KOH-Assisted Molten Salt Route to High-Performance LiNi0.5Mn1.5O4 Cathode Materials
Abstract
:1. Introduction
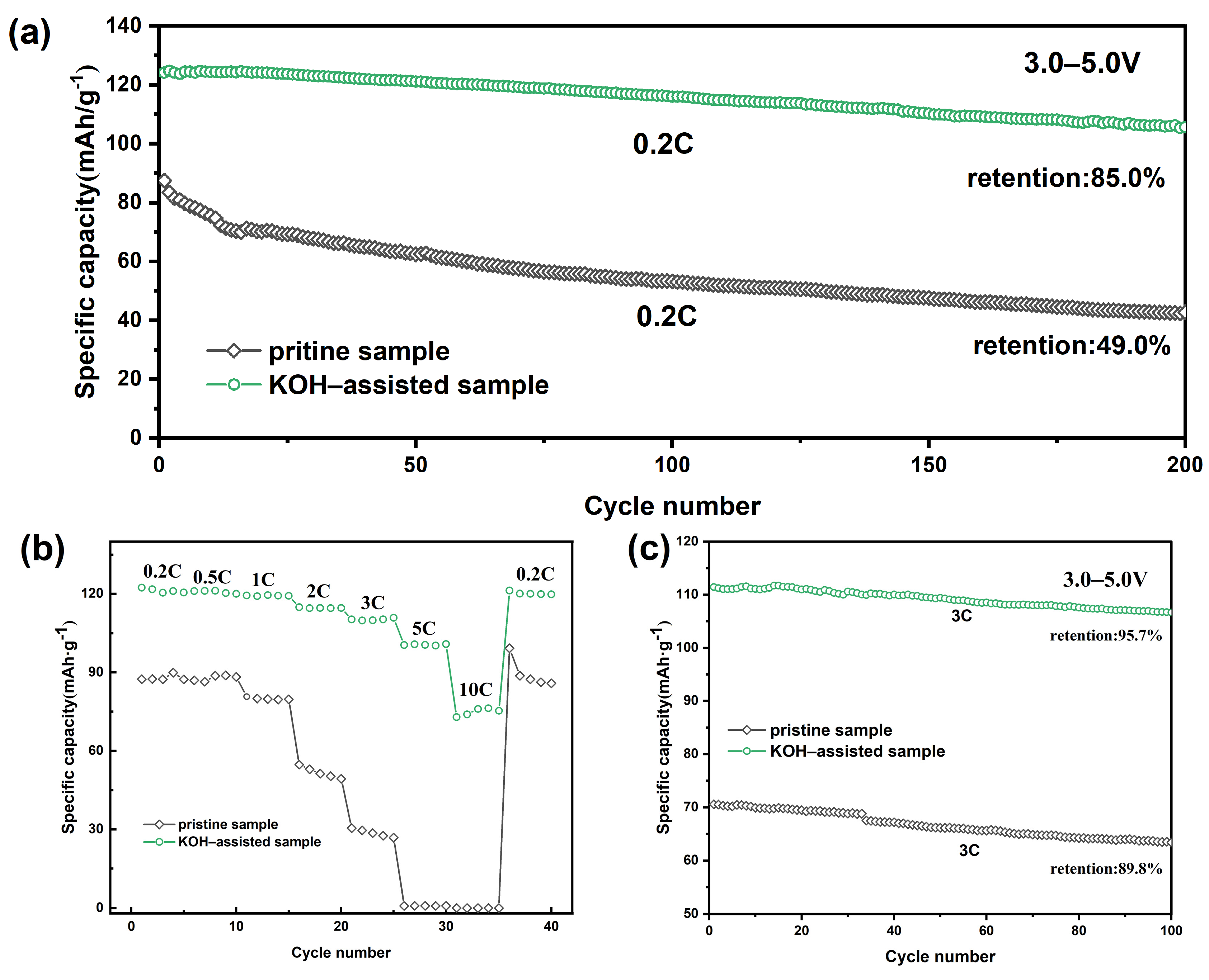
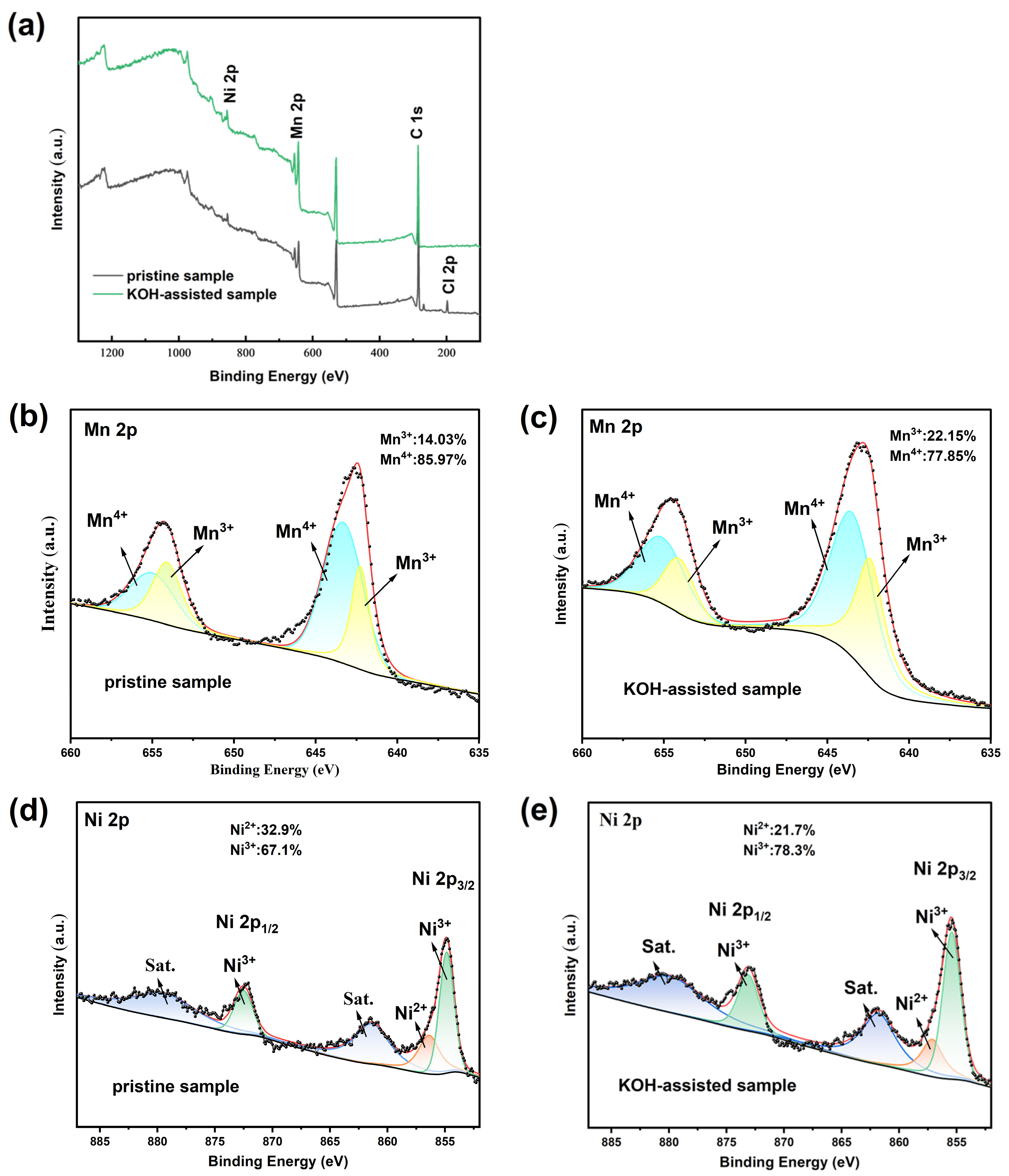
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis Procedure
3.2. Characterizations of LiNi0.5Mn1.5O4 Powder
3.3. Electrochemical Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, C.; Zheng, Z. Toward practical high-energy and high-power lithium battery anodes: Present and future. Adv. Sci. 2022, 9, 2105213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Y.; Yu, M.; Peng, Y.; Ran, F. Electrolyte-wettability issues and challenges of electrode materials in electrochemical energy storage, energy conversion, and beyond. Adv. Sci. 2023, 10, 2300283. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, Z.; Tong, Z.; Wang, Z.; Li, Y.; Wang, L.; Shang, Y.; Bi, J.; Lei, S. Research progress on lithium-rich manganese-based lithium-ion batteries cathodes. Ceram. Int. 2024, 50, 5877–5892. [Google Scholar] [CrossRef]
- Quilty, C.D.; Wu, D.; Li, W.; Bock, D.C.; Wang, L.; Housel, L.M.; Abraham, A.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S. Electron and ion transport in lithium and lithium-ion battery negative and positive composite electrodes. Chem. Rev. 2023, 123, 1327–1363. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.G.; Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A. Rechargeable batteries: Technological advancement, challenges, current and emerging applications. Energy 2023, 266, 126408. [Google Scholar] [CrossRef]
- Ren, Z.; Li, H.; Yan, W.; Lv, W.; Zhang, G.; Lv, L.; Sun, L.; Sun, Z.; Gao, W. Comprehensive evaluation on production and recycling of lithium-ion batteries: A critical review. Renew. Sustain. Energy Rev. 2023, 185, 113585. [Google Scholar] [CrossRef]
- Bai, X.; He, R.; Wei, A.; Li, X.; Zhang, L.; Liu, Z. A Co-Modified strategy for enhanced structural stability and long cycling life of Ni-Rich LiNi0.8Co0.1Mn0.1O2 cathode material. J. Alloys Compd. 2021, 857, 157877. [Google Scholar] [CrossRef]
- Liang, L.; Sun, X.; Zhang, J.; Hou, L.; Sun, J.; Liu, Y.; Wang, S.; Yuan, C. In situ synthesis of hierarchical core double-shell Ti-doped LiMnPO4@NaTi2 (PO4)3@C/3D graphene cathode with high-rate capability and long cycle life for lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1802847. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, L.; Zhao, F.; Liu, Y.; Hou, L.; Yuan, C. Ni-rich LiNi0.8Co0.1Mn0.1O2 coated with Li-ion conductive Li3PO4 as competitive cathodes for high-energy-density lithium ion batteries. Electrochim. Acta 2020, 340, 135871. [Google Scholar] [CrossRef]
- Liu, J.; Hu, X.; Ran, F.; Wang, K.; Dai, J.; Zhu, X. Electrospinning-assisted construction of 3D LiFePO4@rGO/carbon nanofibers as flexible cathode to boost the rate capabilities of lithium-ion batteries. Ceram Int. 2023, 49, 1401–1408. [Google Scholar] [CrossRef]
- Yuan, Y.; Wei, Q.; Yang, S.; Zhang, X.; Jia, M.; Yuan, J.; Yan, X. Towards high-performance phosphate-based polyanion-type materials for sodium-ion batteries. Energy Stor. Mater. 2022, 50, 760–782. [Google Scholar] [CrossRef]
- Xie, H.; Cui, J.; Yao, Z.; Ding, X.; Zhang, Z.; Luo, D.; Lin, Z. Revealing the role of spinel phase on Li-rich layered oxides: A review. Chem. Eng. J. 2022, 427, 131978. [Google Scholar] [CrossRef]
- Haruna, A.B.; Barrett, D.H.; Rodella, C.B.; Erasmus, R.M.; Venter, A.M.; Sentsho, Z.N.; Ozoemena, K.I. Microwave irradiation suppresses the Jahn-Teller distortion in Spinel LiMn2O4 cathode material for lithium-ion batteries. Electrochim. Acta 2022, 426, 140786. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Chen, X.; Koivula, R.; Xu, J. Tunnel manganese oxides prepared using recovered LiMn2O4 from spent lithium-ion batteries: Co adsorption behavior and mechanism. J. Hazard. 2022, 425, 127957. [Google Scholar] [CrossRef]
- Zhang, C.; Lan, X.; Liu, Q.; Yu, L.; Li, Y.; Hu, X. Bi-functional Janus all-nanomat separators for acid scavenging and manganese ions trapping in LiMn2O4 lithium-ion batteries. Mater. Today 2022, 24, 100676. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ariyoshi, K.; Yamamoto, S. Synthesis and characterization of Li[Ni1/2Mn3/2]O4 by two-step solid state reaction. J. Ceram. Soc. Japan 2002, 110, 501–505. [Google Scholar] [CrossRef]
- Bhatia, A.; Cretu, S.; Hallot, M.; Folastre, N.; Berthe, M.; Troadec, D.; Roussel, P.; Pereira-Ramos, J.P.; Baddour-Hadjean, R.; Lethien, C. In Situ Liquid Electrochemical TEM Investigation of LiMn1.5Ni0.5O4 Thin Film Cathode for Micro-Battery Applications. Small Methods 2022, 6, 2100891. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, H.; Lv, Y.; An, S.; Wang, R. ZIF-8/gC3N4 photocatalysts: Enhancing CO2 reduction through improved adsorption and photocatalytic performance. RSC Adv. 2024, 14, 17498–17506. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ji, S.; Lee, H.J.; Lee, S.S.; Song, Y.-C.; Kim, Y.; Choi, S. Tailoring particle size/morphology for the stable cathode performance of polygonal-shaped Li (Ni, Mn)2O4 single crystals. Ceram Int. 2024, 50, 39212–39220. [Google Scholar] [CrossRef]
- Mokhtar, N.; Idris, N.H.; Din, M.M. Molten salt synthesis of disordered spinel LiNi0.5Mn1.5O4 with improved electrochemical performance for Li-ion batteries. Int. J. Electrochem. Sci. 2018, 13, 10113–10126. [Google Scholar] [CrossRef]
- Oney, G.; Olchowka, J.; Demortière, A.; Weill, F.; Croguennec, L. Molten salt synthesis of multifaceted pure-phase Spinel LiNi0.5Mn1.5O4 platelets. ACS Appl. Energy Mater. 2023, 6, 8189–8196. [Google Scholar] [CrossRef]
- Wang, W.-N.; Meng, D.; Qian, G.; Xie, S.; Shen, Y.; Chen, L.; Li, X.; Rao, Q.; Che, H.; Liu, J. Controlling particle size and phase purity of “single-crystal” LiNi0.5Mn1.5O4 in molten-salt-assisted synthesis. J. Phys. Chem. C 2020, 124, 27937–27945. [Google Scholar] [CrossRef]
- Spence, S.L.; Xu, Z.; Sainio, S.; Nordlund, D.; Lin, F. Tuning the Morphology and Electronic Properties of Single-Crystal LiNi0.5Mn1.5O4−δ: Exploring the Influence of LiCl–KCl Molten Salt Flux Composition and Synthesis Temperature. Inorg. Chem. 2020, 59, 10591–10603. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hu, A.; Spence, S.; Kuai, C.; Hou, D.; Mu, L.; Liu, J.; Li, L.; Sun, C.; Sainio, S. Tailoring disordered/ordered phases to revisit the degradation mechanism of high-voltage LiNi0.5Mn1.5O4 spinel cathode materials. Adv. Funct. Mater. 2022, 32, 2112279. [Google Scholar] [CrossRef]
- Yu, X.; Yu, W.A.; Manthiram, A. Advances and prospects of high-voltage spinel cathodes for lithium-based batteries. Small Methods 2021, 5, 2001196. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Z.; Yu, F.; Zhang, Y.; Yin, G. Ethanol-assisted hydrothermal synthesis of LiNi0.5Mn1.5O4 with excellent long-term cyclability at high rate for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 4185–4191. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, C.; Ma, J.; Xu, G.; Chen, Z.; Du, X.; Zhang, S.; Zhou, X.; Cui, G.; Chen, L. Interfacial chemistry of γ-glutamic acid derived block polymer binder directing the interfacial compatibility of high voltage LiNi0.5Mn1.5O4 electrode. Sci. China Chem. 2021, 64, 92–100. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, W.; Qu, Q.; Shi, Q.; Zheng, H.; Huang, Y. Effectively stabilizing 5 V spinel LiNi0.5Mn1.5O4 cathode in organic electrolyte by polyvinylidene fluoride coating. Appl. Surf. Sci. 2018, 455, 349–356. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, L.; Li, J.; Yang, M.; Yu, J.; Cheng, Y.-J.; Huang, Y.; Xia, Y. One thousandth of quaternity slurry additive enables one thousand cycle of 5V LNMO cathode. Energy Stor. Mater. 2024, 64, 103060. [Google Scholar] [CrossRef]
- Fehse, M.; Etxebarria, N.; Otaegui, L.; Cabello, M.; Martín-Fuentes, S.; Cabañero, M.A.; Monterrubio, I.; Elkjær, C.F.; Fabelo, O.; Enkubari, N.A. Influence of Transition-Metal order on the reaction mechanism of LNMO cathode spinel: An operando x-ray absorption spectroscopy study. Chem. Mater. 2022, 34, 6529–6540. [Google Scholar] [CrossRef]
- Liu, T.; Dai, A.; Lu, J.; Yuan, Y.; Xiao, Y.; Yu, L.; Li, M.; Gim, J.; Ma, L.; Liu, J. Correlation between manganese dissolution and dynamic phase stability in spinel-based lithium-ion battery. Nat. Commun. 2019, 10, 4721. [Google Scholar] [CrossRef]
- Hirayama, M.; Ido, H.; Kim, K.; Cho, W.; Tamura, K.; Mizuki, J.; Kanno, R. Dynamic structural changes at LiMn2O4/electrolyte interface during lithium battery reaction. J. Am. Chem. Soc. 2010, 132, 15268–15276. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Shin, D.; Chae, K.H.; Cho, M.K.; Kim, C.; Sohn, S.S.; Lee, M.; Hong, J. Regulating dynamic electrochemical interface of LiNi0.5Mn1.5O4 spinel cathode for realizing simultaneous Mn and Ni redox in rechargeable lithium batteries. Adv. Energy Mater. 2022, 12, 2202049. [Google Scholar] [CrossRef]
- Li, L.; Sui, J.; Chen, J.; Lu, Y. LiNi0.5Mn1.5O4 microrod with ultrahigh Mn3+ content: A high performance cathode material for lithium ion battery. Electrochim. Acta 2019, 305, 433–442. [Google Scholar] [CrossRef]
- Tong, Z.; Ye, Q.; Deng, Y.; She, Q.; Huang, A.; Xu, J.; Zhu, X. Tuning the structural disordering in hierarchical LiNi0.5Mn1.5O4 microrods for stable high-rate electrode performance. J. Alloys Compd. 2023, 937, 168544. [Google Scholar] [CrossRef]
- Zhao, Z.; Fang, H. An Environmentally Friendly and Cost-Effective Route to LiNi0.5Mn1.5O4 Crystals: Structure, Morphology Evolution, and Electrochemical Properties. J. Electrochem. Soc. 2023, 170, 010510. [Google Scholar] [CrossRef]
- Zheng, J.-c.; Yang, Z.; He, Z.-j.; Tong, H.; Yu, W.-j.; Zhang, J.-f. In situ formed LiNi0.8Co0.15Al0.05O2@Li4SiO4 composite cathode material with high rate capability and long cycling stability for lithium-ion batteries. Nano Energy 2018, 53, 613–621. [Google Scholar] [CrossRef]
- Mao, G.; Yu, W.; Zhou, Q.; Li, L.; Huang, Y.; Yao, Y.; Chu, D.; Tong, H.; Guo, X. Improved electrochemical performance of high-nickel cathode material with electronic conductor RuO2 as the protecting layer for lithium-ion batteries. Appl. Surf. Sci. 2020, 531, 147245. [Google Scholar] [CrossRef]
- Seenivasan, M.; Yang, C.C.; Wu, S.-h.; Li, Y.-J.J.; Chien, W.-C.; Piraman, S.; Lue, S.J. Improving structural and thermal stability of LiNi0.8Co0.15Al0.05O2 by a fast-ionic-conductive LiAlSiO4 surface coating for Li-ion batteries. Electrochim. Acta 2021, 387, 138620. [Google Scholar] [CrossRef]
- Xu, C.-L.; Xiang, W.; Wu, Z.-G.; Li, Y.-C.; Xu, Y.-D.; Hua, W.-B.; Guo, X.-D.; Zhang, X.-B.; Zhong, B.-H. A comparative study of crystalline and amorphous Li0.5La0.5TiO3 as surface coating layers to enhance the electrochemical performance of LiNi0.815Co0.15Al0.035O2 cathode. J. Alloys Compd. 2018, 740, 428–435. [Google Scholar]
- Huang, B.; Li, X.; Wang, Z.; Guo, H. A facile process for coating amorphous FePO4 onto LiNi0.8Co0.15Al0.05O2 and the effects on its electrochemical properties. Mater. Lett. 2014, 131, 210–213. [Google Scholar] [CrossRef]
- Chen, C.; Tao, T.; Qi, W.; Zeng, H.; Wu, Y.; Liang, B.; Yao, Y.; Lu, S.; Chen, Y. High-performance lithium ion batteries using SiO2-coated LiNi0.5Co0.2Mn0.3O2 microspheres as cathodes. J. Alloys Compd. 2017, 709, 708–716. [Google Scholar] [CrossRef]
- Yi, X.; Yu, W.-J.; Tsiamtsouri, M.A.; Zhang, F.; He, W.; Dai, Q.; Hu, S.; Tong, H.; Zheng, J.; Zhang, B. Highly conductive C-Si@G nanocomposite as a high-performance anode material for Li-ion batteries. Electrochim. Acta 2019, 295, 719–725. [Google Scholar] [CrossRef]
- Wei, J.; Liang, D.; Ji, Y.; Chen, B.; Jiang, C.; Li, X. Enhanced electrochemical performance of cobalt oxide layers coated LiNi0.8Co0.1Mn0.1O2 by polyvinylpyrrolidone-assisted method cathode for Li-ion batteries. J. Colloid Interface Sci. 2022, 616, 520–531. [Google Scholar] [CrossRef]

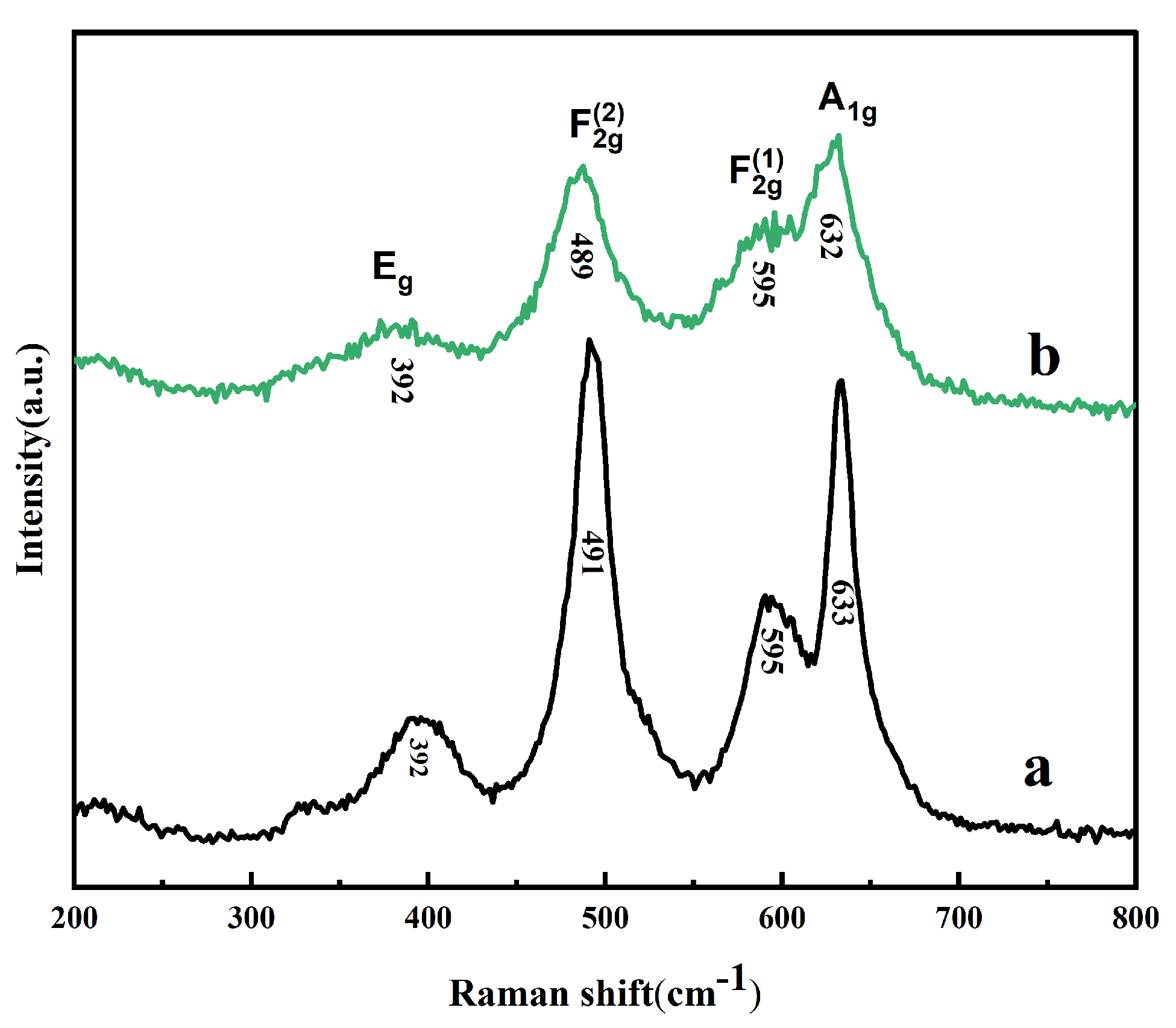
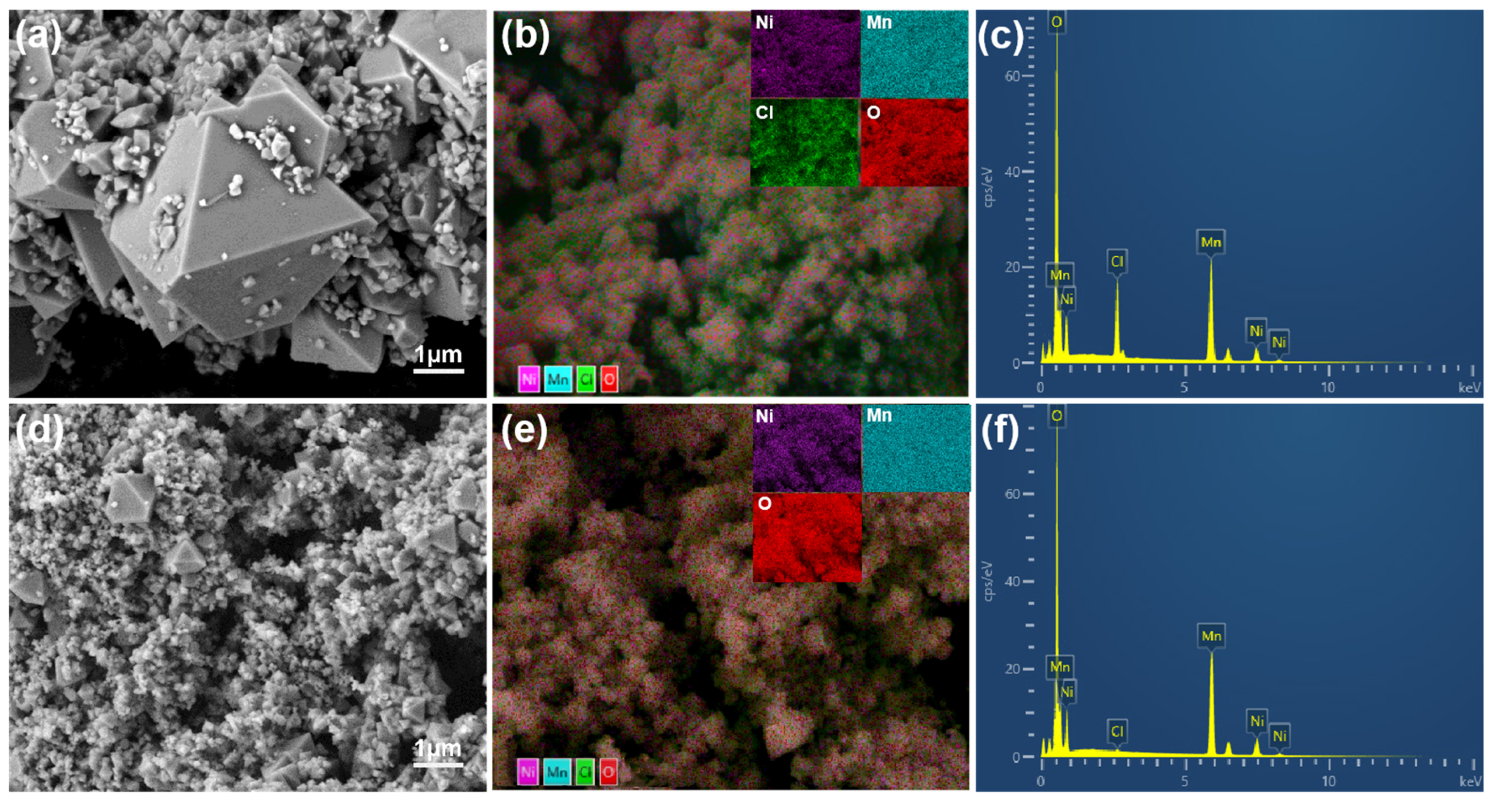
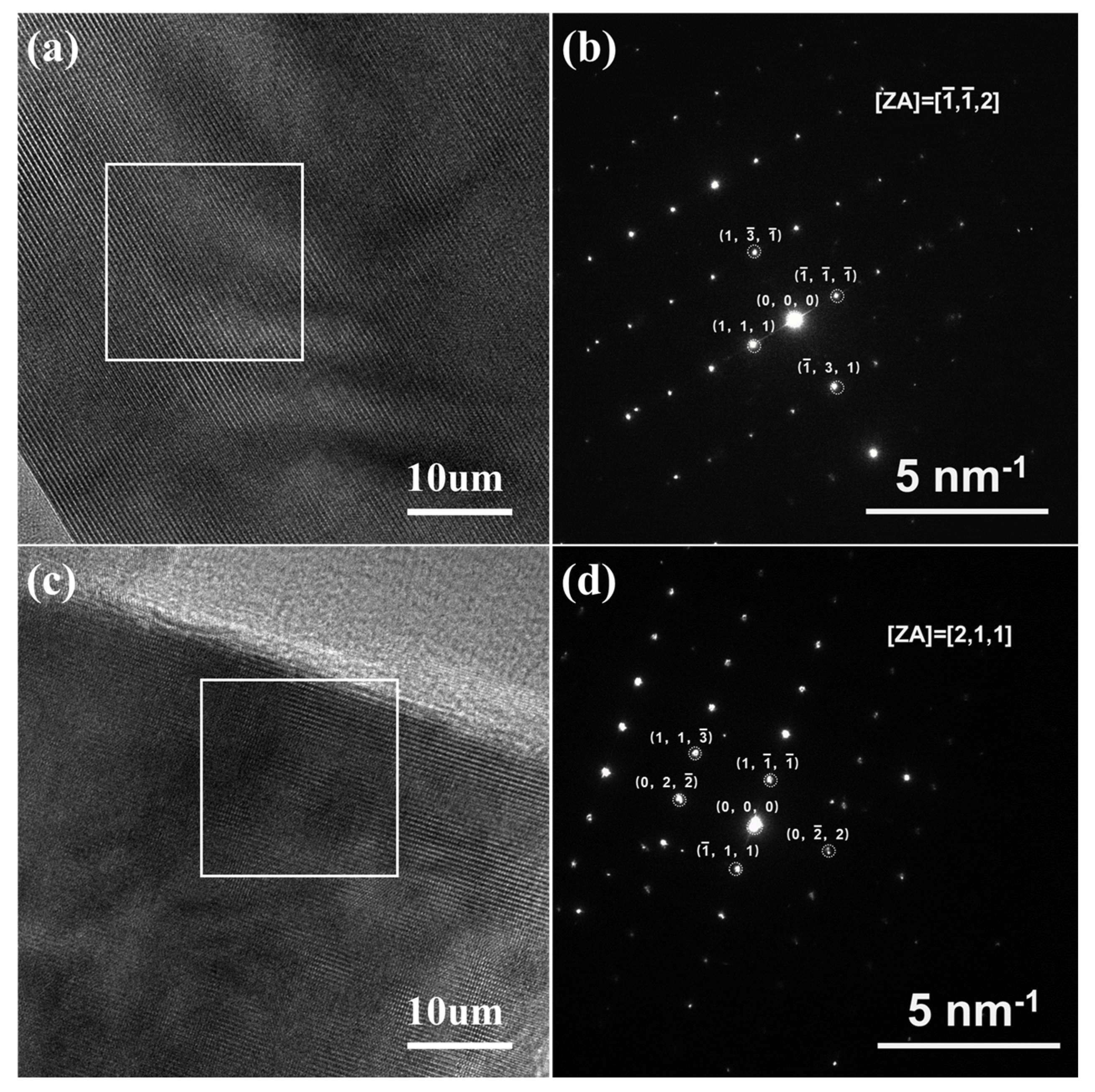
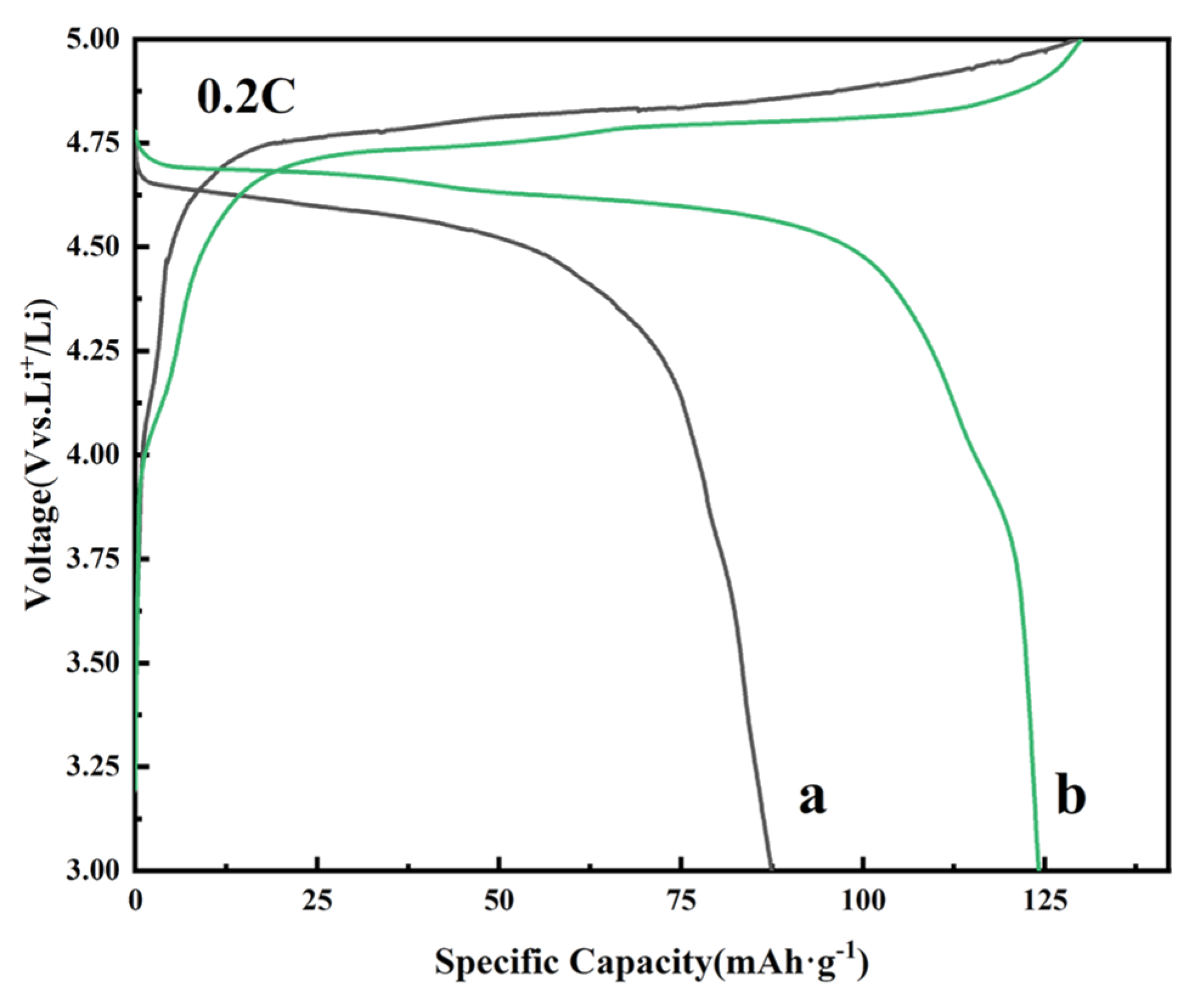


| Samples | D10 (μm) | D50 (μm) | D90 (μm) | Span (Span = (D90−D10)/D50) |
|---|---|---|---|---|
| a | 3.47 | 26.0 | 65.7 | 2.39 |
| b | 3.18 | 13.9 | 38.9 | 2.57 |
| Samples | Rs (Ω) | Rct (Ω) | δ (Ω·s0.5) | DLi + (cm2·s−1) |
|---|---|---|---|---|
| a | 4.178 | 788.364 | 97.629 | 2.770 × 10−13 |
| b | 2.376 | 235.032 | 31.331 | 8.444 × 10−13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, F.; Feng, F.; Zhang, S.; Feng, N.; Cai, C.; An, S. KOH-Assisted Molten Salt Route to High-Performance LiNi0.5Mn1.5O4 Cathode Materials. Molecules 2025, 30, 797. https://doi.org/10.3390/molecules30040797
Pang F, Feng F, Zhang S, Feng N, Cai C, An S. KOH-Assisted Molten Salt Route to High-Performance LiNi0.5Mn1.5O4 Cathode Materials. Molecules. 2025; 30(4):797. https://doi.org/10.3390/molecules30040797
Chicago/Turabian StylePang, Feng, Fushan Feng, Shuyu Zhang, Na Feng, Changkun Cai, and Shengli An. 2025. "KOH-Assisted Molten Salt Route to High-Performance LiNi0.5Mn1.5O4 Cathode Materials" Molecules 30, no. 4: 797. https://doi.org/10.3390/molecules30040797
APA StylePang, F., Feng, F., Zhang, S., Feng, N., Cai, C., & An, S. (2025). KOH-Assisted Molten Salt Route to High-Performance LiNi0.5Mn1.5O4 Cathode Materials. Molecules, 30(4), 797. https://doi.org/10.3390/molecules30040797







