High Sensitivity and Selectivity of PEDOT/Carbon Sphere Composites for Pb2+ Detection
Abstract
:1. Introduction
2. Result and Discussion
2.1. Structural Characterization
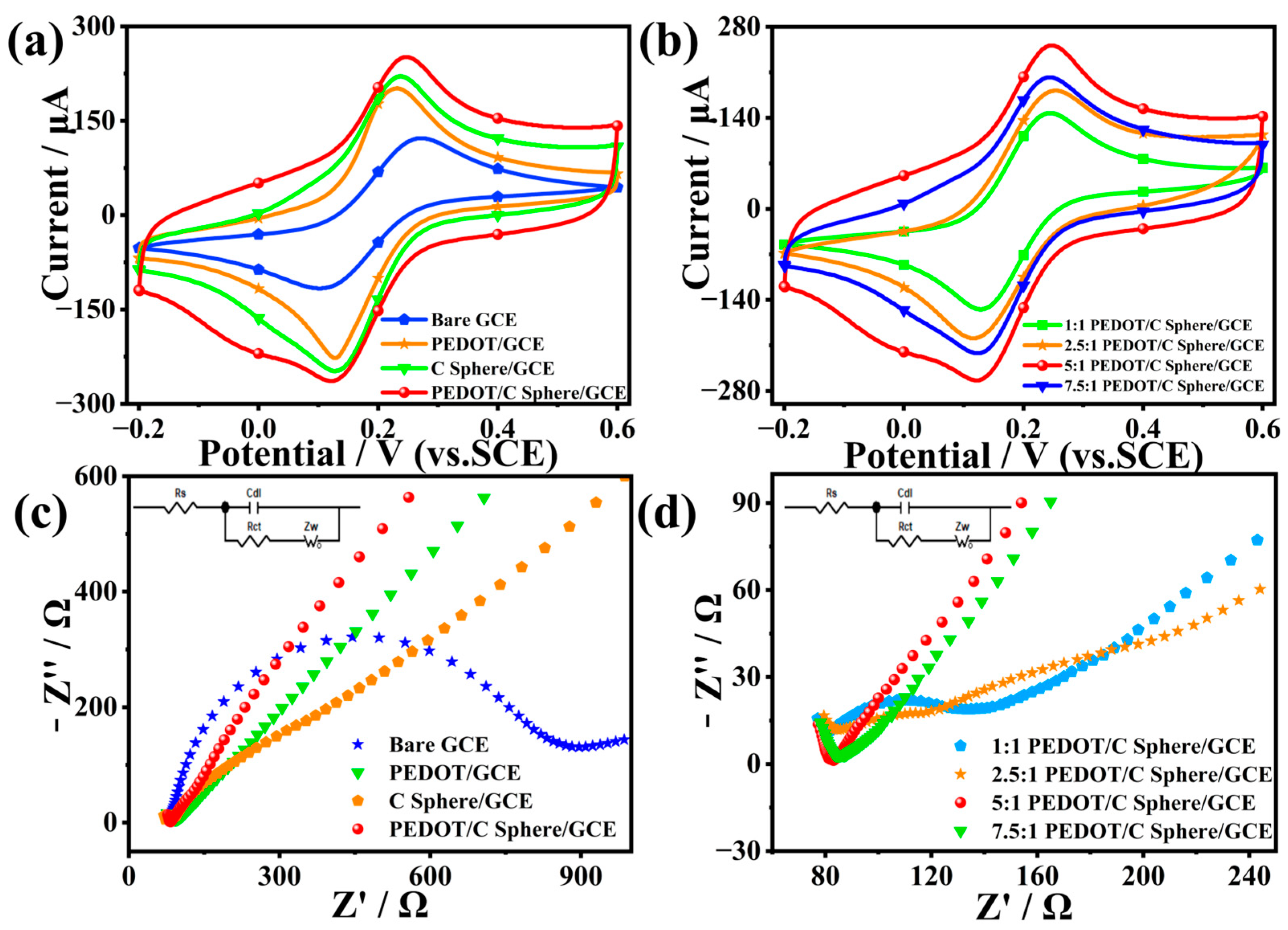
2.2. PEDOT/Carbon Sphere Electrochemical Behavior Analysis
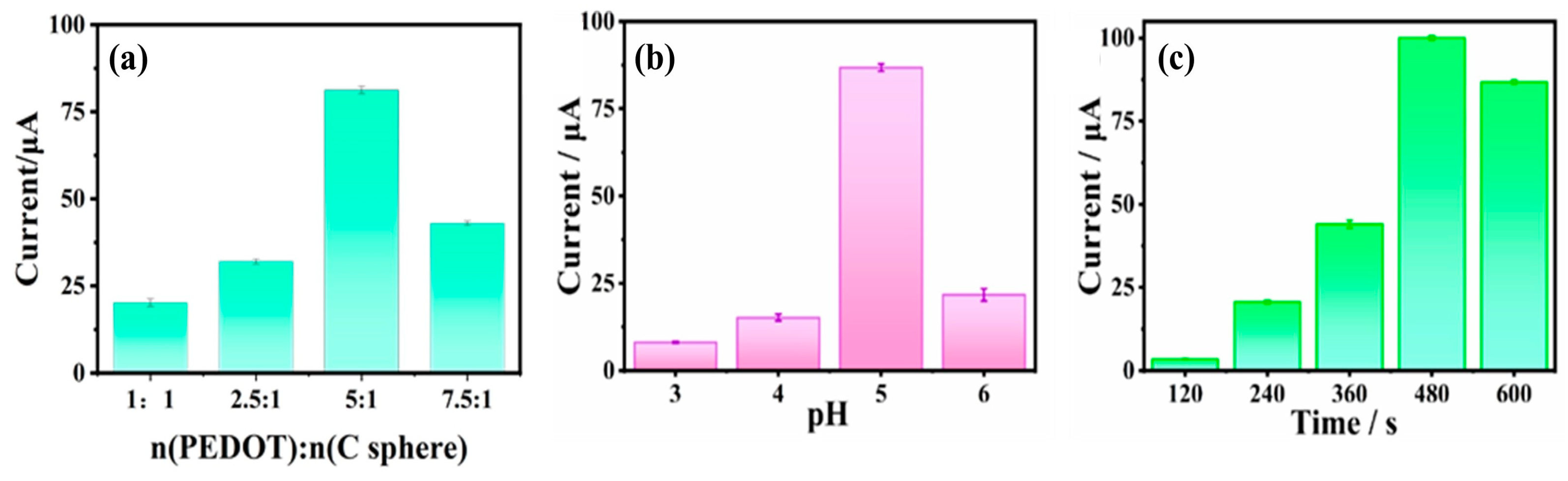
2.3. Optimization of Test Condition for Pb2+ Analysis
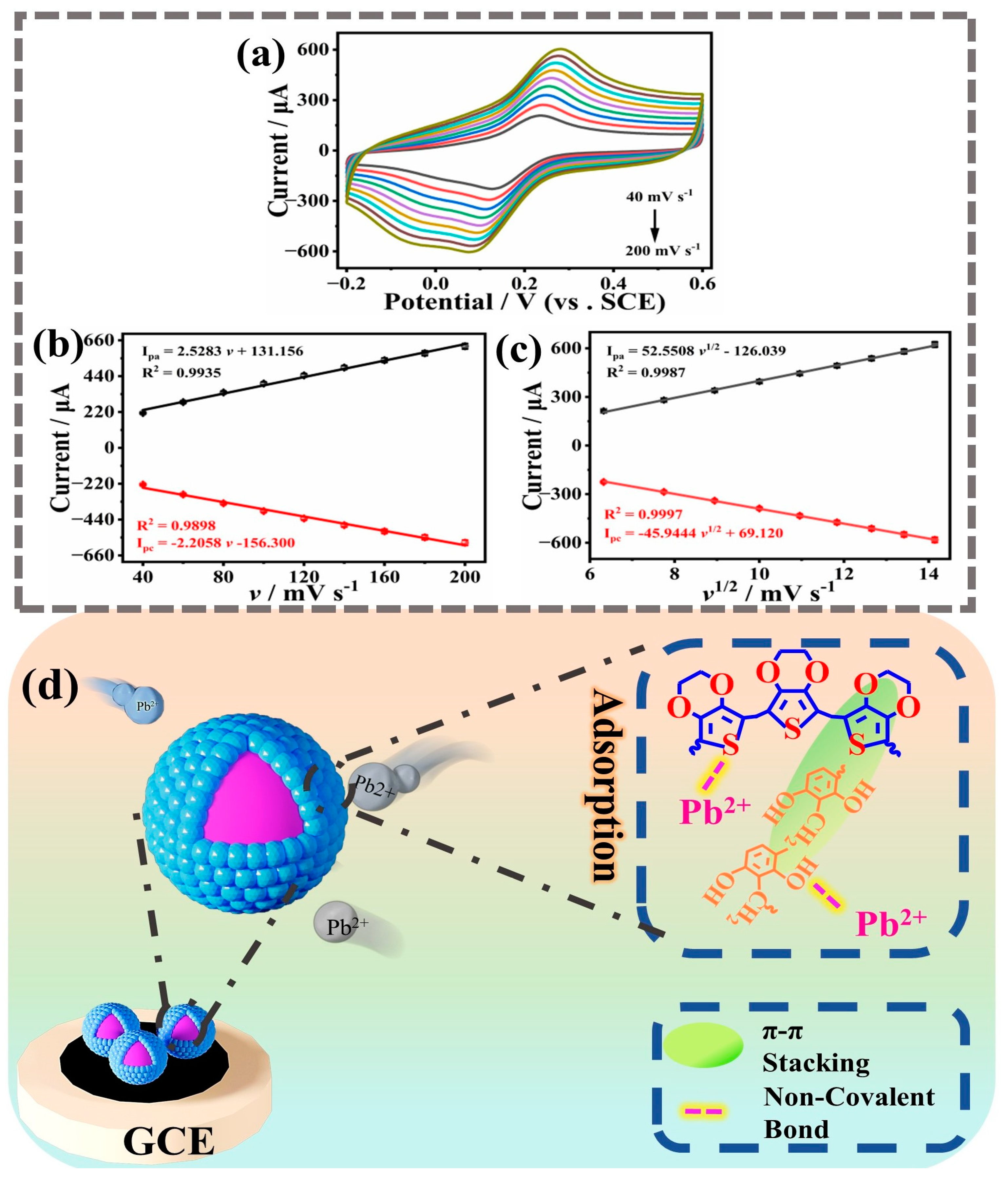
2.4. Electrochemical Mechanism
2.5. Sensing Performance Analysis
3. Experimental Procedure
3.1. Reagents
3.2. Instruments
3.3. Synthesis of Carbon Sphere
3.4. Synthesis of PEDOT/Carbon Sphere Composites
3.5. Preparation of PEDOT/Carbon Sphere/GCE Sensor
3.6. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malik, L.A.; Bashir, A.; Qureashi, A.; Pandith, A.H. Detection and removal of heavy metal ions: A review. Environ. Chem. Lett. 2019, 17, 1495–1521. [Google Scholar] [CrossRef]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Nguyen, H.; Sung, Y.; O’Shaughnessy, K.; Shan, X.; Shih, W.-C. Smartphone Nanocolorimetry for On-Demand Lead Detection and Quantitation in Drinking Water. Anal. Chem. 2018, 90, 11517–11522. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ji, P.; Xu, Y.; Liu, X.; Kong, D. Self-paired dumbbell DNA-assisted simple preparation of stable circular DNAzyme and its application in Pb2+ sensor. Anal. Chim. Acta 2021, 1175, 338733. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xu, C.; Yalikun, N.; Mamat, X.; Li, Y.; Wågberg, T.; Hu, X.; Liu, J.; Luo, J.; Hu, G. Sensitive and Selective Differential Pulse Voltammetry Detection of Cd(II) and Pb(II) Using Nitrogen-Doped Porous Carbon Nanofiber Film Electrode. J. Electrochem. Soc. 2017, 164, H967. [Google Scholar] [CrossRef]
- Wang, G.; Sun, D.; Liang, L.; Wang, G.; Ma, J. Highly sensitive detection of trace lead ions concentration based on a functional film-enhanced optical microfiber sensor. Opt. Laser Technol. 2023, 161, 109171. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R. In-situ sensing of hazardous heavy metal ions through an ecofriendly scheme. Opt. Laser Technol. 2021, 137, 106813. [Google Scholar] [CrossRef]
- Haixia, S.; Zaijun, L.; Ming, L. Ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate as a solvent for extraction of lead in environmental water samples with detection by graphite furnace atomic absorption spectrometry. Microchim. Acta 2007, 159, 95–100. [Google Scholar] [CrossRef]
- Ajab, H.; Ali Khan, A.A.; Nazir, M.S.; Yaqub, A.; Abdullah, M.A. Cellulose-hydroxyapatite carbon electrode composite for trace plumbum ions detection in aqueous and palm oil mill effluent: Interference, optimization and validation studies. Environ. Res. 2019, 176, 108563. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wu, X.; Gong, Z.; Wang, J.; Liu, X.; Wang, Q.; Wang, Y.; Dai, H. Curcumin-based ratiometric electrochemical sensing interface for the detection of Cd2+ and Pb2+ in grain products. Colloids Surf. A 2024, 684, 133125. [Google Scholar] [CrossRef]
- Li, J.; Lu, F.; Umemura, T.; Tsunoda, K.-I. Determination of lead by hydride generation inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2000, 419, 65–72. [Google Scholar] [CrossRef]
- Londonio, A.; Morzán, E.; Smichowski, P. Determination of toxic and potentially toxic elements in rice and rice-based products by inductively coupled plasma-mass spectrometry. Food Chem. 2019, 284, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Noudeng, V.; Pheakdey, D.V.; Xuan, T.D. Toxic heavy metals in a landfill environment (Vientiane, Laos): Fish species and associated health risk assessment. Environ. Toxicol. Pharmacol. 2024, 108, 104460. [Google Scholar] [CrossRef]
- Pu, H.; Ruan, S.; Yin, M.; Sun, Q.; Zhang, Y.; Gao, P.; Liang, X.; Yin, W.; Fa, H.-B. Performance comparison of simultaneo us detection Heavy-Metal ions based on carbon materials electrochemical sensor. Microchem. J. 2022, 181, 107711. [Google Scholar] [CrossRef]
- Song, H.; Huo, M.; Zhou, M.; Chang, H.; Li, J.; Zhang, Q.; Fang, Y.; Wang, H.; Zhang, D. Carbon nanomaterials-based electrochemical sensors for heavy metal detection. Crit. Rev. Anal. Chem. 2024, 54, 1987–2006. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xie, X.; Meng, T.; Guo, X.; Li, Q.; Yang, Y.; Jin, H.; Jin, C.; Meng, X.; Pang, H. Recent advance of nanomaterials modified electrochemical sensors in the detection of heavy metal ions in food and water. Food Chem. 2024, 440, 138213. [Google Scholar] [CrossRef]
- Meng, R.; Zhu, Q.; Long, T.; He, X.; Luo, Z.; Gu, R.; Wang, W.; Xiang, P. The innovative and accurate detection of heavy metals in foods: A critical review on electrochemical sensors. Food Control. 2023, 150, 109743. [Google Scholar] [CrossRef]
- Yang, M.; Li, P.H.; Chen, S.H.; Xiao, X.Y.; Tang, X.H.; Lin, C.H.; Huang, X.J.; Liu, W.Q. Nanometal oxides with special surface physicochemical properties to promote electrochemical detection of heavy metal ions. Small 2020, 16, 2001035. [Google Scholar] [CrossRef]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef]
- Tang, K.; Chen, Y.; Zhao, Y. Exploiting halide perovskites for heavy metal ion detection. Chem. Commun. 2024, 60, 4511–4520. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal–organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Meenakshi, S.; Kaladevi, G.; Pandian, K.; Gopinath, S.C. Metal-polymer-clay nanocomposites based electrochemical sensor for detecting hydrazine in water sources. Colloids Surf. A 2024, 702, 135077. [Google Scholar] [CrossRef]
- Dietrich, M.; Heinze, J.; Heywang, G.; Jonas, F. Electrochemical and spectroscopic characterization of polyalkylenedioxythiophenes. J. Electroanal. Chem. 1994, 369, 87–92. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Qian, Y.; Ma, C.; Zhang, S.; Gao, J.; Liu, M.; Xie, K.; Wang, S.; Sun, K.; Song, H. High performance electrochemical electrode based on polymeric composite film for sensing of dopamine and catechol. Sensor Actuat. B Chem. 2018, 255, 1655–1662. [Google Scholar] [CrossRef]
- Leprince, M.; Regal, S.; Mailley, P.; Sauter-Starace, F.; Texier, I.; Auzély-Velty, R. A cross-linkable and resorbable PEDOT-based ink using a hyaluronic acid derivative as dopant for flexible bioelectronic devices. Mater. Adv. 2023, 4, 3636–3644. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chen, Z.-Y.; Chiu, C.-L.; Huang, T.-T.; Meng, H.-F.; Yu, P. Surface micro-/nanotextured hybrid PEDOT: PSS-silicon photovoltaic cells employing Kirigami graphene. ACS Appl. Mater. Interfaces 2019, 11, 29901–29909. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, J.; Zuo, Y.; Li, Y.; Zhang, J.; Zhou, Y.; Duan, X.; Lu, L.; Jia, H.; Xu, Q. Three-dimensional PEDOT composite based electrochemical sensor for sensitive detection of chlorophenol. J. Electroanal. Chem. 2019, 837, 1–9. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Duan, X.; Zhu, Y.; Xue, T.; Rao, L.; Wen, Y.; Tian, Q.; Cai, Y.; Xu, Q. A novel nanozyme comprised of electro-synthesized molecularly imprinted conducting PEDOT nanocomposite with graphene-like MoS2 for electrochemical sensing of luteolin. Microchem. J. 2021, 168, 106418. [Google Scholar] [CrossRef]
- Meng, R.; Tang, J.; Wu, X.; Zhang, S.; Wang, X.; Li, Q.; Jin, R. Molecularly imprinted electrochemical sensor based on 3D wormlike PEDOT-PPy polymer for the sensitive determination of rutin in flos sophorae immaturus. J. Electrochem. Soc. 2021, 168, 077521. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, G.; Liu, J.; Wang, Y.; Ai, Q.; Huang, J.; Yuan, Z.; Tan, L.; Chen, Y. Effective network formation of pedot by in-situ polymerization using novel organic template and nanocomposite supercapacitor. Electrochim. Acta 2017, 247, 871–879. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, J.; Huang, Y.; Zhang, T. Synthesis and applications of carbon nanospheres: A review. Particuology 2024, 87, 325–338. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Liu, M.; Yu, Y.; Hu, Z.; Liu, B.; Lv, H.; Chen, A. Controllable synthesis of N-doped hollow, yolk-shell and solid carbon spheres via template-free method. J. Alloys Comp. 2019, 778, 294–301. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Xing, W.; Liu, B.; Zhang, J.; Lin, H.; Cui, H.; Zhuo, S. Resorcinol–formaldehyde resin-based porous carbon spheres with high CO2 capture capacities. J. Energy Chem. 2017, 26, 1007–1013. [Google Scholar] [CrossRef]
- Luan, K.; Yao, S.; Zhang, Y.; Zhuang, R.; Xiang, J.; Shen, X.; Li, T.; Xiao, K.; Qin, S. Poly (3, 4-ethyleendioxythiophene) coated titanium dioxide nanoparticles in situ synthesis and their application for rechargeable lithium sulfur batteries. Electrochim. Acta 2017, 252, 461–469. [Google Scholar] [CrossRef]
- Chen, S.; Chen, W.; Wang, Y.; Wang, X.; Ding, Y.; Zhao, D.; Liu, J. Facile one-pot method of AuNPs/PEDOT/CNT composites for simultaneous detection of dopamine with a high concentration of ascorbic acid and uric acid. RSC Advan. 2022, 12, 15038–15045. [Google Scholar] [CrossRef]
- Ravit, R.; Abdullah, J.; Ahmad, I.; Sulaiman, Y. Electrochemical performance of poly (3, 4-ethylenedioxythipohene)/nanocrystalline cellulose (PEDOT/NCC) film for supercapacitor. Carbohydr. Polym. 2019, 203, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Jeon, S.S.; Im, S.S. CNT/PEDOT core/shell nanostructures as a counter electrode for dye-sensitized solar cells. Synth. Met. 2011, 161, 1284–1288. [Google Scholar] [CrossRef]
- Zhou, Y.; Abdurexit, A.; Jamal, R.; Abdiryim, T.; Liu, X.; Liu, F.; Xu, F.; Zhang, Y.; Wang, Z. Highly sensitive electrochemical sensing of norfloxacin by molecularly imprinted composite hollow spheres. Biosens. Bioelectron. 2024, 251, 116119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fang, G.; Liu, G.; Pan, M.; Wang, X.; Kong, L.; He, X.; Wang, S. Electrochemical sensor based on molecularly imprinted polymer film via sol–gel technology and multi-walled carbon nanotubes-chitosan functional layer for sensitive determination of quinoxaline-2-carboxylic acid. Biosens. Bioelectron. 2013, 47, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qi, X.; Wu, J.; Xu, L.; Wan, X.; Liu, Y.; Chen, Y.; Li, Q. Ultrasensitive, label-free voltammetric determination of norfloxacin based on molecularly imprinted polymers and Au nanoparticle-functionalized black phosphorus nanosheet nanocomposite. J. Hazard. Mater. 2022, 436, 129107. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Liu, X.; Cheng, J.; Liu, W.; Liu, T.; Sun, M.; Zhao, L.; Ding, F.; Lu, Z. CuCo2O4/N-Doped CNTs loaded with molecularly imprinted polymer for electrochemical sensor: Preparation, characterization and detection of metronidazole. Biosens. Bioelectron. 2019, 142, 111483. [Google Scholar] [CrossRef]
- Mourya, A.; Mazumdar, B.; Sinha, S.K. Determination and quantification of heavy metal ion by electrochemical method. J. Environ. Chem. Eng. 2019, 7, 103459. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, W.; Gao, X.; Liu, X.; Ma, H. Synthesis of a novel electrode material containing phytic acid-polyaniline nanofibers for simultaneous determination of cadmium and lead ions. Anal. Chim. Acta 2016, 947, 32–41. [Google Scholar] [CrossRef]
- Hassan, K.M.; Gaber, S.E.; Altahan, M.F.; Azzem, M.A. Novel Sensor Based on Poly (1, 2-Diaminoanthraquinone) for Individual and Simultaneous Anodic Stripping Voltammetry of Cd2+, Pb2+, Cu2+ and Hg2+. Electroanalysis 2018, 30, 1155–1162. [Google Scholar] [CrossRef]
- Shen, Y.; Ouyang, H.; Li, W.; Long, Y. Defect-enhanced electrochemical property of h-BN for Pb2+ detection. Microchim. Acta 2021, 188, 40. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.S.B.; Krid, F.; Nacef, M.; Boussaha, E.H.; Chelaghmia, M.L.; Tabet, H.; Selaimia, R.; Atamnia, A.; Affoune, A.M. Green synthesis of copper oxide nanoparticles using Ficus elastica extract for the electrochemical simultaneous detection of Cd2+, Pb2+, and Hg2+. RSC Adv. 2023, 13, 18734–18747. [Google Scholar] [CrossRef]




| Sensor | Detection Method | Linearity Range | Detection Limit | Reference |
|---|---|---|---|---|
| BFS | DPV | 1~5 μM | 1.5 × 10−2 μM | [43] |
| PA−PANI | DPSAV | 0.01~6 mM | 5.0 μM | [44] |
| P1,2-DAAQ | SWASV | 0~12 mM | 58 μM | [45] |
| D−BN | LSASV | 0.05~40 mM | 15 μM | [46] |
| CuONPs/PANI–CPE | SWV | 0.01~0.14 μM | 4.8 × 10−3 μM | [47] |
| PEDOT/C Sphere | DPV | 0.075~1 μM | 3.5 × 10−5 μM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Wang, Z.; Liu, X.; Xu, F.; Abdiryim, T. High Sensitivity and Selectivity of PEDOT/Carbon Sphere Composites for Pb2+ Detection. Molecules 2025, 30, 798. https://doi.org/10.3390/molecules30040798
Ma L, Wang Z, Liu X, Xu F, Abdiryim T. High Sensitivity and Selectivity of PEDOT/Carbon Sphere Composites for Pb2+ Detection. Molecules. 2025; 30(4):798. https://doi.org/10.3390/molecules30040798
Chicago/Turabian StyleMa, Lirong, Zhuangzhuang Wang, Xiong Liu, Feng Xu, and Tursun Abdiryim. 2025. "High Sensitivity and Selectivity of PEDOT/Carbon Sphere Composites for Pb2+ Detection" Molecules 30, no. 4: 798. https://doi.org/10.3390/molecules30040798
APA StyleMa, L., Wang, Z., Liu, X., Xu, F., & Abdiryim, T. (2025). High Sensitivity and Selectivity of PEDOT/Carbon Sphere Composites for Pb2+ Detection. Molecules, 30(4), 798. https://doi.org/10.3390/molecules30040798







