Rare Prenyllipids in Wild St. John’s Wort During Three Harvest Seasons
Abstract
:1. Introduction
2. Results and Discussion
2.1. Tocochromanol Profile in Different Anatomical Parts of St. John’s Wort
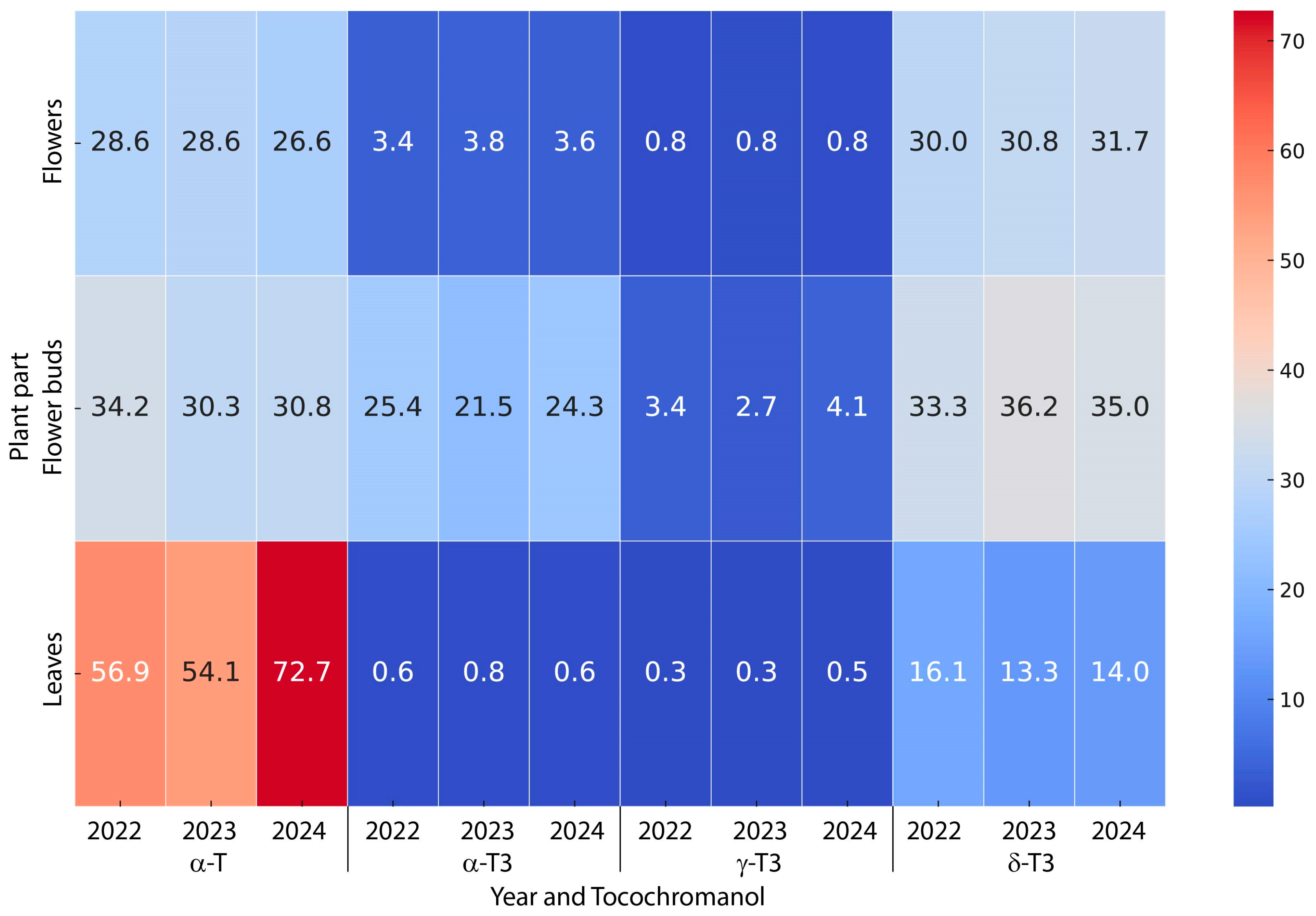
2.2. Variability of Tocopherols and Tocotrienols Contents in St. John’s Wort: Impact of Plant Part and Harvest Year
2.3. The Potential Applications, Challenges, and Future Directions for Tocotrienol-Rich Extracts of H. perforatum
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. Saponification and n-Hexane:Ethyl Acetate Extraction Protocol
3.4. Tocopherol and Tocotrienol Determination by RP-HPLC-FLD
3.5. LC-MS Presence Confirmation of Tocochromanols in St. John’s Wort Aerial Parts
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- World Health Organization. Guidelines on the Conservation of Medicinal Plants; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 1993. [Google Scholar]
- Smith, T.; Kawa, K.; Eckl, V.; Morton, C.; Stredney, R. Herbal supplement sales in US increase 7.7% in 2016. HerbalGram 2017, 115, 56–65. [Google Scholar]
- Crockett, S.L.; Robson, N.K.B. Taxonomy and chemotaxonomy of the genus Hypericum. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 1–13. [Google Scholar] [PubMed]
- Ji, Y.; Zhang, R.; Bensalel, J.; Morcol, T.; Gu, R.; Gallego-Delgado, J.; Kennelly, E.J.; Long, C. Metabolomic and chemometric analyses of St. John’s wort and related Asian Hypericum species linked to bioactivity. J. Ethnopharmacol. 2024, 329, 118163. [Google Scholar] [CrossRef]
- Tanaka, N.; Kashiwada, Y. Characteristic metabolites of Hypericum plants: Their chemical structures and biological activities. J. Nat. Med. 2021, 75, 423–433. [Google Scholar] [CrossRef]
- Büter, B.; Orlacchio, C.; Soldati, A.; Berger, K. Significance of genetic and environmental aspects in the field cultivation of Hypericum perforatum. Planta Medica 1998, 64, 431–437. [Google Scholar] [CrossRef]
- de Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef]
- Lazzara, S.; Carrubba, A.; Napoli, E. Cultivating for the industry: Cropping experiences with Hypericum perforatum L. in a mediterranean environment. Agriculture 2021, 11, 446. [Google Scholar] [CrossRef]
- Süntar, I.P.; Akkol, E.K.; Yılmazer, D.; Baykal, T.; Kırmızıbekmez, H.; Alper, M.; Yeşilada, E. Investigations on the in vivo wound healing potential of Hypericum perforatum L. J. Ethnopharmacol. 2010, 127, 468–477. [Google Scholar] [CrossRef]
- Galeotti, N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef]
- Carrubba, A.; Lazzara, S.; Giovino, A.; Ruberto, G.; Napoli, E. Content variability of bioactive secondary metabolites in Hypericum perforatum L. Phytochem. Lett. 2021, 46, 71–78. [Google Scholar] [CrossRef]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species—A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kladar, N.; Anačkov, G.; Srđenović, B.; Gavarić, N.; Hitl, M.; Salaj, N.; Jeremić, K.; Babović, S.; Božin, B.S. John’s wort herbal teas–Biological potential and chemometric approach to quality control. Plant Foods Hum. Nutr. 2020, 75, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Mišina, I.; Lazdiņa, D. Tocopherol and tocotrienol homologue recovery from Hypericum perforatum L. and extraction residues after hydroethanolic extraction. Ind. Crops Prod. 2025, 224, 120321. [Google Scholar] [CrossRef]
- Inoue, T.; Tatemori, S.; Muranaka, N.; Hirahara, Y.; Homma, S.; Nakane, T.; Takano, A.; Nomi, Y.; Otsuka, Y. The Identification of Vitamin E Homologues in Medicinal Plant Samples Using ESI (+)-LC-MS3. J. Agric. Food Chem. 2012, 60, 9581–9588. [Google Scholar] [CrossRef]
- Hosni, K.; Msaâda, K.; Taârit, M.B.; Marzouk, B. Fatty acid composition and tocopherol content in four Tunisian Hypericum species: Hypericum perforatum, Hypericum tomentosum, Hypericum perfoliatum and Hypericum ericoides ssp. Roberti. Arab. J. Chem. 2017, 10, S2736–S2741. [Google Scholar] [CrossRef]
- Lazdiņa, D.; Mišina, I.; Górnaś, P. Tocotrienols in eleven species of Hypericum genus leaves. Molecules 2025, 30, 662. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef]
- Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Naumovski, N. Tocotrienols, health and ageing: A systematic review. Maturitas 2017, 95, 55–60. [Google Scholar] [CrossRef]
- Younes, M.; Loubnane, G.; Sleiman, C.; Rizk, S. Tocotrienol isoforms: The molecular mechanisms underlying their effects in cancer therapy and their implementation in clinical trials. J. Integr. Med. 2024, 22, 1–11. [Google Scholar] [CrossRef]
- Manu, K.A.; Shanmugam, M.K.; Ramachandran, L.; Li, F.; Fong, C.W.; Kumar, A.P.; Tan, P.; Sethi, G. First evidence that γ-tocotrienol inhibits the growth of human gastric cancer and chemosensitizes it to capecitabine in a xenograft mouse model through the modulation of NF-κB pathway. Clin. Cancer Res. 2012, 18, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Neo, J.R.E.; Wang, C.J.; Chai, N.C.L.; Lieo, E.G.B.; Yeo, M.; Loong, H.Y.; Ung, Y.W.; Yap, W.N. Tocotrienol-rich fraction enhances cell proliferation and memory formation in hippocampal HT22 neuronal cells through BDNF/TrkB pathway. J. Funct. Foods 2024, 116, 106178. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, X.; Zhao, C.; Fan, L.; Yin, S.; Hu, H. Delta-tocotrienol disrupts PD-L1 glycosylation and reverses PD-L1-mediated immune suppression. Biomed. Pharmacother. 2024, 170, 116078. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Wang, L.; Wang, S.; Liu, X.; Wu, D.; Ouyang, Z.; Meng, R.; Shan, Y.; Zhang, S.; Peng, T. Radioprotective effectiveness of a novel delta-tocotrienol prodrug on mouse hematopoietic system against 60Co gamma-ray irradiation through inducing granulocyte-colony stimulating factor production. Eur. J. Med. Chem. 2024, 269, 116346. [Google Scholar] [CrossRef]
- Wang, X.-P.; Li, X.-H.; Lei, J.-J.; Xiao, Y.-W.; Chi, Y.; Sun, Q.; Zhang, H. Polyprenylated acylphloroglucinols from Hypericum sampsonii with cytotoxicity against pancreatic carcinomas. J. Asian Nat. Prod. Res. 2024, 27, 136–142. [Google Scholar] [CrossRef]
- Kanchi, M.M.; Shanmugam, M.K.; Rane, G.; Sethi, G.; Kumar, A.P. Tocotrienols: The unsaturated sidekick shifting new paradigms in vitamin E therapeutics. Drug Discov. Today 2017, 22, 1765–1781. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Waśkiewicz, A.; Perkons, I.; Pugajeva, I.; Segliņa, D. Simultaneous extraction of tocochromanols and flavan-3-ols from the grape seeds: Analytical and industrial aspects. Food Chem. 2025, 462, 140913. [Google Scholar] [CrossRef]
- Siger, A.; Górnaś, P. Free tocopherols and tocotrienols in 82 plant species’ oil: Chemotaxonomic relation as demonstrated by PCA and HCA. Food Res. Int. 2023, 164, 112386. [Google Scholar] [CrossRef]
- Horvath, G.; Wessjohann, L.; Bigirimana, J.; Jansen, M.; Guisez, Y.; Caubergs, R.; Horemans, N. Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry 2006, 67, 1185–1195. [Google Scholar] [CrossRef]
- Muñoz, P.; Munné-Bosch, S. Vitamin E in plants: Biosynthesis, transport, and function. Trends Plant Sci. 2019, 24, 1040–1051. [Google Scholar] [CrossRef]
- Siles, L.; Cela, J.; Munné-Bosch, S. Vitamin E analyses in seeds reveal a dominant presence of tocotrienols over tocopherols in the Arecaceae family. Phytochemistry 2013, 95, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cahoon, R.E.; Hunter, S.C.; Zhang, C.; Han, J.; Borgschulte, T.; Cahoon, E.B. Vitamin E biosynthesis: Functional characterization of the monocot homogentisate geranylgeranyl transferase. Plant J. 2011, 65, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Shibata, A.; Sookwong, P.; Kawakami, Y.; Eitsuka, T.; Asai, A.; Oikawa, S.; Nakagawa, K. Antiangiogenic and anticancer potential of unsaturated vitamin E (tocotrienol). J. Nutr. Biochem. 2009, 20, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Nakagawa, K.; Sookwong, P.; Tsuduki, T.; Asai, A.; Miyazawa, T. α-Tocopherol attenuates the cytotoxic effect of δ-tocotrienol in human colorectal adenocarcinoma cells. Biochem. Biophys. Res. Commun. 2010, 397, 214–219. [Google Scholar] [CrossRef]
- Matsura, T.; Nakaso, K.; Horikoshi, Y. Tocotrienols and Parkinson’s disease: In vitro and in vivo modeling. In Vitamins and Minerals in Neurological Disorders; Elsevier: Amsterdam, The Netherlands, 2023; pp. 513–525. [Google Scholar]
- Chopra, K.; Arora, V.; Kuhad, A. Diabetic nephropathy and tocotrienol. In Diabetes: Oxidative Stress and Dietary Antioxidants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 135–143. [Google Scholar]
- Husain, K.; Malafa, M.P. Role of tocotrienols in chemosensitization of cancer. In Role of nutraceuticals in Cancer Chemosensitization; Elsevier: Amsterdam, The Netherlands, 2018; pp. 77–97. [Google Scholar]
- Górnaś, P.; Segliņa, D.; Lācis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ). LWT Food Sci. Technol. 2014, 59, 211–214. [Google Scholar] [CrossRef]

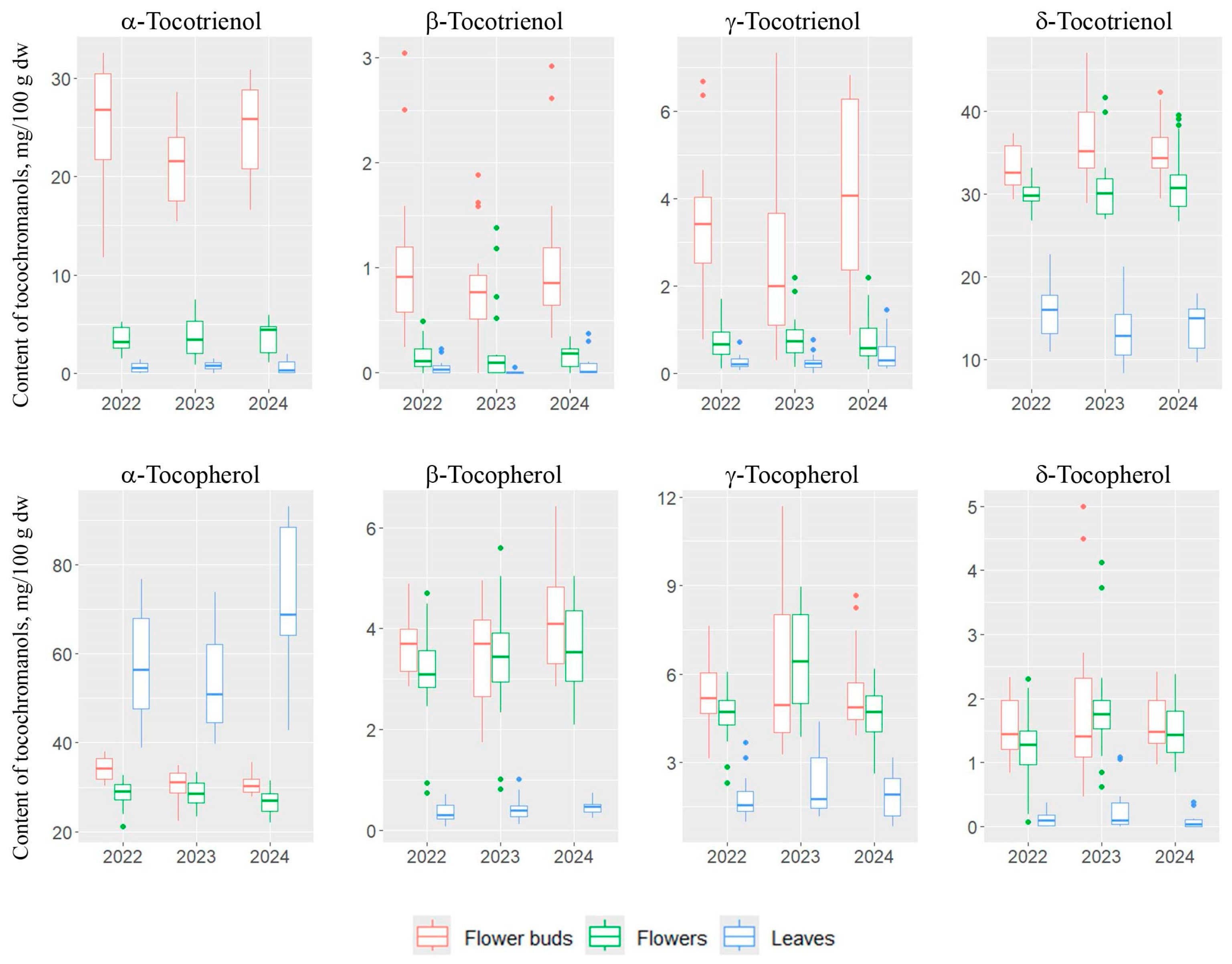
| Tocochromanol | Plant Part | ||
|---|---|---|---|
| Leaves | Flower Buds | Flowers | |
| α-T | 61.3 ± 16.0 c | 31.8 ± 3.4 b | 27.9 ± 3.0 a |
| β-T | 0.4 ± 0.2 a | 3.8 ± 1.0 c | 3.4 ± 1.0 b |
| γ-T | 2.0 ± 0.9 a | 5.6 ± 1.8 b | 5.2 ± 1.5 b |
| δ-T | 0.1 ± 0.2 a | 1.7 ± 0.8 b | 1.5 ± 0.7 b |
| α-T3 | 0.7 ± 0.5 a | 23.7 ± 5.3 c | 3.6 ± 1.6 b |
| β-T3 | 0.0 ± 0.1 a | 1.0 ± 0.7 b | 0.2 ± 0.3 a |
| γ-T3 | 0.3 ± 0.3 a | 3.4 ± 2.0 b | 0.8 ± 0.5 a |
| δ-T3 | 14.5 ± 3.6 a | 34.8 ± 4.0 c | 30.8 ± 3.5 b |
| Tocochromanol | Year | ||
|---|---|---|---|
| 2022 | 2023 | 2024 | |
| α-T | 39.9 ± 14.5 ab | 37.7 ± 13.6 a | 43.4 ± 23.2 b |
| β-T | 2.4 ± 1.6 a | 2.4 ± 1.7 ab | 2.8 ± 1.9 b |
| γ-T | 3.9 ± 1.9 a | 5.0 ± 2.7 b | 4.0 ± 1.9 a |
| δ-T | 1.0 ± 0.8 a | 1.3 ± 1.2 b | 1.1 ± 0.8 ab |
| Plant Part | Year | Tocochromanol | ||
|---|---|---|---|---|
| α-T | α-T3 | δ-T3 | ||
| Leaves | 2022 | 59.9 ± 12.7 b | 0.6 ± 0.5 a | 16.1 ± 3.7 a |
| 2023 | 54.1 ± 11.3 b | 0.8 ± 0.4 a | 13.3 ± 3.7 a | |
| 2024 | 72.7 ± 17.1 c | 0.6 ± 0.6 a | 14.0 ± 2.8 a | |
| Flower buds | 2022 | 34.2 ± 2.4 a | 25.4 ± 5.9 c | 33.3 ± 2.7 bcd |
| 2023 | 30.3 ± 3.7 a | 21.5 ± 4.2 b | 36.2 ± 5.2 d | |
| 2024 | 30.8 ± 2.5 a | 24.3 ± 5.0 bc | 35.0 ± 3.4 cd | |
| Flowers | 2022 | 28.6 ± 3.0 a | 3.4 ± 1.3 a | 30.0 ± 1.6 b |
| 2023 | 28.6 ± 2.3 a | 3.8 ± 2.0 a | 30.8 ± 4.1 b | |
| 2024 | 26.6 ± 2.8 a | 3.6 ± 1.6 a | 31.7 ± 4.2 bc | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górnaś, P.; Siger, A. Rare Prenyllipids in Wild St. John’s Wort During Three Harvest Seasons. Molecules 2025, 30, 901. https://doi.org/10.3390/molecules30040901
Górnaś P, Siger A. Rare Prenyllipids in Wild St. John’s Wort During Three Harvest Seasons. Molecules. 2025; 30(4):901. https://doi.org/10.3390/molecules30040901
Chicago/Turabian StyleGórnaś, Paweł, and Aleksander Siger. 2025. "Rare Prenyllipids in Wild St. John’s Wort During Three Harvest Seasons" Molecules 30, no. 4: 901. https://doi.org/10.3390/molecules30040901
APA StyleGórnaś, P., & Siger, A. (2025). Rare Prenyllipids in Wild St. John’s Wort During Three Harvest Seasons. Molecules, 30(4), 901. https://doi.org/10.3390/molecules30040901









