Study on the Reaction Kinetics of Sulfur Mustard, Nitrogen Mustard and Their Chosen Analogues with Sodium Ethoxide
Abstract
1. Introduction
2. Results and Discussion
2.1. Reactions of the Studied Compounds with Alcoholic Sodium Ethoxide Solution
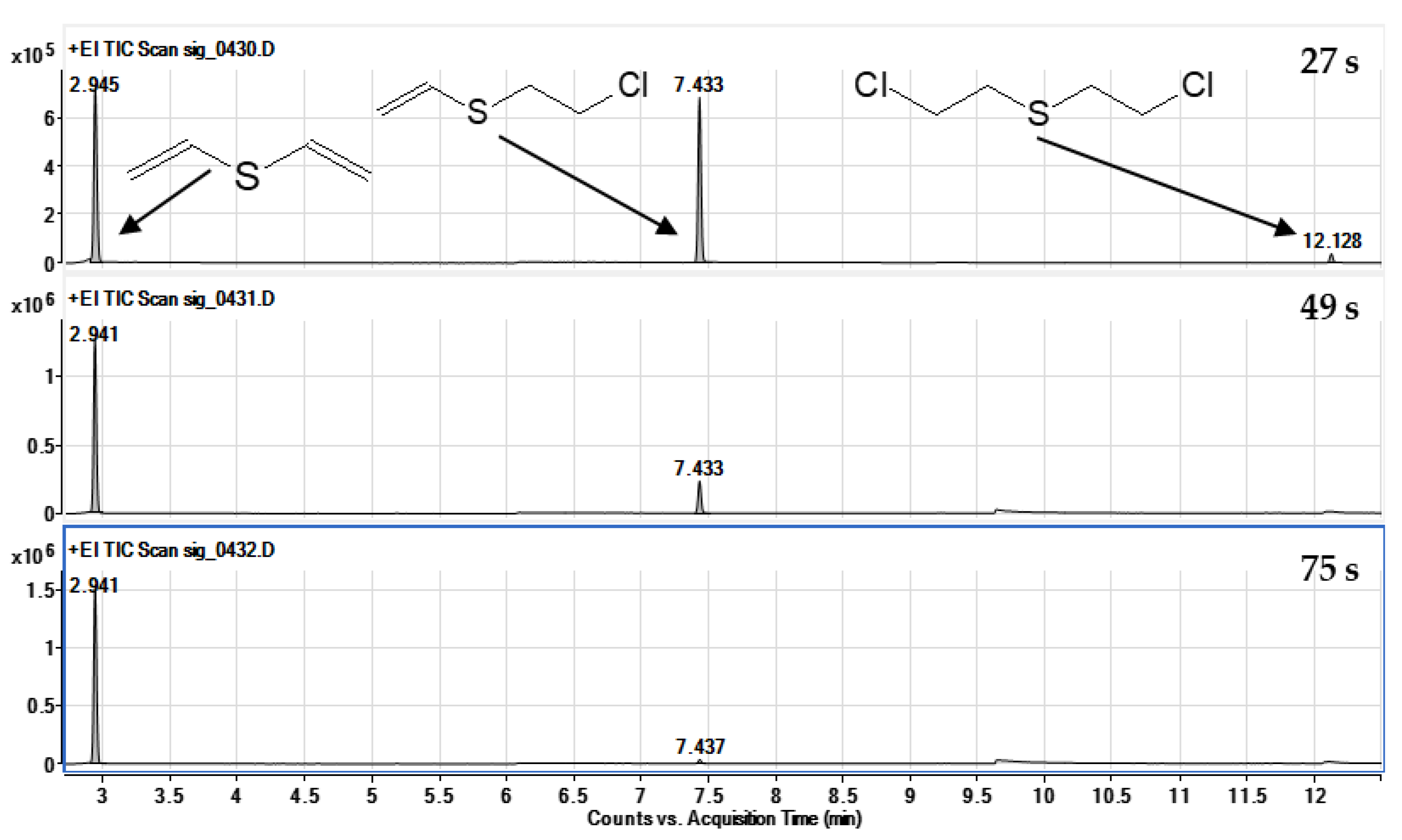
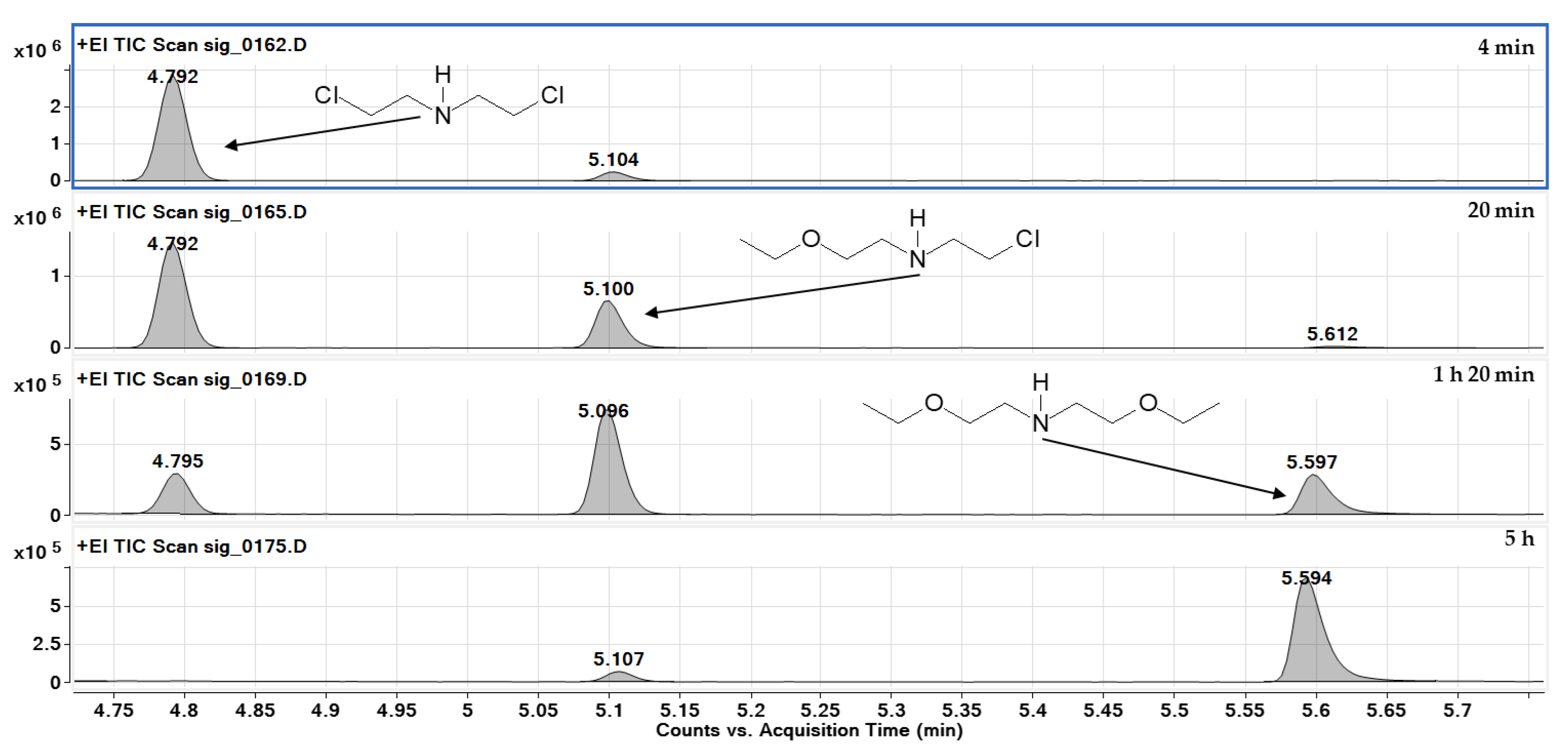
2.2. Identification of Reaction Products
2.3. Influence of Solvent Polarity
- Ethanol is a polar protic solvent, so it can form hydrogen bonds; these bonds can solvate cations present in solution, such as the sodium cation derived from sodium ethoxide; in the elimination reaction, which is dominant in the presence of a strong base such as EtONa, ethanol can stabilise intermediate products.
- Sodium ethoxide in an ethanol environment acts as a strong base, which causes the simultaneous elimination of a proton and a leaving group (in this case, chloride anion) with the formation of a double bond. This type of reaction proceeds better in the presence of an aprotic solvent, but taking into account the studies performed, ethanol is also a suitable solvent. The reaction rate in the case of ethanol is, however, slower.
- Ethanol, as a protic solvent, can partially solvate the ethanoate ion (anion–proton interaction), reducing the anion’s basicity and thus reducing the reaction rate. Despite this, C2H5O- is a strong enough base to abstract a proton from the molecule and promote the elimination mechanism. In some cases (e.g., for CEES), the elimination reaction is slow enough that the competing substitution reaction plays the primary role. In aprotic solvents, the reaction rate for sodium ethanoate is much higher [23].
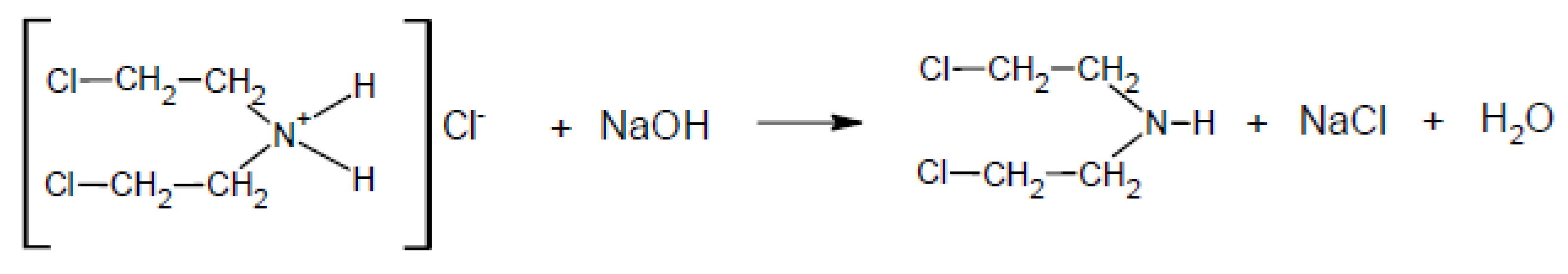
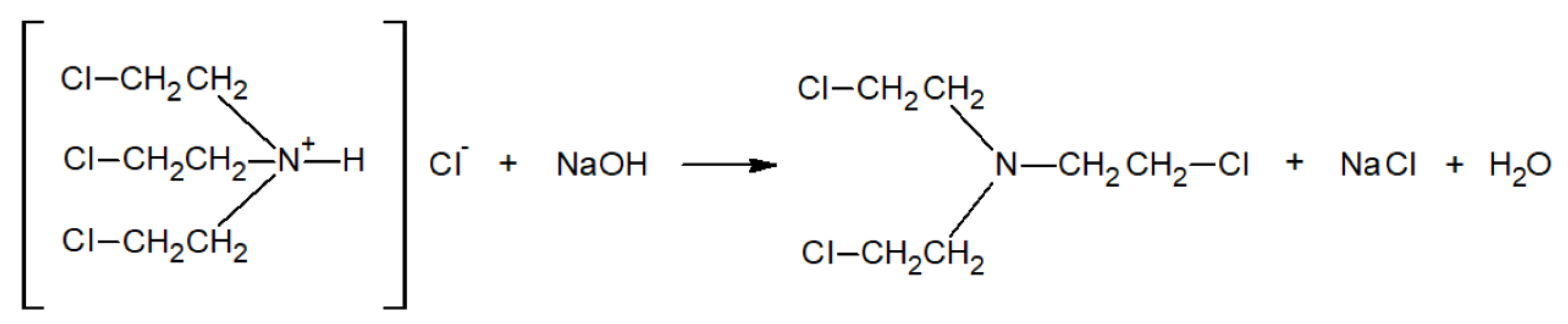
2.4. Reaction Mechanism
3. Materials and Methods
3.1. Reagents
3.2. Instrumentation
3.3. Chromatographic Analysis Conditions
3.4. Bis(2-chloroethyl)amine Synthesis
3.5. Tris(2-chloroethyl)amine Synthesis
3.6. Sodium Ethoxide Synthesis
3.7. Decontamination Mixture Preparation
3.8. Sample Preparation
4. Conclusions
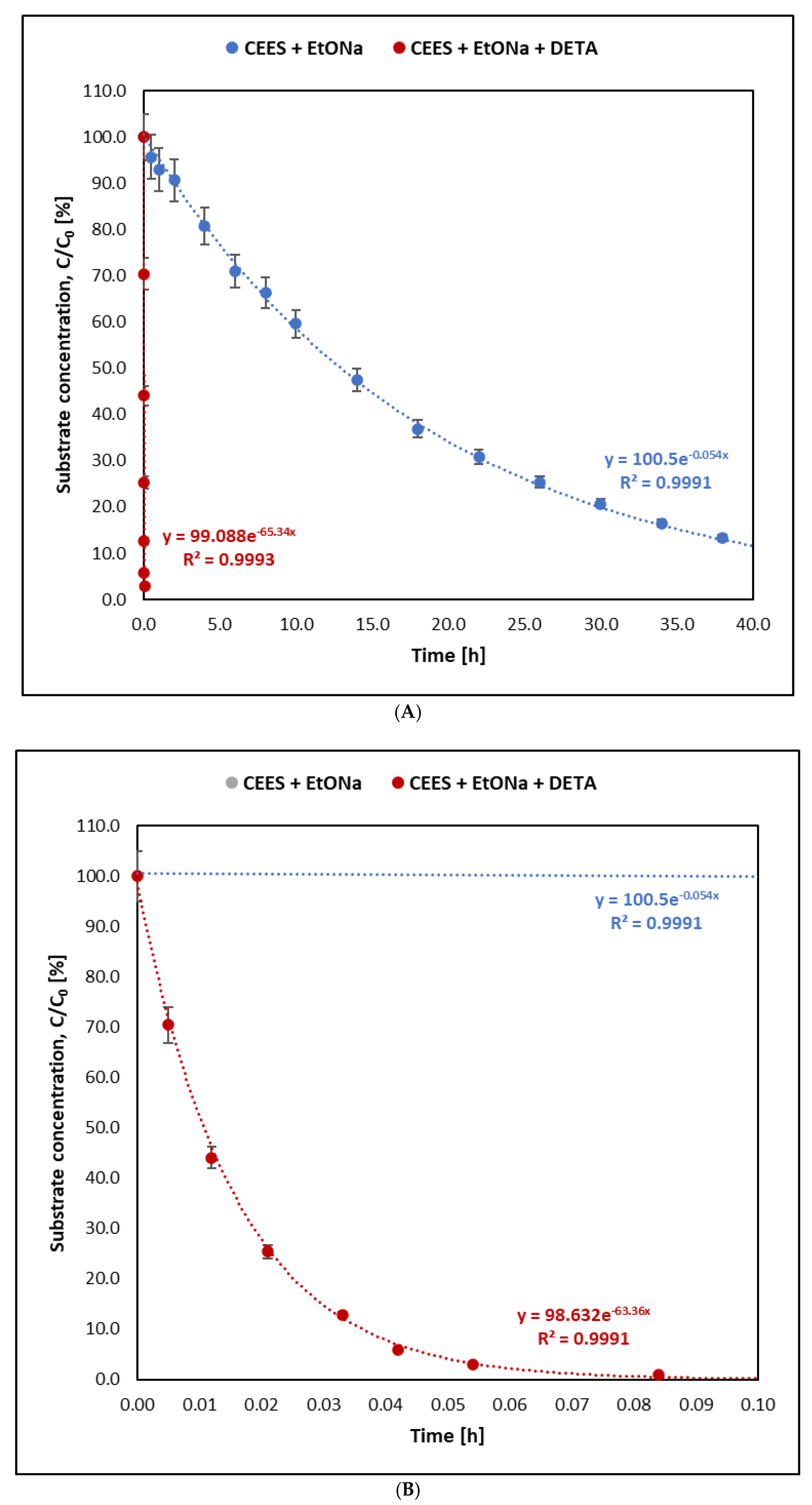
- Adding DETA to the alcoholate significantly increases the rate of decontamination reactions for all five tested compounds. DETA acts as a catalyst regardless of the reaction mechanism, enhancing speed and effectiveness. Its most pronounced impact is observed in the reaction of sodium ethoxide with CEES, where the rate is 1100 times higher compared with reactions without DETA. It is an important additive to commercial decontaminants.
- In reactions of nitrogen mustards (HN-3 and HN-0), the dominant mechanism is nucleophilic substitution, where ethoxy groups replace chlorine atoms. HN-3 forms tris(2-ethoxyethyl)amine, while HN-0 forms bis(2-ethoxyethyl)amine, with both reactions proceeding at similar rates. Due to its similar reactivity, HN-0 can serve as a nitrogen mustard analogue for research purposes.
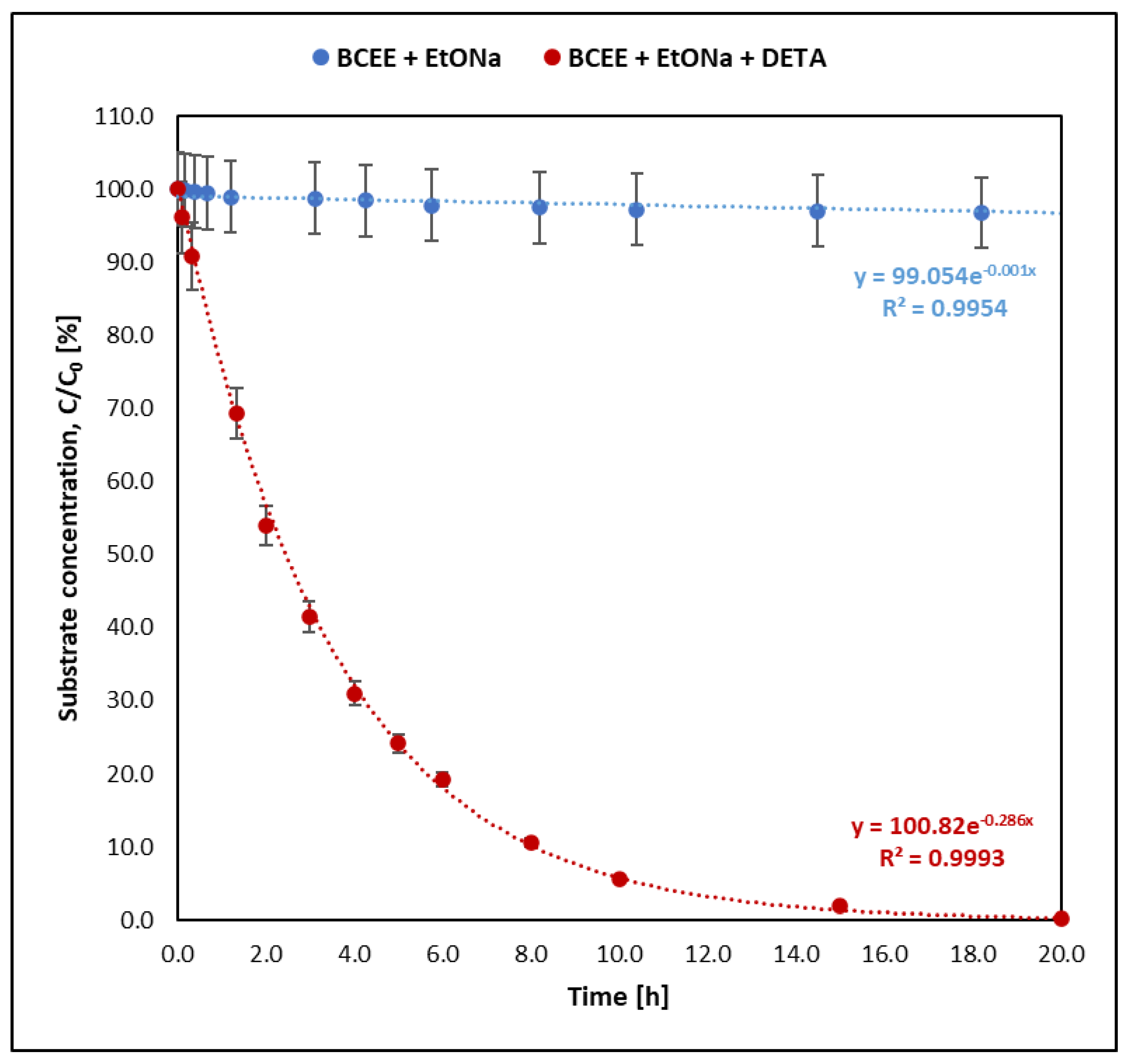
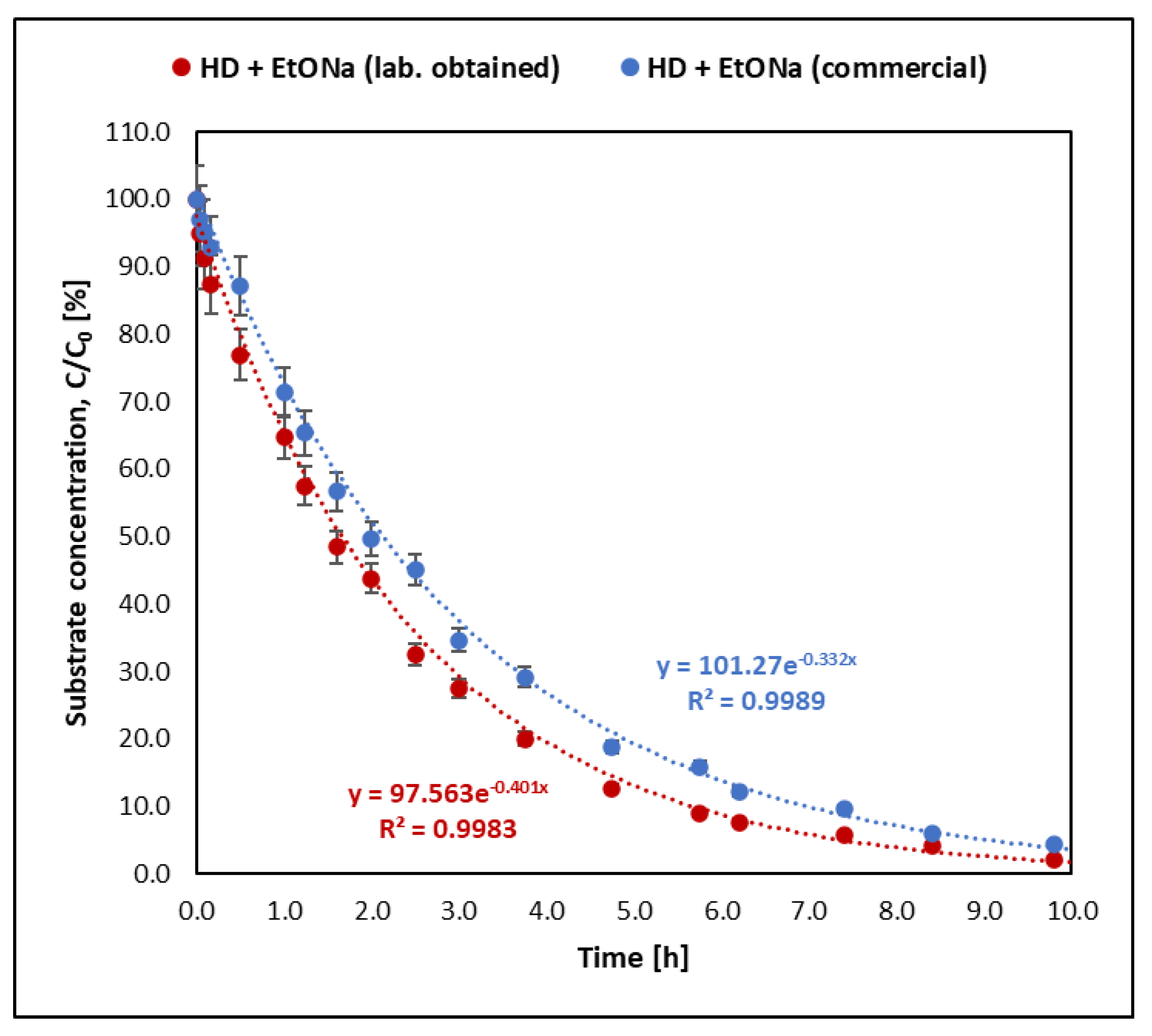
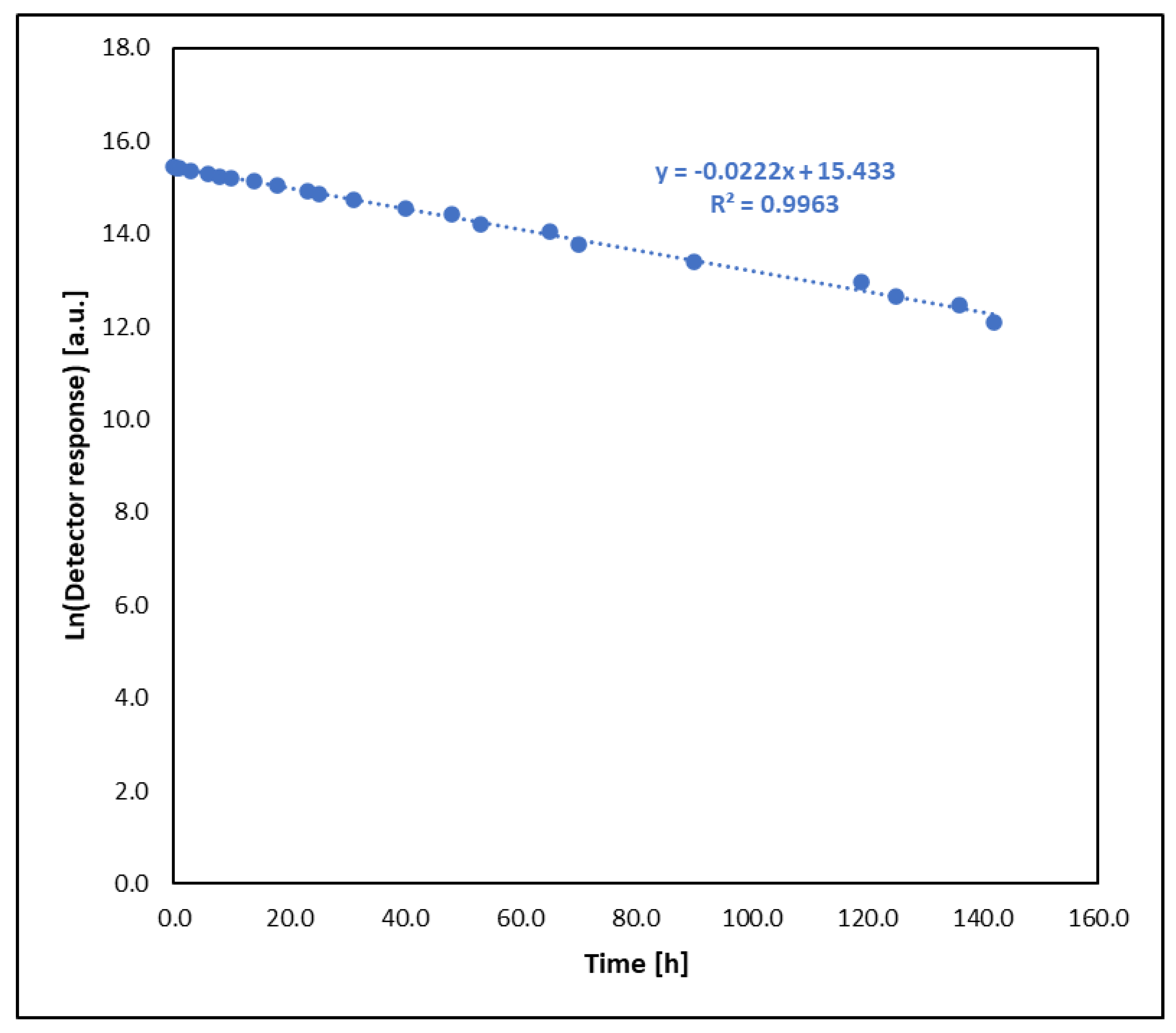
- Sulfur mustard (HD) reacts much faster than its analogues BCEE and CEES. BCEE reacts the slowest (t1/2 = 500 h), while HD and CEES in EtONa + DETA solutions react completely within 1 min. Reaction mechanisms vary: for HD, elimination is dominant, producing divinyl sulfide; for BCEE, nucleophilic substitution occurs, forming bis(2-ethoxyethyl) ether. Due to significant reaction rate differences, BCEE is unsuitable as a sulfur mustard analogue. CEES follows elimination in the presence of amine and substitution without it.
- The method of preparing the sodium ethoxide solution (via synthesis from sodium or by dissolving crystalline EtONa in ethanol) does not significantly influence reaction kinetics. The reaction rate differs only slightly (1.24 times faster with synthesised EtONa), confirming that both preparation methods are equally effective for decontamination.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szinicz, L. History of chemical and biological warfare agents. Toxicology 2005, 214, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Hefazi, M. The pharmacology, toxicology, and medical treatment of sulfur mustard poisoning. Fundam. Clin. Pharmacol. 2005, 19, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Handbook of Toxicology of Chemical Warfare Agents, 3rd ed.; Academic Press: San Diego, CA, USA; London, UK, 2020. [Google Scholar]
- Chemu, H. Report to the 16th Meeting of Helsinki Commission 8–11 March 1994; Danish Environmental Protection Agency: Helsinki, Finland, 1994.
- Pragney, D.; Vijaya Saradhi, U.V.R. Sample-preparation techniques for analysing chemical-warfare agents and related degradation products. TrAC Trends Anal. Chem. 2012, 37, 73–82. [Google Scholar] [CrossRef]
- Vanninen, P.; Östin, A.; Bełdowski, J.; Pedersen, E.A.; Söderström, M.; Szubska, M.; Pączek, B. Exposure status of sea-dumped chemical warfare agents in the Baltic Sea. Mar. Environ. Res. 2020, 161, 105112. [Google Scholar] [CrossRef] [PubMed]
- Popiel, S.; Nawała, J.; Czupryński, K. Preparation and application of sol-gel acrylate and methacrylate solid-phase microextraction fibres for gas chromatographic analysis of organoarsenic compounds. Anal. Chim. Acta 2014, 837, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chang, B.; Lim, T.-T.; Oh, W.-D.; Lei, J.; Mi, J. High-sulfur capacity and regenerable Zn-based sorbents derived from layered double hydroxide for hot coal gas desulfurization. J. Hazard. Mater. 2018, 360, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Saladi, R.N.; Smith, E.; Persaud, A.N. Mustard: A potential agent of chemical warfare and terrorism. Clin. Exp. Dermatol. 2006, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.; Solaymani-dodaran, M.; Ghanei, M.; Abolghasemi, J.; Salesi, M.; Vahedian Azimi, A.; Sahebkar, A. Standardised mortality ratios in people exposed to sulphur mus-tard during the Iran–Iraq war: A retrospective study with 39-year follow-up. Public Health 2024, 227, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Gil-Martín, E.; De Los Ríos, C.; Egea, J.; López-Muñoz, F.; Pita, R.; Juberías, A.; Torrado, J.J.; Serrano, D.R.; Reiter, R.J.; et al. Melatonin as Modulator for Sulfur and Nitrogen Mustard-Induced Inflammation, Oxidative Stress and DNA Damage: Molecular Therapeutics. Antioxidants 2023, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Q.; Begum, R.A.; Day, V.W.; Bowman-James, K. Sulfur, oxygen, and nitrogen mustards: Stability and reactivity. Org. Biomol. Chem. 2012, 10, 8786–8793. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Ramos, E.; López-Muñoz, F.; De Los Ríos, C.; Egea, J.; Gil-Martín, E.; Pita, R.; Torrado, J.J.; Serrano, D.R.; Juberias, A. Toxicology of Blister Agents: Is Melatonin a Potential Therapeutic Option? Diseases 2021, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Labaška, M.; Gál, M.; Mackuľak, T.; Švorec, J.; Kučera, J.; Helenin, J.; Svitková, V.; Ryba, J. Neutralizing the threat: A comprehensive review of chemical warfare agent decontamination strategies. J. Environ. Chem. Eng. 2024, 12, 114243. [Google Scholar] [CrossRef]
- Yang, Y.C.; Baker, J.A.; Ward, J.R. Decontamination of chemical warfare agents. Chem. Rev. 1992, 92, 1729–1743. [Google Scholar] [CrossRef]
- Popiel, S.; Witkiewicz, Z.; Nalepa, T. The reactions of sulfur mustard with the active components of organic decontaminants. J. Hazard. Mater. 2005, 123, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zuo, G.; Qi, L.; Zhou, L.; Zhang, R.; Gao, S.; Liu, Y. Nucleophilic degradation of chemical warfare agents using nonaqueous decontamination formula. Ind. Eng. Chem. Res. 2017, 56, 14933–14939. [Google Scholar] [CrossRef]
- Weetman, C.; Notman, S.; Arnold, P.L. Destruction of chemical warfare agent simulants by air and moisture-stable metal NHC complexes. Dalton Trans. 2018, 47, 2568–2574. [Google Scholar] [CrossRef]
- Doris, E.; Oheix, E.; Gravel, E. Catalytic processes for the neutralization of sulfur mustard. Chem. Eur. J. 2020, 27, 58–64. [Google Scholar]
- Ceremuga, M.; Pirszel, J.; Stela, M.; Czerwiński, P. Decontamination of chemical agents. In CBRN. Security Manager Handbook; Bijak, M., Ed.; WUŁ: Łódź, Polska, 2018; pp. 461–493. [Google Scholar] [CrossRef]
- Prasad, G.K.; Singh, V.V.; Pandey, L.K.; Sharma, P.K.; Baghel, A.; Ganesan, K.; Acharya, J.; Gupta, A.K. A nucleophilic non aqueous decontaminant for degradation of chemical warfare agents. J. Integr. Sci. Technol. 2020, 8, 51–56. [Google Scholar]
- Kim, S.D.; Jung, H. Neutralization and Decontamination of Chemical Warfare Agents using Homogeneous Chemical Solutions. Ind. Eng. Chem. Res. 2023, 62, 6672–6686. [Google Scholar] [CrossRef]
- McMurry, J. Organic Chemistry, 7th ed.; Thomson Brooks/Cole: Boston, MA, USA, 2008. [Google Scholar]

| Tested Compound | t1/2 [h] | kobs [h−1] | ||
|---|---|---|---|---|
| EtONa | EtONa + DETA | EtONa | EtONa + DETA | |
| HD—sulfur mustard | 1.82 ± 0.09 | <0.02 | 0.3815 ± 0.0185 | - |
| BCEE—sulfur mustard analogue | 677.34 ± 36.79 | 2.44 ± 0.05 | 0.0010 ± 0.0001 | 0.284 ± 0.005 |
| CEES—sulfur mustard analogue | 12.53 ± 0.65 | 0.01 ± 0.00 | 0.0553 ± 0.0029 | 65.067 ± 2.091 |
| HN-3—nitrogen mustard | 31.04 ± 1.73 | 0.85 ± 0.02 | 0.0223 ± 0.0012 | 0.795 ± 0.018 |
| HN-0—nitrogen mustard analogue | 22.60 ± 1.51 | 0.55 ± 0.02 | 0.0307 ± 0.0021 | 1.251 ± 0.044 |
| Compound Parameter | Bis(2-chloroethyl) Sulfide | Bis(2-chloroethyl) Ether 2,2′-Dichlorodiethyl Ether | 2-chloroethyl Ethyl Sulfide | Tris(2-chloroethyl)amine Trichlormethine | Bis(2-chloroethyl)amine |
|---|---|---|---|---|---|
| Common name | Sulfur mustard Mustard gas | Sulfur mustard analogue | Half mustard | Nitrogen mustard | Nitrogen mustard analogue |
| Molecular formula | C4H8Cl2S | C4H8Cl2O | C4H9ClS | C6H12Cl3N | C4H9Cl2N |
| Abbreviation/code | H/HD | BCEE | CEES | HN-3 | HN-0 |
| Structure | 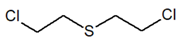 | 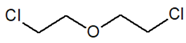 | 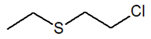 |  | 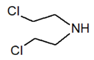 |
| CAS number | 505-60-2 | 111-44-4 | 693-07-2 | 555-77-1 | 334-22-5 |
| Molecular weight | 159.08 | 143.01 | 124.63 | 205.54 | 142.02 |
| Boiling point (760 mm Hg) | 215–217 °C | 178 °C | 156 °C | 230–235 °C, decomp. | 46–50 °C |
| Melting point | 13–14 °C | −50 °C | −48.6 °C | −3.7 °C | Not available |
| Water solubility [g/L] | 0.92 (22 °C) | 10.2 | N.A | 0.16 | Slightly soluble in water |
| Density [g/cm3] | 1.27 (20 °C) | 1.22 (20 °C) | 1.0663 | 1.24 | 1.13 |
| Vapour pressure [mm Hg (20 °C)] | 0.072 | 0.71 | 3.4 (25 °C) | 0.011 | 0.267 (25 °C) |
| Volatility [mg/m3] | 610 | 548 | 16570 | 12 | 205 |
| Log KOW | 2.14 | 1.29 | 2.17 | 2.27 | Not available |
| Van Den Dool–Kratz RI, non-polar column | 1177 | 984 | 895 | 1411 | 1087 |
| Henry’s Law Constant (H, atm × m3/mol) | 2.1 × 10−5 | 2.9 × 10−5 | 4.9 × 10−4 | 3 × 10−7 | Not available |
| LD50 Oral—Rat—[mg/kg] | 2.4 | 75.0 | 252.0 | 5.0 | 1150.0 (hydrochloride) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozon, K.; Nawała, J.; Sura, P.; Popiel, S. Study on the Reaction Kinetics of Sulfur Mustard, Nitrogen Mustard and Their Chosen Analogues with Sodium Ethoxide. Molecules 2025, 30, 780. https://doi.org/10.3390/molecules30040780
Kozon K, Nawała J, Sura P, Popiel S. Study on the Reaction Kinetics of Sulfur Mustard, Nitrogen Mustard and Their Chosen Analogues with Sodium Ethoxide. Molecules. 2025; 30(4):780. https://doi.org/10.3390/molecules30040780
Chicago/Turabian StyleKozon, Klaudia, Jakub Nawała, Paweł Sura, and Stanisław Popiel. 2025. "Study on the Reaction Kinetics of Sulfur Mustard, Nitrogen Mustard and Their Chosen Analogues with Sodium Ethoxide" Molecules 30, no. 4: 780. https://doi.org/10.3390/molecules30040780
APA StyleKozon, K., Nawała, J., Sura, P., & Popiel, S. (2025). Study on the Reaction Kinetics of Sulfur Mustard, Nitrogen Mustard and Their Chosen Analogues with Sodium Ethoxide. Molecules, 30(4), 780. https://doi.org/10.3390/molecules30040780






