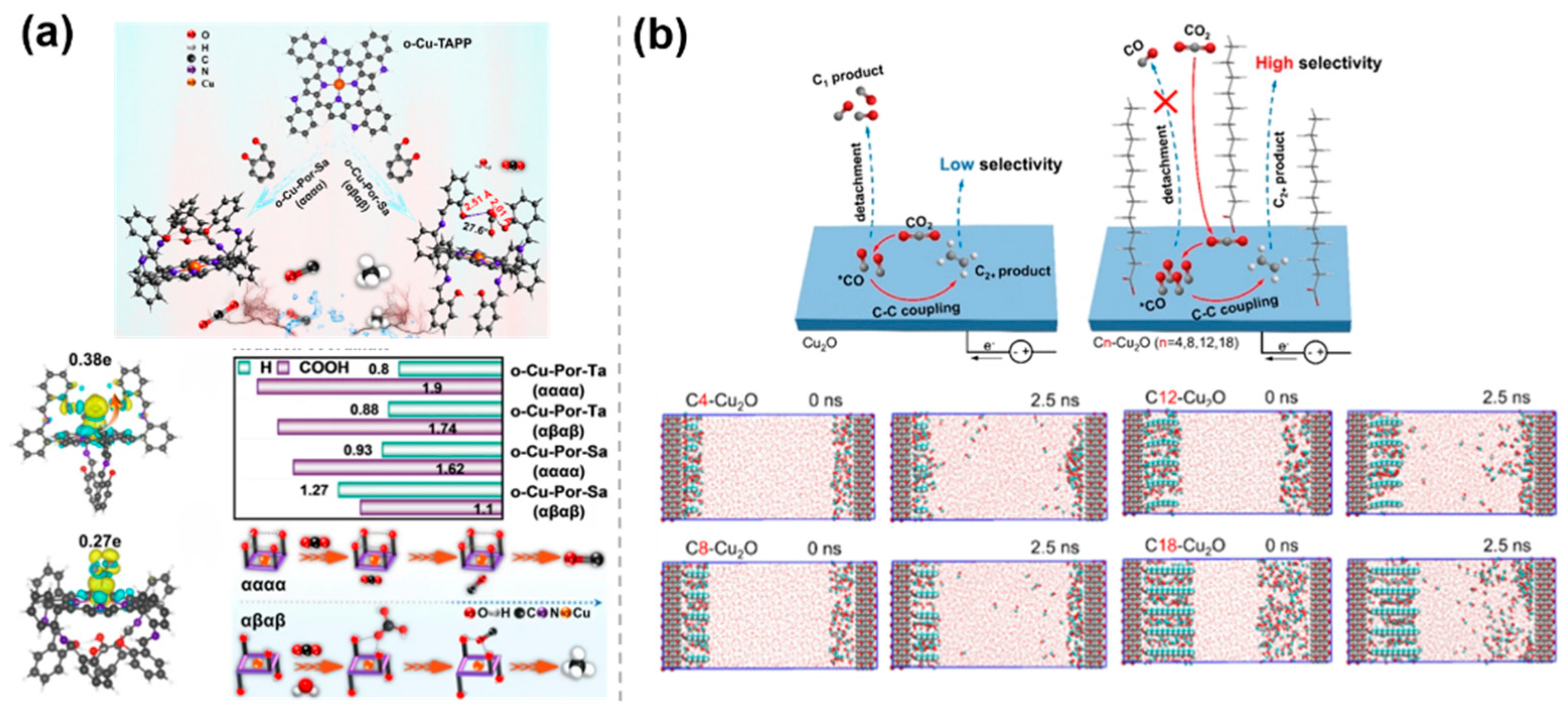
Steric hindrance effect of catalysts, which not only regulates reaction pathways by influencing the adsorption of reactants and the stability of intermediates but also improves catalytic performance by adjusting various factors such as the surface structure of the catalyst, the exposure of active sites, and the stability of the catalyst. Based on the key role of spatial ligand arrangement in inhibiting the competitive HER, Zhang et al. [43] proposed a bifunctional ligand isomerization strategy, which relies on the steric hindrance and hydrogen bonding interactions in o-Cu-Pro-Sa (αααα, αβαβ) to regulate the selectivity of CO2RR (Figure 6a). The study revealed the dual role of the steric hindrance effect in the blocked isomer. On the one hand, the symmetric distribution of the -OH group on o-Cu-Por-Sa (αβαβ) generates a steric hindrance effect that inhibits C-C coupling and promotes protonation reactions, leading to the formation of CH4. On the other hand, -OH aggregates on o-Cu-Por-Sa (αααα) to form a pocketed hydrogen-bonding lattice, which restricts the free adsorption of CO2 and exposes *CO to the formation of CO. To restrict the detachment of C1 intermediates and facilitate C-C coupling, Yan et al. [44] proposed a surface-confinement approach by grafting long alkyl chains onto Cu2O NPs for CO2RR (Figure 6b). The optimized C12-Cu2O sample exhibited an FE of over 63.0% for C2H4. In situ electrochemical attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy shows that the signal intensity of the *CO intermediate on the Cn-Cu2O samples increased more rapidly during the reaction and decreased more slowly after voltage-off, compared with the original Cu2O sample. This suggests that the modified carbon chains limit the diffusion of C1 intermediates. Molecular dynamics simulations further confirmed the limiting effect of the alkyl chains as a key factor, resulting in an extended residence time of *CO on Cn-Cu2O, corresponding to a higher chance of C-C coupling, leading to an increase in C2+ selectivity.
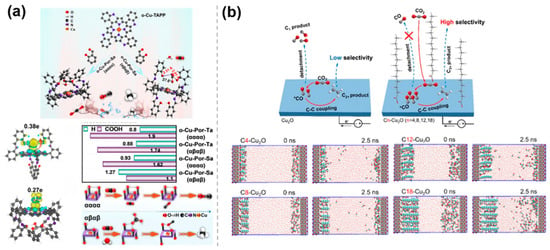
Figure 6.
Steric hindrance effects of molecularly modified materials: (a) o−Cu−Pro−Sa [43], with permission from John Wiley and Sons, copyright 2025; and (b) Cn−Cu2O [44], with permission from American Chemical Society, copyright 2024.
3.3. Promoting Reactant Adsorption and Stabilizing Intermediates
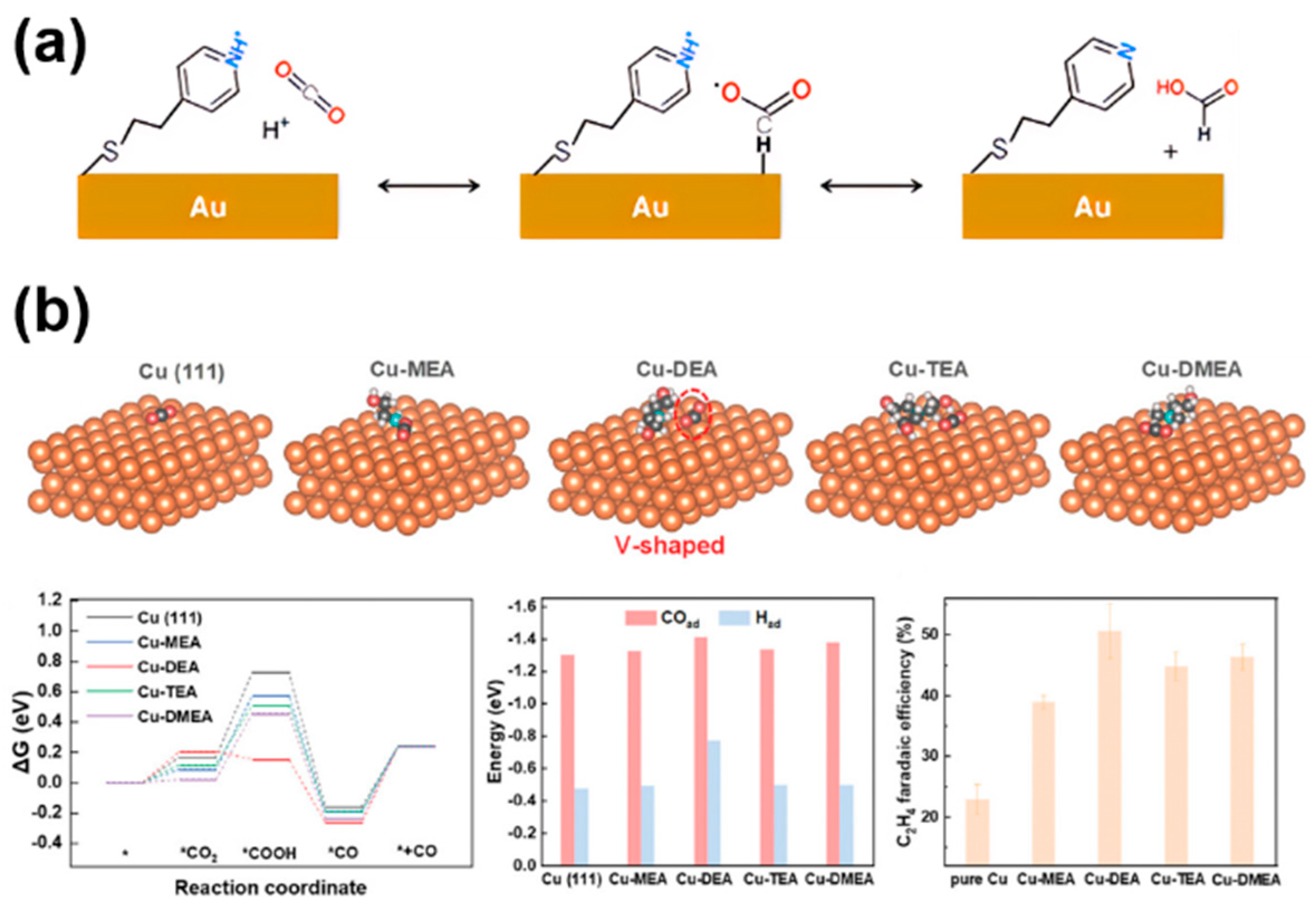
In the process of CO2RR, the interaction of reactants CO2 and intermediates (e.g., *CO, *HCOO, CH3O*, etc.) with the catalyst surface is the key to determining the efficiency of the catalytic reaction. Molecularly modified materials can promote the adsorption of CO2 or stabilize certain intermediates through molecular functionalization, while maintaining the adsorption of intermediates, which can effectively regulate the selectivity of CO2RR and even CORR. Flake et al. [19] carried out CO2 electrocatalytic reduction with 4-pyridineethanethiol (4-PEM) on the surface of a functionalized gold (Figure 7a). Compared to gold foil, the FE on the gold electrode modified with 4-PEM was doubled, and the formate yield increased by three times. This was mainly due to the fact that the hydrogen atom in the pyridine ring of 4-PEM interacts with the oxygen atom in the *HCOO intermediate through hydrogen bonding, thereby stabilizing the intermediate and significantly improving formate selectivity. Similarly, the nitrogen atoms in SPy immobilized on the copper surface could stabilize the *HCOO intermediate through hydrogen bonding, greatly enhancing the FE for formate production [18]. Recently, Wang et al. [45] reported a diethanolamine (DEA)-modified Cu (Cu-DEA) catalyst for the reduction of CO2 to C2H4. They utilized theoretical modeling to understand the effects of alkanolamine-modification on the adsorption behavior of CO2. DFT calculations showed that the CO2 adsorption on Cu-DEA surface transitioned to a V-shaped structure, while CO2 maintained a linear structure on the surfaces of other alkanolamine molecules (monoethanolamine (MEA), triethanolamine (TEA), and N, N-dimethylethanolamine (DMEA)) modified Cu (Figure 7b), indicating that the DEA modification promotes CO2 activation. Although the energy barrier for CO2 adsorption on Cu-DEA is higher than others, the subsequent formation of *COOH intermediates on Cu-DEA possesses self-exothermic reactions, leading to the formation of a large amount of *COOH intermediates. In addition, Cu-DEA has the strongest adsorption energy for *CO and *H, which is beneficial for the C-C coupling and proton coupling step in CO2RR process, resulting in good C2 selectivity. Briefly, molecular modification of catalysts can modulate the binding ability with CO2 molecules or key intermediates, thereby promoting specific reaction pathways to enhance selectivity.
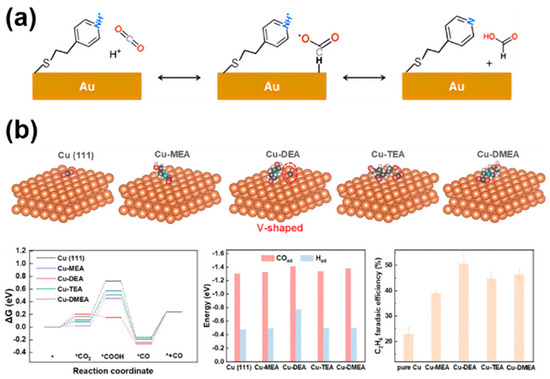
Figure 7.
The effect of reactant adsorption and intermediates stabilization in molecularly modified materials: (a) Au−PEM [19], with permission from American Chemical Society, copyright 2017; and (b) Cu−DEA [45], with permission from John Wiley and Sons, copyright 2024.
3.4. Hydrophobic Environment
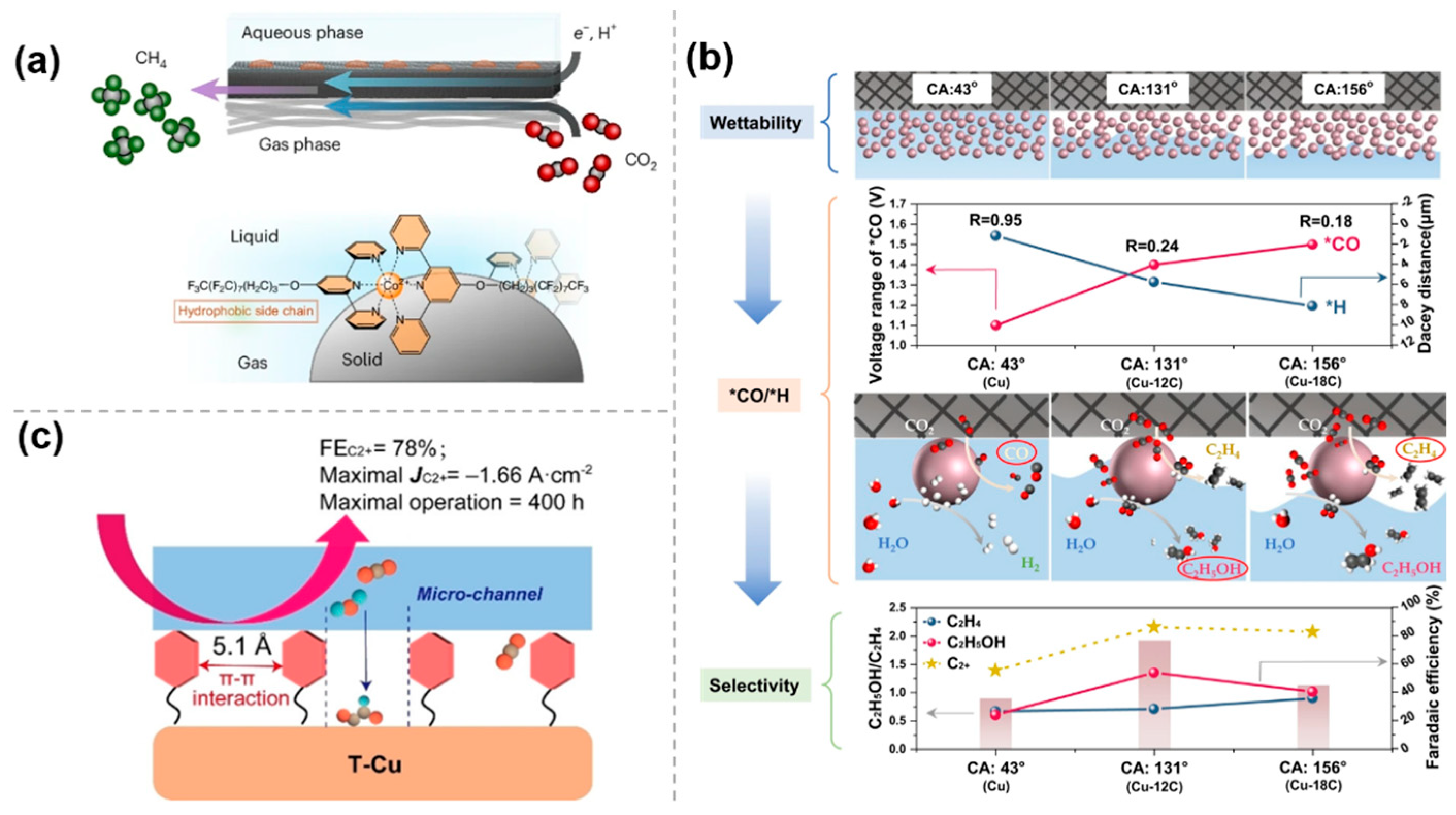
The performance of electrocatalytic CO2 reduction reaction is highly dependent on the microenvironment around the cathode, and molecular modifications are crucial to the catalytic process by creating a hydrophobic environment. Upon introduction of hydrophobic molecular modifications, the hydration on the catalyst surface, the adsorption pattern of reactants, and the reaction pathway can be modulated, which significantly improves the selectivity and efficiency of the CO2 reduction reaction. Kornienko et al. [46] successfully designed a molecular cobalt terpyridine catalyst with perfluorinated alkyl chains (Co Terpy-RF) to assemble the gas–liquid–solid interface. The strong interactions between the perfluoro chains drove the catalyst to assemble in an orderly manner at the gas–liquid–solid interface, forming a hydrophobic microenvironment which can be confirmed by the contact angle (100.7°) of the Co Terpy-RF film. Research has shown that the hydrophobic catalytic pocket can stabilize the intermediates of CO2RR, while the pyridine units act as the proton shuttles, transferring protons to active sites and ultimately promoting the production of CH4 from CO2 (Figure 8a).
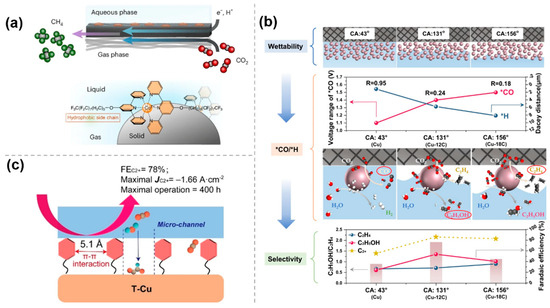
Figure 8.
The effect of hydrophobic environment in molecularly modified materials: (a) Co Terpy−RF [46], with permission from Springer Nature, copyright 2024; (b) Cu−nC [47], with permission from Springer Nature, copyright 2023; and (c) toluene−modified Cu nanosheet (T−Cu) [48], with permission from John Wiley and Sons, copyright 2023.
To investigate the effect of interfacial wettability on the pathway of CO2RR to ethylene and ethanol, Gong et al. [47] proposed to functionalize the Cu surface with alkyl mercaptans with different chain lengths, where the thiol groups coordinated with the substrate to form tunable wettability layers whose hydrophobicity was precisely controlled by the alkyl chain dimensions. The local concentrations of CO2 and H2O are regulated by different interfacial wettability to achieve the optimal equilibrium of kinetically controlled *CO and *H in a controlled manner. The experimental results show that the interfacial structure changes from a liquid–solid interface to a gas–liquid–solid interface and then to a gas–solid interface with increasing hydrophobicity. Different interfacial structures affect CO2 and H2O transport, which may lead to changes in *CO and *H coverage. An increase in hydrophobicity will lead to an increase in *CO coverage and a decrease in *H coverage, which will enhance the C-C coupling to form ethylene; conversely, a decrease in hydrophobicity will increase *H coverage and promote the formation of ethanol. Thus, the creation of a gas–liquid–solid interface by forming a suitably hydrophobic surface can enhance ethanol selectivity, whereas a superhydrophobic surface leads to ethylene production (Figure 8b).
To block the transport of interfacial water, Zheng et al. [48] developed a strategy for surface toluene functionalization of Cu nanosheet catalysts. The surface functionalized toluene molecules could self-assemble into ordered hydrophobic microchannels through π-π interactions of hydrophobic and conjugated benzene rings. The most stable distance between these parallel-arrayed benzene rings is 5.1 A, which is larger than that of the long-chain alkyl groups, and can control the transport of water and CO2 molecules at the same time. Specifically, the hydrophobic microchannels maintain efficient transport of CO2 molecules but delay water supply to the catalyst surface, which in turn facilitates CO2 to C2+ products (Figure 8c).
3.5. Requlation of Interfacial PH
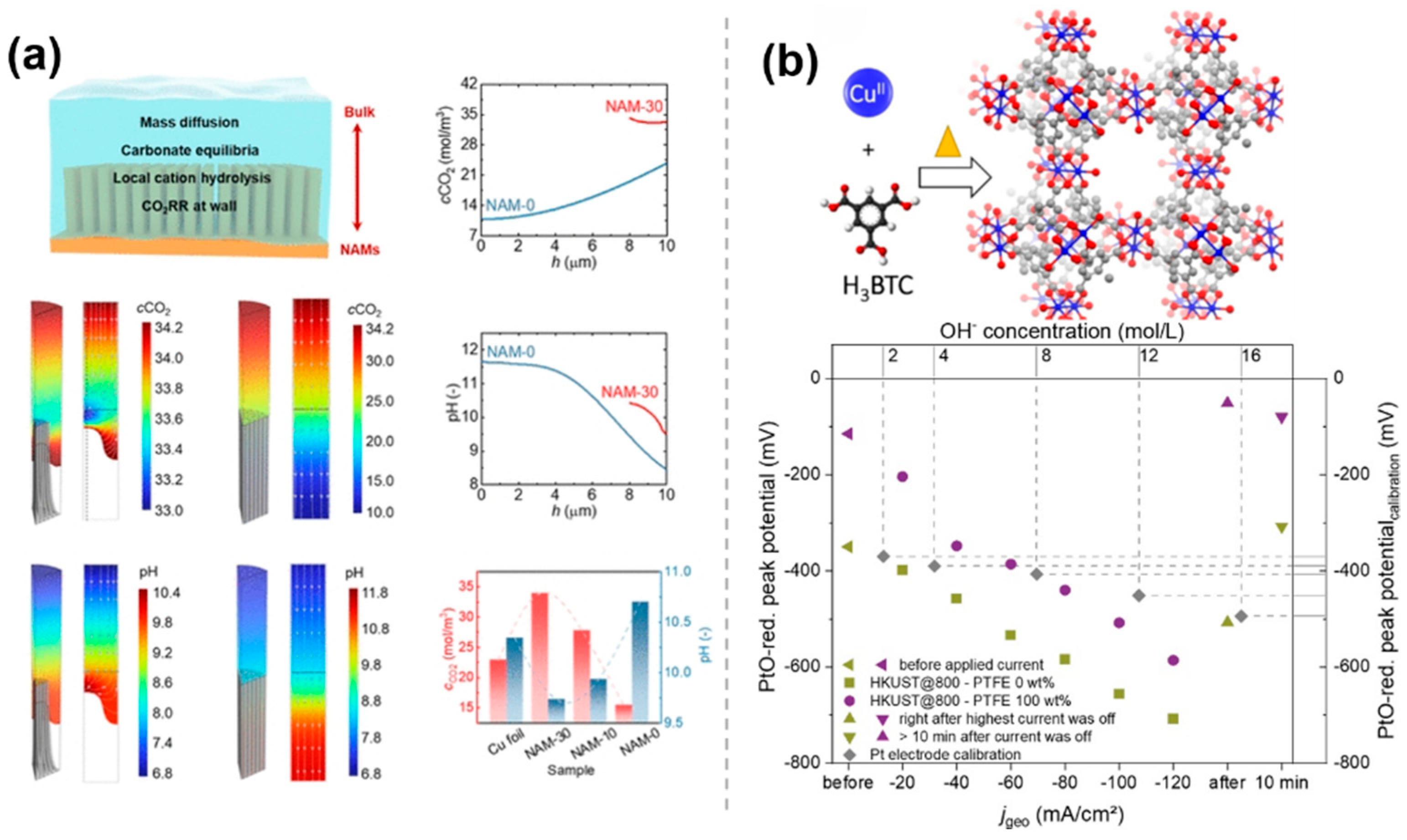
Molecular modifications, in addition to hydrophobicity, play a role in the regulation of the microenvironmental by regulating the interfacial pH. During CO2RR, CO2 is reduced to various products on the catalyst surface. The formation of these products usually depends on the stability of the intermediates, and the formation and conversion of intermediates are affected by acidity and alkalinity, so the mechanism of the reaction may change under different pH environments. In particular, the CO2 electroreduction reaction under acidic conditions can fundamentally solve the problems of CO2 loss and salt precipitation and has more favorable reaction kinetics. However, the acidic environment exacerbates the hydrogen precipitation reaction, which is detrimental to the CO2RR system. It was shown that modulating the pH around the catalyst by molecular modification constructs could improve the performance of the catalyst in CO2RR. Wong et al. [49] designed a tunable superhydrophobic hierarchical Cu nanowire arrays with a microgroove (NAMs), which can be tuned to regulate multiple synergistic effects in the microenvironment by adjusting the structure, including regulating the local pH, stabilizing the microwetting state, increasing the local CO2 concentration, and limiting CO intermediates. This process involves the mass transport processes, carbonate species equilibria of OH–, CO2, HCO3–, and CO32–, the local hydrolysis of K+ in the electrolyte phase, and mass consumption and generation during CO2RR (top left of Figure 9a). The NAMs exhibit different wetting states, resulting in significant differences in the local CO2 concentrations and pH distributions. The computational results of the spatial distributions of the CO2 concentration and pH for NAMs with different interfacial structures are shown in Figure 9a. NAM-30 has a stable gas–liquid interface with an electrolyte intrusion depth of about 2 μm, a small local pH gradient (9.6–10.4), and the neutral environment that promotes proton-electron transfer and suppresses HER. However, for NAM-0, the near one-dimensionality and longer diffusion distances lead to a large pH gradient (8.5–11.7), and the alkaline environment exacerbates HER and reduces C2+ selectivity (Figure 9a). Schuhmann et al. [50] reported a series of CuxOyCz nanostructured electrocatalysts derived from Cu-based MOF as a porous self-sacrificial template, providing variations in the structure and composition of the catalysts. The catalysts were blended with polytetrafluoroethylene (PTFE) on gas diffusion electrodes (GDEs) to inhibit the competitive hydrogen precipitation reaction. Since both CO2RR and competing HER consume water molecules, this leads to the accumulation of hydroxide ions (OH−) locally, thereby altering the local pH value. As the current density increases, the local OH−/H2O activity ratio significantly increases, indicating an increase in the local pH value (Figure 9b). The addition of PTFE can reduce the electrowetting effect on the electrode surface, thereby inhibiting HER and improving the selectivity of CO2RR.
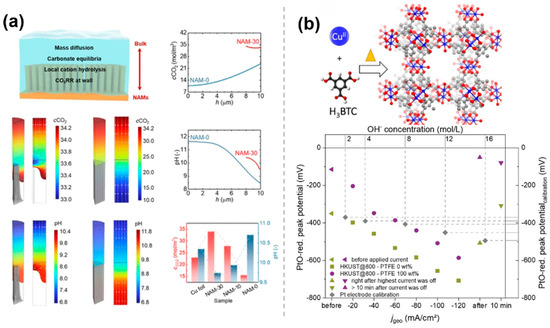
Figure 9.
The pH Regulation effect of molecularly modified materials: (a) NAMs [49], with permission from American Chemical Society, copyright 2025; and (b) CuxOyCz [50], with permission from John Wiley and Sons, copyright 2021.
4. Summary and Outlook
In this work we have summarized molecular modification strategies for enhancing CO2 electroreduction. Specifically, firstly, the strategies for the molecular modification have been summarized, such as metal–organic bonding, weak interactions, and modification with polymer-based materials. Then, the effects of molecularly modified materials on CO2RR are enumerated, including electronic effects, steric hindrance effects, the promotion of reactant adsorption and the stabilization of intermediates, the hydrophobic environment and pH regulation.
In the research of molecularly modified materials, possible future directions may include the following: (1) Structure design and optimization. Through advanced techniques such as computational chemistry simulation and machine learning, molecularly modified materials can be designed and optimized more efficiently to improve their catalytic performance in CO2RR. This approach can accelerate the discovery of new materials and provide guidance for the structural optimization of catalysts. (2) In-depth study of reaction mechanisms. The in-depth study of the reaction mechanisms of molecular modified materials in CO2RR through a combination of experimental and theoretical approaches will help to reveal key steps in the catalytic process and provide a theoretical basis for catalyst design. Particularly, the research to understand electron transfer, reaction pathways, and intermediate stability will be of great importance for catalyst development. (3) The development of multifunctional catalysts by combining different types of molecularly modified materials and developing multifunctional catalysts with synergistic effects may significantly improve the overall performance of CO2RR. The study of this new type of catalyst will not only improve catalytic efficiency but also optimize the reaction selectivity and expand the application areas of CO2RR.
The application of molecular modification in CO2RR enriches the idea of catalyst design and provides an important way to realize efficient and excellent selective CO2 conversion. Through different molecular modification strategies, we were able to modulate the surface properties, electronic structures, and reaction mechanisms of the catalysts, thus optimizing the performance of the CO2RR. Future work can further explore more efficient and sustainable molecular modification strategies, combined with advanced materials synthesis techniques, to promote the commercial application of CO2RR and contribute to solving energy and environmental problems.
Author Contributions
Conceptualization and writing—original draft preparation, Y.W. and H.W.; writing—review and editing, Y.W., H.W., L.C., G.L., J.M., F.Z. and J.L. Project administration and funding acquisition—H.W. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program of China (2023YFA1507903, 2020YFA0710200), and the National Natural Science Foundation of China (22072046).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Han, J.; Bai, X.; Xu, X.; Husile, A.; Zhang, S.; Qi, L.; Guan, J. Advances and challenges in the electrochemical reduction of carbon dioxide. Chem. Sci. 2024, 15, 7870. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.A.; Javed, M.S.; Najam, T.; Molochas, C.; Khan, N.A.; Nazir, M.A.; Xu, M.; Tsiakaras, P.; Bao, S.J. Metal oxides for the electrocatalytic reduction of carbon dioxide: Mechanism of active sites, composites, interface and defect engineering strategies. Coordin. Chem. Rev. 2022, 471, 214716. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.H.; Zhang, X.; Wang, H.; Zhao, Q. Metal-organic frameworks and their derivatives for electrochemical CO2 reduction reaction: Insights from molecular engineering. J. Mater. Chem. A 2024, 12, 20578. [Google Scholar] [CrossRef]
- Guo, W.; Liu, S.; Tan, X.; Wu, R.; Yan, X.; Chen, C.; Zhu, Q.; Zheng, L.; Ma, J.; Zhang, J. Highly efficient CO2 electroreduction to methanol through atomically dispersed Sn coupled with defective CuO catalysts. Angew. Chem. Int. Ed. 2021, 60, 21979. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, J.; Yang, Y.; Hung, S.-F.; Li, J.; Zhang, S.; Zhao, Y.; Zhang, A.; Wang, C.; Appadoo, D. Stabilizing copper sites in coordination polymers toward efficient electrochemical CC coupling. Nat. Commun. 2023, 14, 474. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Fang, Q.; Du, R.; Jin, Y.; Peng, C.; Cheng, D.; Li, T.; Zhao, T.; Zhang, S.; Zheng, Y. Selective electrochemical CO2 reduction to ethylene or ethanol via tuning* OH adsorption. Angew. Chem. Int. Ed. 2025, e202501773. [Google Scholar] [CrossRef]
- Bondue, C.J.; Graf, M.; Goyal, A.; Koper, M.T. Suppression of hydrogen evolution in acidic electrolytes by electrochemical CO2 reduction. J. Am. Chem. Soc. 2020, 143, 279. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Barile, C.J. Electrochemical CO2 reduction to methane with remarkably high Faradaic efficiency in the presence of a proton permeable membrane. Energ. Environ. Sci. 2020, 13, 3567. [Google Scholar] [CrossRef]
- Hirunsit, P.; Soodsawang, W.; Limtrakul, J. CO2 electrochemical reduction to methane and methanol on copper-based alloys: Theoretical insight. J. Phy. Chem. C 2015, 119, 8238. [Google Scholar] [CrossRef]
- Zhou, X.; Shan, J.; Chen, L.; Xia, B.Y.; Ling, T.; Duan, J.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Stabilizing Cu2+ ions by solid solutions to promote CO2 electroreduction to methane. J. Am. Chem. Soc. 2022, 144, 2079. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.-H.; De Luna, P.; Rosas-Hernández, A.; Thevenon, A.; Li, F.; Agapie, T.; Peters, J.C.; Shekhah, O.; Eddaoudi, M.; Sargent, E.H. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 2020, 19, 266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, J.; Liu, Y.; Su, C.; Yang, H.; Huang, Y.; Liu, B. Molecular tuning for electrochemical CO2 reduction. Joule 2023, 7, 1700. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Li, S.-B.; Liang, G.-H.; Xie, Y.-M.; Zhang, Y.; Zhang, H.; Tian, J.-H.; Zheng, S.; Zheng, Q.-N.; Chen, Z. Promoting water activation via molecular engineering enables efficient asymmetric C–C coupling during CO2 electroreduction. J. Am. Chem. Soc. 2024, 146, 32870. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Yang, Z.; Morgan, H.; Shi, J.; Shi, F.; Liu, M.; Wong, H.-W.; Gu, Z.; Che, F. Enhanced CO2 reactive capture and conversion using aminothiolate ligand–metal interface. J. Am. Chem. Soc. 2023, 145, 26038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.D.; Huang, J.R.; Shi, W.; Liao, P.Q.; Chen, X.M. Self-accelerating effect in a covalent-organic framework with Imidazole groups boosts electroreduction of CO2 to CO. Angew. Chem. 2023, 135, e202308195. [Google Scholar] [CrossRef]
- Yu, H.; Han, X.; Hua, Z.; Yang, W.; Wu, X.; Wu, Y.; Chen, S.; Hong, W.; Deng, S.; Zhang, J. Modulating electronic properties of carbon for selective electrochemical reduction of CO2 to methanol on Cu3P@C. ACS Catal. 2024, 14, 12783. [Google Scholar] [CrossRef]
- Yang, H.; Cai, H.; Li, D.; Kong, Y.; Feng, S.; Jiang, X.; Hu, Q.; He, C. Molecular modification enables CO2 electroreduction to methane on platinum surface in acidic media. Nat. Sci. Rev. 2024, 11, nwae361. [Google Scholar] [CrossRef] [PubMed]
- Creissen, C.E.; Rivera de la Cruz, J.G.; Karapinar, D.; Taverna, D.; Schreiber, M.W.; Fontecave, M. Molecular Inhibition for Selective CO2 Conversion. Angew. Chem. Int. Ed. 2022, 61, e202206279. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Flake, J.C. Electrochemical reduction of CO2 at functionalized Au electrodes. J. Am. Chem. Soc. 2017, 139, 3399. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Xi, S.; Zhang, T.; Liu, Z.; Chen, J.; Shen, L.; Kawi, S.; Wang, L. Functionalized Ag with thiol ligand to promote effective CO2 electroreduction. ACS Nano 2022, 16, 13982. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.L.; Lima, F.H.B. Dibenzyldithiocarbamate-functionalized small gold nanoparticles as selective catalysts for the electrochemical r eduction of CO2 to CO. ACS Catal. 2021, 11, 12208. [Google Scholar] [CrossRef]
- Li, J.-K.; Dong, J.-P.; Liu, S.-S.; Hua, Y.; Zhao, X.-L.; Li, Z.; Zhao, S.-N.; Zang, S.-Q.; Wang, R. Promoting CO2 electroreduction to hydrocarbon products via sulfur-enhanced proton feeding in atomically precise thiolate-protected Cu clusters. Angew. Chem. Int. Ed. 2024, 63, e202412144. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Fang, N.; Liu, X.; Ling, Y.; Su, Y.; Tan, T.; Chen, F.; Lin, H.; Zhao, B.; Wang, J.; et al. Observation of metal-organic interphase in Cu-based electrochemical CO2-to-ethanol conversion. Nat. Commun. 2025, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, F.; Ren, X.; Liu, Y.; Li, Y.; Shen, Z.; Wang, T.; Wang, W.; Wang, Y.-G.; Cui, Y.; et al. Molecular tuning boosts asymmetric C-C coupling for CO conversion to acetate. Nat. Commun. 2024, 15, 3641. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, E.; Jin, Q.; Zeraati, A.S.; Dorakhan, R.; Goncalves, T.J.; Abed, J.; Lee, B.-H.; Rasouli, A.S.; Wicks, J.; Zhang, J.; et al. Ligand-modified nanoparticle surfaces influence CO electroreduction selectivity. Nat. Commun. 2024, 15, 2995. [Google Scholar] [CrossRef] [PubMed]
- Bhoumik, N.C.; Padovan, Q.A.; Akter, T.; Stem, D.K.; Barile, C.J. Gold self-assembly on copper electrodes promotes n-propanol formation in electrochemical CO2 reduction. Angew. Chem. Int. Ed. 2025, 64, e202423882. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Qin, Y.; Huang, H.; Lv, Z.; Cai, M.; Su, Y.; Huang, F.; Yan, Y. Stabilized Cu0-Cu1+ dual sites in a cyanamide framework for selective CO2 electroreduction to ethylene. Nat. Commun. 2024, 15, 7820. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, T.; Zhao, W.; Wang, J.; Zhang, Y.; Zhang, S.; Yang, Y.; Yang, C.; Teng, W.; Chen, Z.; et al. Substituent tuning of Cu coordination polymers enables carbon-efficient CO2 electroreduction to multi-carbon products. Nat. Commun. 2024, 15, 9706. [Google Scholar] [CrossRef] [PubMed]
- Abdinejad, M.; Irtem, E.; Farzi, A.; Sassenburg, M.; Subramanian, S.; van Montfort, H.-P.I.; Ripepi, D.; Li, M.; Middelkoop, J.; Seifitokaldani, A.; et al. CO2 electrolysis via surface-engineering electrografted pyridines on silver catalysts. ACS Catal. 2022, 12, 7862. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, T.; Shi, H.; Pan, H.; Kang, P. Grafting amine-functionalized ligand layer on catalyst for electrochemical CO2 capture and utilization. Appl. Catal. B Environ. 2024, 343, 123456. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lu, X.; Liang, Y.; Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 2019, 575, 639. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Q.; Chang, A.; Cheon, S.; Gao, Y.; Shang, B.; Li, H.; Rooney, C.L.; Ren, L.; Jiang, Z.; et al. Molecular-scale CO spillover on a dual-site electrocatalyst enhances methanol production from CO2 reduction. Nat. Nanotechnol. 2025, 20, 515. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Duan, X.; Xu, X.; Win, P.E.P.; Ren, S.-B.; Wang, J. Noncovalent construction of hangman cobalt phthalocyanine for enhanced electrochemical carbon dioxide reduction. Chem. Mater. 2025, 37, 360. [Google Scholar] [CrossRef]
- Su, L.; Hua, Q.; Feng, G.; Yang, Y.; Mei, H.; Yu, Y.; Chang, X.; Huang, Z. Multifunctional conductive polymer modification for efficient CO2 electroreduction in acidic electrolyte. Adv. Funct. Mater. 2025, 2425636. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Zhou, X.; Zhang, L.; Fu, Z.; Wu, Y.; Lv, X.; Zheng, G.; Chen, H. Bio-inspired engineering of Bi2S3-PPy composite for the efficient electrocatalytic reduction of carbon dioxide. Energ. Environ. Sci. 2023, 16, 3885. [Google Scholar] [CrossRef]
- Xin, Z.; Liu, J.; Wang, X.; Shen, K.; Yuan, Z.; Chen, Y.; Lan, Y.-Q. Implanting polypyrrole in metal-porphyrin MOFs: Enhanced electrocatalytic performance for CO2RR. ACS Appl. Mater. Interf. 2021, 13, 54959. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Jia, S.; Chen, C.; Jiao, J.; Chen, X.; Xue, C.; Xia, W.; Xing, X.; Zhu, Q.; Wu, H.; et al. Polymer modification strategy to modulate reaction microenvironment for enhanced CO2 electroreduction to ethylene. Angew. Chem. Int. Ed. 2024, 63, e202313796. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Qin, B.; Zhang, Q.; Yang, G.; Du, S.; Liu, Z.; Peng, F. Influence of electron-inducted effect of ligand on electrocatalytic reduction of CO2 by copper phthalocyanine. Chem. Eng. J. 2025, 506, 160154. [Google Scholar] [CrossRef]
- Wang, K.; Huang, K.; Wang, Z.; An, G.; Zhang, M.; Liu, W.; Fu, S.; Guo, H.; Zhang, B.; Lian, C.; et al. Functional group engineering of single-walled carbon nanotubes for anchoring copper nanoparticles toward selective CO2 electroreduction to C2 products. Small 2025, 21, 2502733. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, G.; Dong, Y.; Ji, J.; Li, L.; Xue, M.; Zhang, X.; Cheng, H.-M. Controlling the polarity of metal-organic frameworks to promote electrochemical CO2 reduction. Angew. Chem. Int. Ed. 2024, 64, e202416367. [Google Scholar] [CrossRef] [PubMed]
- Kolding, K.N.; Bretlau, M.; Zhao, S.; Ceccato, M.; Torbensen, K.; Daasbjerg, K.; Rosas-Hernández, A. NHC-CDI ligands boost multicarbon production in electrocatalytic CO2 reduction by increasing accumulated charged intermediates and promoting *CO dimerization on Cu. J. Am. Chem. Soc. 2024, 146, 13034. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Wang, Z.; Song, Z.; Wang, W.; Wang, X.; Chen, Z.; Ren, Z. Microenvironment modulation induced by ethylene-glycol modification enables high activity in selective CO2 electroreduction over lead-based catalysts. Chem. Eng. J. 2025, 512, 161963. [Google Scholar] [CrossRef]
- Wang, H.; Ma, C.; Lu, Q.; Gu, M.; Jiang, L.; Hao, Y.; Hu, F.; Li, L.; Wang, G.; Peng, S.; et al. Precise tuning of functional group spatial distribution on porphyrin rings for enhanced CO2 electroreduction selectivity. Angew. Chem. Int. Ed. 2025, 64, e202501091. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.; Guo, C.; Jia, S.; Zhou, Y.; Liu, Z.; Jiang, E.; Chen, X.; Zou, Y.; Huo, P.; et al. Confined intermediates boost C2+ selectivity in CO2 electroreduction. ACS Catal. 2024, 14, 13400. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, C.; Liu, W.; Liu, R.; Liu, Y.; Feng, X.; Yang, W.; Wang, B. Enhanced CO2 adsorption and conversion in diethanolamine-Cu interfaces achieving stable neutral ethylene electrosynthesis. Adv. Energy Mater. 2024, 14, 2402551. [Google Scholar] [CrossRef]
- McKee, M.; Kutter, M.; Wu, Y.; Williams, H.; Vaudreuil, M.-A.; Carta, M.; Yadav, A.K.; Singh, H.; Masson, J.-F.; Lentz, D.; et al. Hydrophobic assembly of molecular catalysts at the gas-liquid-solid interface drives highly selective CO2 electromethanation. Nat. Chem. 2025, 17, 92. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, T.; Zhang, L.; Zhang, G.; Li, L.; Chang, Q.; Pang, Z.; Gao, H.; Huang, K.; Zhang, P.; et al. Tunable CO2 electroreduction to ethanol and ethylene with controllable interfacial wettability. Nat. Commun. 2023, 14, 3575. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, X.; Kong, S.; Liu, M.; Liu, K.; Zhang, J.; Wu, B.; Zhang, Q.; Tang, Y.; Qian, L.; et al. Interfacial water tuning by intermolecular spacing for stable CO2 electroreduction to C2+ products. Angew. Chem. Int. Ed. 2023, 62, e202309319. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, Q.; Salaman, M.I.B.; Wei, C.; Wang, Q.; Ma, X.; Liu, B.; Wong, A.B. Microenvironment tailoring for electrocatalytic CO2 reduction: Effects of interfacial structure on controlling activity and selectivity. J. Am. Chem. Soc. 2025, 147, 12438. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, N.; Junqueira, J.R.C.; Dieckhöfer, S.; Quast, T.; Braun, M.; Song, Y.; Aiyappa, H.B.; Seisel, S.; Weidner, J.; Öhl, D.; et al. A metal-organic framework derived CuxOyCz catalyst for electrochemical CO2 reduction and impact of local pH change. Angew. Chem. Int. Ed. 2021, 60, 23427. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).