Tuning the d-Band Center of Nickel Bimetallic Compounds for Glycerol Chemisorption: A Density Functional Study
Abstract
:1. Introduction
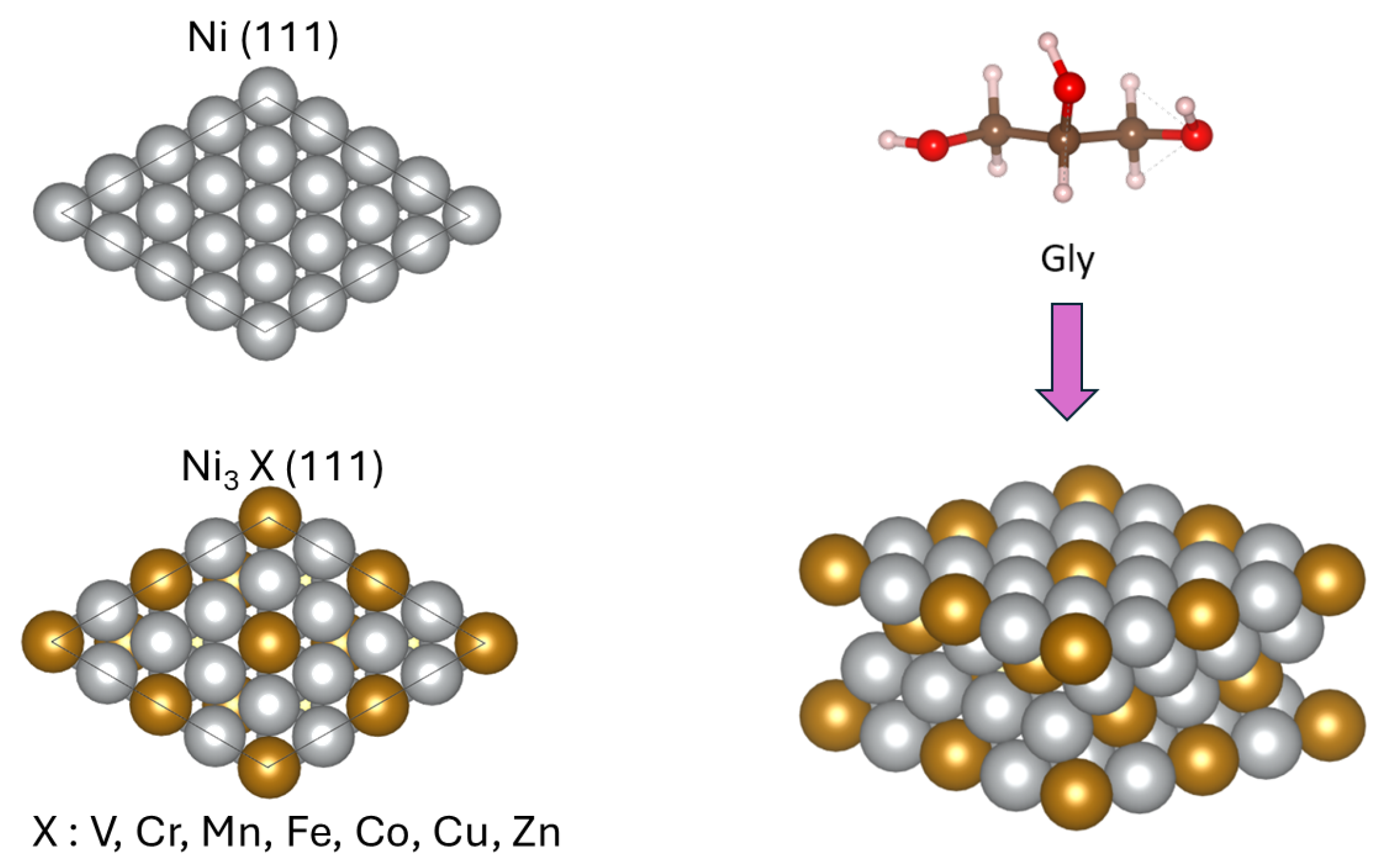
2. Computational Methods
3. Results
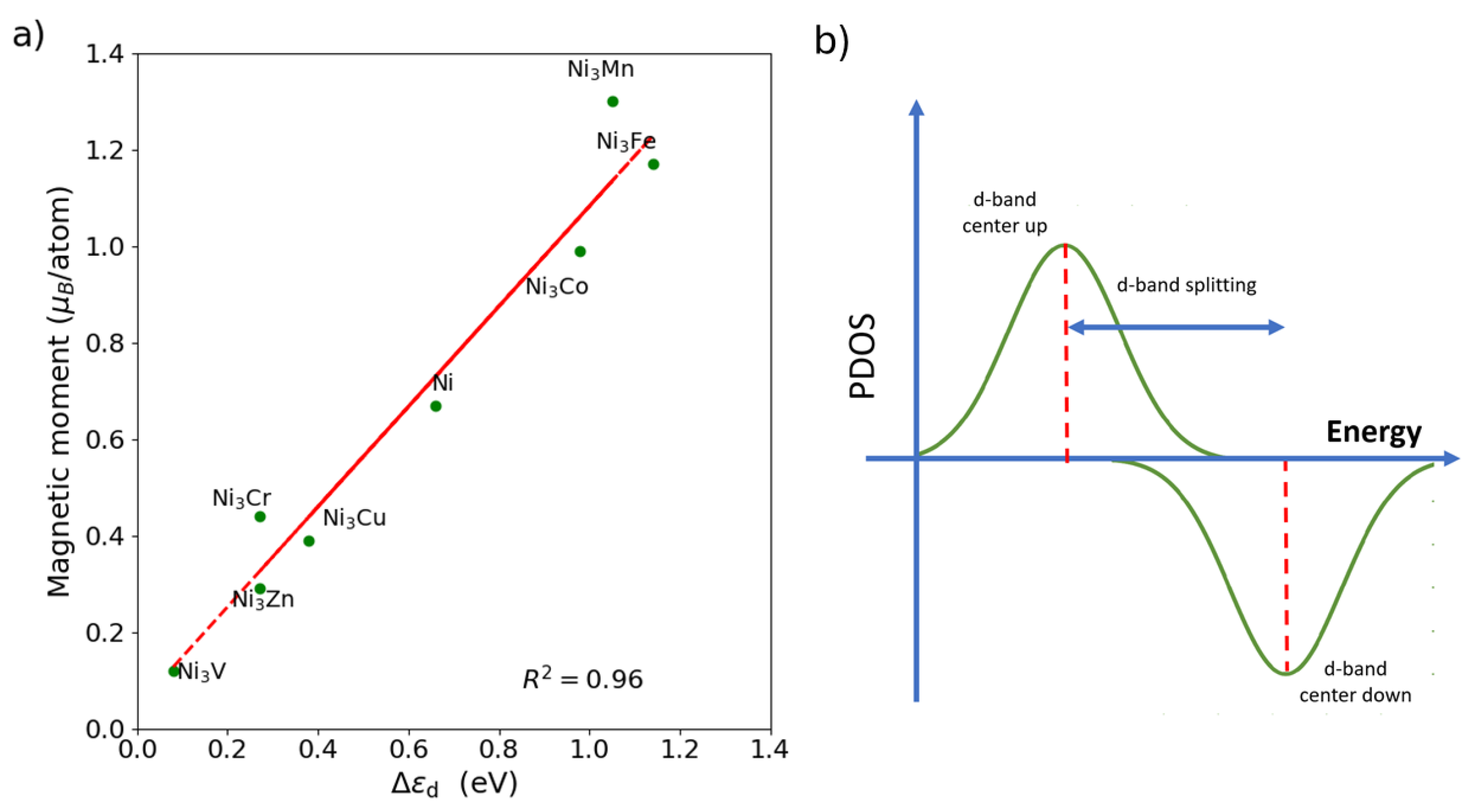
3.1. Influence of the Promoter on Electronic Properties
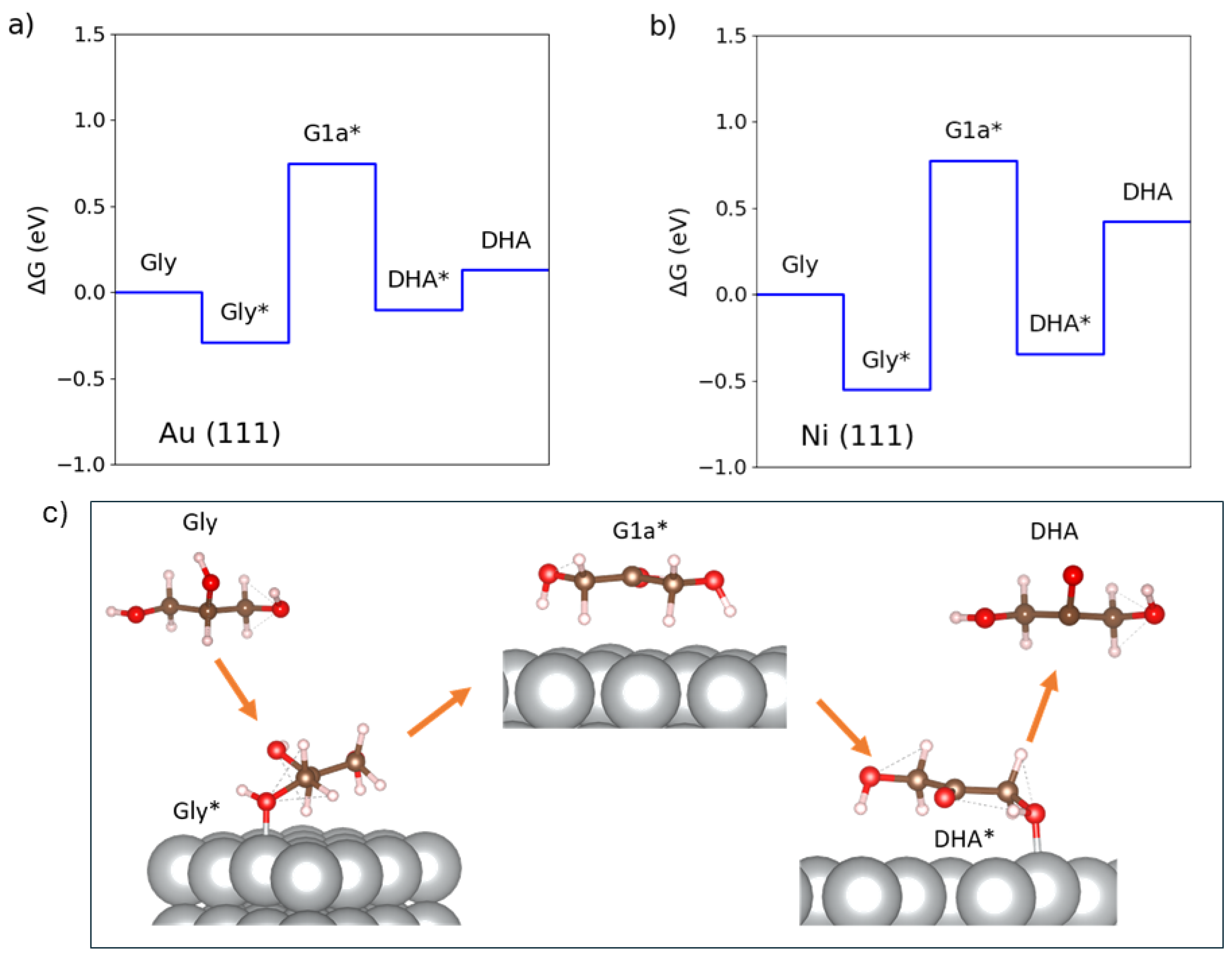
3.2. Influence on the Adsorption of Glycerol Molecules
3.3. d-Band Model Analysis
3.4. Intermediates of the Reaction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panizza, M.; Cerisola, G. Direct and Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, P.; Wang, S.; Chen, Y.H.; Zhang, H.; Lei, A. Direct Electrochemical Oxidation of Alcohols with Hydrogen Evolution in Continuous-Flow Reactor. Nat. Commun. 2019, 10, 2796. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, C.; Shen, P.K. Palladium-Based Electrocatalysts for Alcohol Oxidation in Half-Cell and Direct Alcohol Fuel Cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Hannagan, R.T.; Giannakakis, G.; Flytzani-Stephanopoulos, M.; Sykes, E.C.H. Single-Atom Alloy Catalysis. Chem. Rev. 2020, 120, 12044–12088. [Google Scholar] [CrossRef] [PubMed]
- Reina, A.; Serrano-Maldonado, A.; Teuma, E.; Martin, E.; Gomez, M. Palladium Nanocatalysts in Glycerol: Tuning the Reactivity by Effect of the Stabilizer. Catal. Commun. 2018, 104, 22–27. [Google Scholar] [CrossRef]
- Wu, K.; Mao, X.B.; Liang, Y.; Chen, Y.; Tang, Y.W.; Zhou, Y.M.; Lin, J.; Ma, C.N.; Lu, T.H. Multiwalled Carbon Nanotubes Supported Palladium-Phosphorus Nanoparticles for Ethanol Electrooxidation in Alkaline Solution. J. Power Sources 2012, 219, 258–262. [Google Scholar] [CrossRef]
- Liu, J.P.; Zhou, H.H.; Wang, Q.Q.; Zeng, F.Y.; Kuang, Y.F. Reduced Graphene Oxide Supported Palladium-Silver Bimetallic Nanoparticles for Ethanol Electro-Oxidation in Alkaline Media. J. Mater. Sci. 2012, 47, 2188–2194. [Google Scholar] [CrossRef]
- Zhao, L.; Thomas, J.P.; Heinig, N.F.; Abd-Ellah, M.; Wang, X.; Leung, K.T. Au–Pt Alloy Nanocatalysts for Electro-Oxidation of Methanol and Their Application for Fast-Response Non-Enzymatic Alcohol Sensing. J. Mater. Chem. C 2014, 2, 2707–2714. [Google Scholar] [CrossRef]
- Yang, D.; Liu, X.; Song, F.; Dai, Y.; Wan, X.; Zhou, C.; Yang, Y. Chemoselective Oxidation of Glycerol over Platinum-Based Catalysts: Toward the Role of Oxide Promoter. ChemCatChem 2022, 14, 10. [Google Scholar] [CrossRef]
- Wu, G.; Dong, X.; Mao, J.; Li, G.; Zhu, C.; Li, S.; Chen, A.; Feng, G.; Song, Y.; Chen, W.; et al. Anodic Glycerol Oxidation to Formate Facilitating Cathodic Hydrogen Evolution with Earth-Abundant Metal Oxide Catalysts. Chem. Eng. J. 2023, 468, 143640. [Google Scholar] [CrossRef]
- Massaneiro, J.; Valério, T.L.; Pellosi, D.S.; Gonçalves da Silva, B.J.; Vidotti, M. Electrocatalytic Oxidation of Glycerol Performed by Nickel/Cobalt Alloys: Adding Value to a Common Subproduct of Chemical Industry. Electrochim. Acta 2024, 506, 145013. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A. Ag/Co3O4 as an Effective Catalyst for Glycerol Electro-Oxidation in Alkaline Medium. Int. J. Hydrogen Energy 2021, 46, 4788–4797. [Google Scholar] [CrossRef]
- Liang, D.; Gao, J.; Wang, J.; Chen, P.; Wei, Y.; Hou, Z. Bimetallic Pt–Cu Catalysts for Glycerol Oxidation with Oxygen in a Base-Free Aqueous Solution. Catal. Commun. 2011, 12, 1059–1062. [Google Scholar] [CrossRef]
- Fan, L.; Ji, Y.; Wang, G.; Chen, J.; Chen, K.; Liu, X.; Wen, Z. High Entropy Alloy Electrocatalytic Electrode toward Alkaline Glycerol Valorization Coupling with Acidic Hydrogen Production. J. Am. Chem. Soc. 2022, 144, 7224–7235. [Google Scholar] [CrossRef] [PubMed]
- Houache, M.S.E.; Hughes, K.; Ahmed, A.; Safari, R.; Liu, H.; Botton, G.A.; Baranova, E.A. Electrochemical Valorization of Glycerol on Ni-Rich Bimetallic NiPd Nanoparticles: Insight into Product Selectivity Using in Situ Polarization Modulation Infrared-Reflection Absorption Spectroscopy. ACS Sustain. Chem. Eng. 2019, 7, 14425–14434. [Google Scholar] [CrossRef]
- Mohd-Nasir Nor Shafiqah, Y.; Jiao, Y.; Nguyen, V.C.; Abidin, S.Z. Promoted Ni–Co Bimetallic Catalysts for Glycerol Dry Reforming: Understanding the Physiochemical Properties and Carbon Formation. Int. J. Hydrogen Energy 2024, 56, 651–666. [Google Scholar] [CrossRef]
- Mohamed, R.; Rizk, M.G.; Abd El-Moghny, H.H.; Abdelhady, W.M.; Ragheb, A.H.; Mohamed, H.F.; Fouad, M.; Mohsen, M.; Kamel, A.S.; El-Deab, M.S. Tailor-Designed Bimetallic Co/Ni Macroporous Electrocatalyst for Efficient Glycerol Oxidation and Water Electrolysis. Int. J. Hydrogen Energy 2022, 47, 32145–32157. [Google Scholar] [CrossRef]
- Ghaith, M.E.; Abd El-Moghny, M.G.; El-Nagar, G.A.; Alalawy, H.H.; El-Shakre, M.E.; El-Deab, M.S. Tailor-Designed Binary Ni–Cu Nano Dendrites Decorated 3D-Carbon Felts for Efficient Glycerol Electrooxidation. RSC Adv. 2023, 13, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Campos dos Santos, E.; Araujo, R.B.; Valter, M.; Salazar-Alvarez, G.; Johnsson, M.; Bajdich, M.; Abild-Pedersen, F.; Pettersson, L.G.M. Efficient Screening of Bi–Metallic Electrocatalysts for Glycerol Valorization. Electrochim. Acta 2021, 398, 139283. [Google Scholar] [CrossRef]
- Abild-Pedersen, F.; Greeley, J.; Studt, F.; Rossmeisl, J.; Munter, T.R.; Moses, P.G.; Skúlason, E.; Bligaard, T.; Nørskov, J.K. Scaling Properties of Adsorption Energies for Hydrogen-Containing Molecules on Transition-Metal Surfaces. Phys. Rev. Lett. 2007, 99, 016105. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Nørskov, J.K. Theoretical surface science and catalysis—Calculations and concepts. Adv. Catal. 2000, 45, 71–129. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Saini, S.; Halldin Stenlid, J.; Abild-Pedersen, F. Electronic structure factors and the importance of adsorbate effects in chemisorption on surface alloys. npj Comput. Mater. 2022, 8, 163. [Google Scholar] [CrossRef]
- Zhang, T.; Walsh, A.G.; Yu, J.; Zhang, P. Single-atom alloy catalysts: Structural analysis, electronic properties, and catalytic activities. Chem. Soc. Rev. 2021, 50, 569–588. [Google Scholar] [CrossRef]
- Mavrikakis, M.; Stoltze, P.; Nørskov, J.K. Making gold less noble. Catal. Lett. 2000, 64, 101–106. [Google Scholar] [CrossRef]
- Nilsson, A.; Pettersson, L.G.; Hammer, B.; Bligaard, T.; Christensen, C.H.; Nørskov, J.K. The electronic structure effect in heterogeneous catalysis. Catal. Lett. 2005, 100, 111–114. [Google Scholar] [CrossRef]
- Greeley, J.; Nørskov, J.K.; Mavrikakis, M. Electronic structure and catalysis on metal surfaces. Annu. Rev. Phys. Chem. 2002, 53, 319–348. [Google Scholar] [CrossRef]
- Ma’Mari, F.A.; Moorsom, T.; Teobaldi, G.; Deacon, W.; Prokscha, T.; Luetkens, H.; Lee, S.; Sterbinsky, G.E.; Arena, D.A.; MacLaren, D.A.; et al. Beating the Stoner criterion using molecular interfaces. Nature 2015, 524, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mtangi, W.; Kiran, V.; Fontanesi, C.; Naaman, R. Role of the electron spin polarization in water splitting. J. Phys. Chem. Lett. 2015, 6, 4916–4922. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Nørskov, J.K. Spin effects in chemisorption and catalysis. ACS Catal. 2023, 13, 3456–3462. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Waghmare, U.; Lee, S.C. An improved d-band model of the catalytic activity of magnetic transition metal surfaces. Sci. Rep. 2016, 6, 35916. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab-initio total-energy calculations using a plane-wave basis set. Phys. Rev. B—Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab-initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric, and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPkit: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Verma, A.M.; Laverdure, L.; Melander, M.M.; Honkala, K. Mechanistic origins of the pH dependency in Au-catalyzed glycerol electro-oxidation: Insight from first-principles calculations. ACS Catal. 2022, 12, 662–675. [Google Scholar] [CrossRef]
- Kwon, Y.-K.; Schouten, K.J.P.; Koper, M.T.M. Mechanism of the Catalytic Oxidation of Glycerol on Polycrystalline Gold and Platinum Electrodes. ChemCatChem 2011, 3, 1176–1185. [Google Scholar] [CrossRef]
- Valter, M.; Busch, M.; Wickman, B.; Groenbeck, H.; Baltrusaitis, J.; Hellman, A. Electrooxidation of Glycerol on Gold in Acidic Medium: A Combined Experimental and DFT Study. J. Phys. Chem. C 2018, 122, 10489–10494. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Kouame, R.S.B. Selective Electrooxidation of Glycerol into Value-Added Chemicals: A Short Overview. Front. Chem. 2019, 7, 100. [Google Scholar] [CrossRef]
- Velázquez-Hernández, I.; Zamudio, E.; Rodríguez-Valadez, F.J.; García-Gómez, N.A.; Álvarez-Contreras, L.; Guerra-Balcázar, M.; Arjona, N. Electrochemical Valorization of Crude Glycerol in Alkaline Medium for Energy Conversion Using Pd, Au and PdAu Nanomaterials. Fuel 2020, 262, 116556. [Google Scholar] [CrossRef]



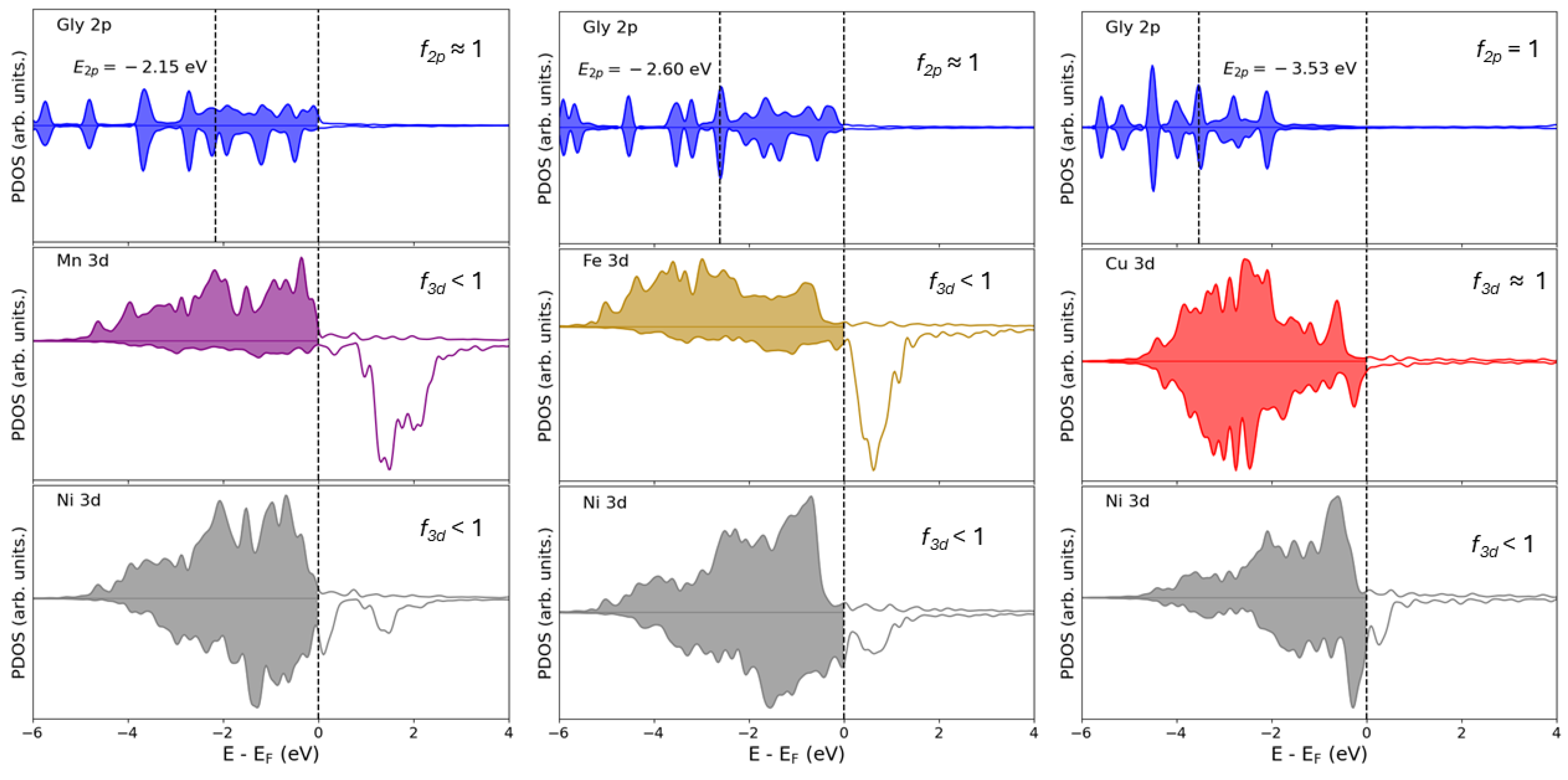


| Species | 3d Occupation () | O-2p Occupation () |
|---|---|---|
| Ni3Cr | 0.76/0.73 | 0.95/0.96 |
| Ni3V | 0.79/0.74 | 0.94/0.95 |
| Ni3Mn | 0.88/0.62 | 0.93/0.95 |
| Ni3Fe | 0.90/0.68 | 0.96/0.94 |
| Ni3Co | 0.93/0.72 | 0.97/0.97 |
| Ni | 0.92/0.78 | 0.98/0.96 |
| Ni3Cu | 0.94/0.87 | 1.00/1.00 |
| Ni3Zn | 0.95/0.90 | 1.00/1.00 |
| Cu | 1.00/1.00 | 1.00/1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Castillo, C.M.; Torres-Pacheco, L.; Álvarez-Contreras, L.; Arjona, N.; Guerra-Balcázar, M. Tuning the d-Band Center of Nickel Bimetallic Compounds for Glycerol Chemisorption: A Density Functional Study. Molecules 2025, 30, 744. https://doi.org/10.3390/molecules30030744
Ramos-Castillo CM, Torres-Pacheco L, Álvarez-Contreras L, Arjona N, Guerra-Balcázar M. Tuning the d-Band Center of Nickel Bimetallic Compounds for Glycerol Chemisorption: A Density Functional Study. Molecules. 2025; 30(3):744. https://doi.org/10.3390/molecules30030744
Chicago/Turabian StyleRamos-Castillo, Carlos M., Luis Torres-Pacheco, Lorena Álvarez-Contreras, Noé Arjona, and Minerva Guerra-Balcázar. 2025. "Tuning the d-Band Center of Nickel Bimetallic Compounds for Glycerol Chemisorption: A Density Functional Study" Molecules 30, no. 3: 744. https://doi.org/10.3390/molecules30030744
APA StyleRamos-Castillo, C. M., Torres-Pacheco, L., Álvarez-Contreras, L., Arjona, N., & Guerra-Balcázar, M. (2025). Tuning the d-Band Center of Nickel Bimetallic Compounds for Glycerol Chemisorption: A Density Functional Study. Molecules, 30(3), 744. https://doi.org/10.3390/molecules30030744





