Synthesis and Optoelectronic Properties of Perylene Diimide-Based Liquid Crystals
Abstract
1. Introduction
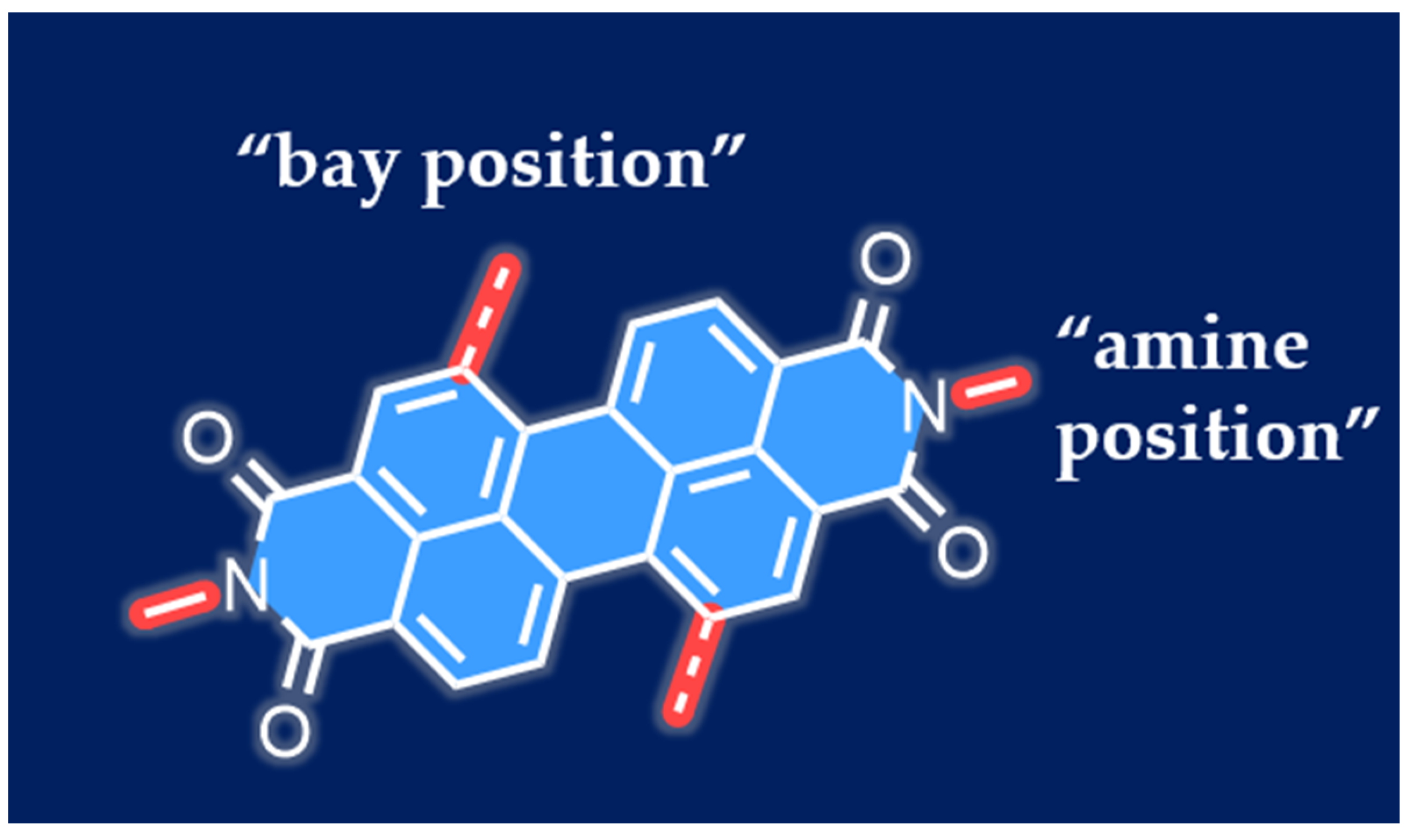
2. Design and Synthesis of PDI Derivatives
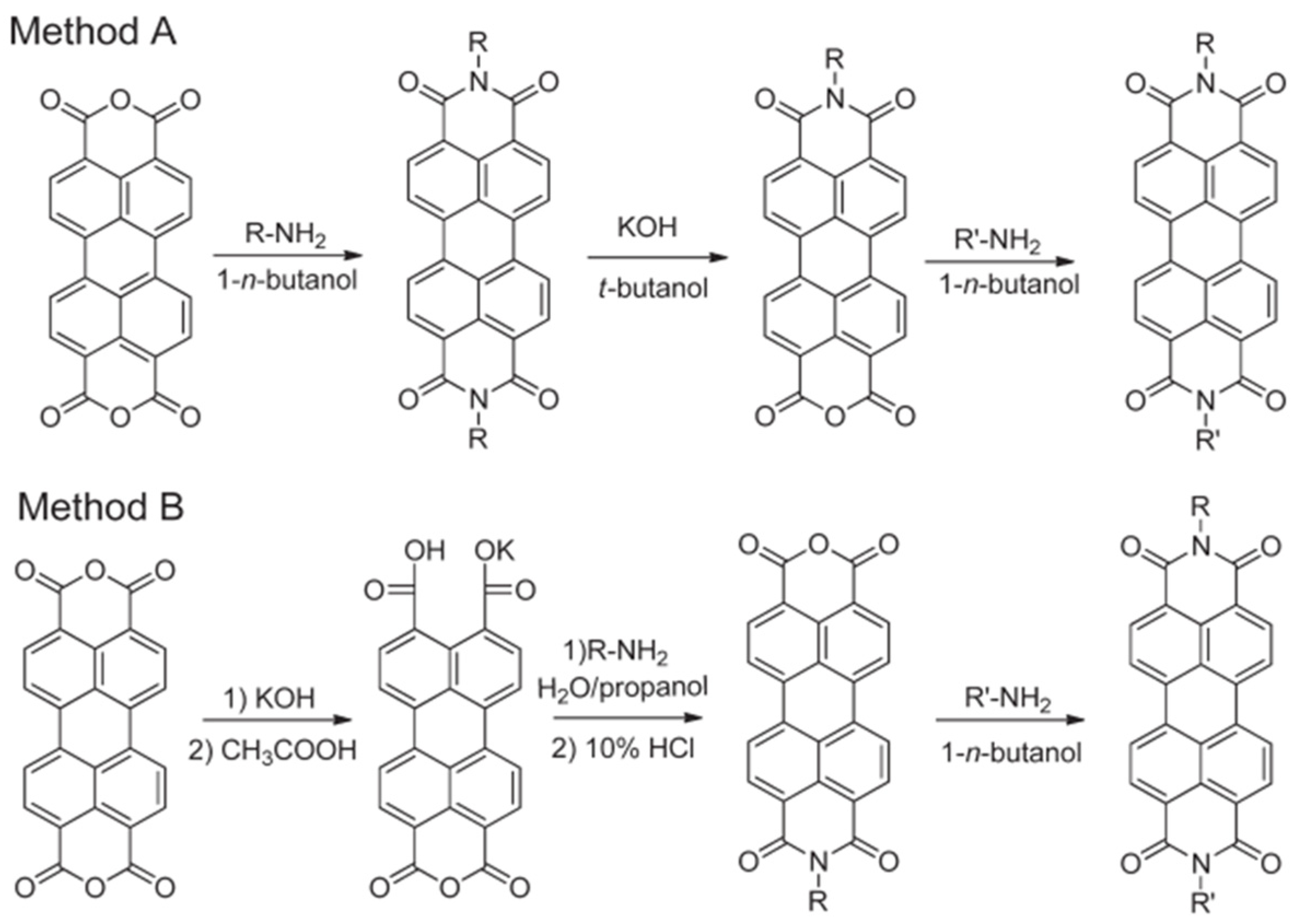
2.1. PDI Derivatives Modified at the Amine Position
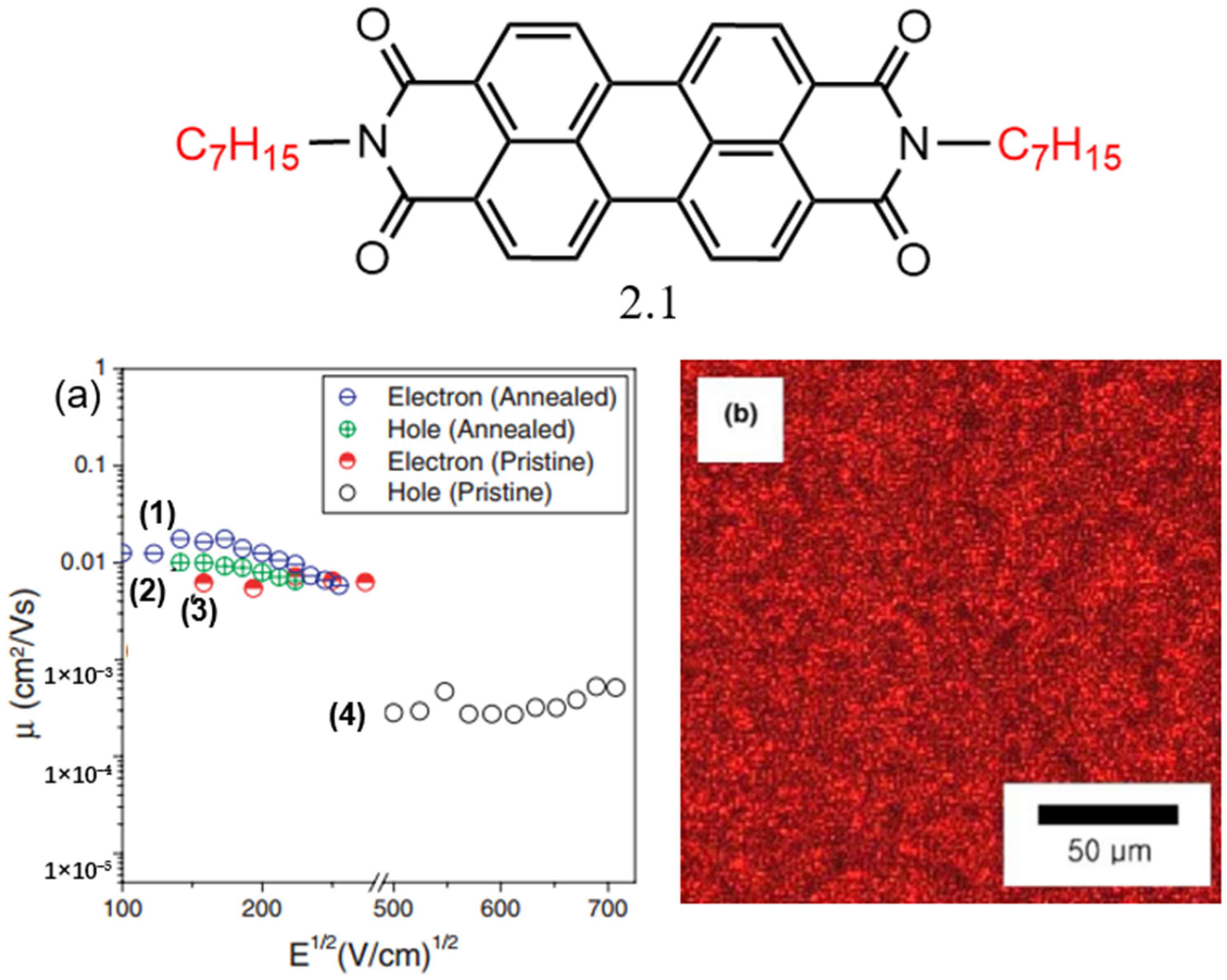
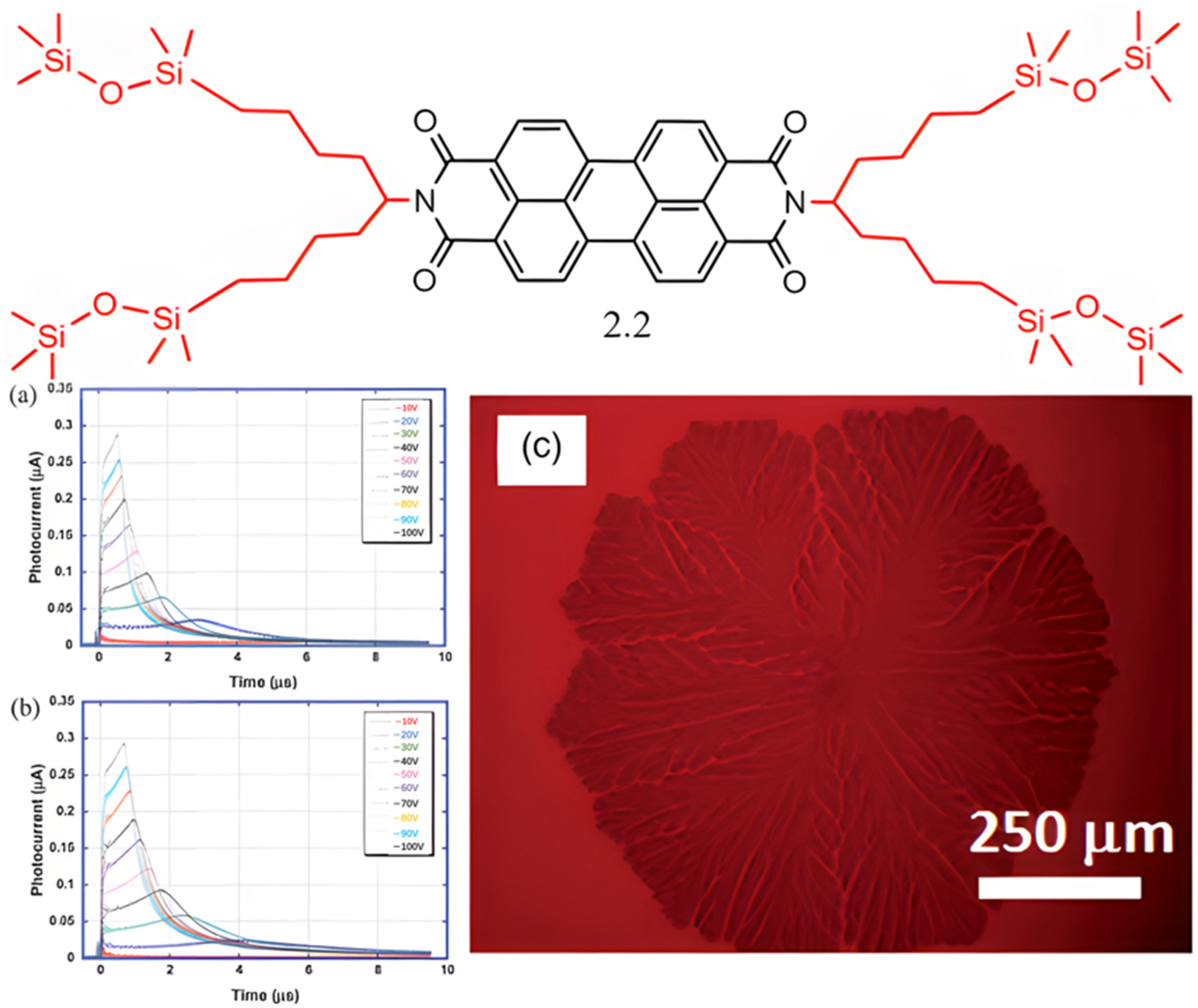
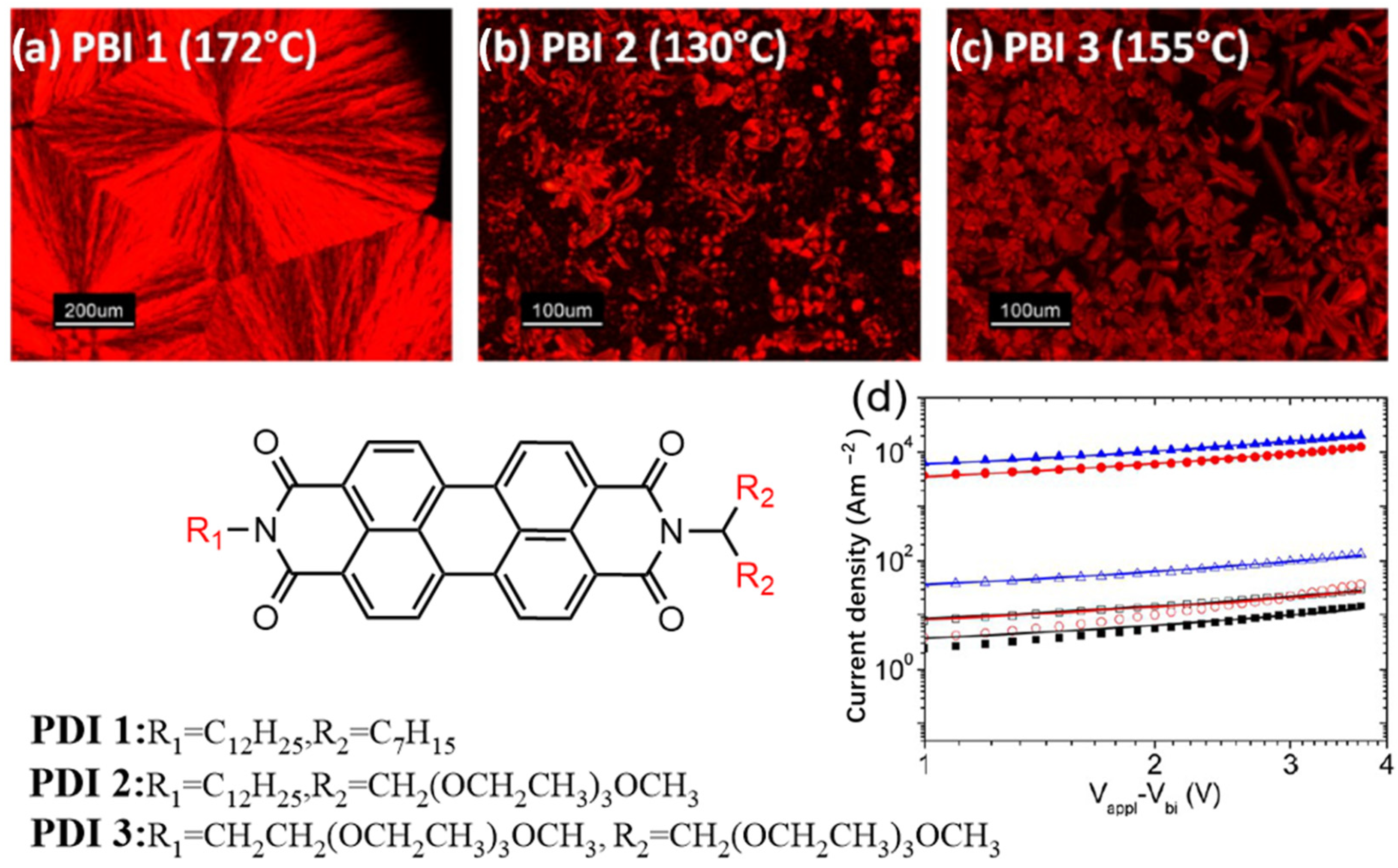
2.1.1. PDI Derivatives with Symmetrical Substitution at the Amine Position
2.1.2. PDI Derivatives with Non-Symmetric Substitution at the Amine Position
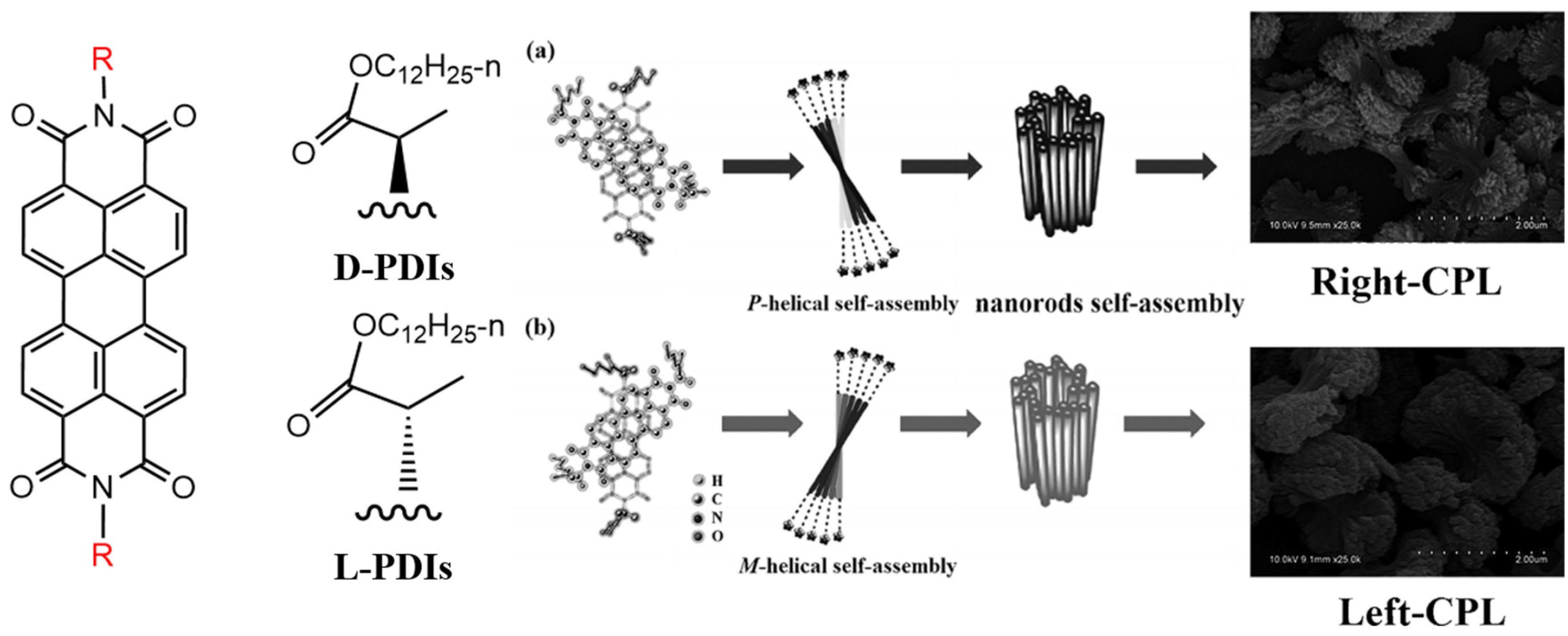
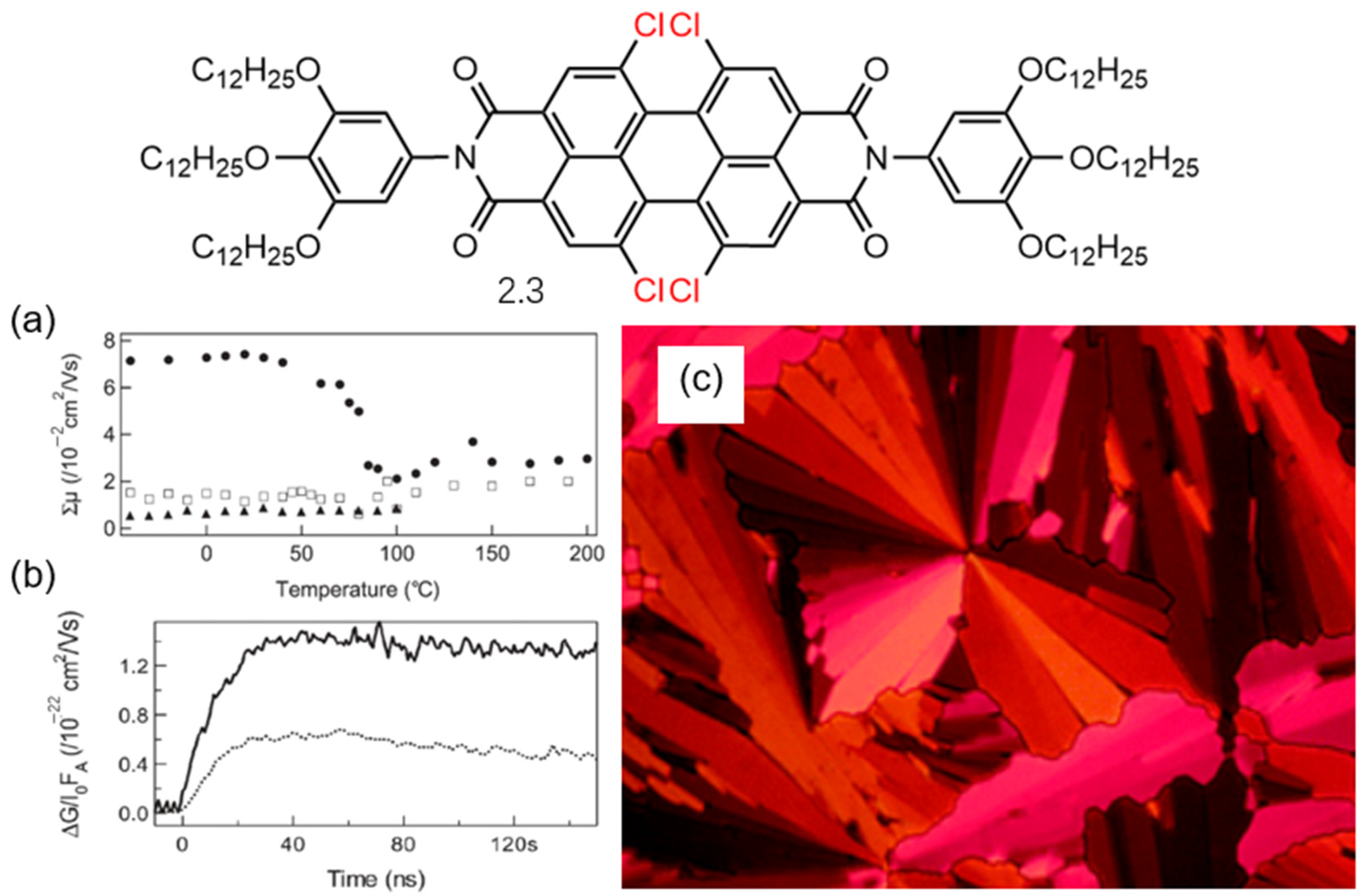
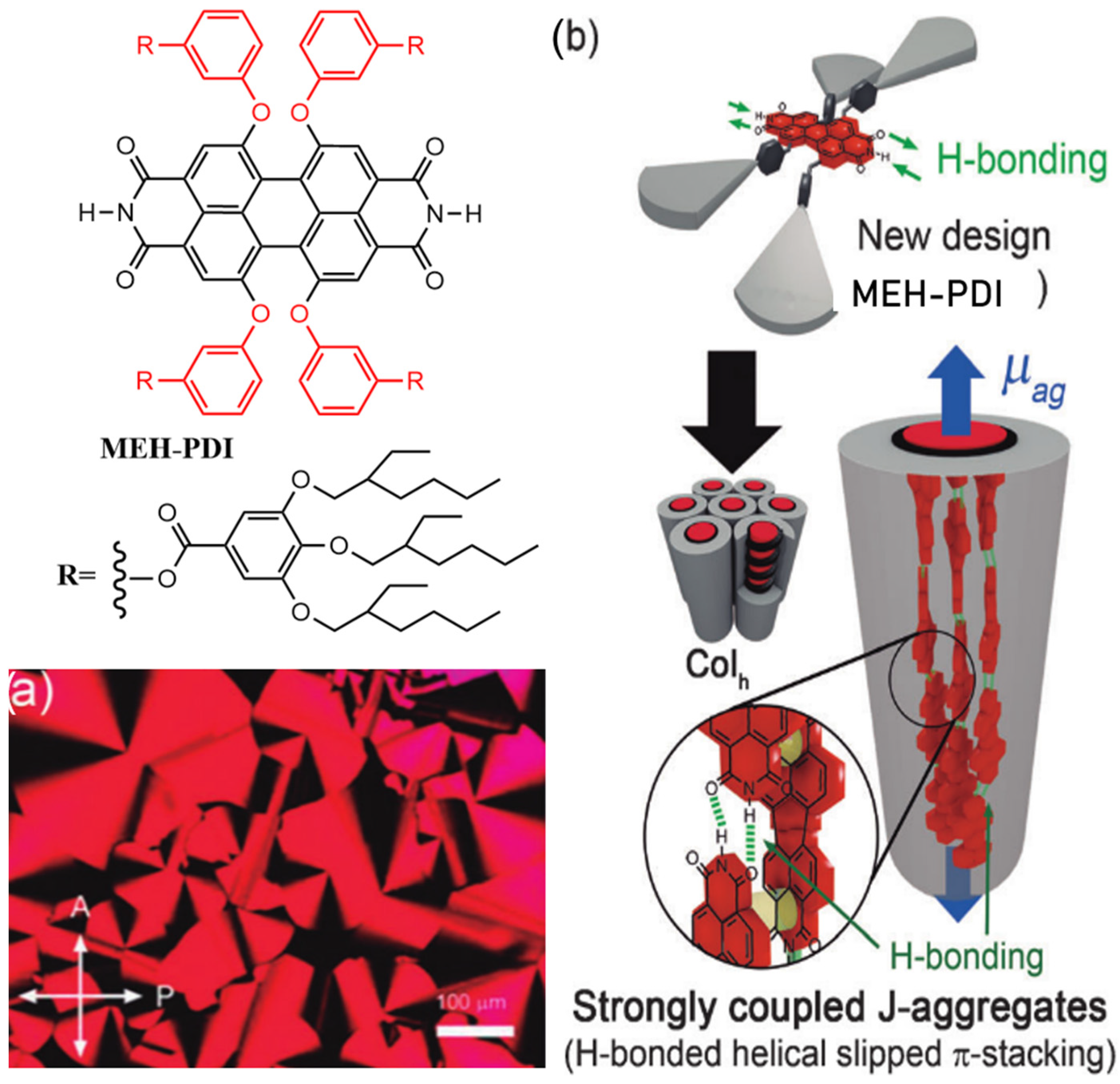
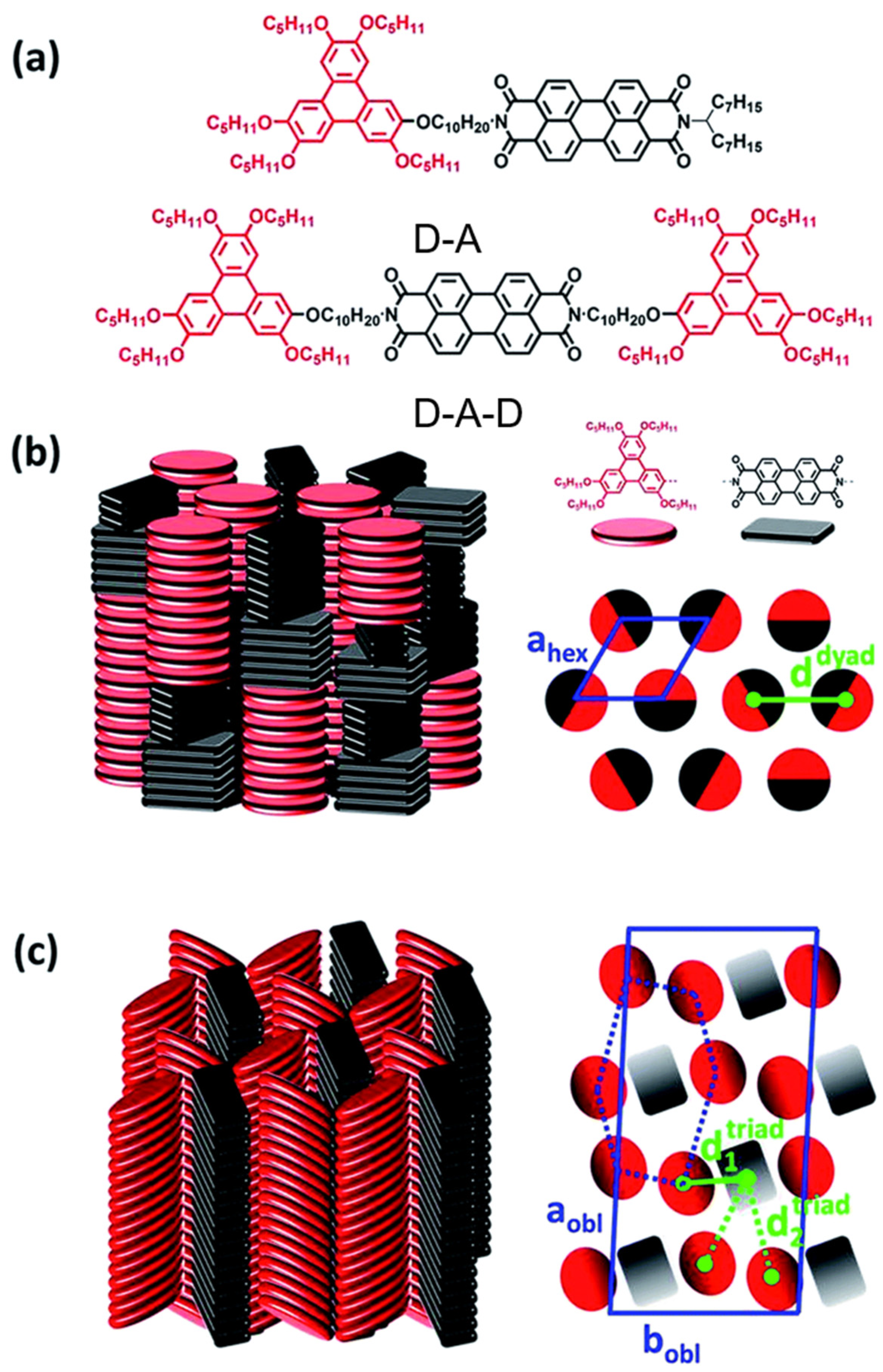
2.2. Both the Amine Position and the Bay Position Are Modified in the PDI Derivative
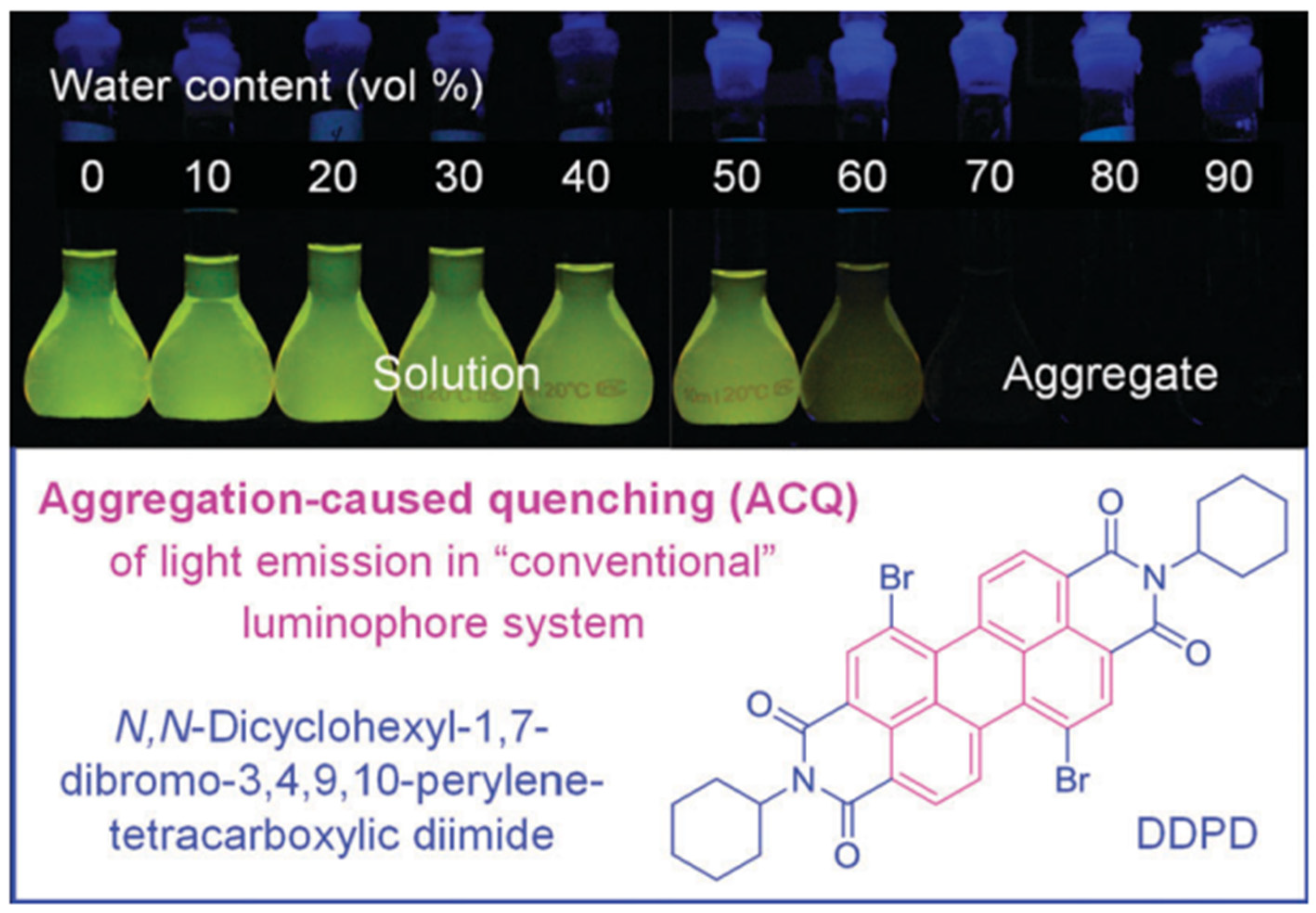
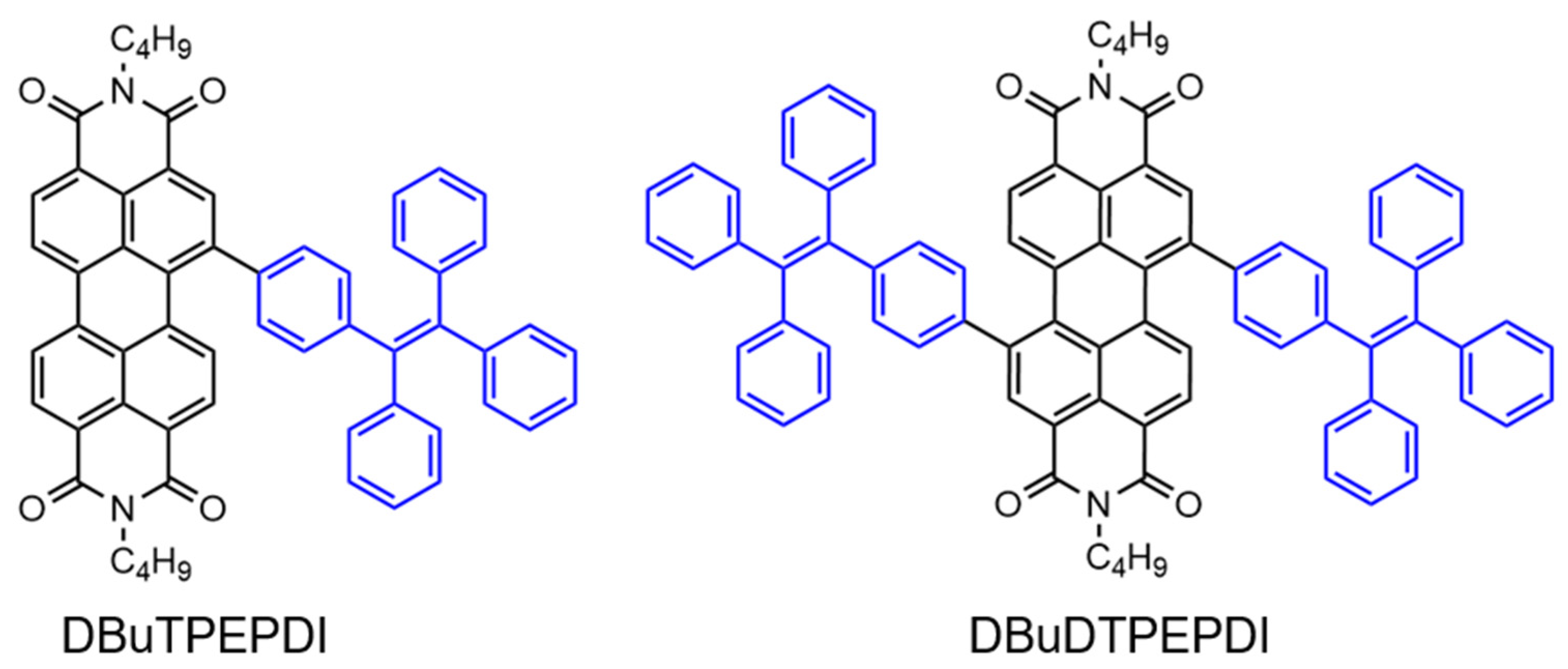
3. AIE-Modified PDI-Based Discotic Liquid Crystals
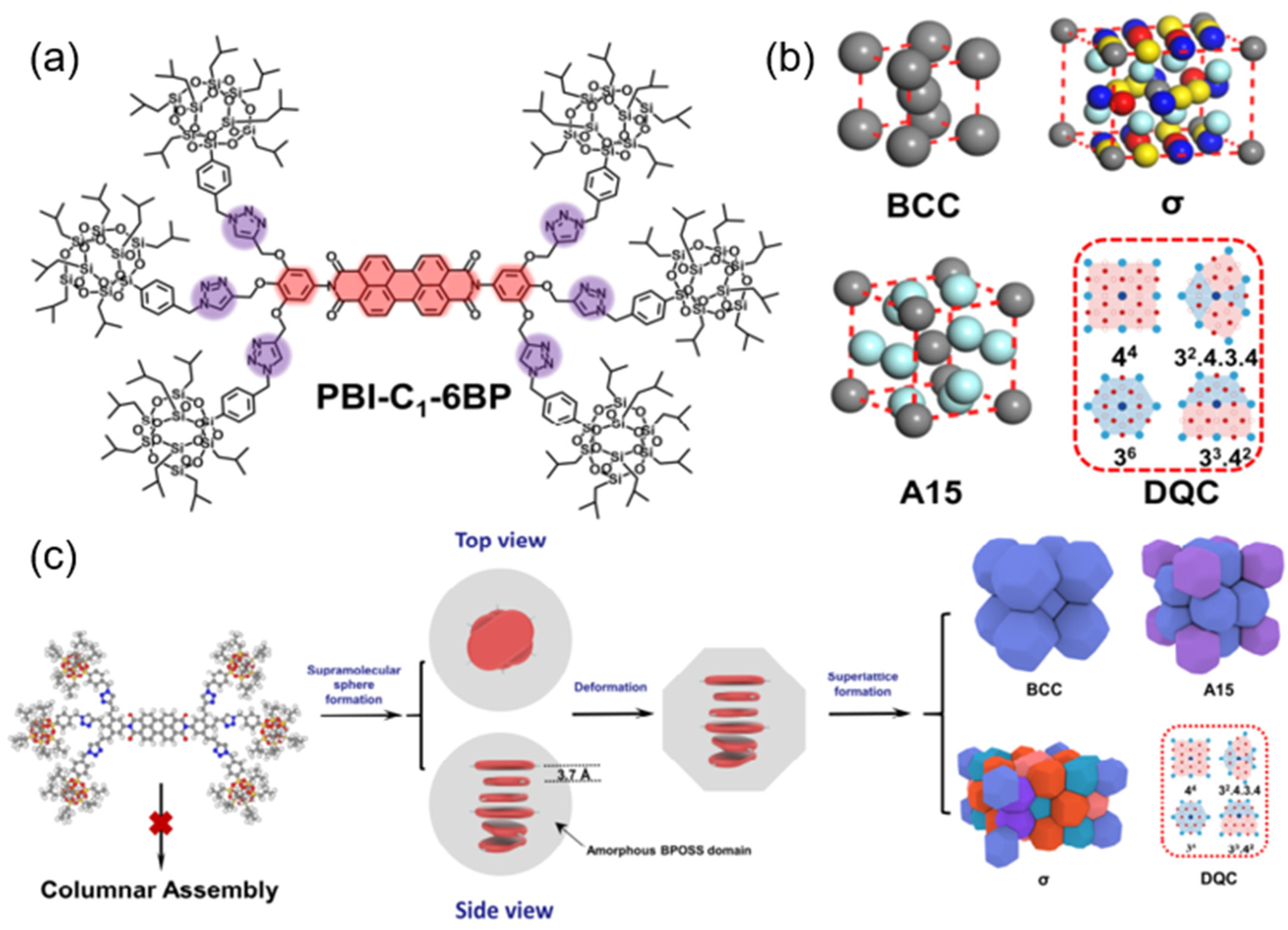
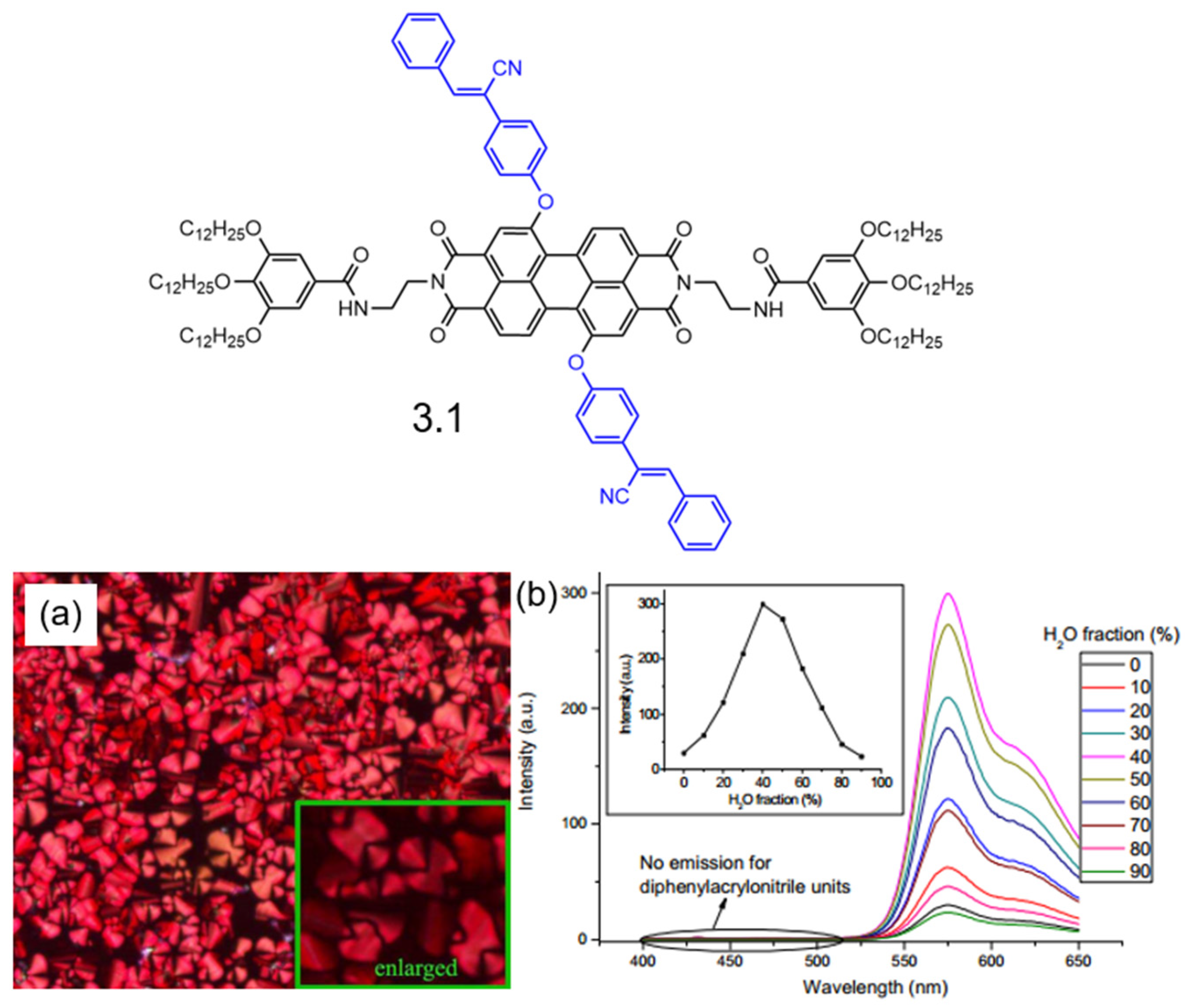
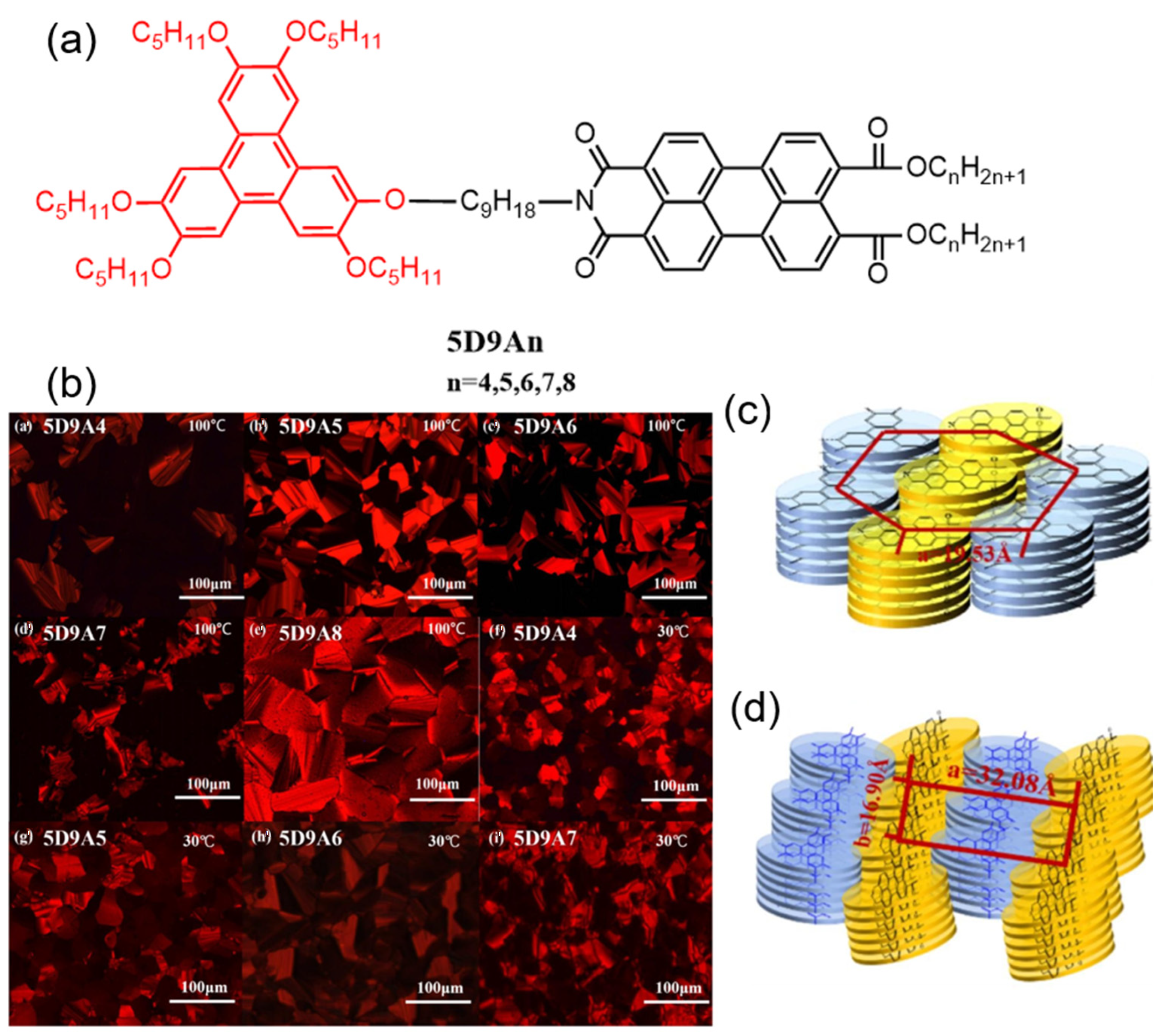
4. Discotic Liquid Crystal-Modified PDI Derivatives
5. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liebermann, C.; Kardos, M. Über die bei der Kernmethylierung aromatischer Basen entstehenden Acridin-Homologen. Berichte Dtsch. Chem. Ges. 1914, 47, 2. [Google Scholar] [CrossRef][Green Version]
- Langhals, H. Synthese von hochreinen Perylen-Fluoreszenzfarbstoffen in großen Mengen—Gezielte Darstellung von Atrop-Isomeren. Chem. Berichte 1985, 118, 4641–4645. [Google Scholar] [CrossRef]
- Liebermann, C.; Kardos, M. Über das Verhalten des Aceanthrenchinons gegen verdünntes Alkali. Berichte Dtsch. Chem. Ges. 1914, 47, 1203–1210. [Google Scholar] [CrossRef]
- Aleshinloye, A.O. Multichromophoric Perylene Bisimide Dyes; Eastern Mediterranean University (EMU): Gazimağusa, Cyprus, 2009. [Google Scholar]
- Demmig, S.; Langhals, H. Leichtlösliche, lichtechte Perylen-Fluoreszenzfarbstoffe. Chem. Berichte 1988, 121, 225–230. [Google Scholar] [CrossRef]
- Langhals, H. Cyclic carboxylic imide structures as structure elements of high stability. Novel developments in perylene dye chemistry. Heterocycles 1995, 1, 477–500. [Google Scholar] [CrossRef]
- Langhals, H.; von Unold, P.; Speckbacher, M. Balanced Decarboxylation of Aromatic Polyacids–A One-Step Synthesis of Perylene-3, 4-dicarboxylic Anhydride. Liebigs Ann. 1997, 1997, 467–468. [Google Scholar] [CrossRef]
- Cormier, R.A.; Gregg, B.A. Synthesis and characterization of liquid crystalline perylene diimides. Chem. Mater. 1998, 10, 1309–1319. [Google Scholar] [CrossRef]
- Würthner, F.; Saha-Möller, C.R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Perylene bisimide dye assemblies as archetype functional supramolecular materials. Chem. Rev. 2016, 116, 962–1052. [Google Scholar] [CrossRef]
- Pressner, D.; Gültner, C.; Spiess, H.W.; Müllen, K. Liquid crystalline perylene derivatives: Orientation and phase variation of discotic dyes. Berichte Bunsenges. Phys. Chem. 1993, 97, 1362–1365. [Google Scholar] [CrossRef]
- Cormier, R.A.; Gregg, B.A. Self-organization in thin films of liquid crystalline perylene diimides. J. Phys. Chem. B 1997, 101, 11004–11006. [Google Scholar] [CrossRef]
- Görl, D.; Soberats, B.; Herbst, S.; Stepanenko, V.; Würthner, F. Perylene bisimide hydrogels and lyotropic liquid crystals with temperature-responsive color change. Chem. Sci. 2016, 7, 6786–6790. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Bauer, C.; Stepanenko, V.; Yagai, S. A black perylene bisimide super gelator with an unexpected J-type absorption band. Adv. Mater. 2008, 20, 1695–1698. [Google Scholar] [CrossRef]
- Safont-Sempere, M.M.; Stepanenko, V.; Lehmann, M.; Würthner, F. Impact of core chirality on mesophase properties of perylene bisimides. J. Mater. Chem. 2011, 21, 7201–7209. [Google Scholar] [CrossRef]
- Würthner, F.; Thalacker, C.; Diele, S.; Tschierske, C. Fluorescent J-type aggregates and thermotropic columnar mesophases of perylene bisimide dyes. Chem.—Eur. J. 2001, 7, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Baumeister, U.; Tschierske, C.; Würthner, F. Effect of core twisting on self-assembly and optical properties of perylene bisimide dyes in solution and columnar liquid crystalline phases. Chem.—Eur. J. 2007, 13, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Hecht, M.; Wurthner, F. Supramolecularly engineered J-aggregates based on perylene bisimide dyes. Acc. Chem. Res. 2020, 54, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Sudhakar, A.A. Perylene-based liquid crystals as materials for organic electronics applications. Langmuir 2018, 35, 2455–2479. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.K.; El-Qarra, L.H.; Smith, D.K. Chirality-directed hydrogel assembly and interactions with enantiomers of an active pharmaceutical ingredient. Chem. Commun. 2022, 58, 3941–3944. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Fan, M.; Zheng, X.; Guan, J.; Yin, M. Chirality of Perylene Diimides: Design strategies and applications. Angew. Chem. 2022, 134, e202202532. [Google Scholar] [CrossRef]
- Molla, M.R.; Ghosh, S. Aqueous self-assembly of chromophore-conjugated amphiphiles. Phys. Chem. Chem. Phys. 2014, 16, 26672–26683. [Google Scholar] [CrossRef]
- Würthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 14, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Debije, M.G.; Chen, Z.; Piris, J.; Neder, R.B.; Watson, M.M.; Müllen, K.; Würthner, F. Dramatic increase in charge carrier lifetime in a liquid crystalline perylene bisimide derivative upon bay substitution with chlorine. J. Mater. Chem. 2005, 15, 1270–1276. [Google Scholar] [CrossRef]
- Ahrens, M.J.; Fuller, M.J.; Wasielewski, M.R. Cyanated perylene-3, 4-dicarboximides and perylene-3, 4: 9, 10-bis (dicarboximide): Facile chromophoric oxidants for organic photonics and electronics. Chem. Mater. 2003, 15, 2684–2686. [Google Scholar] [CrossRef]
- Zhan, X.; Tan, Z.A.; Domercq, B.; An, Z.; Zhang, X.; Barlow, S.; Li, Y.; Zhu, D.; Kippelen, B.; Marder, S.R. A high-mobility electron-transport polymer with broad absorption and its use in field-effect transistors and all-polymer solar cells. J. Am. Chem. Soc. 2007, 129, 7246–7247. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.M.; Kaufmann, N.; Thelakkat, M. Nanostructured semiconductor block copolymers: π–π Stacking, optical and electrochemical properties. Org. Electron. 2007, 8, 69–75. [Google Scholar] [CrossRef]
- Jung, B.J.; Tremblay, N.J.; Yeh, M.-L.; Katz, H.E. Molecular design and synthetic approaches to electron-transporting organic transistor semiconductors. Chem. Mater. 2011, 23, 568–582. [Google Scholar] [CrossRef]
- Jiménez, Á.J.; Spänig, F.; Rodriguez-Morgade, M.S.; Ohkubo, K.; Fukuzumi, S.; Guldi, D.M.; Torres, T. A tightly coupled bis (zinc (II) phthalocyanine)-perylenediimide ensemble to yield long-lived radical ion pair states. Org. Lett. 2007, 9, 2481–2484. [Google Scholar] [CrossRef] [PubMed]
- Law, K.Y. Organic photoconductive materials: Recent trends and developments. Chem. Rev. 1993, 93, 449–486. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Mo, X.; Shi, M.; Wang, M.; Bao, Z. Air stable n-channel organic semiconductors for thin film transistors based on fluorinated derivatives of perylene diimides. Chem. Mater. 2007, 19, 816–824. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; Fechtenkotter, A.; Mullen, K.; Moons, E.; Friend, R.H.; MacKenzie, J.D. Self-organized discotic liquid crystals for high-efficiency organic photovoltaics. Science 2001, 293, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Peng, J.; Zhong, T.; Sun, J.; Wang, X.; Yue, C. Biocompatible elastomer of waterborne polyurethane based on castor oil and polyethylene glycol with cellulose nanocrystals. Carbohydr. Polym. 2012, 87, 2068–2075. [Google Scholar] [CrossRef]
- Anthony, J.E. Small-molecule, nonfullerene acceptors for polymer bulk heterojunction organic photovoltaics. Chem. Mater. 2011, 23, 583–590. [Google Scholar] [CrossRef]
- Figueira-Duarte, T.M.; Mullen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef]
- Würthner, F.; Chen, Z.; Dehm, V.; Stepanenko, V. One-dimensional luminescent nanoaggregates of perylene bisimides. Chem. Commun. 2006, 16, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Barlow, S.; Marder, S.R. Perylene-3, 4, 9, 10-tetracarboxylic acid diimides: Synthesis, physical properties, and use in organic electronics. J. Org. Chem. 2011, 76, 2386–2407. [Google Scholar] [CrossRef] [PubMed]
- Behera, P.K.; Chen, F.-R.; Mondal, I.; Lenka, S.; Gautam, P.; Khatiwoda, N.; Siddiqui, I.; Krishnaprasad, V.; Ahmed, R.; Rao, D.S.S. Superior electron mobility, red electroluminescence with high quantum efficiency from printable room temperature columnar liquid crystalline perylene bisimide. Chem. Eng. J. 2024, 488, 150762. [Google Scholar] [CrossRef]
- Ishii, T.; Bencheikh, F.; Forget, S.; Chénais, S.; Heinrich, B.; Kreher, D.; Sosa Vargas, L.; Miyata, K.; Onda, K.; Fujihara, T. Enhanced light–matter interaction and polariton relaxation by the control of molecular orientation. Adv. Opt. Mater. 2021, 9, 2101048. [Google Scholar] [CrossRef]
- Maki, T.; Hashimoto, H. Vat dyes of acenaphthene series. IV. Condensation of perylenetetracarboxylic acid anhydride with o-phenylenediamine. Bull. Chem. Soc. Jpn. 1952, 25, 411–413. [Google Scholar] [CrossRef]
- Maki, T.; Hashimoto, H. Vat Dyes of Acenaphthene Series. VI. Derivatives of Acenaphthene Violet. Bull. Chem. Soc. Jpn. 1954, 27, 602–605. [Google Scholar] [CrossRef]
- Rademacher, A.; Märkle, S.; Langhals, H. Loesliche perylen-Fluoreszenzfarbstoffe mit hoher photostabilitaet. Chem. Berichte 1982, 8, 2927–2934. [Google Scholar] [CrossRef][Green Version]
- Lindner, S.M.; Hüttner, S.; Chiche, A.; Thelakkat, M.; Krausch, G. Charge separation at self-assembled nanostructured bulk interface in block copolymers. Angew. Chem. Int. Ed. 2006, 45, 3364–3368. [Google Scholar] [CrossRef] [PubMed]
- Hüttner, S.; Sommer, M.; Thelakkat, M. n-type organic field effect transistors from perylene bisimide block copolymers and homopolymers. Appl. Phys. Lett. 2008, 92, 093302. [Google Scholar] [CrossRef]
- Sommer, M.; Lang, A.S.; Thelakkat, M. Crystalline–crystalline donor–acceptor block copolymers. Angew. Chem. 2008, 120, 8019–8022. [Google Scholar] [CrossRef]
- Iverson, I.K.; Tam-Chang, S.-W. Cascade of molecular order by sequential self-organization, induced orientation, and order transfer processes. J. Am. Chem. Soc. 1999, 121, 5801–5802. [Google Scholar] [CrossRef]
- Kim, J.Y.; Chung, I.J.; Kim, Y.C.; Yu, J.-W. Mobility of electrons and holes in a liquid crystalline perylene diimide thin film with time of flight technique. Chem. Phys. Lett. 2004, 398, 367–371. [Google Scholar] [CrossRef]
- Struijk, C.W.; Sieval, A.B.; Dakhorst, J.E.; van Dijk, M.; Kimkes, P.; Koehorst, R.B.; Donker, H.; Schaafsma, T.J.; Picken, S.J.; van de Craats, A.M. Liquid crystalline perylene diimides: Architecture and charge carrier mobilities. J. Am. Chem. Soc. 2000, 122, 11057–11066. [Google Scholar] [CrossRef]
- Funahashi, M.; Sonoda, A. High electron mobility in a columnar phase of liquid-crystalline perylene tetracarboxylic bisimide bearing oligosiloxane chains. J. Mater. Chem. 2012, 22, 25190–25197. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Wei, G.; Wang, Y.; Li, S.; Cheng, Y. Circularly Polarized Luminescence of Chiral Perylene Diimide Based Enantiomers Triggered by Supramolecular Self-Assembly. Chem.—Eur. J. 2016, 22, 12910–12915. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Partridge, B.E.; Huang, N.; Olsen, J.T.; Sahoo, D.; Zeng, X.; Ungar, G.; Graf, R.; Spiess, H.W.; Percec, V. Extraordinary acceleration of cogwheel helical self-organization of dendronized perylene bisimides by the dendron sequence encoding their tertiary structure. J. Am. Chem. Soc. 2020, 142, 9525–9536. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Su, Z.; Huang, M.; Zhang, R.; Wang, J.; Feng, X.; Zhang, R.; Zhang, R.; Shan, W.; Yan, X.Y. Spherical supramolecular structures constructed via chemically symmetric perylene bisimides: Beyond columnar assembly. Angew. Chem. 2020, 132, 18722–18730. [Google Scholar] [CrossRef]
- Muth, M.-A.; Gupta, G.; Wicklein, A.; Carrasco-Orozco, M.; Thurn-Albrecht, T.; Thelakkat, M. Crystalline vs. liquid crystalline perylene bisimides: Improved electron mobility via substituent alteration. J. Phys. Chem. C 2014, 118, 92–102. [Google Scholar] [CrossRef]
- Rogovik, V.; Gutnik, L. Chemistry of perylene-halo-derivatives of perylene-3, 4, 9, 10-tetracarboxylic acid. Zh. Org. Khim. 1988, 24, 635–639. [Google Scholar]
- Seybold, G.; Wagenblast, G. New perylene and violanthrone dyestuffs for fluorescent collectors. Dyes Pigments 1989, 11, 303–317. [Google Scholar] [CrossRef]
- Benning, S.; Kitzerow, H.-S.; Bock, H.; Achard, M.-F. Fluorescent columnar liquid crystalline 3, 4, 9, 10-tetra-(n-alkoxycarbonyl)-perylenes. Liq. Cryst. 2000, 27, 901–906. [Google Scholar] [CrossRef]
- Li, S.; Ye, D.; Henzen, A.; Deng, Y.; Zhou, G. Novel perylene-based organic dyes for electro-fluidic displays. New J. Chem. 2020, 44, 415–421. [Google Scholar] [CrossRef]
- Hill, Z.B.; Rodovsky, D.B.; Leger, J.M.; Bartholomew, G.P. Synthesis and utilization of perylene-based n-type small molecules in light-emitting electrochemical cells. Chem. Commun. 2008, 48, 6594–6596. [Google Scholar] [CrossRef]
- Rajasingh, P.; Cohen, R.; Shirman, E.; Shimon, L.J.; Rybtchinski, B. Selective bromination of perylene diimides under mild conditions. J. Org. Chem. 2007, 72, 5973–5979. [Google Scholar] [CrossRef]
- Aivali, S.; Anastasopoulos, C.; Andreopoulou, A.K.; Pipertzis, A.; Floudas, G.; Kallitsis, J.K. A “Rigid–Flexible” Approach for Processable Perylene Diimide-Based Polymers: Influence of the Specific Architecture on the Morphological, Dielectric, Optical, and Electronic Properties. J. Phys. Chem. B 2020, 124, 5079–5090. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Osswald, P.; Schmidt, R.; Kaiser, T.E.; Mansikkamäki, H.; Könemann, M. Synthesis and optical and electrochemical properties of core-fluorinated perylene bisimides. Org. Lett. 2006, 8, 3765–3768. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.d.; Oh, J.H.; Sun, Y.-S.; Deppisch, M.; Krause, A.-M.; Radacki, K.; Braunschweig, H.; Könemann, M.; Erk, P.; Bao, Z. High-performance air-stable n-channel organic thin film transistors based on halogenated perylene bisimide semiconductors. J. Am. Chem. Soc. 2009, 131, 6215–6228. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Ling, M.M.; Oh, J.H.; Winkler, M.; Könemann, M.; Bao, Z.; Würthner, F. Core-Fluorinated Perylene Bisimide Dyes: Air Stable n-Channel Organic Semiconductors for Thin Film Transistors with Exceptionally High On-to-Off Current Ratios. Adv. Mater. 2007, 19, 3692–3695. [Google Scholar] [CrossRef]
- Zhao, Y.; Wasielewski, M.R. 3, 4: 9, 10-Perylenebis (dicarboximide) chromophores that function as both electron donors and acceptors. Tetrahedron Lett. 1999, 40, 7047–7050. [Google Scholar] [CrossRef]
- Zhen, Y.; Wang, C.; Wang, Z. Tetrachloro-tetra (perylene bisimides): An approach towards N-type graphene nanoribbons. Chem. Commun. 2010, 46, 1926–1928. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, Y.; Yue, W.; Zhen, Y.; Qu, J.; Wang, Z. One-pot facile synthesis of pyridyl annelated perylene bisimides. Org. Lett. 2010, 12, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Sivamurugan, V.; Kazlauskas, K.; Jursenas, S.; Gruodis, A.; Simokaitiene, J.; Grazulevicius, J.; Valiyaveettil, S. Synthesis and photophysical properties of glass-forming bay-substituted perylenediimide derivatives. J. Phys. Chem. B 2010, 114, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Hao, X.; Ma, T.; Zhang, Z.; Wang, L.; Tian, W. Lamellarly Segregated Columnar Liquid Crystalline Alternating Copolymers with High Ambipolar Charge Mobilities. Macromolecules 2023, 56, 4845–4854. [Google Scholar] [CrossRef]
- Yang, W.; Liu, D.; Liu, Y.; Yang, S.; Liu, Y.; Shen, Z.; Yang, H.; Fan, X.H.; Zhou, Q.F. Large-Area Uniaxially Oriented Sub-5 nm Line Patterns of Hybrid Liquid Crystals Constructed by Perylene Diimide and Oligo (Dimethylsiloxane). Chem.—Eur. J. 2023, 29, e202203702. [Google Scholar] [CrossRef]
- Rohr, U.; Schlichting, P.; Böhm, A.; Gross, M.; Meerholz, K.; Bräuchle, C.; Müllen, K. Liquid crystalline coronene derivatives with extraordinary fluorescence properties. Angew. Chem. Int. Ed. 1998, 37, 1434–1437. [Google Scholar] [CrossRef]
- Zhan, X.; Tan, Z.a.; Zhou, E.; Li, Y.; Misra, R.; Grant, A.; Domercq, B.; Zhang, X.-H.; An, Z.; Zhang, X. Copolymers of perylene diimide with dithienothiophene and dithienopyrrole as electron-transport materials for all-polymer solar cells and field-effect transistors. J. Mater. Chem. 2009, 19, 5794–5803. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, S.; Sun, X.; Liu, Y.; Zhu, D. Suzuki coupling reaction of 1, 6, 7, 12-tetrabromoperylene bisimide. Org. Lett. 2006, 8, 867–870. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Pschirer, N.G.; Baumgarten, M.; Mullen, K. Polyphenylene-based materials for organic photovoltaics. Chem. Rev. 2010, 110, 6817–6855. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, M.; Guo, H.; Yang, F. Fluorescein-bridged perylene bisimide dimer for use as liquid crystal: Studies on mesomorphic and fluorescence properties. J. Fluoresc. 2021, 31, 1555–1565. [Google Scholar] [CrossRef]
- Lin, J.; Ji, X.; Guo, H.; Yang, F. Novel perylene columnar liquid crystal tuned by azo isomerization on bay-positions. J. Mol. Liq. 2019, 290, 111228. [Google Scholar] [CrossRef]
- Prehm, M.; Liu, F.; Zeng, X.; Ungar, G.; Tschierske, C. Axial-bundle phases-new modes of 2D, 3D, and helical columnar self-assembly in liquid crystalline phases of bolaamphiphiles with swallow tail lateral chains. J. Am. Chem. Soc. 2011, 133, 4906–4916. [Google Scholar] [CrossRef]
- Herbst, S.; Soberats, B.; Leowanawat, P.; Lehmann, M.; Würthner, F. A Columnar Liquid-Crystal Phase Formed by Hydrogen-Bonded Perylene Bisimide J-Aggregates. Angew. Chem. Int. Ed. 2017, 56, 2162–2165. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Guo, H.; Yang, F.; Wang, Z. Synthesis, mesomorphic and photophysical properties of novel triads and pentads of perylene liquid crystals with cholesterol units at the bay-position. RSC Adv. 2017, 7, 4320–4328. [Google Scholar] [CrossRef]
- Herbst, S.; Soberats, B.; Leowanawat, P.; Stolte, M.; Lehmann, M.; Würthner, F. Self-assembly of multi-stranded perylene dye J-aggregates in columnar liquid-crystalline phases. Nat. Commun. 2018, 9, 2646. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, H.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: New vistas at the aggregate level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lam, J.W.; Kwok, R.T.; Liu, B.; Tang, B.Z. Aggregation-induced emission: Fundamental understanding and future developments. Mater. Horiz. 2019, 6, 428–433. [Google Scholar] [CrossRef]
- Zhao, Z.; He, B.; Tang, B.Z. Aggregation-induced emission of siloles. Chem. Sci. 2015, 6, 5347–5365. [Google Scholar] [CrossRef]
- Birks, J. Photophysics of Aromatic Molecules; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1970. [Google Scholar]
- Zhelev, Z.; Ohba, H.; Bakalova, R. Single quantum dot-micelles coated with silica shell as potentially non-cytotoxic fluorescent cell tracers. J. Am. Chem. Soc. 2006, 128, 6324–6325. [Google Scholar] [CrossRef]
- Würthner, F. Aggregation-induced emission (AIE): A historical perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef] [PubMed]
- Bakalova, R.; Zhelev, Z.; Aoki, I.; Ohba, H.; Imai, Y.; Kanno, I. Silica-shelled single quantum dot micelles as imaging probes with dual or multimodality. Anal. Chem. 2006, 78, 5925–5932. [Google Scholar] [CrossRef]
- Niu, G.; Zhang, R.; Shi, X.; Park, H.; Xie, S.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. AIE luminogens as fluorescent bioprobes. TrAC Trends Anal. Chem. 2020, 123, 115769. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Dou, C.; Sun, H.; Xu, P.; Ye, K.; Zhang, J.; Jiang, S.; Li, F.; Wang, Y. Alkyl and dendron substituted quinacridones: Synthesis, structures, and luminescent properties. J. Phys. Chem. B 2007, 111, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.; Fréchet, J.M. Dendritic encapsulation of function: Applying nature’s site isolation principle from biomimetics to materials science. Angew. Chem. Int. Ed. 2001, 40, 74–91. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Gautrot, J.E.; Ji, C.; Brunner, P.-L.; Nguyen, M.T.; Zhu, X. Enhancing the photoluminescence intensity of conjugated polycationic polymers by using quantum dots as antiaggregation reagents. Langmuir 2006, 22, 4799–4803. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, B.S.; Tang, B.Z. Molecular design, circularly polarized luminescence, and helical self-assembly of chiral aggregation-induced emission molecules. Chem.—Asian J. 2019, 14, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.Z.; Lu, P.; Chen, S.; Lam, J.W.; Wang, Z.; Liu, Y.; Kwok, H.S.; Ma, Y.; Tang, B.Z. Changing the behavior of chromophores from aggregation-caused quenching to aggregation-induced emission: Development of highly efficient light emitters in the solid state. Adv. Mater. 2010, 22, 2159–2163. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Zhang, Z.; Quan, X.; Hao, X.; Tian, W. Perylene Bisimide-Based Luminescent Liquid Crystals with Tunable Solid-State Light Emission. ACS Appl. Mater. Interfaces 2021, 13, 57786–57795. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, S.; McBranch, D.; Whitten, D. Tuning the properties of conjugated polyelectrolytes through surfactant complexation. J. Am. Chem. Soc. 2000, 122, 9302–9303. [Google Scholar] [CrossRef]
- Taylor, P.N.; O’Connell, M.J.; McNeill, L.A.; Hall, M.J.; Aplin, R.T.; Anderson, H.L. Insulated molecular wires: Synthesis of conjugated polyrotaxanes by Suzuki coupling in water. Angew. Chem. Int. Ed. 2000, 39, 3456–3460. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, E.; Lam, J.W.; Tang, B.Z. AIE luminogens: Emission brightened by aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Chen, J.; Xu, B.; Ouyang, X.; Tang, B.Z.; Cao, Y. Aggregation-induced emission of cis, cis-1, 2, 3, 4-tetraphenylbutadiene from restricted intramolecular rotation. J. Phys. Chem. A 2004, 108, 7522–7526. [Google Scholar] [CrossRef]
- Tong, H.; Hong, Y.; Dong, Y.; Ren, Y.; Häussler, M.; Lam, J.W.; Wong, K.S.; Tang, B.Z. Color-tunable, aggregation-induced emission of a butterfly-shaped molecule comprising a pyran skeleton and two cholesteryl wings. J. Phys. Chem. B 2007, 111, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Dong, Y.; Hong, Y.; Häussler, M.; Lam, J.W.; Sung, H.H.-Y.; Yu, X.; Sun, J.; Williams, I.D.; Kwok, H.S. Aggregation-induced emission: Effects of molecular structure, solid-state conformation, and morphological packing arrangement on light-emitting behaviors of diphenyldibenzofulvene derivatives. J. Phys. Chem. C 2007, 111, 2287–2294. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Alam, P.; Bai, H.; Bhalla, V.; Bryce, M.R.; Cao, M.; Chen, C.; Chen, S.; Chen, X. Aggregation-induced emission (AIE), life and health. ACS Nano 2023, 17, 14347–14405. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Kang, Y.; Tang, B.Z. Recent advances in AIE polymers. Polym. J. 2016, 48, 359–370. [Google Scholar] [CrossRef]
- Hu, R.; Leung, N.L.; Tang, B.Z. AIE macromolecules: Syntheses, structures and functionalities. Chem. Soc. Rev. 2014, 43, 4494–4562. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, X.A.; Wei, Q.; Wang, J.; Shen, X.Y.; Qin, A.; Sun, J.Z.; Tang, B.Z. Tetraphenylethene modified perylene bisimide: Effect of the number of substituents on AIE performance. Chem. Commun. 2012, 48, 11671–11673. [Google Scholar] [CrossRef]
- Hong, Y.; Häußler, M.; Lam, J.W.; Li, Z.; Sin, K.K.; Dong, Y.; Tong, H.; Liu, J.; Qin, A.; Renneberg, R. Label-free fluorescent probing of G-quadruplex formation and real-time monitoring of DNA folding by a quaternized tetraphenylethene salt with aggregation-induced emission characteristics. Chem.—Eur. J. 2008, 14, 6428–6437. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lam, J.W.; Qin, A.; Li, Z.; Liu, J.; Sun, J.; Dong, Y.; Tang, B.Z. Endowing hexaphenylsilole with chemical sensory and biological probing properties by attaching amino pendants to the silolyl core. Chem. Phys. Lett. 2007, 446, 124–127. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, S.; Lam, J.W.; Wang, Z.; Lu, P.; Mahtab, F.; Sung, H.H.; Williams, I.D.; Ma, Y.; Kwok, H.S. Pyrene-substituted ethenes: Aggregation-enhanced excimer emission and highly efficient electroluminescence. J. Mater. Chem. 2011, 21, 7210–7216. [Google Scholar] [CrossRef]
- Zhu, M.; Zhuo, Y.; Cai, K.; Guo, H.; Yang, F. Novel fluorescent perylene liquid crystal with diphenylacrylonitrile groups: Observation of a large pseudo stokes shift based on AIE and FRET effects. Dyes Pigments 2017, 147, 343–349. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, Y.; Zhang, X.; Chen, M.; Guo, H.; Yang, F. Perylene bisimide with diphenylacrylonitrile on side-chain: Strongly fluorescent liquid crystal with large pseudo Stokes shift based on AIE and FRET effect. Soft Matter 2018, 14, 6737–6744. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Sadashiva, B.; Suresh, K. Liquid crystals of disc-like molecules. Pramana 1977, 9, 471–480. [Google Scholar] [CrossRef]
- Park, L.Y.; Hamilton, D.G.; McGehee, E.A.; McMenimen, K.A. Complementary C 3-Symmetric Donor—Acceptor Components: Cocrystal Structure and Control of Mesophase Stability. J. Am. Chem. Soc. 2003, 125, 10586–10590. [Google Scholar] [CrossRef]
- Sergeyev, S.; Pisula, W.; Geerts, Y.H. Discotic liquid crystals: A new generation of organic semiconductors. Chem. Soc. Rev. 2007, 36, 1902–1929. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.; Schuhmacher, P.; Simmerer, J.; Häussling, L.; Siemensmeyer, K.; Etzbachi, K.; Ringsdorf, H.; Haarer, D. Fast photoconduction in the highly ordered columnar phase of a discotic liquid crystal. Nature 1994, 371, 141–143. [Google Scholar] [CrossRef]
- Kumar, S. Recent developments in the chemistry of triphenylene-based discotic liquid crystals. Liq. Cryst. 2004, 31, 1037–1059. [Google Scholar] [CrossRef]
- Shin, W.S.; Jeong, H.-H.; Kim, M.-K.; Jin, S.-H.; Kim, M.-R.; Lee, J.-K.; Lee, J.W.; Gal, Y.-S. Effects of functional groups at perylene diimide derivatives on organic photovoltaic device application. J. Mater. Chem. 2006, 16, 384–390. [Google Scholar] [CrossRef]
- Käfer, D.; Bashir, A.; Dou, X.; Witte, G.; Müllen, K.; Wöll, C. Evidence for Band-Like Transport in Graphene-Based Organic Monolayers. Adv. Mater. 2010, 3, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Debije, M.G.; Dabaerdemaker, T.; Osswald, P.; Würthner, F. Tetrachloro-substituted perylene bisimide dyes as promising n-type organic semiconductors: Studies on structural, electrochemical and charge transport properties. ChemPhysChem 2004, 5, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.Q.; An, L.L.; Zhang, X.B.; Yu, W.H.; Hu, P.; Wang, B.Q.; Xu, J.; Zeng, Q.D.; Monobe, H.; Shimizu, Y. Highly Segregated Lamello-Columnar Mesophase Organizations and Fast Charge Carrier Mobility in New Discotic Donor—Acceptor Triads. Chem.—Eur. J. 2015, 21, 10379–10390. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Facchetti, A.; Barlow, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R.; Marder, S.R. Rylene and related diimides for organic electronics. Adv. Mater. 2011, 23, 268–284. [Google Scholar] [CrossRef]
- Alibert-Fouet, S.; Seguy, I.; Bobo, J.F.; Destruel, P.; Bock, H. Liquid-Crystalline and Electron-Deficient Coronene Oligocarboxylic Esters and Imides By Twofold Benzogenic Diels–Alder Reactions on Perylenes. Chem.—Eur. J. 2007, 13, 1746–1753. [Google Scholar] [CrossRef]
- Hayashi, H.; Nihashi, W.; Umeyama, T.; Matano, Y.; Seki, S.; Shimizu, Y.; Imahori, H. Segregated donor–acceptor columns in liquid crystals that exhibit highly efficient ambipolar charge transport. J. Am. Chem. Soc. 2011, 133, 10736–10739. [Google Scholar] [CrossRef]
- An, Z.; Yu, J.; Jones, S.C.; Barlow, S.; Yoo, S.; Domercq, B.; Prins, P.; Siebbeles, L.D.; Kippelen, B.; Marder, S.R. High electron mobility in room-temperature discotic liquid-crystalline perylene diimides. Adv. Mater. 2005, 17, 2580–2583. [Google Scholar] [CrossRef]
- Lee, K.J.; Woo, J.H.; Xiao, Y.; Kim, E.; Mazur, L.M.; Kreher, D.; Attias, A.-J.; Matczyszyn, K.; Samoc, M.; Heinrich, B. Structure–charge transfer property relationship in self-assembled discotic liquid-crystalline donor–acceptor dyad and triad thin films. RSC Adv. 2016, 6, 57811–57819. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, R.; Zhang, A.; Fang, Y.; Song, X.; Xie, M.; Zhang, C.; Feng, Y.; Yu, H. Design of bipolar transport DA discotic liquid crystals based on triphenylene and perylene cores: Effect of the chain length of ester groups. J. Mol. Liq. 2024, 409, 125502. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, R.; Zhang, A.; Fang, Y.; Song, X.; Xie, M.; Wu, T.; Zhang, C.; Yang, H.; Yu, H. Optoelectronic properties of ambipolar transport triphenylene-perylene donor–acceptor discotic liquid crystals. New J. Chem. 2024, 48, 11173–11182. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, S.; Liao, R.; Xie, M.; Song, X.; Zhang, A.; Fang, Y.; Zhang, C.; Yu, H. Synthesis and Optoelectronic Properties of Perylene Diimide-Based Liquid Crystals. Molecules 2025, 30, 799. https://doi.org/10.3390/molecules30040799
Qiao S, Liao R, Xie M, Song X, Zhang A, Fang Y, Zhang C, Yu H. Synthesis and Optoelectronic Properties of Perylene Diimide-Based Liquid Crystals. Molecules. 2025; 30(4):799. https://doi.org/10.3390/molecules30040799
Chicago/Turabian StyleQiao, Shiyi, Ruijuan Liao, Mingsi Xie, Xiaoli Song, Ao Zhang, Yi Fang, Chunxiu Zhang, and Haifeng Yu. 2025. "Synthesis and Optoelectronic Properties of Perylene Diimide-Based Liquid Crystals" Molecules 30, no. 4: 799. https://doi.org/10.3390/molecules30040799
APA StyleQiao, S., Liao, R., Xie, M., Song, X., Zhang, A., Fang, Y., Zhang, C., & Yu, H. (2025). Synthesis and Optoelectronic Properties of Perylene Diimide-Based Liquid Crystals. Molecules, 30(4), 799. https://doi.org/10.3390/molecules30040799






